Abstract
The adrenal gland comprises two endocrine tissues of distinct origin, the catecholamine-producing medulla and the steroid-producing cortex. The inner adrenocortical zone, which is in direct contact with the adrenomedullary chromaffin cells, produces dehydroepiandrostendione (DHEA) and DHEA sulfate (DHEAS). These two androgens exhibit potential effects on neurogenesis, neuronal survival, and neuronal stem cell proliferation. Unlike the closely related sympathetic neurons, chromaffin cells are able to proliferate throughout life. The aim of this study was to investigate the effect of DHEA and DHEAS on proliferation of bovine chromaffin cells from young and adult animals. We demonstrated that graded concentrations of leukemia inhibitory factor induced proliferation of chromaffin cells from young animals, whereas EGF had no effect. On the contrary, EGF increased the cell proliferation in cells from adult animals, whereas leukemia inhibitory factor was inactive. In both cases, DHEA decreased the proliferative effect induced by the growth factors. Surprisingly, DHEAS enhanced, in a dose-dependent-manner, the effect of growth factors on proliferation in cells from adult animals but not from young animals. Flutamide, ICI 182,780, and RU 486 had no effect on the action of DHEA or DHEAS on chromaffin cell proliferation. These data show that DHEA and its sulfated form, DHEAS, differentially regulate growth-factor-induced proliferation of bovine chromaffin cells. In addition, the sensitivity of chromaffin cells to different growth factors is age-dependent. Furthermore, these two androgens may act through a receptor other than the classical steroid receptors.
Keywords: aging, adrenal medulla, neurosteroid, paracrine interactions
The mammalian adrenal gland consists of two anatomically distinct parts derived from different embryological origins. The outer cortex, which synthesizes steroid hormones, and the central medulla, which contains catecholamine-producing chromaffin cells, are functionally and structurally closely connected (1–3). Axelrod and Wurtman's groups have demonstrated the key role of adrenal glucocorticoids on the induction of catecholamine enzymes in chromaffin cells (4, 5). However, little is known about the role of dehydroepiandrostendione (DHEA) and DHEA sulfate (DHEAS) on chromaffin cell function. In particular, DHEA-producing adrenal cortical cells are tightly intermingled with adrenomedullary cells, providing ample contact surface for paracrine interaction (Fig. 1). DHEA and DHEAS, the most abundant steroids in the human body, have been shown to play a neuroprotective role against excitatory amino acid-induced neurotoxicity (6) while increasing neurogenesis (7) in the adult rodent hippocampus in vivo. Together with the age-dependent decline of DHEA levels, there is a decline in adrenomedullary function over life. In addition, it has previously been shown that anti-androgen treatment may cause cardiovascular effects by altering catecholamine biosynthesis pathways in the adrenal medulla (8). Hyperandrogenism as seen in patients with 21-hydroxylase deficiency is associated with structural and functional alterations of the adrenal medulla (9, 10). Furthermore, recent evidence suggests an antiapoptotic effect of DHEA and DHEAS on chromaffin cells (11) and opposite effects of these two androgens on catecholamine secretion (12, 13).
Fig. 1.
Close contact of chromaffin cells with androgen-producing cortical cells. (A) Section of bovine adrenal gland showing intermingling of cortical cells and chromaffin cells (arrows). (B) Cryosection of human adrenal gland showing zona reticularis adrenocortical cells immunostained (brown) with an antibody against D-11 in close contact with the adrenomedullary cells (asterisks). (C) Electron micrograph of human adrenal gland exhibiting DHEA-producing cells of the zona reticularis (ZR) in direct contact with chromaffin cells of the medulla (Ch). Arrows indicate filopodia. V, vessel; SG, secretory granule; Nuc, nucleus.
Interestingly, chromaffin cells, in contrast to closely related sympathetic neurons, are able to proliferate throughout life (14). Proliferation of chromaffin cells constantly requires the presence of growth factors, including nerve growth factor, insulin-like growth factor II, and fibroblast growth factor type II (14). EGF is expressed by the adrenal cortex, and EGF binding sites have been found on chromaffin cells (15) suggesting that EGF acts as a paracrine factor in the adrenal gland. There is now plentiful evidence that EGF enhances cell proliferation in PC12 cells, a cell line established from a rat pheochromocytoma (16); however, little is known about the action of EGF on normal chromaffin cells. Leukemia inhibitory factor (LIF) is a pleiotropic cytokine that belongs to the IL-6 family. LIF is released by various types of immune cells (17) and adrenocortical cells (18). This cytokine is known to be strongly involved in the development and maintenance of hypothalamic–pituitary–adrenal axis (18–20) as well as sympathetic neurons and adrenal medullary development (21). To explore the role of adrenal androgens on adrenal development, we investigated the age-dependent effect of DHEA and DHEAS on chromaffin cell proliferation induced by major adrenal growth factors, including EGF and LIF.
Results
Bovine Chromaffin Cells Are Able to Grow in Vitro.
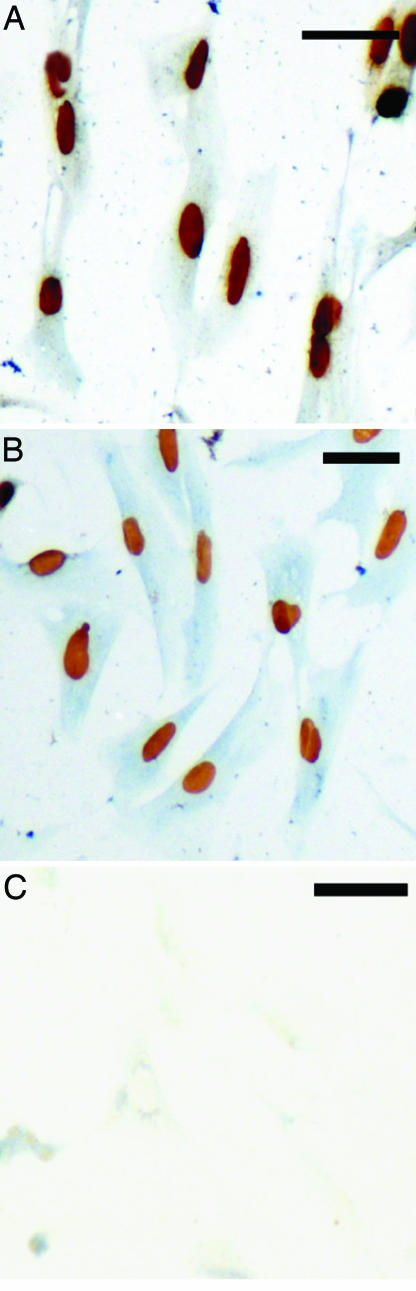
The capacity of bovine chromaffin cells in primary culture to proliferate in vitro has been evaluated by incorporation of BrdU. Chromaffin cells isolated from young and adult animals (Fig. 2A and B, respectively) were both able to incorporate BrdU. To discriminate labeled and unlabeled chromaffin cells from other cell types, a double-labeling of the cells was performed with an antibody directed against phenylethanolamine N-methyltransferase (PNMT). In the presence of serum, bovine chromaffin cells were able to incorporate BrdU in vitro. No labeling was observed when BrdU and PNMT antisera were replaced by PBS (Fig. 2C).
Fig. 2.
Capacity of bovine chromaffin cells to grow in vitro. (A and B) Double labeling for BrdU (brown nuclei) and PNMT (blue cytoplasm) of chromaffin cells from juvenile (A) or adult (B) cattle incubated for 72 h in the presence of 10% FBS. (C) Control section incubated in the absence of primary antibodies. (Scale bar, 50 μm.)
Effect of LIF and EGF on the Proliferation of Chromaffin Cells Is Age-Dependent.
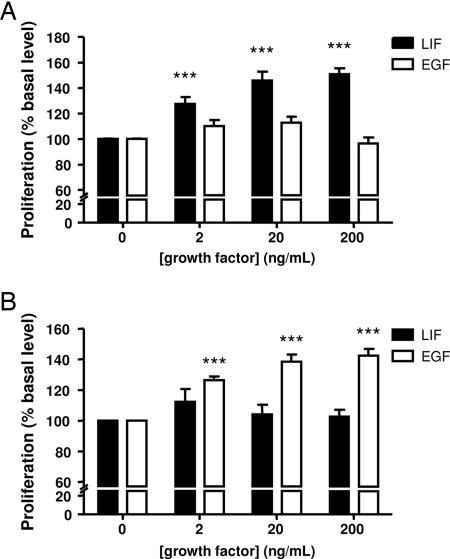
To investigate the effects of LIF and EGF on cell proliferation, we first evaluated proliferative activity with a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Graded concentrations of LIF (2–200 ng·ml−1) significantly increased the proliferation of chromaffin cells from young animals incubated for 4 days in serum-free medium, whereas EGF (2–200 ng·ml−1) had no effect (Fig. 3A). On the contrary, in the same range of concentrations, EGF significantly increased the cell proliferation of cells from adult animals in primary culture, whereas LIF was inactive (Fig. 3B).
Fig. 3.
Effects of LIF and EGF on the proliferation of chromaffin cells from young (A) or adult (B) cattle. Cells were cultured for 96 h in DMEM/F12 containing the indicated growth factors. Cell proliferation was then assessed with MTS reagent and measurement of absorbance at 490 nm. The results are expressed as the mean ± SEM of three to six independent experiments. ∗∗∗, P < 0.001 vs. control.
Differential Action of DHEA and DHEAS on Induced Chromaffin Cell Proliferation.
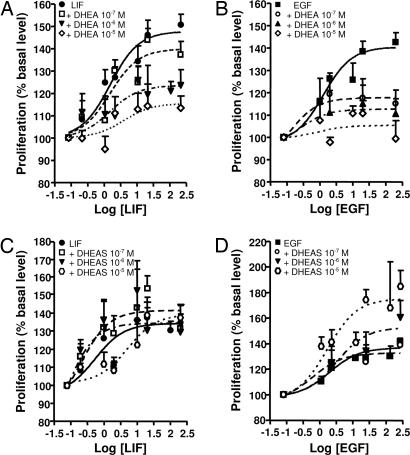
In cells from young animals, as in cells from adult animals, DHEA alone had no effect on bovine chromaffin cell proliferation (data not shown), but decreased proliferation induced by LIF and EGF, respectively, in a dose-dependent manner (Fig. 4A and B). In the same way, dexamethasone (Dex), which also exhibited no real effect on chromaffin cell proliferation, reduced proliferation induced by the growth factors in both populations of cells (data not shown).
Fig. 4.
Effect of DHEA and DHEAS on chromaffin cell proliferation induced by LIF in cells from young animals (A and C) or by EGF in cells from adult animals (B and D). Cell proliferation was assessed with MTS reagent and measurement of absorbance at 490 nm. The results are expressed as the mean ± SEM of three to six independent experiments.
The sulfate ester of DHEA, DHEAS, did not induce any modifications of the basal proliferation of young or adult chromaffin cells (data not shown). However, DHEAS did not affect LIF-evoked chromaffin cell proliferation in young animals but enhanced at high concentration (10−5 M) the EGF-induced proliferation of chromaffin cell in adults (Fig. 4 C and D).
DHEA Has No Effect on Chromaffin Cell Death.
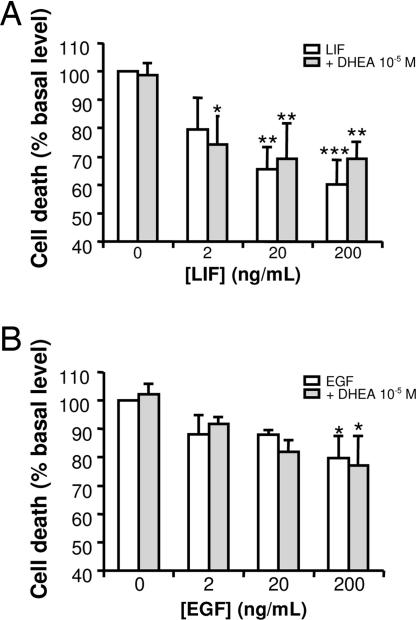
The cytotoxic effect of DHEA on chromaffin cells was evaluated by using a lactate dehydrogenase release assay. We observed that LIF and EGF in serum-free medium both decreased the cell death of chromaffin cells from young and adult animals, respectively (Fig. 5). In addition, DHEA at high concentrations (10−5 M) had no effect alone on chromaffin cell death (data not shown). Furthermore, DHEA did not induce any changes in the decrease of cell death induced by growth factors in serum-free medium (Fig. 5).
Fig. 5.
Effect of DHEA on chromaffin cell death. Cells were cultured for 96 h in DMEM/F12 containing LIF in cells from young animal culture (A) or EGF in cells from adult animals (B) in the absence (white bar) or presence (gray bar) of DHEA at 10−5 M. The results are expressed as the mean ± SEM of at least three independent experiments. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 vs. control.
Effect of DHEA and DHEAS on Chromaffin-Stimulated Proliferation Was Not Affected by Steroid Receptor Antagonists.
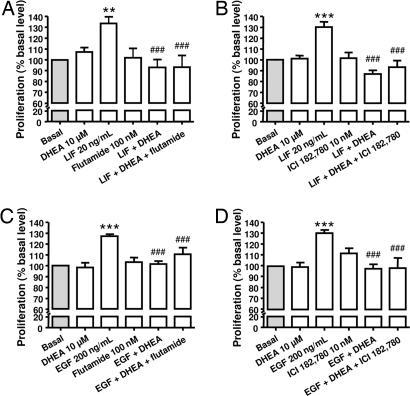
The inhibitory effect of DHEA (10 μM) on LIF/EGF-induced chromaffin cell proliferation was not blocked either by androgen receptor antagonist flutamide (10 nM) or estrogen receptor antagonist ICI 182,780 (10 nM) (Fig. 6). As expected, glucocorticoid receptor antagonist RU 486 (1 μM) completely abolished the inhibitory effect of Dex (10 μM) on growth factor-induced chromaffin cells proliferation but had no effect on DHEA action (data not shown). Flutamide, ICI 182,780, and RU had no effect alone on cell proliferation.
Fig. 6.
Effect of 10−5 M DHEA on 20 ng/ml LIF- and on 200 ng/ml EGF-induced chromaffin cell proliferation after 96 h of pharmacological inhibition of androgen receptor flutamide (A and C) and estrogen receptor ICI 182,780 (B and D). Cell proliferation was assessed with MTS reagent and measurement of absorbance at 490 nm. The results are expressed as the mean ± SEM of three to seven independent experiments. ∗∗∗, P < 0.001 vs. control; ###, P < 0.001 vs. response to LIF (A and B) or EGF (C and D).
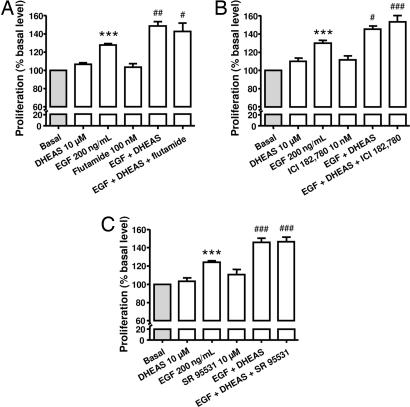
In the same way, the stimulatory effect of DHEAS on EGF-induced adult chromaffin cell proliferation was not modified by both androgen and estrogen receptors antagonists (Fig. 7A and B) as well as GABAA receptor antagonist (Fig. 7C).
Fig. 7.
Effect of 10−5 M DHEAS on 200 ng/ml EGF-induced chromaffin cell proliferation after 96 h of pharmacological inhibition of androgen receptor by flutamide (A), estrogen receptor by ICI 182,780 (B), and GABAA receptor by SR 95531(C). Cell proliferation was assessed with MTS reagent and measurement of absorbance at 490 nm. The results are expressed as the mean ± SEM of three to eight independent experiments. ∗∗∗, P < 0.001 vs. control; #, P < 0.05; ##, P < 0.01; ###, P < 0.001 vs. response to EGF.
Discussion
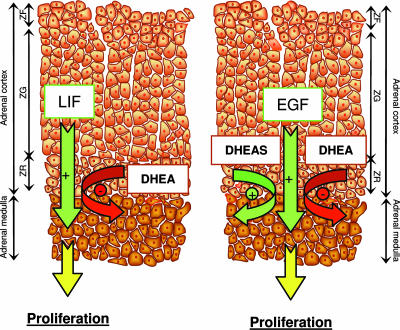
DHEA- and DHEAS-producing cortical cells of the zona reticularis and the chromaffin cells are tightly intermingled, suggesting strong paracrine interactions. Our data suggest that DHEA attenuates the effect of growth factors during life; whereas, in adulthood, DHEAS acts as a local pro-survival agent by increasing the effect of growth factors required for chromaffin cell proliferation, such as EGF (Fig. 8).
Fig. 8.
Artistic rendition depicting potential interactions between adrenal cortex and medulla during life. (Left) Young animals. (Right) Adult animals. ZG, zona glomerulosa, ZF, zona fasciculata, ZR, zona reticularis.
Adrenal chromaffin cells and sympathetic neurons are derived from a common sympathoadrenal precursor during embryogenesis. However, in contrast to sympathetic neurons, chromaffin cells retain the ability to proliferate in response to a variety of mitogens (22). Nevertheless, depending on the species studied, there is a marked disparity in chromaffin cell proliferation in vitro. Rat chromaffin cells proliferate in the presence of neuronal growth factor (23), basic fibroblast growth factor (24), neuturin, or glial cell line-derived neurotrophic factor (25), whereas mouse chromaffin cells do not respond to any mitogens (26). Our data show that, like rat chromaffin cells, young bovine and adult bovine chromaffin cells are able to proliferate in vitro in the presence of serum or in serum-free medium supplemented with growth factors such as LIF or EGF.
As previously discussed, adrenomedullary cell proliferation is regulated by various bioactive factors. In mice, the LIF receptor is expressed during embryological development and in adulthood. LIF has been shown to be strongly involved in the PNMT expression and in adrenal medulla innervation by sympathetic neurons during embryogenesis (21). Our data strongly suggest that LIF is also involved in adrenomedullary cell proliferation in juvenile cattle but not in adult cattle. In contrast to LIF, EGF does not induce any changes in young chromaffin cell proliferation in our model, but increases proliferation of cells from adult animals. Juvenile and adult bovine adrenals medulla did not present any difference at the histological level. In addition, no changes were observed in the expression of classical chromaffin cells markers, such as tyrosine hydroxylase, PNMT, synaptophysin, or chromogranin A, by immunohistochemistry (data not shown). The major difference between both tissues was the size: juvenile adrenals are <4 cm long, whereas adult adrenals usually measured at least 5 cm. Adrenal medulla rapidly grows during infancy and childhood and constitutes <1% of the whole gland at birth and ≈9% in young adults. The growth then reduces in adulthood and declines with senescence (27). These data suggest a decrease in growth factors levels in the medulla and/or a decrease in the chromaffin cell's sensitivity to growth factors. Our results suggest a switch in the response of chromaffin cells to growth factors with aging.
The proliferation of various tissues, such as vascular muscle cells (28), endothelial cells (29), fibroblasts (30), T-lymphocytes (31), and preadipocytes (32), is influenced by DHEA. Because of the anatomical structure of the adrenal gland, steroid hormones secreted by adrenal cortex have been suggested to interact with chromaffin cell biology. In neuronal cell types, previous studies have shown both the neuroprotective (7, 33–36) and neurotoxic action of DHEA (37). However, these disparities in the action of DHEA may reflect differences in cell models or experimental paradigm. The present study has revealed that, independently of age, DHEA decreases proliferation of sympathoadrenal cells induced by LIF or EGF in young and adult populations of cells, respectively. The antiproliferative action of DHEA was not due to a cytotoxic effect of DHEA on bovine chromaffin cells. In addition, some reports have shown opposite effects of DHEA and Dex, in particular in the regulation of catecholamines secretion (38), in our model, DHEA and Dex exhibited the same inhibitory activity on chromaffin cell-induced proliferation. In contrast, DHEAS has been shown to display various neuroprotective activities (6, 39, 40), and a different action of DHEA and DHEAS on neuronal cell viability has been suggested (37). Nevertheless, DHEA and DHEAS exhibit the same protective effect against serum deprivation-induced apoptosis in rat chromaffin cells (11). The results presented in the present study show that DHEAS enhances proliferation of bovine chromaffin cells from adult but not from young animals, suggesting that DHEAS activity depends on age and/or growth factors.
Levels of DHEA and DHEAS produced by the fetal adrenal are very high in humans. After birth, there is a rapid decrease in serum levels, and serum levels remain low until 6 years of age, when levels start to rise again. DHEA sulfotransferase (SULT2A1) is responsible for the sulfonation of DHEA to DHEAS. This enzyme is highly expressed in fetal adrenal, in particular in fetal and transitional zones. During childhood, an increase in DHEAS production occurring before the puberty is associated with accelerated expression of SULT2A1 in adrenal reticularis (41). Changes in the expression of SULT2A1 and consequently of DHEAS levels during development might be important in the development of adrenal medulla.
The different actions of DHEA and DHEAS on chromaffin cell proliferation appear to be independent of androgen and estrogen receptors. Some effects of DHEAS in the brain are mediated by the GABAA receptor (42), which is expressed by bovine chromaffin cells (43). However, SR 95531, a GABAA receptor antagonist, androgen, and estrogen receptor antagonists all failed to reverse the action of DHEA and DHEAS on chromaffin cell proliferation. There is growing evidence for DHEA/DHEAS action via specific receptors. Recently, a DHEA-specific Gα protein-coupled receptor has been identified in human and bovine endothelial cells. Nevertheless, this receptor does not bind DHEAS (44). Furthermore, Gq/11 protein-coupled membrane DHEAS binding sites, which are sensitive to endocrine disrupting chemicals, have been identified on RBL-2H3 rat mast cells (45). In addition, another DHEA-specific Gi protein-coupled receptor has been found in PC12 cells and in human chromaffin cells (46). However, these potential plasma membrane receptors have not yet been isolated. Definitive molecular and/or pharmacological studies have to be done before we can draw any implication from one of these receptors in its effects of DHEA/DHEAS in our model.
In conclusion, we have shown in this study that chromaffin cells from young and adult cattle are able to grow in vitro. These cells present age-dependent sensitivity to LIF and EGF. In addition, DHEA and DHEAS are able to modulate in a differential manner the proliferation induced by these growth factors. DHEA reduces the proliferation in both populations of cells, whereas DHEAS exclusively increases the proliferation provoked by EGF in adult cell culture. These effects are not mediated through androgen or estrogen receptors. These data support the view that adrenomedullary cells are under the control of complex interactions between several factors released by the adrenal cortex, such as growth factors and steroid hormones. The aging process is associated with declines in the levels of hormones and trophic factors, and the loss of adrenomedullary functions with advancing age and tumor formation could be due to an imbalance in the equilibrium of the paracrine pro-/antiproliferative factors.
Materials and Methods
Cell Preparation and Culture.
Bovine adrenal glands were obtained from freshly slaughtered juvenile (1 year old) and adult (from 2 to 4 years old) male and female cattle. Juvenile adrenals are <4 cm long, and adult adrenals are usually >5 cm long. Adrenals were trimmed free of adipose tissue and transported to the laboratory in ice-cold PBS. The adrenals were put into 70% ethanol for 10 s, and connective tissue was removed.
Primary cultures of bovine adrenochromaffin cells were obtained after retrograde perfusion of bovine adrenal glands with 0.3% collagenase (Sigma–Aldrich, Munich, Germany) and 30 units/ml DNase I (Sigma–Aldrich), followed by dissociation of the digested adrenal medulla. The cells were cultured in DMEM-F12 (GIBCO, Paisley, U.K.) supplemented with 10% FBS (GIBCO), 1% antibiotic-antimycotic solution (GIBCO), and 1% gentamicin solution (GIBCO). Chromaffin cells were purified by differential plating to remove adherent nonchromaffin cells as described previously (47). They were plated at a density of 105 cells per milliliter in poly-l-lysine-coated 96-well plates (Becton Dickinson, Bedford, MA). The cells were incubated in a humidified atmosphere at 37°C (95% O2/5% CO2). After 24 h, the medium was changed with the same medium to which 5% FBS was added. Cultures were used 2–3 days after plating. The serum-free medium that was used contained 10−7 M ascorbic acid, 0.001% (wt/vol) transferrin, and 0.01% (wt/vol) bacitracin.
Electron Microscopy.
Adrenal glands were fixed in 2% formaldehyde and 2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.3. Tissue slices were postfixed for 90 min (2% OsO4 in 0.1 M cacodylate buffer, pH 7.3), dehydrated in ethanol, and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate and examined at 80 kV in a CM 10 electron microscope (Philips, Eindhoven, The Netherlands).
Immunohistochemistry.
Adrenal tissue was fixed in 4% paraformaldehyde and processed as previously described (48). We incubated 10-μm sections overnight with mouse anti-D11 antiserum. For detection of primary antibodies, a horseradish peroxidase system was used (DAKO-Cytomation, Hamburg, Germany), and the signal was visualized with 3,3′-diaminobenzidine (DAB tablet set; DAKO-Cytomation).
BrdU Staining.
Chromaffin cells were plated at a density of 2 × 106 cells per milliliter on poly-l-lysine-coated glass coverslips and incubated for 4 days in serum-containing medium with 10 μM BrdU (Sigma–Aldrich). The cells were washed twice with PBS at room temperature and fixed with 4% paraformaldehyde in PBS for 30 min. After three washes with PBS, DNA was denatured by adding of 2 M HCL for 1 h at 37°C. Acid was then aspirated, and coverslips were neutralized by three washes with 0.1 M borate buffer (pH 8.5). The endogenous peroxidase was then neutralized with 3% H2O2 for 15 min, and the sections were blocked with 10% FBS diluted in PBS containing 1% BSA (Sigma–Aldrich) and 0.3% Triton X-100 (Sigma–Aldrich). The immunostaining procedure was performed with a mouse monoclonal anti-BrdU antibody diluted to 1:500 (Sigma–Aldrich) and horseradish peroxidase-coupled goat anti-mouse diluted to 1:100 (DAKO-Cytomation). Color reactions were performed with peroxidase substrate 3,3′-diaminobenzidine. After three washes, a second staining was performed with a sheep monoclonal anti-PNMT antibody diluted to 1:1500 (Chemicon, Temecula, CA) and horseradish peroxidase-coupled rabbit anti-sheep diluted to 1:100 (DAKO-Cytomation) using peroxidase substrate 3,3′,5,5′ tetramethylbenzidine (TMB; Vector Laboratories, Burlingame, CA) as a chromogen. All antibodies were diluted in PBS containing 1% BSA and 0.3% Triton X-100. To study the specificity of the immunoreaction, primary antibodies were substituted with PBS.
Measurement of Cell Proliferation.
Chromaffin cells were stimulated for 4 days in serum-free medium containing various concentrations of EGF (Sigma–Aldrich), LIF (Sigma–Aldrich), and/or steroids. DHEA, Dex, and DHEAS (Sigma–Aldrich) were initially diluted in ethanol (DHEA and Dex) or in DMSO (DHEAS). The final concentration of ethanol/DMSO in each well was ≤0.01%. After stimulation time, 20 μl of MTS rea-gent (Promega, Madison, WI) was added to each well (containing cells in 100 μl of culture medium), and the plate was incubated for 4 h at 37°C under a humidified atmosphere of 5% CO2. The absorbance of each well was then measured at 492 nm with a microplate reader (Mithras LB940; Berthold Technologies, Bad Wildbad, Germany). Relative cell numbers were quantified on the basis of the concentration of the formazan product of MTS.
Evaluation of Cell Death.
Chromaffin cells were stimulated for 4 days in serum-free medium containing various concentrations of growth factors, cytokines, and/or DHEA. After stimulation time, DHEA toxicity was evaluated by measuring lactate dehydrogenase (LDH) activity using the CytoTox96 nonradioactive assay (Promega) and quantitated by measuring wavelength absorbance at 490 nm with a microplate reader (Mithras LB940; Berthold Technologies). Cell death was assessed by the ratio of LDH release in the medium to cytosolic LDH content.
Acknowledgments
We thank Dr. D. Burel for helpful discussions. This work was supported by Deutsche Forschungsgemeinschaft Grants SFB 655 A6 (to M.E.-B. and S.R.B.) and SFB 655 A13 (to D.C.).
Abbreviations
- Dex
dexamethasone
- DHEA
dehydroepiandrostendione
- DHEAS
DHEA sulfate
- LIF
leukemia inhibitory factor
- PNMT
phenylethanolamine N-methyltransferase
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
Footnotes
The authors declare no conflict of interest.
References
- 1.Bornstein SR, Ehrhart-Bornstein M, Usadel H, Bockmann M, Scherbaum WA. Cell Tissue Res. 1991;265:1–9. doi: 10.1007/BF00318133. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd SP, Holzwarth MA. Am J Physiol. 2001;280:C61–C71. doi: 10.1152/ajpcell.2001.280.1.C61. [DOI] [PubMed] [Google Scholar]
- 3.Hodel A. J Neuroendocrinol. 2001;13:216–220. doi: 10.1046/j.1365-2826.2001.00628.x. [DOI] [PubMed] [Google Scholar]
- 4.Thoenen H, Mueller RA, Axelrod J. Biochem Pharmacol. 1970;19:669–673. doi: 10.1016/0006-2952(70)90229-7. [DOI] [PubMed] [Google Scholar]
- 5.Pohorecky LA, Piezzi RS, Wurtman RJ. Endocrinology. 1970;86:1466–1468. doi: 10.1210/endo-86-6-1466. [DOI] [PubMed] [Google Scholar]
- 6.Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Proc Natl Acad Sci USA. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karishma KK, Herbert J. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- 8.Kumai T, Tanaka M, Tateishi T, Watanabe M, Nakura H, Asoh M, Kobayashi S. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:620–624. doi: 10.1007/pl00005216. [DOI] [PubMed] [Google Scholar]
- 9.Merke DP, Chrousos GP, Eisenhofer G, Weise M, Keil MF, Rogol AD, Van Wyk JJ, Bornstein SR. N Engl J Med. 2000;343:1362–1368. doi: 10.1056/NEJM200011093431903. [DOI] [PubMed] [Google Scholar]
- 10.Merke DP, Bornstein SR. Lancet. 2005;365:2125–2136. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- 11.Charalampopoulos I, Tsatsanis C, Dermitzaki E, Alexaki VI, Castanas E, Margioris AN, Gravanis A. Proc Natl Acad Sci USA. 2004;101:8209–8214. doi: 10.1073/pnas.0306631101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu PS, Lin MK, Hsieh HL. Neurosci Lett. 1996;204:181–184. doi: 10.1016/0304-3940(96)12350-8. [DOI] [PubMed] [Google Scholar]
- 13.Charalampopoulos I, Dermitzaki E, Vardouli L, Tsatsanis C, Stournaras C, Margioris AN, Gravanis A. Endocrinology. 2005;146:3309–3318. doi: 10.1210/en.2005-0263. [DOI] [PubMed] [Google Scholar]
- 14.Unsicker K. J Anat. 1993;183:207–221. [PMC free article] [PubMed] [Google Scholar]
- 15.Chabot JG, Walker P, Pelletier G. Acta Endocrinol. 1986;113:391–395. doi: 10.1530/acta.0.1130391. [DOI] [PubMed] [Google Scholar]
- 16.Huff K, End D, Guroff G. J Cell Biol. 1981;88:189–198. doi: 10.1083/jcb.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gearing DP. Adv Immunol. 1993;53:31–58. doi: 10.1016/s0065-2776(08)60497-6. [DOI] [PubMed] [Google Scholar]
- 18.Bamberger AM, Schulte HM, Wullbrand A, Jung R, Beil FU, Bamberger CM. Mol Cell Endocrinol. 2000;162:145–149. doi: 10.1016/s0303-7207(00)00200-8. [DOI] [PubMed] [Google Scholar]
- 19.Kariagina A, Zonis S, Afkhami M, Romanenko D, Chesnokova V. Am J Physiol. 2005;289:E857–E863. doi: 10.1152/ajpendo.00577.2004. [DOI] [PubMed] [Google Scholar]
- 20.Ware CB, Kariagina A, Zonis S, Alon D, Chesnokova V. FEBS Lett. 2005;579:4465–4469. doi: 10.1016/j.febslet.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Oberle S, Schober A, Meyer V, Holtmann B, Henderson C, Sendtner M, Unsicker K. J Neurosci. 2006;26:1823–1832. doi: 10.1523/JNEUROSCI.4127-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unsicker K, Krieglstein K. Prog Neurobiol. 1996;48:307–324. doi: 10.1016/0301-0082(95)00045-3. [DOI] [PubMed] [Google Scholar]
- 23.Tischler AS, Riseberg JC, Hardenbrook MA, Cherington V. J Neurosci. 1993;13:1533–1542. doi: 10.1523/JNEUROSCI.13-04-01533.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stemple DL, Mahanthappa NK, Anderson DJ. Neuron. 1988;1:517–525. doi: 10.1016/0896-6273(88)90182-1. [DOI] [PubMed] [Google Scholar]
- 25.Powers JF, Schelling KH, Tischler AS. Neuroscience. 2001;108:341–349. doi: 10.1016/s0306-4522(01)00410-9. [DOI] [PubMed] [Google Scholar]
- 26.Tischler AS, Powers JF, Shahsavari M, Ziar J, Tsokas P, Downing J, McClain RM. Fundam Appl Toxicol. 1997;35:216–220. doi: 10.1006/faat.1996.2277. [DOI] [PubMed] [Google Scholar]
- 27.Kreiner E. Virchows Arch A Pathol Anat Histol. 1982;397:7–15. doi: 10.1007/BF00430889. [DOI] [PubMed] [Google Scholar]
- 28.Yoneyama A, Kamiya Y, Kawaguchi M, Fujinami T. Life Sci. 1997;60:833–838. doi: 10.1016/s0024-3205(97)00011-8. [DOI] [PubMed] [Google Scholar]
- 29.Williams MR, Dawood T, Ling S, Dai A, Lew R, Myles K, Funder JW, Sudhir K, Komesaroff PA. J Clin Endocrinol Metab. 2004;89:4708–4715. doi: 10.1210/jc.2003-031560. [DOI] [PubMed] [Google Scholar]
- 30.Saenger P, New M. Experientia. 1977;33:966–967. doi: 10.1007/BF01951309. [DOI] [PubMed] [Google Scholar]
- 31.Meikle AW, Dorchuck RW, Araneo BA, Stringham JD, Evans TG, Spruance SL, Daynes RA. J Steroid Biochem Mol Biol. 1992;42:293–304. doi: 10.1016/0960-0760(92)90132-3. [DOI] [PubMed] [Google Scholar]
- 32.McIntosh M, Hausman D, Martin R, Hausman G. Am J Physiol. 1998;275:E285–E293. doi: 10.1152/ajpendo.1998.275.2.E285. [DOI] [PubMed] [Google Scholar]
- 33.Compagnone NA, Mellon SH. Proc Natl Acad Sci USA. 1998;95:4678–4683. doi: 10.1073/pnas.95.8.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastianetto S, Ramassamy C, Poirier J, Quirion R. Brain Res Mol Brain Res. 1999;66:35–41. doi: 10.1016/s0169-328x(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 35.Aragno M, Parola S, Brignardello E, Mauro A, Tamagno E, Manti R, Danni O, Boccuzzi G. Diabetes. 2000;49:1924–1931. doi: 10.2337/diabetes.49.11.1924. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Li B, Ma W, Barker JL, Chang YH, Zhao W, Rubinow DR. Brain Res Mol Brain Res. 2002;98:58–66. doi: 10.1016/s0169-328x(01)00315-1. [DOI] [PubMed] [Google Scholar]
- 37.Gil-ad I, Shtaif B, Eshet R, Maayan R, Rehavi M, Weizman A. Isr Med Assoc J. 2001;3:639–643. [PubMed] [Google Scholar]
- 38.Lai GJ, McCobb DP. Proc Natl Acad Sci USA. 2002;99:7722–7727. doi: 10.1073/pnas.112619799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lapchak PA, Chapman DF, Nunez SY, Zivin JA. Stroke. 2000;31:1953–1956. doi: 10.1161/01.str.31.8.1953. and discussion (2000) 31:1957. [DOI] [PubMed] [Google Scholar]
- 40.Kurata K, Takebayashi M, Morinobu S, Yamawaki S. J Pharmacol Exp Ther. 2004;311:237–245. doi: 10.1124/jpet.104.067629. [DOI] [PubMed] [Google Scholar]
- 41.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Trends Endocrinol Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- 42.Monnet FP, Maurice T. J Pharmacol Sci. 2006;100:93–118. doi: 10.1254/jphs.cr0050032. [DOI] [PubMed] [Google Scholar]
- 43.Castro E, Gonzalez MP, Oset-Gasque MJ. J Neurosci Res. 2003;71:375–382. doi: 10.1002/jnr.10488. [DOI] [PubMed] [Google Scholar]
- 44.Liu D, Dillon JS. J Biol Chem. 2002;277:21379–21388. doi: 10.1074/jbc.M200491200. [DOI] [PubMed] [Google Scholar]
- 45.Mizota K, Yoshida A, Uchida H, Fujita R, Ueda H. Br J Pharmacol. 2005;145:545–550. doi: 10.1038/sj.bjp.0706213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charalampopoulos I, Alexaki VI, Lazaridis I, Dermitzaki E, Avlonitis N, Tsatsanis C, Calogeropoulou T, Margioris AN, Castanas E, Gravanis A. FASEB J. 2006;20:577–579. doi: 10.1096/fj.05-5078fje. [DOI] [PubMed] [Google Scholar]
- 47.Unsicker K, Muller TH. J Neurosci Methods. 1981;4:227–241. doi: 10.1016/0165-0270(81)90034-0. [DOI] [PubMed] [Google Scholar]
- 48.Bornstein SR, Gonzalez-Hernandez JA, Ehrhart-Bornstein M, Adler G, Scherbaum WA. J Clin Endocrinol Metab. 1994;78:225–232. doi: 10.1210/jcem.78.1.7507122. [DOI] [PubMed] [Google Scholar]