Abstract
DNA rearrangements, including insertions, deletions, and inversions, control gene expression in numerous prokaryotic and eukaryotic systems, ranging from phase variation of surface antigens in pathogenic bacteria to generation of Ig diversity in human B cells. We report here that precise excision of the mobile element IS492 from one site on the Pseudoalteromonas atlantica chromosome directly correlates with phase variation of peripheral extracellular polysaccharide (pEPS) production from OFF (epsG::IS492) to ON (epsG+). In a previously undescribed application of quantitative PCR, we determined that the frequency of this transposase-dependent precise excision is remarkably high, ranging from 10−3 to 10−2 per cell per generation. High-frequency excision resulting in nonmutagenic repair of donor DNA is extremely unusual for classical transposable elements. Interestingly, high-frequency precise excision of IS492 does not occur at four different insertion sites on the P. atlantica chromosome, despite identity in the IS492 nucleotide sequences and 5- to 7-bp flanking DNA. The genome sequence revealed that epsG-associated IS492 is the only element inserted within a gene. Quantitative RT-PCR assays for externally derived transposase transcripts from each IS492 copy showed that IS492 at epsG has higher levels of host-initiated transcription through the element, suggesting that transcription per se or an increase in transposase (mooV) expression is responsible for the effect of chromosomal position on element excision. MooV levels and excision activity for IS492 inserted in forward and reverse orientations relative to plac and pT7 in Escherichia coli support that external transcription of mooV boosts transposase to a critical level required for detectable excision.
Keywords: DEDD-motif recombinases, IS110 family, phase-variation frequency, transposition, quantitative PCR
Transposons are genetic elements that can move to multiple sites in a host genome and generate DNA rearrangements that contribute to genome diversity. These mobile elements use a variety of mechanisms for movement, including rolling circle transposition and nonreplicative transposition mediated by tyrosine (Y)- and serine (S)-site-specific recombinases (1–4). However, the majority of the characterized transposons move by a common mechanism involving hydrolysis of phosphodiester bonds at element–host junctions and DNA strand transfer (one-step transesterification) catalyzed by a classical DDE transposase (Asp, Asp, Glu catalytic motif). Although there are various intermediates in the transposition reactions of the classical transposons, the chemistries of the DNA cleavage and strand-transfer reactions are conserved (reviewed in ref. 5). Because the transposon is excised from donor DNA by hydrolysis reactions, precise excision in which the host DNA is restored to the original target sequence is rarely observed. In eukaryotes, nonhomologous end-joining host functions can restore the donor sequence (6). In prokaryotes, composite transposons can be excised precisely by a DNA replication-dependent process (RecA- and transposase-independent) that utilizes the short direct repeats that flank the element and the long terminal inverted repeats of the transposon (7). However, processes that can result in precise excision of mobile elements are often mutagenic, generating deletions and small insertions.
Insertion element IS492 controls peripheral extracellular polysaccharide (pEPS) expression in the marine bacterium Pseudoalteromonas atlantica by insertion and precise excision at a single site within a predicted glucosyl-transferase gene (epsG) (Fig. 1; see also refs. 8 and 9). IS492 is a member of the unusual IS110 family of insertion elements that lack terminal inverted repeats and encode novel recombinases that mediate transposition by an undetermined mechanism (10). The transposase of IS492, MooV, is one of the defining members of the DEDD DNA recombinase family, which includes the site-specific invertase Piv from Moraxella lacunata and Moraxella bovis, as well as the transposases of the IS110 family (11). MooV is required for precise excision of IS492 (9). Although MooV and Piv appear to mediate conservative site-specific excision and inversion, respectively, these recombinases show no identity or similarity to Y- or S-site-specific recombinases. Instead, the recombinases of the Piv-MooV family have a conserved DEDD motif, shown to be essential for Piv-mediated DNA inversion, and are predicted to have a tertiary structural motif common to the DDE transposases and the DEDD-motif Holliday junction resolvases (RuvC-related; see refs. 12 and 13).
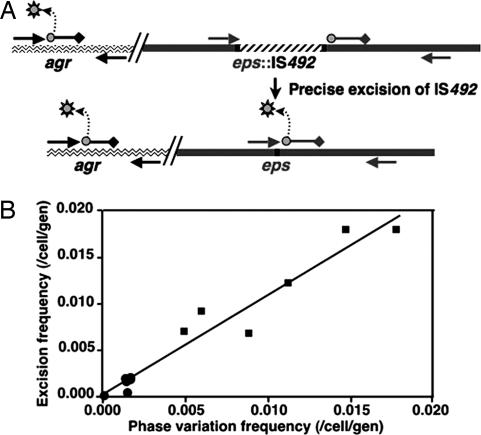
Fig. 1.
pEPS phase variation in P. atlantica. Colony morphology switching between mucoid (pEPS+) and crenated (pEPS−) colonies is controlled by IS492 (white and cross-hatched bar) reversible insertion into a single site in epsG. Insertion and the precise excision of IS492 are mediated by the transposase, encoded by mooV (cross-hatched bar). The inserted element is flanked by a 5-bp direct repeat of the target sequence (black bar); precise excision of the element restores eps (9).
Many of the unusual features of IS492 and its transposition products suggest a novel transposition mechanism, and here we focus on the precise excision of IS492. We directly assayed precise excision from a single site within epsG and determine that the frequency of precise excision approaches 2 × 10−2 per cell per generation on solid medium, correlating well with the frequency of colony-phase variation measured for the same cell populations. This level of precise excision from the chromosome is unprecedented for classical transposable elements (14, 15). We further show that the high frequency of IS492 precise excision is unique to the eps-associated copy of IS492 and positively correlates with the level of transcription that initiates upstream of the element and passes through the mooV gene. Both the stimulation of excision by transcription through IS492 and the frequent repair of donor sequence in excision are unexpected features for a classical IS and strongly suggest that IS492 utilizes a novel mechanism for transposition.
Results
Precise Excision of IS492 from epsG in P. atlantica Occurs at an Unusually High Frequency.
It has been reported that P. atlantica DB27 colony switching from crenated (pEPS−) to mucoid (pEPS+) is variable depending on growth conditions, including colony density on marine agar (MA; ref. 8). Therefore, in our determination of the frequencies of precise excision of IS492 from epsG (PEX) and pEPS− to pEPS+ phase variation (PPV) in P. atlantica, we used equal numbers of cells from colonies plated at low-colony density (LCD) and high-colony density (HCD) on MA.
We developed a multiplex quantitative PCR (qPCR) assay to directly measure both the total number of chromosomes and the number in which IS492 has precisely excised from the epsG site (Fig. 2A). Two different dual-labeled fluorogenic probes [Taqman technology (16)] were used to measure amplification of the stable β-agarase gene (agrA; ref. 17), which signifies each chromosome, and the restored epsG gene. Release of the different 5′-fluorophore (FAM-490 or HEX-530) from each probe resulting from 5′-3′ exonuclease activity of Taq polymerase as it extends from the forward primer was monitored by increased fluorescence. The presence of IS492 within epsG separates the forward primer and eps probe by 1.2 kb (Fig. 2A), and the extension conditions of the qPCR do not allow Taq polymerase to reach the probe (see Materials and Methods). Precise excision of IS492 moves the primer to within 3 bp of the 5′ end of the probe, which allows Taq polymerase to reach the probe and release FAM-490. The agr probe is positioned 2 bp from the forward primer, so that the HEX-530 fluorophore is released with every extension reaction. Representative data from one set of reactions, including the epsG and agrA standards, and the corresponding standard DNA plot for determining the quantities of agrA and restored epsG in the DNA aliquots are shown in supporting information (SI) Fig. 6. These template quantities are used in calculating the PEX for each sample (see Materials and Methods). Because the frequency of IS492 insertion is 3–4 orders of magnitude lower than excision (B.P.H., unpublished data), the back rate does not affect the calculation of precise excision frequency.
Fig. 2.
Correlation of IS492 precise excision from epsG, directly measured by qPCR, with pEPS phase variation in P. atlantica. (A) Two Taqman probes with different fluorophores (circles) and black hole quenchers (diamonds) are designed to separately measure amplification by the forward and reverse primers (arrows) of agrA (wavy line; base pairs 2849088–2850606) and restored epsG (dark gray line; base pairs 1303974–1304985). Release of the fluorophore (gray sun) from the probe by the 5′-3′ exonuclease activity of Taq polymerase fluorophore is measured (Bio-Rad iCycler Real Time PCR System). (B) The frequency of IS492 PEX mean values are plotted vs. the frequency of PPV mean values; the data are from six independent experiments.
The PPV and PEX values, determined for cells grown at HCD and LCD, fit a simple linear regression model with a r2 value of 0.945 and slope of 1.08 (Fig. 2B), indicating there is one precise excision event for each colony-morphology phase-variation event. Multiple regression analyses show that PEX and PPV are strongly correlated with each other, and this relationship is independent of colony density (SI Text). The average PEX and PPV at LCD are 1.2 ± 0.5 × 10−2 and 1.0 ± 0.5 × 10−2 per cell per generation, respectively, and at HCD, they are 1.2 ± 0.8 × 10−3 and 1.3 ± 0.6 × 10−3 per cell per generation, respectively.
IS492 Copies at Locations Other Than the eps Site on the P. atlantica Chromosome Do Not Exhibit High-Frequency Precise Excision.
In light of the surprisingly high-frequency precise excision of the eps-associated IS492, we asked whether copies of IS492 located at different sites on the P. atlantica chromosome exhibit precise excision. Five chromosomal copies of IS492, identified by inverse PCR (9) and designated here as copies 1, 2, 3, 4, and eps, were PCR-amplified from chromosomal DNA of crenated cells using primers that correspond to the unique flanking sequences. Sequence analysis revealed that all five copies of IS492 are identical at the nucleotide level; thus, all five elements have the potential to excise precisely.
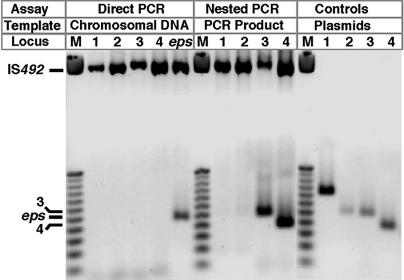
In a PCR-based assay for precise excision of each IS492 copy, we used P. atlantica DB27recA chromosomal DNA from the qPCR assays as template because the frequency of excision for the eps-associated copy determined for these samples was a convenient reference point. Each reaction used a set of inwardly directed primers unique to one chromosomal site. A long extension time was used in this PCR assay, so the flanking sequence and the IS492 element are amplified, and upon excision of IS492, the restored integration site is amplified (Fig. 3). The products observed from this direct PCR assay for precise excision suggested that only the eps-associated IS492 excised precisely at a detectable level (Fig. 3). However, amplification with nested PCR primers from the PCR products of the direct PCR reproducibly yielded precise excision products for copies 3 and 4 (Fig. 3). Nested PCR inconsistently gave PCR products for copies 1 or 2; these products were refractory to cloning or sequencing, and therefore precise excision of copies 1 and 2 could not be confirmed. The positive-control DNA for each repaired insertion site (see Materials and Methods) gave product by direct PCR at the lowest quantity that was detected for the repaired eps site (2.5 fg) in these chromosomal DNA samples, demonstrating that the direct PCR assay could detect each repaired site if generated at a frequency of at least 10−4 per cell per generation. Thus, copies 3 and 4 excise precisely at a frequency <10−4 per cell per generation, and copies 1 and 2 may not ever excise. Because all of the copies of IS492 are identical, the altered levels of precise excision for copies 1-4 relative to the eps copy suggest that the context of the insertion site determines the mechanism or level of transposition.
Fig. 3.
Precise excision of IS492 from different P. atlantica chromosomal locations. Forward and reverse primers that flank IS492 at each insertion site were used in PCRs with chromosomal DNA template from HCD-1. For those reactions that yielded no PCR product (Direct PCR, Locus 1–4), the sensitivity of the PCR was increased by using aliquots of the Direct PCR assay with nested forward and reverse primers (Nested PCR). The predicted products for precise excision of copies 1–4 from their respective sites were generated and cloned into pCR2.1 (SI Table 2) for use as template to confirm the activity of the nested primers (Controls). The mobilities of the amplified unexcised IS492 elements with flanking DNA and of the amplified insertion sites for the precisely excised eps-associated element, and copies 3 and 4 are indicated. The DNA size marker (M) is the Promega (Madison, WI) 25-bp ladder.
mooV Transcript Levels at epsG::IS492 in P. atlantica Are Significantly Higher Than at Other IS492 Insertion Sites.
To relieve regulatory constraints on movement of IS492 that might be unique to the P. atlantica chromosome, each copy of IS492, along with 116–201 bp of flanking chromosomal sequence, was inserted into pCR2.1 and transformed into Escherichia coli DH5α. The transformants were assayed for repaired plasmids that result from precise excision of IS492 by using a direct PCR protocol with the same primers used in the assays for precise excision from P. atlantica chromosomal DNA. Like the epsG-associated IS492, which in E. coli excises precisely to form a circular IS492 product and a repaired plasmid (9), copies 1–4 showed precise excision (SI Fig. 7).
These results suggest that it is the chromosomal environment in P. atlantica that controls the frequency of precise excision of IS492. Therefore, we examined the chromosomal context of each element using the sequence and annotation of the P. atlantica genome that was obtained through collaboration with the Joint Genome Institute and the Department of Energy (GenBank accession no. NC008220). Based on the identified candidate gene models in regions surrounding the IS492 elements, copies 1–4 (base pairs 4526674–4525473, 3218003–3219204, 1015462–1014261, and 2234900–2233700, respectively) are not inserted into identifiable genes, and only copies 2 and 4 have ORFs immediately upstream that are in the same orientation as mooV. In contrast, the eps-associated copy of IS492 is inserted in the glucosyl transferase gene at base pair 1304919 such that mooV may be expressed from the epsG transcript. Quantitative RT-PCR (qRT-PCR) assays were performed to ask whether the level of host-initiated mooV transcript at each insertion site corresponds to the level of precise excision of IS492 from each site in P. atlantica.
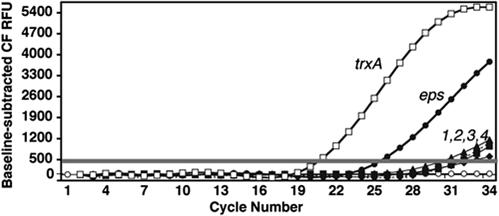
qRT-PCRs were set up under the same conditions used for the qPCR assay for IS492 excision (R-HCD1–4 and R-LCD1–4). Representative results from the qRT-PCR assay for host-initiated mooV transcripts from the five different chromosomal locations and the P. atlantica thioredoxin A (trxA) reference transcript (18), are shown in Fig. 4. The eps-associated mooV transcript is present at a level 100- to 100,000-fold higher than the level of mooV transcript from the IS492 element at each of the other four sites. Although the absolute amount of mooV transcript from each site varied in independent repetitions of this assay at HCD and LCD, the same trend was seen (Table 1). These results suggest that transcription through IS492 at the eps locus distinguishes it from the other IS492 loci and may account for the significantly higher frequency of IS492 precise excision from the eps locus.
Fig. 4.
qRT-PCR assay for host-initiated mooV transcripts. The curve-fit fluorescence units (CF RFU), adjusted for the signal base line, are plotted vs. the PCR cycle number from the qRT-PCRs with the cDNA generated from host-initiated mooV transcripts for each of the five IS492 copies on the P. atlantica chromosome (filled symbols) and for the reference trxA mRNA (open squares). The threshold bar determines the threshold cycle used to calculate the amount of starting template; the control reactions lacking reverse transcriptase (open circles) demonstrate the absence of detectable DNA contamination of the RNA preparations used. The results for RNA from crenated colonies at HCD (R-HCD1) are shown.
Table 1.
Levels of externally initiated mooV transcripts
| Source* | Copy 1 | Copy 2 | Copy 3 | Copy 4 | eps-associated |
|---|---|---|---|---|---|
| LCD1 | 7.0 × 10−4 | 3.2 × 10−3 | 7.0 × 10−4 | 4.4 × 10−3 | 1 |
| LCD2 | 1 × 10−5 | 2.0 × 10−4 | 6 × 10−5 | 5.0 × 10−4 | 1 |
| HCD1 | 6 × 10−5 | 4.0 × 10−4 | 1.0 × 10−4 | 1.4 × 10−2 | 1 |
| HCD2 | 2.0 × 10−4 | 6.0 × 10−4 | 1.3 × 10−4 | 1.3 × 10−3 | 1 |
Quantities of mooV transcripts measured by qRT-PCR. Values are normalized against trxA cDNA and divided by the value for mooV transcript from epsG::IS492; the ratios are shown.
*RNA isolated from pooled low- and high-colony-density cells.
Higher Level of IS492 Excision Corresponds to Increased Expression of mooV from External Promoters in E. coli.
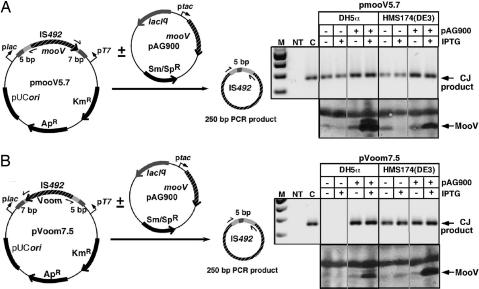
The role of external transcription in precise excision of IS492 from epsG is most likely a physical process, such as increasing positive supercoiling or raising in cis levels of MooV. To address this mechanism, the element and flanking sequence that are conserved at all of the P. atlantica insertion sites (9) were inserted in both orientations between plac and pT7 on pCR2.1, allowing transcription to impinge on IS492 from either or both directions, depending on the host strain. Precise excision of IS492 from these constructs (pmooV5.7 and pVoom7.5) was assayed by PCR for the circle junction of the IS492 circular excision product (9) (Fig. 5). IS492 precise excision could be detected for pmooV5.7 in DH5α and HMS174(DE3) when mooV is expressed from plac (Fig. 5A). Convergent transcription from both plac and pT7 in HMS174(DE3), in which the T7 polymerase gene is under control of plac on DE3 λ lysogen, resulted in reduced expression of MooV from pmooV5.7, as measured by Western blot analysis, but still supported precise excision of IS492 (Fig. 5A). However, no precise excision could be detected from pVoom7.5 in DH5α, where pT7 is silent, and expression of mooV would depend on an internal IS492 promoter. In fact, within the limited sensitivity of the Western blot assays, no MooV appears to be expressed (Fig. 5B). Excision is restored in the presence of MooV provided in trans from a compatible expression plasmid, pAG900, which has mooV under control of ptac and encodes lacIq. This result suggests that transcription through the element is not needed for IS492 precise excision, because plac is repressed by LacI, and pT7 is inactive in the absence of T7 polymerase. In HMS174(DE3), transcription initiated from pT7 expresses mooV on pVoom7.5, resulting in IS492 precise excision (Fig. 5B). The sum of these results indicates there is a transitional level of MooV, which is near the detection limit of our Western blot assay, required for precise excision; at low in cis levels of MooV, excision can be restored by providing MooV in trans. The same results were obtained when additional flanking sequence was included from the epsG or copy 1 insertion sites on the pmooV and pVoom constructs (data not shown).
Fig. 5.
PCR assay for precise excision of IS492 under varying external transcription conditions in E. coli. Precise excision of IS492 is linked to the formation of a circularized IS492 (9). A PCR assay to detect circle junction (CJ) formation under different levels of external transcription through the element from upstream or downstream is depicted. IS492 (mooV) is in two orientations (A and B) between plac and pT7 on pCR2.1. PCR primers to detect CJ are shown as half arrows. Isopropyl β-d-thiogalactoside-inducible MooV is provided in trans from the expression vector, pAG900. The position of the PCR product is marked by an arrow. Western blot analysis revealed the relative expression of MooV under the different transcription conditions.
Discussion
The frequency of precise excision of IS492 was directly measured by using qPCR and shown by statistical analyses to correlate with the high occurrence of pEPS phase variation (10−3 to 10−2 per cell per generation). This is the highest frequency of nonmutagenic repair of the donor DNA described for any classical transposon or IS. Another IS element from the IS110/IS492 family (19) and a few IS elements from the IS3 (20, 21), IS4 (22), IS5 (23, 24), and IS256 (25) families have been reported to excise precisely. The best-characterized of these elements is IS256, which exhibits reversible insertion that regulates expression of extracellular polysaccharide in Staphylococcus epidermidis (25, 26). The frequency of precise excision of this element has not been determined directly but, based on phase variation of polysaccharide production, it is ≈10−4 per cell per generation. The DDE-motif transposase of IS256 is required for circle formation by the element (27) but has not been shown to mediate precise excision of the element. Precise excision of prokaryotic mobile elements is usually mediated by Y or S recombinases, which use a covalent recombinase–DNA intermediate to conserve the energy of the phosphodiester bond (reviewed in ref. 5). MooV is the only reported prokaryotic DDE- or DEDD-motif transposase demonstrated to be required for precise excision of the transposable element (9).
The DEDD tetrad of the Piv-MooV recombinases is predicted to coordinate divalent metal cations that direct DNA hydrolysis at the element–donor DNA junction and mediate strand transfer through one-step transesterification (11–13). The proposed mechanism for conservative inversion mediated by Piv, which involves a Holliday junction intermediate (13), may be applied to the precise excision reaction mediated by MooV if the 5-bp direct repeats that flank the inserted element serve as the core cross-over sequences. However, because IS492 has no terminal inverted repeats, it is difficult to predict how MooV recognizes both ends to set up the appropriate synaptic complex.
To define factors that influence precise excision of IS492, we assayed transposition of IS492 from four different P. atlantica chromosomal sites (copies 1–4) as compared with the eps site. Precise excision of IS492 could not be reliably detected from two of the alternate insertion sites and could be measured at very low levels from the other two sites. These results are consistent with previous Southern blot analyses of crenated and mucoid-phase variants of P. atlantica by Bartlett et al. (8), which suggested that insertion of IS492 into the eps site does not result in loss of one of the other copies of IS492 on the chromosome. These elements may move by replicative transposition or cut-and-paste transposition with efficient double-strand-break repair from a sister chromosome, such that the element would be found at both the donor site and the target (for review, see ref. 5). Each of the elements at the five chromosomal insertion sites (including the eps site) are identical in nucleotide sequence, and all have the same 5- and 7-bp sequence immediately flanking the left and right of the element, respectively (9, 13). In addition, copies of IS492 with the different flanking sequences from the five P. atlantica chromosomal sites are competent for precise excision when cloned into pCR2.1 and introduced into E. coli. Taking all these results into consideration, we examined the context of each IS492 insertion site on the P. atlantica chromosome.
The organization of genes at each IS492 insertion site suggested that the level of transcription from external promoters through the elements might differ substantially between the eps site and the other insertion sites. qRT-PCR confirmed that the levels of host-initiated mooV transcripts for copies 1–4 were 100- to 100,000-fold lower than for IS492 at the eps insertion site, suggesting a coupling between external transcription through IS492 and precise excision of the element. Such coupling could result from an increased concentration of MooV at the eps locus; alternatively, transcription through the eps locus could facilitate IS492 transposition from this site. Our results from IS492 excision assays in E. coli indicate impinging transcription that increases expression of mooV raises MooV to a critical level that is required for precise excision of IS492, but transcription through the element is not essential for excision when MooV is provided in trans.
When MooV expression is increased at the eps locus, why does it affect only excision of IS492 at the eps locus and not copies 1–4? The probable answer is that MooV preferentially acts in cis like the transposases of IS1, IS10, IS50, IS903, and IS911 (reviewed in ref. 28). Multiple factors contribute to cis activity of these transposases, including message instability; MooV may act in trans in the E. coli complementation assays, because the mooV transcript from the expression vector is more stable than that from IS492. An alternative explanation is that MooV from the eps-associated copy does act in trans to support excision of other IS492 copies, but reinsertion of the element is inhibited at the highly transcribed epsG site, thus resulting in detectable precise excision from epsG only. The strong negative effect of transcription through a target site on insertion of a transposable element has clearly been demonstrated in replicative transposition of bacteriophage Mu (29, 30).
Many mobile elements have evolved mechanisms to attenuate impinging transcription from host DNA or inhibit insertion into a transcriptionally active gene to prevent increased expression of transposase and higher levels of transposition that can be lethal to the host (reviewed in ref. 28). However, IS492 precise excision appears to require external transcription of the transposase, which is consistent with the regulatory role of IS492 in pEPS phase variation. Our working model is that an environmental signal turns on transcription of the eps operon, which increases MooV levels and promotes precise excision of IS492 to restore a functional epsG gene. To protect the host from increased insertion of the element at new sites, the transposase levels required for precise excision of the element may also inhibit reinsertion into the chromosome. This result is supported by the results of Southern blot analyses with chromosomal DNA from clonally derived crenated and mucoid colonies, which show excision of IS492 from epsG does not result in insertion at a new site on the P. atlantica chromosome (8).
Materials and Methods
Bacterial Strains, Primers, and Plasmids.
P. atlantica T6c (31), DB27 (hsd1, Rifr), and DB27recA (DB50) were gifts from D. Bartlett (Scripps Institute of Oceanography, La Jolla, CA), and E. coli DH5α and HMS174 (DE3) were cultured as described (8, 9). Sequences for all oligonucleotides used in PCR, qPCR, and qRT-PCR are given in SI Table 3. Plasmids used as control templates in PCR assays and as substrates in excision assays in E. coli were created by TOPO TA cloning (Invitrogen, Carlsbad, CA) of PCR products (plasmid names with corresponding primers/products are listed in SI Table 2). All plasmids were sequenced to confirm inserted PCR products.
Assays for Frequencies of pEPS Phase Variation and IS492 Excision in P. atlantica.
One colony was resuspended in 40 μl of MB, plated on marine agar at LCD (≤100 colonies per 100-mm dish) and HCD (≥1,000 colonies per dish), and incubated 7 days at 25°C. One thousand colonies from HCD plates or 100 colonies from LCD plates were resuspended in 10 ml of MB, washed, resuspended in 1 ml of MB, and three separate aliquots were removed for serial dilutions and platings to determine the ratio of pEPS+ to total colonies. Genomic DNA was isolated (32) from an aliquot corresponding to ≈1.5 × 109 cells, digested with NgoMIV, and the DNA concentration was determined by absorbance at 260/280 nm. qPCRs were performed by using 18 replicates from each genomic DNA sample. The forward and reverse primers for the epsG target site and for agrA are designated FWEPS/RVEPS and AGARL/AGARR, respectively; the probes for the restored epsG site and for agrA are EPSPRB3 (FAM-490 fluorophore on the 5′-end and Black Hole Quencher1 on the 3′end) and AGARPRB (HEX-530 fluorophore on the 5′-end and Black Hole Quencher2 on the 3′end), respectively. Real-time PCRs (50 μl) contained 100 ng of digested chromosomal DNA, 1× TaqPCR Buffer B (Fisher Scientific, Hampton, NH), 2 units of Taq polymerase, 400 nM each dNTP, 6 mM MgCl2, 300 nM AGARL and AGARR, 500 nM FWEPS and RVEPS, 5 nM AGARPRB, and 200 nM EPSPRB3. Primer and probe concentrations for optimal PCR efficiency and good correlation coefficients were determined empirically. To generate a standard curve for quantification of template in the reactions, pBHG126 (pCR2.1 encoding both 107 bp of eps and 100 bp of agr sequences to be amplified) was prepared and used in a dilution series corresponding to 100 pg to 1 fg of target DNA (three replicates of each dilution was used for the standard curve in each experiment). The extension time with Taq at 57°C, which results in release of the fluorophore for the restored epsG template but not for epsG::IS492, was determined empirically in qPCRs with pCR2.1 containing eps (pBHG115) and eps::IS492mooV− (pAG990); extension times of 5 min or longer were required with pAG990 to detect eps probe signal above the threshold or to detect the 1.3-kb PCR product on an ethidium bromide-stained agarose gel (data not shown). Cycling conditions for standards and experimental reactions were 50°C for 2 min, 95°C for 3 min, and 30 cycles of 95°C for 30 s and 57°C for 2 min. Products of the qPCRs were cloned and sequenced to confirm IS492 precise excision.
Standard plots of CT vs. log starting quantity (SQ) were generated with the BioRad (Hercules, CA) iCycler iQ software ver. 3.0 using the data from the pBHG126 dilution series; the SQ of restored eps site and total chromosomal copies in each genomic sample (HCD1–6 and LCD1–6) were extrapolated from this plot. The ratio of SQ of restored eps locus present in each sample to the SQ of agr locus present in the same sample (XEX) is used to determine PEX: PEX = 1−(1 − XEX)1/n, where n is the number of generations in the sample population (33). For calculating n, the inocula were 1,000 cells for HCD samples and 100 cells for LCD samples (each colony in the pooled samples started from a single cell). The fraction of cells switching from pEPS− to pEPS+ (XPV) is used in the calculation of PPV.
PCR Assays for IS492 Precise Excision from Different Sites in P. atlantica.
P. atlantica chromosomal DNA from the qPCR assays described above (HCD 1 and 2 and LCD 1 and 2) was used as template for the direct PCR, and 6 μl of the products from the direct PCR were used in the nested PCR. The 50-μl reactions contained 200 ng of template DNA, a 600 nM concentration of the paired primers that specifically amplify the individual IS492 insertion sites (loci 1, 2, 3, and 4; eps, direct PCR, 1PDB1L/R, PDB6L/NOR, PDB9L/R, PDB12L/R, and L58/R76; nested PCR, PB1NL/NR, PDB6NL/NIR, PDB9NL/NR, and PDB12NL/NR, respectively), 1× Taq buffer, 3 mM MgCl2/200 nM dNTPs/1 unit Taq polymerase. Cycling conditions were 95°C for 5 min and 40 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 15 s. Products were electrophoresed on 3% agarose (FMC, Rockville, ME) gels and visualized by ethidium bromide staining. The positive-control plasmids that contained the restored sequence for each insertion site were used as template at 2.5, 25, and 250 fg per reaction; all SQ gave the same end products in the direct PCR (data not shown).
RNA Isolation and qRT-PCR.
Total RNA isolated from ≈5 × 109 P. atlantica cells using RNeasy Midi Kit (Qiagen, Valencia, CA) was treated with RNase-free DNase I followed by phenol/chloroform extraction and ethanol precipitation (32). For each RNA sample, three separate reverse transcription reactions (Invitrogen First Strand Synthesis Kit) were performed, one with ISL270 (for all mooV-specific transcripts), another with TRXAR (for the reference gene trxA), and the third with both primers but no reverse transcriptase. Each reverse transcription reaction contained 2–5 μg of RNA.
qPCRs with the resulting cDNA (performed in triplicate for each target transcript from each RNA sample) contained 1 μl of RT-PCR (20-μl reaction), 1× SYBR green (BioWhittaker, Walkersville, MD), 1 unit of Taq polymerase, 3 mM MgCl2, 0.5 μM forward primer for each insertion site (PDB1L, PDB6NL, PDB9L, PDB12L, or EPSL58), and 0.5 μM reverse primer (ISL270). Reactions for the reference gene had 0.5 μM TRXAL and TRXAR. Cycling conditions were 3 min at 95°C, 40 cycles of 30 s at 95°C, 30 s at 56°C, and 60 s at 72°C. Two negative controls included in each qPCR run had as template either water or the RT-PCR that had no reverse transcriptase added. Amplification of the correct target was confirmed by melt-curve analysis for each qPCR using the BioRad iCycler, and products were confirmed by gel electrophoresis.
The quantity of the cDNA for each targeted transcript was determined as described for the qPCR for IS492 excision. The standard plot for each target sequence, generated with the corresponding plasmid (pBHG117: trxA, pBHG119–123: copies 1–4 and eps-associated copy; SI Table 2), indicates the PCR efficiency for that reaction (based on the slope) and is used to accurately calculate the unknown target quantity in each reaction, because it takes into account the different efficiencies of each qPCR with different primer sets.
Circle Junction PCR Assay for IS492 Precise Excision and Western Blot for MooV Expression from IS492 in E. coli.
Electrocompetent E. coli, DH5α or HMS174(DE3) were transformed with pmooV5.7 or pVoom7.5 and incubated overnight at 37°C on LBAp or LBAp with 0.05 mM isopropyl β-d-thiogalactoside. In addition, DH5α or HMS174(DE3) that already contained either pmooV5.7 or pVoom7.5 were transformed with pAG900 and incubated under the same conditions, except 50 μg/ml spectinomycin (Sp; Sigma, St. Louis, MO) was added to the plates. Three transformants from each plate were pooled in 40 μl of TE buffer, boiled for 10 min, and the lysate cleared by centrifugation. Cleared lysate (10 μl) from each sample was used in a 50-μl PCR containing 1× Taq polymerase Buffer B (Fisher Scientific), 2 units of Taq polymerase, 200 nM each dNTP, 3 mM MgCl2, 600 nM CJ250A, and 600 nM CJ250B. Cycling conditions were 3 min at 95°C and 30 cycles at 95°C for 30 s, 58°C for 30 s, and 72°C for 30 s. Aliquots (10 μl) of each reaction were electrophoresed on a 2% agarose gel and visualized with ethidium bromide.
Transformed DH5α or HMS174(DE3) cells were also inoculated into LBAp or LBApSc, grown to midlogarithmic phase, induced with 0.05 mM isopropyl β-d-thiogalactoside (or not induced), and grown for an additional 2 h at 37°C. Western blots to detect MooV expressed in cells harvested from these cultures were performed as described in Buchner et al. (13) by using chicken polyclonal α-MooV.His6 at 1:2,000 (9).
Supplementary Material
Acknowledgments
We thank D. Promislow and N. Wurzburger for assistance with statistical analysis; R. Karls, T. Hoover, M. Schell, S. Kushner, B. Mohanty, and M. Powers for productive discussions; and A. Popkowski and P. Caruana for assistance in phase variation assays. This work was supported by National Science Foundation Grant MCB-0004123 (to A.C.K.), National Institutes of Health Grant GM49794 (to A.C.K.), and the University of Georgia Office of the Vice President for Research. Sequencing and annotation of the P. atlantica genome were performed under the auspices of the U.S. Department of Energy Office of Science, Biological, and Environmental Research Program (Joint Genome Project ID 4000130).
Abbreviations
- pEPS
peripheral extracellular polysaccharide
- pEPS−
crenated pEPS+, mucoid
- PPV
frequency of phase variation
- PEX
frequency of precise excision
- HCD
high colony density
- LCD
low colony density
- IS
insertion sequence
- qPCR
quantitative PCR
- SQ
starting quantity
- qRT-PCR
quantitative RT-PCR.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. NC 008228).
This article contains supporting information online at www.pnas.org/cgi/content/full/0608633104/DC1.
References
- 1.del Pilar Garcillan-Barcia M, Bernales I, Mendiola MV, de la Cruz F. Mol Microbiol. 2001;39:494–501. doi: 10.1046/j.1365-2958.2001.02261.x. [DOI] [PubMed] [Google Scholar]
- 2.Rudy C, Taylor KL, Hinerfeld D, Scott JR, Churchward G. Nucleic Acids Res. 1997;25:4061–4066. doi: 10.1093/nar/25.20.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ton-Hoang B, Guynet C, Ronning DR, Cointin-Marty B, Dyda F, Chandler M. EMBO J. 2005;24:3325–3338. doi: 10.1038/sj.emboj.7600787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyras D, Adams V, Lucet I, Rood JI. Mol Microbiol. 2004;51:1787–1800. doi: 10.1111/j.1365-2958.2003.03950.x. [DOI] [PubMed] [Google Scholar]
- 5.Curcio MJ, Derbyshire KM. Nat Rev Mol Cell Biol. 2003;4:865–877. doi: 10.1038/nrm1241. [DOI] [PubMed] [Google Scholar]
- 6.Izsvak Z, Stuwe EE, Fiedler D, Katzer A, Jeggo PA, Ivics Z. Mol Cell. 2004;13:279–290. doi: 10.1016/s1097-2765(03)00524-0. [DOI] [PubMed] [Google Scholar]
- 7.Lovett ST. Mol Microbiol. 2004;52:1243–1253. doi: 10.1111/j.1365-2958.2004.04076.x. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett DH, Wright ME, Silverman M. Proc Natl Acad Sci USA. 1988;85:3923–3927. doi: 10.1073/pnas.85.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins-Balding D, Duval-Valentin G, Glasgow AC. J Bacteriol. 1999;181:4937–4948. doi: 10.1128/jb.181.16.4937-4948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandler M, Mahillon J. In: Mobile DNA II. Craig NL, Craigie R, Gellert M, Lambowitz AM, editors. Washington, DC: Am Soc Microbiol; 2002. pp. 305–366. [Google Scholar]
- 11.Lenich AG, Glasgow AC. J Bacteriol. 1994;176:4160–4164. doi: 10.1128/jb.176.13.4160-4164.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobiason DM, Buchner JM, Thiel WH, Gernert KM, Karls AC. Mol Microbiol. 2001;39:641–651. doi: 10.1046/j.1365-2958.2001.02276.x. [DOI] [PubMed] [Google Scholar]
- 13.Buchner JM, Robertson AE, Poynter DJ, Denniston SS, Karls AC. J Bacteriol. 2005;187:3431–3437. doi: 10.1128/JB.187.10.3431-3437.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidwell MG, Lisch DR. In: Mobile DNA II. Craig NL, Craigie R, Gellert M, Lambowitz AM, editors. Washington, DC: Am Soc Microbiol; 2002. pp. 59–90. [Google Scholar]
- 15.Godoy VG, Fox MS. Proc Natl Acad Sci USA. 2000;97:7393–7398. doi: 10.1073/pnas.130186597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heid CA, Stevens J, Livak KJ, Williams PM. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 17.Belas R. J Bacteriol. 1989;171:602–605. doi: 10.1128/jb.171.1.602-605.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim CJ, Daws T, Gerami-Nejad M, Fuchs JA. Biochim Biophys Acta. 2000;1491:1–6. doi: 10.1016/s0167-4781(00)00026-9. [DOI] [PubMed] [Google Scholar]
- 19.Partridge SR, Hall RM. J Bacteriol. 2003;185:6371–6384. doi: 10.1128/JB.185.21.6371-6384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullin DA, Zies DL, Mullin AH, Caballera N, Ely B. Mol Gen Genet. 1997;254:456–463. doi: 10.1007/s004380050439. [DOI] [PubMed] [Google Scholar]
- 21.Kusumoto M, Nishiya Y, Kawamura Y. Appl Environ Microbiol. 2000;66:1133–1138. doi: 10.1128/aem.66.3.1133-1138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brass S, Ernst A, Boger P. Appl Environ Microbiol. 1996;62:1964–1968. doi: 10.1128/aem.62.6.1964-1968.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammerschmidt S, Hilse R, van Putten JP, Gerardy-Schahn R, Unkmeir A, Frosch M. EMBO J. 1996;15:192–198. [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz R, Lopez R, Garcia E. J Bacteriol. 1998;180:1381–1388. doi: 10.1128/jb.180.6.1381-1388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziebuhr W, Krimmer V, Rachid S, Lossner I, Gotz F, Hacker J. Mol Microbiol. 1999;32:345–356. doi: 10.1046/j.1365-2958.1999.01353.x. [DOI] [PubMed] [Google Scholar]
- 26.Conlon KM, Humphreys H, O'Gara JP. J Bacteriol. 2004;186:6208–6219. doi: 10.1128/JB.186.18.6208-6219.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loessner I, Dietrich K, Dittrich D, Hacker J, Ziebuhr W. J Bacteriol. 2002;184:4709–4714. doi: 10.1128/JB.184.17.4709-4714.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy Z, Chandler M. Res Microbiol. 2004;155:387–398. doi: 10.1016/j.resmic.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Manna D, Breier AM, Higgins NP. Proc Natl Acad Sci USA. 2004;101:9780–9785. doi: 10.1073/pnas.0400745101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manna D, Higgins NP. Mol Microbiol. 1999;32:595–606. doi: 10.1046/j.1365-2958.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 31.Corpe WA. In: Adsorption of Microorganisms to Surfaces. Bitton G, Marshall KC, editors. New York, NY: Wiley; 1980. pp. 105–144. [Google Scholar]
- 32.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. Hoboken, NJ: Wiley; 2005. [Google Scholar]
- 33.Gally DL, Bogan JA, Eisenstein BI, Blomfield IC. J Bacteriol. 1993;175:6186–6193. doi: 10.1128/jb.175.19.6186-6193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.