Abstract
Chronic ethanol feeding to mice and rats decreases serum adiponectin concentration and adiponectin treatment attenuates chronic ethanol-induced liver injury. While it is clear that lowered adiponectin has pathophysiological importance, the mechanisms by which chronic ethanol decreases adiponectin are not known. Here we have investigated the impact of chronic ethanol feeding on adiponectin expression and secretion by adipose tissue. Rats were fed a 36% Lieber-DeCarli ethanol-containing liquid diet or pair-fed control diet for 4 weeks. Chronic ethanol feeding decreased adiponectin mRNA, but had no effect on adiponectin protein, in subcutaneous adipose tissue. Chronic ethanol feeding also reduced adiponectin secretion by isolated subcutaneous and retroperitoneal adipocytes, despite the maintenance of equivalent intracellular concentrations of adiponectin between subcutaneous adipocytes from ethanol- and pair-fed rats. Treatment with brefeldin A suppressed adiponectin secretion by subcutaneous adipocytes from pair-fed rats, but had little effect after ethanol feeding. In subcutaneous adipocytes from pair-fed rats, adiponectin was enriched in endoplasmic reticulum (ER)/Golgi relative to plasma membrane; however, after chronic ethanol feeding, adiponectin was equally distributed between plasma membrane and ER/Golgi fractions. In conclusion, chronic ethanol feeding impaired adiponectin secretion by subcutaneous and retroperitoneal adipocytes; impaired secretion likely contributes to decreased adiponectin concentrations after chronic ethanol feeding.
Keywords: Adiponectin, secretion, chronic ethanol feeding, trafficking, adipocytes
Introduction
Adiponectin (also known as adipocyte complement-related protein 30 kDa, Acrp30) is an adipokine exclusively secreted by adipose tissue (28). Adiponectin is abundantly expressed by adipose tissues, composing approximately 0.01% of the total serum protein (3). Two forms of adiponectin, a low molecular weight (LMW) trimer and a high molecular weight (HMW) complex are primarily found in serum (24). Two isoforms of adiponectin receptors, AdipoR1 and AdipoR2, are predominantly expressed in skeletal muscle and liver, respectively (42). Adiponectin has important functions in the regulation of glucose and lipid metabolism in both liver and skeletal muscle; it improves insulin action in the liver by suppressing hepatic glucose production (4). Unlike other adipokines, such as leptin, expression of adiponectin is lower in obese humans (3). Serum adiponectin concentrations are also decreased in both humans and murine models of insulin resistance or type 2 diabetes (13; 14; 37). Targeted disruption of adiponectin expression in mice increases their sensitivity to diet-induced insulin resistance compared to wild-type mice (19). Adiponectin also has potent anti-inflammatory functions, decreasing the production of inflammatory cytokines such as TNF-α and IL-6 by macrophages and adipocytes (1; 39; 43).
Chronic alcohol consumption is characterized by an increase in the expression of a number of inflammatory mediators, including cytokines, reactive oxygen species and nitrogen species. This pro-inflammatory state is due to an increased exposure to gut-derived endotoxin/lipopolysaccharide (LPS), as well as enhanced sensitivity of macrophages to activation by LPS (21; 33). Increased production of pro-inflammatory mediators, in particular TNF-, is an important contributor to the progression of ethanol-induced liver injury (21; 33). Recent studies have shown that chronic ethanol consumption decreases serum adiponectin concentration in mice (41; 44) and rats (32). Importantly, treatment of mice with adiponectin during chronic ethanol exposure prevents the development of ethanol-induced liver injury (41) by increasing fatty acid oxidation in the liver, thus preventing ethanol-induced steatosis, as well as decreasing TNF-α expression in mice chronically-exposed to ethanol (41). Further, decreased serum adiponectin concentrations after chronic ethanol feeding are dependent on the type of fat in the diet (44). You, et al reported that ethanol-containing diets high in unsaturated fats contribute to ethanol-induced decreases in adiponectin, while inclusion of saturated fats in the ethanol feeding protocol prevent decreased adiponectin (44). Importantly, the maintenance of serum adiponectin concentrations during ethanol feeding in the context of a high saturated fat diet are associated with aprotective effect of saturated fats on the progression of ethanol-induced fatty liver injury (44).
While these data demonstrate that decreased serum adiponectin concentration contributes to chronic ethanol-induced liver injury, the mechanisms by which chronic ethanol decreases serum adiponectin concentrations have not been studied. Here we have investigated the effects of chronic ethanol consumption to rats on the production and secretion of adiponectin by adipocytes. We demonstrate that chronic ethanol feeding decreased adiponectin mRNA in subcutaneous adipose tissue. In addition, chronic ethanol feeding disrupted the intracellular distribution of adiponectin peptide and impaired adiponectin secretion by subcutaneous adipocytes.
Materials and Methods:
Materials.
Adult male Wistar rats weighing 170-180g were purchased from Harlan Sprague-Dawley, Inc (Indianapolis, IN). Lieber-DeCarli high-fat ethanol diet was purchased from Dyets (Bethlehem, PA). Cell culture reagents were purchased from GIBCO (Grand Island, NY). Lactate dehydrogenase (LDH) kit was from Promega (Madison, WI). PicoGreen dsDNA kit was from Invitrogen (Eugene, OR). Antibody against adiponectin was from Alpha Diagnostic (San Antonio, TX); anti-ERK1/2 antibody was from Upstate (Charlottesville, VA), anti-NaK-ATPase was from the Developmental Hybridoma Studies Bank (University of Iowa) and antibodies against calreticulin and syntaxin 6 were from BD Bioscience (San Jose, CA).
Chronic ethanol feeding.
The chronic ethanol feeding model used in this study has been previously described (27). Randomly-selected rats assigned to the ethanol-fed group were provided an ad libitum liquid diet containing ethanol as 36% of total caloric value for four weeks. Control rats were pair-fed a liquid diet with maltose dextrin isocalorically substituted for ethanol. The type of fat included in the Lieber-Decarli diet used in this study was 8.5 g/L corn oil, 28.4 g/L olive oil and 2.7 g/L safflower oil. All animal protocols were approved by the Case Western Reserve University Institutional Animal Care and Use Committee. After the feeding period, ethanol-fed and their specific pair-fed controls were anaesthetized and adipose depots were removed and used immediately to isolate adipocytes or flash frozen in liquid nitrogen and then stored at -80°C. Blood was collected and serum was isolated and stored at -80°C. Experiments on ethanol-fed rats and their specific pair-fed controls were carried out in parallel.
Adipose tissue homogenization.
Adipose tissue was homogenized (3ml homogenate buffer per g tissue) in a glass homogenizer with a buffer containing 1% Triton-X100, 50mM Tris-HCl, 6.4mM NaCl, 1mM EDTA, 1mM sodium pyrophosphate, 1mM activated sodium vanadate, 10 mM NaF and protease inhibitor cocktail (Complete-EDTA free™, Roche Molecular Biochemicals, Indianapolis IN). The homogenate was gently rotated for 15 minutes at 4°C and then centrifuged at 16,000g for 5 minute. The infranatant was used to measure adiponectin by ELISA and then normalized to dsDNA by Picogreen dsDNA kit according to manufacturer’s instructions.
Adipocyte isolation, incubation and lysate.
Adipocytes were isolated as previously described (29) except that cells were washed and incubated in DMEM/F12 with 25mM HEPES, 0.1% BSA (pH 7.4). After isolation, adipocytes were diluted to 0.5 × 106 cells/ml in wash buffer. 0.1 × 106 cells were aliquoted to 5ml polypropylene round bottom tubes and incubated at 37°C in a shaking water bath. At the end of the treatment periods, adipocyte suspensions were centrifuged briefly and medium was removed for adiponectin ELISA or stored at -20 °C. Adipocytes were washed once with ice-cold PBS and lysed with ice-cold RIPA buffer (50mM Tris-HCl, 1% NP-40, 0.25% Na-deoxycholate, 150mM NaCl, 1mM EDTA and protease inhibitor cocktail). Cell lysates were centrifuged at 7,000g for 5 minutes. Infranatant was collected, flash frozen in liquid nitrogen and then used for adiponectin ELISA or stored at -80 °C.
Real-time PCR for adiponectin and TNF-α mRNA expression.
Total RNA was isolated from fat pads by using the RNeasy lipid tissue mini kit (Qiagen, Valencia, CA) and DNA digestion was performed by using RNase-free DNase set (Qiagen, Valencia, CA) according to manufacturer′s instructions. One microgram of total RNA were reverse transcribed by using the RETROscript kit (Ambion, Austin, TX) with random primers according to manufacturer′s protocol. Real-time polymerase chain reactions (real-time PCR) were performed by using the SYBR Green Core reagents (Applied Biosystems, Warrington, UK) and three sets of primers specific for adiponectin (forward: 5′-GAC ACG CAG GTG TTC TTG-3′, reverse: 5′-CCT ACG CTG AAT GCT GAG-3′), TNF-α (forward: 5′-CAA GGA GGA GAA GTT CCC AA-3′, reverse: 5′-CTC TGC TTG GTG GTT TGC TA-3′) and α-actin (forward: 5′-CGG TCA GGT CAT CAC TAT CG-3′, reverse: 5′-TTC CAT ACC CAG GAA GGA AG-3′). Primers were intron-spanning to avoid interference with genomic DNA. Data were analyzed by delta Ct method (18).
Adiponectin ELISA.
Adiponectin concentrations in rat serum, incubation medium, adipocyte lysates and adipose tissue homogenates were measured by ELISA (B-Bridge, San Diego CA) according to manufacturer′s protocol. Two different lots of this ELISA kit were used, the first lot was used for studies in Figures 2A and 3C, while the second lot was used for all other experiments. The absolute values for adiponectin concentration varied slightly between these two different lots of the ELISA kit (data not shown).
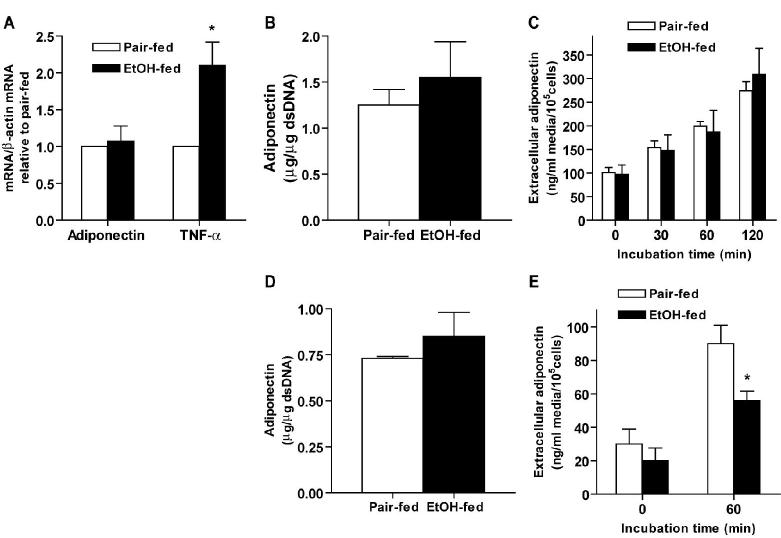
Figure 2.
Chronic ethanol feeding decreased adiponectin secretion by isolated subcutaneous adipocytes without affecting intracellular adiponectin content. (A) Extracellular (medium) and (B) intracellular adiponectin in isolated subcutaneous adipocytes was measured by ELISA. (C) Subcutaneous adipocytes were incubated at 37°C or on ice for 60 minutes. After incubation, extracellular adiponectin concentration was measured by ELISA. Quantity of adiponectin was normalized to cell number. Values represent means ± SEM, n=8 (A), n=6 (B) and n=4(C), *p<0.05, chronic ethanol-fed compared to pair-fed controls
Figure 3.
Chronic ethanol feeding had no effect on adiponectin protein content in epididymal or retroperitoneal adipose tissue but decreased adiponectin secretion by retroperitoneal adipocytes. Adiponectin and TNF-α mRNA level (A) in epididymal adipose tissue were measured by real-time PCR and then normalized to -actin mRNA level. mRNA levels in ethanol-fed rats were then expressed relative to the mRNA levels in their specific pair-fed controls. Adiponectin protein content in subcutaneous (B) and retroperitoneal (D) adipose tissue was measured by ELISA and then normalized to dsDNA. Extracellular adiponectin concentration after incubation of epididymal (C) or retroperitoneal (E) adipocytes was measured by ELISA and then normalized to cell number. Values represent means ± SEM, n=10 (A) and (B), n=8 (C), n=4 (D) and n=8 (E), *p<0.05, chronic ethanol-fed compared to pair-fed controls.
Western blotting.
Each sample with 1-4 microgram of total protein was boiled 5 minutes and separated on a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel before being transferred to a polyvinylidene fluoride (PVDF) membrane by using a semidry transfer technique. Blots were blocked with 3% BSA in Tris-buffered saline, pH 7.6, containing 0.1% Tween-20 before the overnight incubation at 4°C with primary antibody. Dilutions of primary antibodies used were: 1:1000 for adiponectin, 1μg/ml for NaK-ATPase, 1:2500 for calreticulin, 1:5000 for syntaxin 6 and 1:10,000 for ERK1/2. After 1-hour incubation with secondary antibody and subsequent washes, bound antibodies were visualized using enhanced chemiluminescence reagent.
Subcellular fractionation.
Subcellular fractions were prepared from isolated adipocytes as previously described with minor modifications (26; 31). After isolation, subcutaneous adipocytes were homogenized 10 strokes in a Potter-Elvehjem tissue grinder with TSE buffer containing 20mM Tris HCl/1mM EDTA/255 mM sucrose plus protease inhibitor cocktail (6 million cells/ml TSE). All procedures were performed at 4°C. In brief, homogenates were centrifuged at 16,000g for 15 min. The pellet (P1) was resuspended in 500μl TSE and layered over 750μl sucrose pad (20mM Tris/HCl/1mM EDTA/1.12M sucrose, pH 7.4) and centrifuged at 100,000g for 70 min. The resulting interface, enriched in plasma membrane (PM), was collected and pelleted at 16,000g for 15 min, and then resuspended in 50μl of RIPA buffer. The supernatant (S1) was centrifuged at 48,000g for 20 min and the pellet, containing high density microsomes (HDM) enriched in endoplasmic reticulum and Golgi, was resuspended in 40μl of RIPA. The supernatant (S2) was centrifuged at 200,000g for 70 min and the pellet, containing low density microsomes (LDM) enriched in Golgi, was resuspended in 40μl RIPA. The remaining supernatant, enriched in cytosol, was collected. Protein content was measured in all fractions and then used for Western blotting. Yield and enrichment for each subcellular fraction was monitored using specific marker proteins: Na-K ATPase for plasma membrane, calreticulin for endoplasmic reticulum, syntaxin 6 for Golgi and ERK1/2 for cytosol.
Data analysis.
Values are expressed as means ± SEM. Data were analyzed by Student′s t-test using InStat (GraphPad Software, Inc) or general linear models procedure followed by least square means analysis of differences between groups (SAS, Carey, IN). Data were log transformed, if needed, to obtain a normal distribution.
Results
Chronic ethanol consumption decreases serum adiponectin concentration in mice (41; 44) and rats (32). Consistent with previous studies, serum adiponectin concentration was reduced by 30% in rats after chronic ethanol feeding (Figure 1A). Since adiponectin is mainly secreted by adipose tissue, we hypothesized that decreased serum adiponectin concentration was due to the decreased adiponectin expression by adipose tissue after chronic ethanol feeding. To test this hypothesis, we first investigated the effect of chronic ethanol feeding on adiponectin mRNA and protein level in subcutaneous adipose tissue. Real-time PCR analysis showed that adiponectin mRNA was decreased by 40% in subcutaneous adipose tissue from ethanol-fed compared to pair-fed rats (Figure 1B), while TNF-α mRNA was increased two- to three-fold by chronic ethanol feeding. In contrast to the decrease in adiponectin mRNA, adiponectin protein content in subcutaneous adipose tissue did not differ between pair- and ethanol-fed rats (Figure 1C).
Figure 1.
Chronic ethanol feeding to rats decreased serum adiponectin concentration and adiponectin mRNA in subcutaneous adipose tissue. Rats were allowed free access to an ethanol-containing diet or pair-fed control diet for 4 weeks. Serum adiponectin concentrations were measured by ELISA (A). Adiponectin and TNF-α mRNA level in subcutaneous adipose tissue were measured by real-time PCR (B) and then normalized to -actin mRNA level. mRNA levels in ethanol-fed rats were then expressed relative to the mRNA levels in their specific pair-fed controls. Adiponectin protein content (C) in subcutaneous adipose tissue was measured by ELISA and normalized to dsDNA. Values represent means ± SEM, n=7 (A), n=10 (B), n=8 (C). *p<0.05, chronic ethanol-fed compared to pair-fed controls.
Since chronic ethanol feeding had no effect on total adiponectin protein content in subcutaneous adipose tissue, despite a suppression in adiponectin mRNA, we hypothesized that chronic ethanol might decrease serum adiponectin level by impairing the secretion of adiponectin from adipocytes. In order to test this hypothesis, we utilized an ex vivo model of isolated adipocytes. Isolated adipocytes provide a homogeneous population of cells that retain many physiological activities of adipocytes in vivo (12; 38) where secretion of adiponectin can be monitored independent of potential changes in clearance from the circulation. While adiponectin secretion increased over time in adipocytes from both pair-fed and ethanol-fed rats, chronic ethanol feeding decreased adiponectin secretion by 40% from subcutaneous adipocytes compared to pair-fed controls (Figure 2A). However, intracellular adiponectin content was not changed by either time of incubation or chronic ethanol feeding (Figure 2B). More than 60% of the total adiponectin content (the sum of intracellular adiponectin and adiponectin released into the medium) remained in the intracellular compartment, suggesting that impaired adiponectin secretion by chronic ethanol feeding was not due to reduced intracellular adiponectin peptide in adipocytes.
Several control experiments were conducted to insure that the integrity of the isolated adipocytes was maintained over the two-hour incubation and did not differ between adipocytes isolated from pair-fed and ethanol-fed rats. If the release of adiponectin is a regulated process, then incubation of adipocytes on ice should decrease the rate of release. When subcutaneous adipocytes were incubated on ice for 60 min, no increase in extracellular adiponectin over baseline concentrations was observed in subcutaneous adipocytes isolated from either pair-fed or ethanol-fed rats (Figure 2C). Furthermore, while some LDH, an intracellular/cytosolic enzyme, was detected in the extracellular media from adipocyte incubations, there were no differences in either LDH concentration after incubation for 60 min compared to baseline or between adipocytes from pair- and ethanol-fed rats (0.31± 0.1 in pair-fed rats, 0.27± 0.09 units of activity/105 cells in ethanol-fed rats, n=6) after 60 min incubation.
We also measured the effect of chronic ethanol feeding on adiponectin expression and secretion by epididymal and retroperitoneal adipose tissue, as well as adipocytes isolated from epididymal or retroperitoneal adipose tissue. Chronic ethanol feeding had no effect on adiponectin mRNA or protein content in epididymal adipose tissue (Figure 3A, 3B). Epididymal adipose tissue was not completely insensitive to the chronic effects of ethanol since TNF-α mRNA was increased by chronic ethanol feeding in epididymal adipose tissue (Figure 3A). Adiponectin secreted by epididymal adipocytes from pair-fed rats increased over time, however, chronic ethanol feeding did not affect adiponectin secretion by epididymal adipocytes (Figure 3C). Chronic ethanol feeding had no effect on the total intracellular adiponectin content in retroperitoneal adipose tissue (Figure 3D), but decreased secretion of adiponectin over 60 min in isolated retroperitoneal adipocytes compared to adipocytes from pair-fed controls (Figure 3E). Taken together, these data suggest that the inhibition of adiponectin secretion by chronic ethanol feeding was adipose tissue depot specific.
Brefeldin A (BFA), a drug which disrupts protein trafficking through the Golgi apparatus, severely inhibits adiponectin secretion by 3T3-L1 adipocytes (40). We hypothesized that chronic ethanol feeding impaired adiponectin secretion by affecting its trafficking in subcutaneous adipocytes. Treatment with BFA strongly inhibited adiponectin secretion by subcutaneous adipocytes isolated from pair-fed rats (Figure 4), but had no effect on adipocytes from ethanol-fed rats. In contrast, treatment with cycloheximide (CHX), a drug which inhibits protein synthesis, had no effect on adiponectin secretion by subcutaneous adipocytes from either pair- or ethanol-fed rats, suggesting that secreted adiponectin was mainly derived from an already synthesized pool of adiponectin. Taken together, these data suggest that impaired adiponectin secretion after chronic ethanol feeding might be due, at least in part, to impaired adiponectin trafficking in subcutaneous adipocytes.
Figure 4.
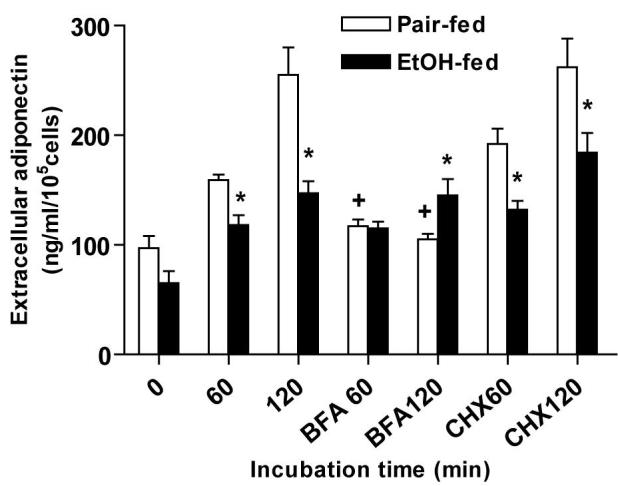
Effect of chronic ethanol feeding, brefeldin A (BFA) and cyclohexamide (CHX) on adiponectin secretion by subcutaneous adipocytes. Subcutaneous adipocytes were incubated with or without BFA or CHX at 37°C for 60 minutes. After incubation, extracellular adiponectin concentration was measured by ELISA. Quantity of adiponectin was normalized to cell number. Values represent means ± SEM, *p<0.05, chronic ethanol-fed compared to pair-fed controls; +p<0.05, compared to cells not treated with BFA.
The lack of sensitivity of adiponectin secretion to BFA after chronic ethanol feeding suggests that chronic ethanol disrupts the trafficking of adiponectin through the Golgi itself and/or upstream of the Golgi in the endoplasmic reticulum. If chronic ethanol impaired trafficking of adiponectin through the endoplasmic reticulum and Golgi, we would expect to observe an abnormal intracellular distribution of adiponectin after chronic ethanol feeding. In order to test this hypothesis, adipocytes were fractionated by standard procedures into four compartments: plasma membrane-enriched, high density microsomes (HDM; enriched in endoplasmic reticulum and Golgi), light density microsomes (LDM; enriched in Golgi) and cytosol. In adipocytes from pair-fed rats, adiponectin was mainly detected in the plasma membrane and HDM fractions, but barely detectable in LDM or cytosol fractions. Adiponectin was most abundant in the HDM fractions, exhibiting a four-fold enrichment over the plasma membrane in adipocytes from pair-fed rats. This enrichment in ER/Golgi is consistent with previous reports in 3T3-L1 adipocytes (40) and adipose tissue explants from mice (8). Chronic ethanol feeding had no effect on the distribution of either total proteins (data not shown) or marker proteins (Figure 5A) to each subcellular fraction. However, after chronic ethanol feeding, the amount of adiponectin in HDM fraction was decreased, resulting in a loss of enrichment of adiponectin in the HDM fraction (Figure 5B). This decrease of adiponectin in the endoplasmic reticulum/Golgi compartment was consistent with the inability of BFA to suppress adiponectin secretion after chronic ethanol feeding.
Figure 5.
Subcellular distribution of adiponectin in subcutaneous adipocytes. Subcutaneous adipocytes were homogenized and separated by differential centrifugation into four subcellular fractions: plasma membrane (PM), high density microsomes (HDM), low density microsomes (LDM) and cytosol. (A) Adiponectin protein content was detected by western blotting. The marker proteins for plasma membrane, ER, Golgi and cytosol were Na-K ATPase, calreticulin, syntaxin 6 and ERK1/2, respectively. The images are representative of 5-7 different subcellular fractionations per experimental group. P: pair-fed, E: ethanol-fed. (B) Adiponectin content in HDM and PM fractions from ethanol-fed rats was normalized to the quantity of adiponectin in HDM from adipocytes from their specific pair-fed rats. Values represent means ± SEM, n=5-7 rats per group, *p<0.05, chronic ethanol-fed compared to pair-fed controls. +p<0.05, HDM compared to PM within an experimental group.
Discussion:
Chronic ethanol consumption decreases serum adiponectin concentration in mice (41; 44) and rats (32)(Figure 1). This decrease in adiponectin contributes to the development of ethanol-induced liver injury, as delivery of exogenous adiponectin via osmotic pump (41) protects mice from ethanol-induced liver injury. Thus, while it is clear that decreased adiponectin concentrations are important to the pathophysiology of chronic ethanol-induced liver injury, the mechanisms by which chronic ethanol feeding decreases serum adiponectin concentration have not been investigated. Since adipose tissue is the major source of adiponectin in the circulation, we hypothesized that chronic ethanol feeding decreased adiponectin production by adipose tissue. In this study, we identify two mechanisms by which chronic ethanol decreases adiponectin production: first, chronic ethanol decreases adiponectin mRNA in subcutaneous adipose tissue and second, chronic ethanol decreased the secretion of adiponectin from subcutaneous and retroperitoneal adipocytes. Decreased secretion from subcutaneous adipocytes was associated with a disruption in the intracellular trafficking of adiponectin after chronic ethanol feeding.
Chronic ethanol feeding decreased adiponectin mRNA in subcutaneous adipose tissue without affecting the intracellular pool of adiponectin peptide. A number of DNA binding sites have been identified in the adiponectin promoter region, including sites for peroxisome proliferator-activated receptor-γ (PPAR-γ), CCAAT/enhancer binding protein-β (C/EBP-β), nuclear factor-Y (NF-Y) and sterol regulatory element-binding protein 1c (SREBP1c) (15; 25; 30). Mutation analysis of the SREBP1c, C/EBP-β and PPAR-γ sites showed that these elements are required for basal promoter activity of adiponectin (15; 25; 30). Therefore, it is likely that decreased adiponectin mRNA in subcutaneous adipose tissue in response to chronic ethanol feeding is regulated by one or more of these elements. Data from HeLa cells transfected with the adiponectin promoter suggest that ethanol may interact with the specific fatty acids in the media to down-regulate adiponectin promoter activity (44). Also of particular relevance may be the interaction between the inflammatory cytokine TNF-α and adiponectin, which suppress each other′s expression both in vivo and in vitro (19; 41). Suppression of adiponectin expression by TNF-α has been associated with inactivation of C/EBP-β (17), as well as regulation of PPAR-γ DNA binding activity (30). After chronic ethanol exposure, increased circulating TNF-α is observed both in rodent models and humans (32; 33). Thus, it is likely that increased TNF-α in the circulation creates an “inflammatory environment” for adipocytes, contributing to the down-regulation of adiponectin expression.
Adiponectin gene expression is also inhibited by -adrenergic stimulation via protein kinase A (PKA) both in vivo and in vitro (8; 11). However, chronic ethanol feeding decreases -adrenergic receptor-mediated activation of PKA in isolated adipocytes (16). Therefore, it is unlikely that the suppression of adiponectin mRNA in response to chronic ethanol feeding involves inhibition by PKA.
The second major mechanism for decreased adiponectin production by adipocytes identified here is a decrease in the secretion of adiponectin from adipocytes isolated from subcutaneous, as well as retroperitoneal, adipose tissue. Secretion was suppressed despite the maintenance of a large intracellular pool of adiponectin peptide. This intracellular pool of adiponectin was not reduced either by chronic ethanol feeding or incubation for 2 hours ex vivo. Furthermore, inhibition of adiponectin synthesis by treatment with CHX did not reduce adiponectin secretion, indicatingthat the pre-synthesized pool of adiponectin was sufficient to support secretion for at least 2 hours. This observation is consistent with a previous report demonstrating that treatment with CHX does not affect adiponectin secretion by 3T3-L1 adipocytes for 6 hours (40). Based on these results, it appears unlikely that impaired adiponectin secretion after chronic ethanol feeding is due to a decrease in the total intracellular pool of adiponectin. Since the intracellular concentration of adiponectin is determined by the rate of synthesis of adiponectin peptide and by its rate of secretion from the adipocyte, we hypothesize that the intracellular pool of adiponectin was maintained after chronic ethanol feeding due to the combined impact of decreased quantity of adiponectin mRNA in response to chronic ethanol feeding and the ethanol-induced decrease in adiponectin secretion.
While the total intracellular content of adiponectin was not decreased, the intracellular distribution of adiponectin was disrupted by chronic ethanol feeding. The mechanisms by which adiponectin is trafficked through the adipocyte and secreted are not well understood. Several lines of evidence suggest that adiponectin localized in the Golgi is an important source of intracellular adiponectin available for secretion. Adiponectin is predominantly co-localized with Golgi marker proteins in 3T3-L1 adipocytes (5) and is found in HDM fractions, which are enriched in Golgi and endoplasmic reticulum, in mouse adipose explants (8) and isolated rat subcutaneous adipocytes (Figure 5). When adipocytes are treated with BFA, adiponectin is shifted from the Golgi compartment and adiponectin secretion is inhibited in 3T3-L1 adipocytes (40), as well as subcutaneous adipocytes from pair-fed rats (Figure 4). Adiponectin secretion is also dependent on GGA proteins (for Golgi localizing adaptin ear homology domain ARF binding protein) (40) and Vti1a, a v-Snare protein found in the trans-Golgi network (6) in 3T-L1 adipocytes.
In contrast to the predominant localization of adiponectin to Golgi/Golgi-enriched compartments in control adipocytes, adiponectin was shifted from the HDM fraction to a plasma membrane-enriched fraction after chronic ethanol feeding (Figure 5). Further, adiponectin secretion was no longer sensitive to inhibition by BFA after chronic ethanol feeding (Figure 4). These data suggest that chronic ethanol feeding acts to disrupt the normal trafficking of adiponectin through the Golgi apparatus. Ethanol exposure disrupts protein trafficking in a number of cell types, targeting multiple steps in protein trafficking, including receptor-mediated endocytosis and the flow of newly synthesized proteins to the plasma membrane (22; 35). In adipocytes, chronic ethanol consumption disrupts insulin-stimulated GLUT4 trafficking in rat adipocytes (26), as well as localization of membrane proteins to lipid rafts (29). In other cell types, trafficking of both endogenous and viral marker proteins through the Golgi apparatus is disrupted by chronic ethanol exposure (20; 34). This impairment may be related to the effect of chronic ethanol on required post-translational modifications, including glycosylation, that occur within the endoplasmic reticulum and Golgi apparatus (7) and/or changes in the activity of small GTP binding proteins required for Golgi function (20; 34) since post-transcriptional glycosylation, as well as formation of disulfide bonds within the adiponectin trimer, are required for the functional activity of adiponectin (24; 36). It is possible that impairment of either appropriate glycosylation and/or trimer formation contributes to the abnormal intracellular trafficking of adiponectin, as well as the reduced rates of secretion observed after chronic ethanol feeding.
Chronic ethanol feeding differentially regulated adiponectin mRNA expression in subcutaneous compared to epididymal adipose tissue. In contrast, TNF-α mRNA was increased by chronic ethanol in both adipose depots. Individual adipose depots exhibit varied rates of lipolysis and respond differentially to nutrient or insulin action; these depot-specific responses are thought to relate to the specific metabolic demands required by the individual organs in the body associated with each depot (9; 10; 23). Very little data is available regarding the depot specific expression of adiponectin; although one study suggests that a shift in the depot-specific expression of adiponectin may be a contributing factor for the development of insulin resistance in Zucker fatty rats (2). In that study, Zucker fatty rats had lower adiponectin mRNA in both subcutaneous and visceral adipose than lean controls. Further, the relative expression of adiponectin is shifted from higher relative expression in visceral fat in lean rats, to a higher expression of adiponectin in visceral fat in the fatty rats (2). In contrast to the suppression of adiponectin mRNA in several adipose depots in obesity (2), the effects of chronic ethanol on adiponectin mRNA were not global, in that adiponectin mRNA was decreased specifically in subcutaneous adipose tissue.
Chronic ethanol feeding specifically suppressed adiponectin secretion by adipocytes isolated from retroperitoneal adipose, considered a visceral fat depot, as well as subcutaneous adipocytes, but not epididymal adipocytes. Since chronic ethanol feeding decreased adiponectin secretion from the two major types of adipose tissue, subcutaneous and visceral, it is likely that these changes contribute to decreased serum adiponectin concentration after chronic ethanol feeding. It is also possible that chronic ethanol feeding increases the clearance of adiponectin by the kidneys or liver, which could also contribute to the decrease in circulating adiponectin observed after chronic ethanol feeding.
In summary, here we have demonstrated that chronic ethanol feeding to rats decreases adiponectin mRNA in subcutaneous adipocytes, as well as disrupts the typical intracellular distribution of adiponectin from an endoplasmic reticulum/Golgi-enriched compartment to the plasma membrane. This impaired intracellular trafficking was associated with a suppression in the rate of adiponectin secretion by subcutaneous adipocytes. Because decreased adiponectin concentration contributes to the progression of chronic-ethanol induced liver injury (41), it is likely that the suppression of adiponectin secretion reported here contributes to the pathophysiological effects of chronic ethanol feeding.
Acknowledgment
We thank Dr. Michele T. Pritchard for her help with the real-time PCR setup and primer design. We are also grateful to Megan R. McMullen and Brian T. Pratt for their technical support. The monoclonal antibody against 1 subunit of Na-K ATPase (6F), developed by D. M. Fambrough, was obtained from the Developmental Studies Hybridoma Bank, maintained by the University of Iowa, Department of Biological Sciences, under Contract NO1-HD-7-3263.
Footnotes
Grants
This work was supported in part by NIH grant RO1-AA 11876.
Reference List
- 1.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1220–5. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 2.Altomonte J, Harbaran S, Richter A, Dong H. Fat depot-specific expression of adiponectin is impaired in Zucker fatty rats. Metabolism. 2003;52:958–63. doi: 10.1016/s0026-0495(03)00092-1. [DOI] [PubMed] [Google Scholar]
- 3.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 4.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 5.Bogan JS, Lodish HF. Two compartments for insulin-stimulated exocytosis in 3T3- L1 adipocytes defined by endogenous ACRP30 and GLUT4. J.Cell Biol. 1999;146:609–620. doi: 10.1083/jcb.146.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bose A, Guilherme A, Huang S, Hubbard AC, Lane CR, Soriano NA, Czech MP. The v-SNARE Vti1a regulates insulin-stimulated glucose transport and Acrp30 secretion in 3T3-L1 adipocytes. J Biol Chem. 2005;280:36946–51. doi: 10.1074/jbc.M508317200. [DOI] [PubMed] [Google Scholar]
- 7.Cottalasso D, Domenicotti C, Traverso N, Pronzato M, Nanni G. Influence of chronic ethanol consumption on toxic effects of 1,2-dichloroethane: glycolipoprotein retention and impairment of dolichol concentration in rat liver microsomes and Golgi apparatus. Toxicology. 2002;178:229–240. doi: 10.1016/s0300-483x(02)00235-4. [DOI] [PubMed] [Google Scholar]
- 8.Delporte ML, Funahashi T, Takahashi M, Matsuzawa Y, Brichard SM. Pre- and post-translational negative effect of beta-adrenoceptor agonists on adiponectin secretion: in vitro and in vivo studies. Biochem J. 2002;367:677–85. doi: 10.1042/BJ20020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Einstein FH, Atzmon G, Yang XM, Ma XH, Rincon M, Rudin E, Muzumdar R, Barzilai N. Differential responses of visceral and subcutaneous fat depots to nutrients. Diabetes. 2005;54:672–8. doi: 10.2337/diabetes.54.3.672. [DOI] [PubMed] [Google Scholar]
- 10.Engfeldt P, Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Horm Metab Res Suppl. 1988;19:26–9. [PubMed] [Google Scholar]
- 11.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Adiponectin gene expression is inhibited by beta-adrenergic stimulation via protein kinase A in 3T3-L1 adipocytes. FEBS Lett. 2001;507:142–6. doi: 10.1016/s0014-5793(01)02960-x. [DOI] [PubMed] [Google Scholar]
- 12.Honnor RC, Dhillon GS, Londos C. cAMP-dependent protein kinase and lipolysis in rat adipocytes. I. Cell preparation, manipulation, and predictability in behavior. J Biol Chem. 1985;260:15122–9. [PubMed] [Google Scholar]
- 13.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 14.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 15.Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–63. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 16.Kang L, Nagy LE. Chronic ethanol feeding suppresses {beta}-adrenergic receptor-stimulated lipolysis in adipocytes isolated from epididymal fat. Endocrinology. 2006 doi: 10.1210/en.2006-0120. in press (PMID: 16794014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kita A, Yamasaki H, Kuwahara H, Moriuchi A, Fukushima K, Kobayashi M, Fukushima T, Takahashi R, Abiru N, Uotani S, Kawasaki E, Eguchi K. Identification of the promoter region required for human adiponectin gene transcription: Association with CCAAT/enhancer binding protein-beta and tumor necrosis factor-alpha. Biochem Biophys Res Commun. 2005;331:484–90. doi: 10.1016/j.bbrc.2005.03.205. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 20.Marmillot P, Rao MN, Lakshman MR. Chronic ethanol exposure in rats affects rabs-dependent hepatic trafficking of apolipoprotein E and transferrin. Alcohol. 2001;25:195–200. doi: 10.1016/s0741-8329(01)00179-3. [DOI] [PubMed] [Google Scholar]
- 21.Nagy LE. New insights into the role of the innate immune response in the development of alcoholic liver disease. Expt Biol Med. 2003;228:882–890. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- 22.Nagy LE, Lakshman MR, Casey CA, Bearer CF. Ethanol and membrane protein trafficking: diverse mechanisms of ethanol action. Alcohol Clin Exp Res. 2002;26:287–93. [PubMed] [Google Scholar]
- 23.Ostman J, Arner P, Engfeldt P, Kager L. Regional differences in the control of lipolysis in human adipose tissue. Metabolism. 1979;28:1198–205. doi: 10.1016/0026-0495(79)90131-8. [DOI] [PubMed] [Google Scholar]
- 24.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–85. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 25.Park SK, Oh SY, Lee MY, Yoon S, Kim KS, Kim JW. CCAAT/enhancer binding protein and nuclear factor-Y regulate adiponectin gene expression in adipose tissue. Diabetes. 2004;53:2757–66. doi: 10.2337/diabetes.53.11.2757. [DOI] [PubMed] [Google Scholar]
- 26.Poirier LA, Rachdaoui N, Nagy LE. GLUT4 vesicle trafficking in rat adipocytes after ethanol feeding: regulation by heterotrimeric G-proteins. Biochem J. 2001;354:323–330. doi: 10.1042/0264-6021:3540323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rachdaoui N, Sebastian BM, Nagy LE. Chronic ethanol feeding impairs endothelin-1-stimulated glucose uptake via decreased Gα11 expression in rat adipocytes. Am J Physiol. 2003;285:E303–310. doi: 10.1152/ajpendo.00547.2002. [DOI] [PubMed] [Google Scholar]
- 28.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 29.Sebastian BM, Nagy LE. Decreased insulin-dependent glucose transport by chronic ethanol feeding is associated with dysregulation of the Cbl/TC10 pathway in rat adipocytes. Am J Physiol. 2005;289:E1077–84. doi: 10.1152/ajpendo.00296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo JB, Moon HM, Noh MJ, Lee YS, Jeong HW, Yoo EJ, Kim WS, Park J, Youn BS, Kim JW, Park SD, Kim JB. Adipocyte determination- and differentiation-dependent factor 1/sterol regulatory element-binding protein 1c regulates mouse adiponectin expression. J Biol Chem. 2004;279:22108–17. doi: 10.1074/jbc.M400238200. [DOI] [PubMed] [Google Scholar]
- 31.Simpson EA, Yver DR, Hissin PJ, Wardzala LJ, Darnieli E, Salans LB, Cushman SW. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cell: Characterization of subcellular fractions. Biochim. Biophys. Acta. 1983;763:393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- 32.Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-alpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G998–1007. doi: 10.1152/ajpgi.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thurman RG. Mechanisms of Hepatic Toxicity II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am. J. Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 34.Tomas M, Marin P, Megias L, Egea G, Renau-Piqueras J. Ethanol perturbs the secretory pathway in astrocytes. Neurobiol Dis. 2005;20:773–84. doi: 10.1016/j.nbd.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Tuma DJ, Casey CA, Sorrell MF. Effects of ethanol on hepatic protein trafficking: impairment of receptor-mediated endocytosis. Alcohol Alcohol. 1990;25:117–25. doi: 10.1093/oxfordjournals.alcalc.a044986. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Xu A, Knight C, Xu LY, Cooper GJ. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem. 2002;277:19521–9. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- 37.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 38.Wilkes JJ, DeForrest LL, Nagy LE. Chronic ethanol feeding in a high-fat diet decreases insulin-stimulated glucose transport in rat adipocytes. Am. J. Physiol. 1996;271:E477–484. doi: 10.1152/ajpendo.1996.271.3.E477. [DOI] [PubMed] [Google Scholar]
- 39.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–9. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 40.Xie L, Boyle D, Sanford D, Scherer PE, Pessin JE, Mora S. Intracellular trafficking and secretion of adiponectin is dependent on GGA-coated vesicles. J Biol Chem. 2006;281:7253–9. doi: 10.1074/jbc.M511313200. [DOI] [PubMed] [Google Scholar]
- 41.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 43.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–32. [PubMed] [Google Scholar]
- 44.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–77. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]