Abstract
The voltage-gated potassium (Kv) channel Kv1.5 mediates the IKur repolarizing current in human atrial myocytes and regulates vascular tone in multiple peripheral vascular beds. Understanding the complex regulation of Kv1.5 function is of substantial interest because it represents a promising pharmacological target for the treatment of atrial fibrillation and hypoxic pulmonary hypertension. Herein we demonstrate that posttranslational modification of Kv1.5 by small ubiquitin-like modifier (SUMO) proteins modulates Kv1.5 function. We have identified two membrane-proximal and highly conserved cytoplasmic sequences in Kv1.5 that conform to established SUMO modification sites in transcription factors. We find that Kv1.5 interacts specifically with the SUMO-conjugating enzyme Ubc9 and is a target for modification by SUMO-1, -2, and -3 in vivo. In addition, purified recombinant Kv1.5 serves as a substrate in a minimal in vitro reconstituted SUMOylation reaction. The SUMO-specific proteases SENP2 and Ulp1 efficiently deconjugate SUMO from Kv1.5 in vivo and in vitro, and disruption of the two identified target motifs results in a loss of the major SUMO-conjugated forms of Kv1.5. In whole-cell patch-clamp electrophysiological studies, loss of Kv1.5 SUMOylation, by either disruption of the conjugation sites or expression of the SUMO protease SENP2, leads to a selective ≈15-mV hyperpolarizing shift in the voltage dependence of steady-state inactivation. Reversible control of voltage-sensitive channels through SUMOylation constitutes a unique and likely widespread mechanism for adaptive tuning of the electrical excitability of cells.
Keywords: electrical excitability, posttranslational modification, transmembrane protein, ubiquitin-like modifier
Voltage-gated potassium (Kv) channels play critical roles in the highly regulated electrical responses throughout the cardiovascular system. By regulating potassium ion fluxes in response to alterations of the membrane potential, Kv channels are responsible for establishing the resting membrane potential and cellular repolarization in the heart and in peripheral vascular beds (1–3). Defects in members of this class of channels are thus linked to a wide range of cardiovascular diseases (4–6). The Shaker family of Kv channels has been extensively characterized both functionally and structurally (7, 8), and within this group Kv1.5 is widely represented in the cardiovascular system (9). In the human heart Kv1.5 is selectively expressed in atrial myocytes and mediates the ultrarapid K+ current (IKur) central to action potential repolarization (10). Recently, a familial form of atrial fibrillation has been attributed to a loss-of-function mutation in Kv1.5 (6). In the pulmonary vasculature Kv1.5 plays a critical role in the oxygen-sensitive regulation of arterial tone (11). Therefore, there is significant interest in Kv1.5 as a potential pharmacological target for diseases such as chronic atrial fibrillation and chronic hypoxic pulmonary arterial hypertension.
Allosteric channel modulators that selectively alter a gating property or that have preference for a particular conformational state are often preferable for therapeutic applications. An important mechanism of allosteric modulation of channel function is through posttranslational modifications. Despite the recent progress on the structural and functional properties of Kv channels (8), the nature and significance of posttranslational modifications remain poorly understood. For a subset of Kv channels, a role for phosphorylation (12–14) and glycosylation (15) in the modulation of channel function is emerging, but additional forms of posttranslational modification, such as conjugation of ubiquitin-like family members, remain to be investigated and represent a potentially rich area of regulation as illustrated recently for the K2P1 channel (16).
The SUMO family of proteins belongs to a growing class of ubiquitin-like (Ubl) proteins that share a common ancestry, structural fold, and core enzymological pathway of conjugation yet have very distinct sequences and functional properties (17). After an initial C-terminal proteolytic processing step, SUMO is activated in an ATP-dependent manner by the E1-activating enzyme (SAE1/SAE2) and transferred to Ubc9, the E2 SUMO-conjugating enzyme (18). Ubc9 interacts directly with substrates to catalyze the formation of an isopeptide bond between the C terminus of SUMO and the amino group of the target lysine. SUMO E3 ligases such as members of the PIAS family (19–22) enhance conjugation. SUMOylation is a reversible process, and SUMO-specific proteases such as Ulp (in yeast) and SENP family members (in mammals) release the SUMO moiety (23). Lysine residues targeted for modification are often embedded within specific sequences. The features of these preferred modification sites are dictated by the mode used by Ubc9 to recognize them (24). They include a large hydrophobic residue (ψ) preceding the modification site and a negatively charged amino acid two residues downstream (ψ-K-X-E/D). Interestingly, a detailed description of these properties, including the additional feature that this core sequence is often flanked by proline or glycine residues, was already established in the definition of synergy control or SC motifs. These regulatory motifs, found in many transcription factors, were initially defined by our group (25) and soon afterward were shown to function as sites of SUMOylation (26, 27). As with other modifications, the function of SUMOylation varies depending on the target protein. Unlike ubiquitination, however, SUMOylation does not directly target proteins for proteasomal degradation. Instead, SUMO modification can modulate a protein's activity or intracellular localization (17). Many of these effects appear to require a distinct surface in SUMO (28) capable of recognizing SUMO-interacting motifs in partner proteins (29). To date, SUMOylation has been most extensively studied in the context of nuclear proteins, and, in the case of sequence-specific transcription factors, SUMOylation exerts a direct and context-dependent inhibitory role (27, 28). SUMOylation, however, is not restricted to this compartment because the SUMO conjugation machinery is found throughout the cell and multiple proteins not associated with the nuclear compartment, such as the plasma membrane K+ channel K2P1 (16), are targets for SUMO modification (30–32).
Using a bioinformatic approach, we identified a number of Kv channels harboring sequences conforming to the definition of SC motifs. In Kv1.5, two such sequences are located in membrane-proximal regions of the channel. Concurrent with this initial observation, the potassium channel associated protein (KChAP), which can modulate the surface expression and whole-cell current densities of several Kv isoforms (33–35), was found to be a member of the PIAS family of SUMO E3 ligases (36). These observations raised the intriguing possibility that the consensus SUMOylation motifs we identified in Kv1.5 could serve as targets for SUMO modification.
Results
Kv1.5 Harbors Conserved SUMOylation Motifs and Interacts with Ubc9.
Scanning of the human Kv1.5 sequence using a search profile based on functionally characterized synergy control motifs in transcription factors identified two high-scoring sequences. The first, centered on Lys-221, lies between the T1 tetramerization domain and the first transmembrane segment. The second, centered on Lys-536, is located in the C-terminal tail just beyond the sixth transmembrane segment (Fig. 1A). Structural modeling of Kv1.5 using the recent crystal structure of Kv1.2 (8) places the motifs of each α-subunit (colored red in Fig. 1B) in close proximity to each other exposed to the side portals that provide cytoplasmic access to the pore of the channel. The model suggests that SUMO attachment to either K221 or K536 can be accommodated without undue alterations of the overall structure. The predicted SUMOylation sequences (Fig. 1A) conform to the canonical ψ-K-X-E/D pattern, and the core sequence is flanked by nearby proline or glycine residues, a criterion included in our search model. Sequence comparison across vertebrate species revealed that they lie within regions of high local conservation because surrounding sequences, especially those C-terminal to the second motif, are far more divergent (data not shown). Interestingly, whereas both sites are conserved in mammalian sequences, only one of the sites is present in avian, amphibian, and fish species. Comparison to other human Kv family members reveals that the first motif is intact in Kv1.1 and Kv1.2.
Fig. 1.
Kv1.5 contains two conserved consensus SUMOylation motifs and interacts with Ubc9. (A) A schematic representation of Kv1.5. T1, tetramerization domain; S1–S6, transmembrane domains. Aligned vertebrate Kv1.5 sequences centered on human K221 (Motif 1) and human K536 (Motif 2) are on the left, and the corresponding regions in human Kv channels are on the right. The core and flanking Gly/Pro residues of the motif are boxed. Asterisks indicate channels with predicted SUMOylation motifs. A SUMOylation consensus is shown above. (B) Structural model of Kv1.5 using the coordinates of Kv1.2-β2 (24). Modeled consensus SUMOylation motifs on each of the three depicted α-subunits are highlighted in red. SUMO-2 is shown for comparison. (C) In vitro interaction between Kv1.5 and Ubc9. Kv1.5 N556 corresponds to a C-terminal truncation lacking the last 57 aa but retaining both SUMOylation motifs. Load corresponds to 10% of applied material.
To determine whether Kv1.5 can interact with the E2 SUMO-conjugating enzyme Ubc9, we subjected extracts from cells expressing V5/His epitope-tagged hKv1.5 to affinity chromatography using immobilized recombinant GST-Ubc9 or GST. Kv1.5 was retained efficiently on the GST-Ubc9 resin but failed to interact with GST alone (Fig. 1C). Titration of the GST-Ubc9 fusion indicates that the affinity of interaction is ≈400 nM (data not shown). Consistent with the location of the two predicted modification sites, a 57-aa C-terminal truncation of Kv1.5 (N556) that preserves both motifs also interacts efficiently (Fig. 1C Right). The presence of putative modification sites and the interaction with Ubc9 make Kv1.5 a likely target of SUMOylation.
Kv1.5 Is SUMO-Modified in Vivo and in Vitro.
To evaluate whether Kv1.5 is SUMOylated in live cultured cells (in vivo), we isolated V5/His-tagged hKv1.5 from cells coexpressing HA-SUMO-3 and Ubc9 by Ni+-chelate chromatography under denaturing conditions. Western blot analysis of the preparations using anti-HA antibodies (Fig. 2A) indicates that two major HA-immunoreactive bands (marked with arrows) are detected only in samples derived from cells coexpressing SUMO and Kv1.5. These bands correspond to SUMO-modified Kv1.5 because they are also visible as minor V5-immunoreactive species (arrows on anti-V5 blot). As in the case of most SUMOylated proteins (17), the extent of Kv1.5 modification appears to be relatively low (≈1%). For the N556 C-terminal deletion mutant of Kv1.5, we detected a corresponding shift in the mobility of the HA- and V5-immunoreactive species, confirming that the SUMO conjugates do indeed derive from Kv1.5 and that the C-terminal region is dispensable for SUMOylation. Omission of N-ethylmaleimide (NEM), an inhibitor of SUMO-specific deconjugating enzymes, during sample preparation led to reduced recovery of both HA- and V5-immunoreactive SUMO-modified Kv1.5 species (Fig. 2B). Likewise, exposure of Kv1.5 to purified recombinant Ulp1, a yeast SUMO isopeptidase (37), during the Ni+-chelate chromatography released substantial amounts of the SUMO moiety from Kv1.5 (Fig. 2B).
Fig. 2.
Kv1.5 is SUMO modified in vivo and in vitro. (A) Cos-7 cells were cotransfected as described in Materials and Methods with 200 ng of pCDNA3 Ubc9 and, as indicated, 200 ng of pCDNA3 HA-SUMO3, 200 ng of pCDNA3.1 hKv1.5 V5-His, 160 ng of pCDNA3.1 hKv1.5 N556 V5-His, or an equimolar amount of the corresponding empty vector. His-tagged proteins were purified and detected by immunoblotting with anti-HA (Top) and anti-V5 (Middle and Bottom) antibodies. Middle is an overexposed version of Bottom. SUMO-modified species of Kv1.5 are indicated with arrows, and nonspecific bands are indicated with asterisks. (B) Samples were transfected and processed as in A except cells were harvested in the presence or absence of NEM as indicated, and samples were treated with GST or the SUMO-specific protease GST-Ulp1. Arrows indicate SUMO-modified species of Kv1.5. (C) Purified hKv1.5 was incubated with the indicated SUMO conjugation machinery components as described in Materials and Methods. Arrows indicate SUMO-modified species of Kv1.5.
To examine whether Kv1.5 can serve as a substrate for SUMO modification in vitro, we obtained purified preparations of recombinant WT V5/His-tagged hKv1.5 after baculovirus expression in Sf9 cells. Incubation of this material in a reconstituted SUMOylation reaction containing homogenous, recombinant E1-activating (SAE1/SAE2) and E2-conjugating (Ubc9) enzymes along with purified SUMO-2 and an ATP-regenerating system led to the appearance of slow-migrating, SUMO-2-conjugated forms of Kv1.5 (Fig. 2C, first lane). The two lower bands (marked by arrows) correspond in size to the species seen in Fig. 2 A and B. The additional higher-molecular-weight species likely represent multiple SUMOylated forms due to the abundant SUMO chain formation that occurs under these in vitro conditions (data not shown). Although not as extensive, a similar pattern of conjugation is observed with SUMO-1 (Fig. 2, leftmost lanes). Notably, when any one component is omitted from the reaction, no SUMO-modified species could be detected. These results provide strong evidence that Kv1.5 is covalently modified by SUMO both in vivo and in vitro.
Modification of Kv1.5 by Multiple SUMO Isoforms Requires Intact SUMOylation Motifs.
In humans, three SUMO isoforms competent for conjugation have been identified. Whereas SUMO-2 and SUMO-3 are nearly identical, they share only 50% amino acid identity with SUMO-1 and there is mounting evidence for isoform-selective subcellular localization (17) and function (27). We find that each of these SUMO isoforms can be conjugated to Kv1.5 in vivo; however, they do so to different extents (Fig. 3A). In contrast to SUMO-3 modified forms, SUMO-2 and especially SUMO-1 conjugates are substantially less abundant and require longer exposures to be detected. Although this preference may be intrinsic to Kv1.5, it also reflects the relative levels of expression of the isoforms because free SUMO-3 accumulates to substantially higher levels than SUMO-2 or SUMO-1 (data not shown). To determine whether the two putative SUMOylation motifs that we identified serve as sites of conjugation, we replaced the predicted target lysines with non-SUMOylatable arginine residues. Mutation of the first (K221R) or second (K536R) motifs leads to a preferential loss of the higher and lower migrating SUMO-conjugated species, respectively (Fig. 3B, arrows). Although we have not assessed the stoichiometry or topology of the linkage within individual species, modification at different positions may lead to distinct migration patterns. The double mutant (K221/536R), however, leads to loss of the two major SUMO-modified Kv1.5 species. Consistent with the SUMOylation consensus, replacement of the conserved Glu-538 at the fourth position of the second motif by Arg (in the context of K221R) or mutation of the first hydrophobic position of both motifs to Asn (I220N/L535N) also severely compromised Kv1.5 SUMOylation [supporting information (SI) Fig. 6]. In contrast, disruption of the motifs with the K221/536R mutations did not alter the recovery of ubiquitinated species in the presence of a proteasomal inhibitor (SI Fig. 7). These findings indicate that the proposed motifs in Kv1.5 serve as the major sites of SUMO conjugation.
Fig. 3.
Modification of Kv1.5 by multiple SUMO isoforms requires intact SUMOylation motifs. (A) Cos-7 cells were cotransfected with 3 μg each of pCDNA3 Ubc9, pCDNA3 HA-SUMO1/2/or 3, and 1 μg of pCDNA3.1 hKv1.5 V5-His or an equimolar amount of the corresponding empty vector. Samples were analyzed as in Fig. 2A. Relative exposure times of the anti-HA immunoblots are indicated below each blot. Arrows indicate SUMO-modified species of Kv1.5. (B) Cos-7 cells were transfected with pCDNA3-based vectors for the indicated proteins. Transfection and sample analysis were as in Fig. 2A. SUMO-modified species are indicated with arrows, and nonspecific bands observed occasionally are indicated with an asterisk.
Disruption of SUMOylation Motifs Alters Kv1.5 Function.
To probe the functional role of the SUMOylation motifs in Kv1.5, we characterized the voltage-dependent potassium currents elicited by expression of Kv1.5 forms harboring intact or disrupted SUMOylation motifs. Analysis of whole-cell patch-clamp recordings indicated that the K221/536R substitutions had no effect on the total current density (Fig. 4A) or on the voltage dependence of channel activation (Fig. 4B). In contrast, the substitutions led to a specific ≈15-mV hyperpolarizing shift in the half-maximal voltage of inactivation (V50) from the WT value of 0.33 ± 1.2 to −14.8 ± 1.4 mV (P < 0.0001). This shift occurs without appreciable alterations in the extent of inactivation (53.8% and 50.9% for WT and K221/536R, respectively). Notably, reintroduction of the WT sequence at either position returned the V50 value to nearly WT levels (Fig. 4C). This indicates that the motifs are functionally equivalent and that the presence of a single intact motif is sufficient to exert a nearly complete effect. Moreover, mutation of motif residues other than the lysines also had a functional effect. In the context of the K221R mutant, addition of the E538R substitution in the second motif led to a significant shift in V50 from −4.0 ± 0.9 to −8.8 ± 1.2 mV (P < 0.0005). Likewise, mutation of the first position in both motifs to Asn (I220N/L535N) led to a shift indistinguishable from that of the K221/536R mutant (−17.44 ± 1.6 vs. −14.8 ± 1.4 mV; P = 0.32) (SI Fig. 6). Taken together, these data suggest that, although not essential for assembly of functional Kv1.5 channels, the motifs selectively regulate their biophysical properties.
Fig. 4.
Disruption of SUMOylation motifs alters Kv1.5 inactivation. Cos-7 cells were cotransfected with 0.1 μg each of pEGFP-C1 (to identify transfected cells) and a pCDNA3-based vector for the expression of the indicated Kv1.5 variant. Recordings were obtained as described in Materials and Methods. (A) Current–voltage curves. Data represent average ± SEM of 11 WT and 11 K221/536R cells. (B) Voltage dependence of steady-state activation (n = 11 and 10 for WT and K221/536R, respectively). (C) Voltage dependence of steady-state inactivation (n = 7–10 for each construct). The key shows calculated V50 values.
SUMOylation Regulates Kv1.5 Inactivation.
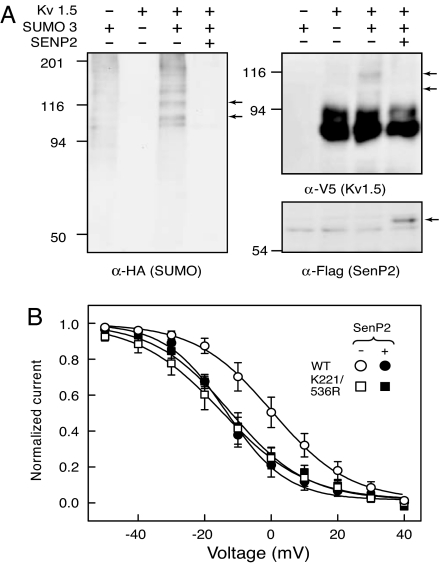
The concurrent loss of SUMO modification and shift in the voltage dependence of inactivation in multiple SUMOylation motif mutants argues strongly in favor of SUMOylation (over other lysine-directed modifications) as the mechanism responsible for the functional effects exerted by the motifs. If this is indeed the case, reducing the levels of SUMOylation from WT Kv1.5 should lead to a similar alteration in the voltage dependence of inactivation. To test this hypothesis we took advantage of a variant of the SUMO protease SENP2 lacking the first 70 N-terminal residues (FLAG-SENP2 71–590). This form displays enhanced activity against multiple SUMOylated substrates (38) and localizes to the cytoplasm (39). Coexpression of this SUMO protease led to the complete loss of Kv1.5 SUMO conjugates (Fig. 5A). In parallel, coexpression of SENP2 with WT Kv1.5 produced an ≈14-mV hyperpolarizing shift in the voltage dependence of steady-state inactivation, altering the V50 from 0.33 ± 1.2 mV in its absence to −13.5 ± 0.9 mV in its presence (P < 0.0001) (Fig. 5B). This effect is indistinguishable from that of the K221/536R mutant (V50 = −14.8 ± 1.4 mV; P > 0.4). Importantly, the effects of SENP2 require the integrity of the SUMOylation motifs in Kv1.5 because coexpression of SENP2 did not alter the V50 of the SUMOylation-deficient K221/536R mutant (V50 = −12.3 ± 0.9 mV; P > 0.16). Taken together, these findings demonstrate that loss of SUMO modification of Kv1.5, by either point mutations at the SUMOylation motifs or coexpression with the SUMO-specific protease SENP2, leads to a significant hyperpolarizing shift in the voltage dependence of inactivation without altering either the total current density or voltage dependence of activation of the channel.
Fig. 5.
SENP2-mediated loss of Kv1.5 SUMOylation alters inactivation. (A) Cos-7 cells were cotransfected as in Fig. 2A with an additional 200 ng of pCMV or pCMV FLAG SENP2 (71–590). Samples were analyzed as in Fig. 2A. Whole-cell lysates were resolved by SDS/PAGE and probed with anti-FLAG antibodies (Lower Right). Arrows indicate SUMO-modified species of Kv1.5 in the anti-HA and anti-V5 immunoblots and SENP2 in the anti-FLAG blot. (B) Cos-7 cells were cotransfected with 0.1 μg of the indicated Kv1.5 plasmid, 0.1 μg of pEGFP-C1, and 0.5 μg of pCMV FLAG SENP2 (71–590). Voltage dependence of steady-state inactivation in the presence of SENP2 was obtained from seven and six cells for WT and K221/536R, respectively. Data in the absence of SENP2 are from Fig. 4C.
Discussion
The present study identifies SUMOylation as a previously undescribed form of posttranslational modification of Kv1.5 and establishes that it exerts a selective regulatory function. Although this is the first member of the voltage-gated ion family that we have found to serve as a target for SUMO modification, multiple Kv channel α-subunits, including Kv1.1 and Kv1.2, contain similarly located consensus SUMOylation motifs. SUMOylation may thus be a widespread mechanism of channel regulation. The recent description of SUMOylation of the glucose transporters GLUT1 and GLUT4 (30, 40), the two-pore potassium leak channel K2P1 (16), and the G protein-coupled receptor mGluR8 (31) further supports the view that SUMOylation is an important regulatory mechanism for integral membrane proteins.
In this report we have demonstrated that disruption of Kv1.5 SUMO modification has a significant effect on the biophysical properties of the channel. Notably, loss of SUMOylation elicits a substantial phenotype even though only a small fraction of the total cellular Kv1.5 appears to be SUMOylated. Interestingly, neither Ubc9 nor SUMO appear to be limiting, because overexpression of SUMO3 and Ubc9 did not shift the V50 of WT Kv1.5 in the depolarizing direction (data not shown). Given the oligomeric structure of the channel, however, modification of only one of the eight sites per tetramer may be sufficient to exert a functional effect. In addition, the small fraction of channels that are active at the plasma membrane may be selectively targeted for SUMOylation. Alternatively, transient SUMOylation may be sufficient to establish a long-lived assembly or functional state of the channel. The ability of SUMO to exert substantial regulatory effects even at low stoichiometries is also observed for other target proteins such as transcription factors and appears to be more the norm than the exception. There are, however, proteins that are modified at high levels, such as the K2P1 channel (16), RanGap (41), and the A40R vaccinia virus protein (42). For this second class, SUMO may be functioning as an essential structural assembly element as opposed to serving a transient modulatory one. Thus, in the case of the K2P1 channel, SUMOylation appears to be an absolute gatekeeper preventing any access to conducting states of the channel (16), whereas in Kv1.5 this modification plays a more modulatory role.
Although the basis for the effects of SUMOylation on inactivation of Kv1.5 is not fully understood, the juxtamembrane location of the modification sites may be instructive. The electrostatic environment in this region is thought to influence the voltage-sensitivity of Kv channels (43). The surface of SUMO is highly charged. Notably, one hemisphere is rich in basic residues whereas the opposite face is predominantly acidic. Conjugation of SUMO may modulate the local electrostatic environment and thereby influence voltage-sensitive steps. The selective effects of SUMOylation on the voltage dependence of inactivation, but not activation, argue that SUMOylation affects steps other than the voltage-sensitive transitions along the usual path toward activation. Certain Kv channel β-subunits confer rapid N-type inactivation to Kv1.5 by providing an N-terminal inactivation peptide. Intriguingly, the first SUMOylation site (K221) is three residues downstream from an acidic region in the T1–S1 linker that is thought to facilitate N-type inactivation by positioning the inactivating peptide in close vicinity to the pore (44). SUMOylation could regulate β-subunit-dependent N-type inactivation through direct steric hindrance or by altering the local electrostatic environment. The current experiments were conducted in cells that do not express β-subunits, so exploring the role of SUMO modification of Kv1.5 in the context of various α/β-subunit combinations will likely be informative. The effects of SUMO modification in contexts such as transcriptional regulation are likely due to the promotion of protein–protein interactions (27, 45, 46). Notably, a critical surface in SUMO essential for transcriptional inhibition (28) is the site of interaction for I/L/V rich SUMO-interacting motifs present in multiple proteins (29, 47), including some associated with membrane proteins (40). Because Kv1.5 functions as part of a multiprotein/lipid complex comprising proteins with scaffolding, cytoskeletal, and enzymatic activities, SUMO modification of Kv1.5 may function by modulating these interactions.
Given the functional effects of SUMOylation, SUMO proteases or E3 enzymes that target Kv1.5 are likely to exert important regulatory functions. In this regard, the K+ channel-associated protein KChAP (35) has been shown to be a member of the PIAS family of proteins originally identified as negative regulators of several transcriptional responses (33). PIAS proteins possess intrinsic SUMO-specific E3 activity (48), and most of their transcriptional effects depend on this function. Although the regulatory consequences of KChAP association with Kv1.5 remain to be determined, the effects on surface expression observed for other Kv channels occur in a transcription-independent manner (33). In addition, because the SUMOylation pathway is sensitive to redox signals (49), SUMOylation may contribute to the oxygen-sensing properties of Kv1.5 in the pulmonary vasculature (50). Clearly, alterations in the SUMOylation of Kv1.5 have the potential to alter the excitability of both atrial myocytes and vascular smooth muscle cells by modulating either the action potential duration or the resting membrane potential. Thus, mechanisms that regulate Kv1.5 SUMOylation afford a novel link between signaling pathways and cellular excitability.
Materials and Methods
Expression Plasmids, Cell Culture, and in Vivo SUMOylation Assays.
The expression vectors pCDNA3.1 hKv1.5 V5-His and pCDNA3.1 hKv1.5 N556 V5-His consist of the full-length or the first 556 residues of human Kv1.5 inserted at the EcoRI and XhoI sites of pCDNA3.1 V5-HIS A (Invitrogen, Carlsbad, CA). Derivatives bearing K221R or K536R substitutions alone or in combination were generated by PCR. pCMV FLAG SENP2 (71–590) was a gift of Mary Dasso (National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD), and pEGFP-C1 was from Clontech (Mountain View, CA). Other vectors are described in ref. 21. COS-7 cells were maintained and transfected as described (27). For in vivo SUMOylation assays, 1.2 × 105 cells were seeded in six-well plates and transfected with the indicated amounts of expression plasmids. The total amount of DNA per transfection was supplemented to 1.3 μg per well with pBSKS (−). For the data in Fig. 3A, 2 × 106 cells were seeded in 10-cm plates, and the total amount of DNA was 12 μg per plate. In vivo SUMOylation assays were adapted from ref. 21. Cells were harvested 48 h after transfection and homogenized in 300 μl of CHAPS lysis buffer [50 mM sodium phosphate, pH 8.0/500 mM NaCl/10 mM imidazole/1% CHAPS/20 mM NEM, supplemented with complete EDTA-free miniprotease inhibitor tablets (Roche, Indianapolis, IN); one tablet per 10 ml]. After centrifugation (16,000 × g for 15 min), solubilized proteins were incubated (1 h at 25°C) with 0.1 ml of Ni-NTA-agarose (Qiagen, Valencia, CA). The resin was sequentially washed (2 ml three times) with buffer 1 (50 mM sodium phosphate, pH 6.5/400 mM NaCl/10 mM imidazole/0.1% CHAPS), buffer 2 (buffer 1 with 150 mM NaCl, no imidazole, and 8 M urea), and buffer 3 (50 mM sodium phosphate, pH 8.0/50 mM NaCl/0.1% CHAPS). Ulp1 treatment was carried out between washes one and two and consisted of 1-h incubations at 30°C with 242 pmol of purified GST or GST-Ulp1 after equilibration in buffer 2 without urea. For the data in Fig. 3A, the cells were lysed in 750 μl of urea lysis buffer (CHAPS lysis buffer with 8 M urea instead of 1% CHAPS and no NEM) and washed in urea buffer 1 (wash buffer 1 with 8 M urea and 0.2% Nonidet P-40 instead of 0.1% CHAPS) and Nonidet P-40 buffer 2 (wash buffer 2 with no urea and 0.2% Nonidet P-40 instead of 0.1% CHAPS). Proteins were eluted by incubating (55°C for 20 min) in SDS sample buffer (50 mM Tris·HCl, pH 6.8/2% SDS/5% glycerol/3.3 mM EDTA/0.0075% bromophenol blue/6.5 mM DTT), resolved by SDS/PAGE (7.5%), and processed for immunoblotting by using anti-V5 (Invitrogen), anti-HA (HA-11; Covance), or anti-FLAG (Sigma, St. Louis, MO) mouse monoclonal antibodies. Detection was achieved with goat anti-mouse IgG peroxidase conjugate (Bio-Rad, Hercules, CA) and Super Signal West Femto substrates (Pierce, Rockford, IL). All experiments were performed at least three times with similar results.
Purification of hKv1.5, Protein–Protein Interaction, and in Vitro SUMO Conjugation Assays.
hKv1.5 V5/His was expressed in Sf-9 cells and purified from detergent extracts by sequential Ni2+-NTA Sepharose affinity, ion exchange, and gel filtration columns. (Procedural details can be found in SI Materials and Methods.) For protein–protein interaction assays, 2 × 106 cells were seeded in 10-cm plates and transfected with 5 μg of either pCDNA3.1 Kv1.5 V5-His or pCDNA3.1 Kv1.5 N556 V5-His. Cells were harvested 48 h after transfection in 750 μl of CHAPS lysis buffer, sonicated, and centrifuged (16,000 × g for 15 min, 4°C). Supernatant (4.5 μl) was processed as described (21) by using buffers supplemented with 0.1% DDM. Samples were resolved by SDS/PAGE and processed for V5 epitope immunoblotting. In vitro SUMOylation assays (27) included 400 nM SAE1/SAE2, 4 μM Ubc9, 15 μM SUMO, and purified hKv1.5 V5-His preparations as indicated. Reactions were carried out at 30°C for 2 h. Samples were resolved by SDS/PAGE and processed for V5 epitope immunoblotting.
Electrophysiology.
For electrophysiological studies, 1 × 106 cells were seeded in 35-mm plates and cotransfected with 0.1 μg each of pEGFP-C1 and the indicated pCDNA3.1 hKv1.5 plasmid. Electrophysiological recordings and analysis were conducted as previously described (51). The current–voltage relationships and activation curves were measured by using 250-ms voltage-clamp pulses applied in 10-mV steps between −80 mV and +60 mV. Current was measured at the end of the 250-ms depolarization to obtain the steady-state current–voltage relationships. Tail current amplitude immediately after the capacitive transient was measured to obtain the voltage dependence of activation curves. Steady-state inactivation was measured by using a 5-s conditioning pulse applied in 10-mV steps from −60 mV to +40 mV followed by a 500-ms test pulse at +30 mV. Current was measured at the end of the 500-ms test pulse. Normalized activation and inactivation curves were fitted to the Boltzmann equation, and results are expressed as mean ± standard error. Comparison of V50 values was carried out with an F test applied to global fits of pooled vs. separate sets.
Supplementary Material
Acknowledgments
We thank Dr. Mary Dasso for the SENP2 expression plasmid. This work was supported by U.S. Public Health Service Grants DK61656-01 and HL070973, National Institutes of Health Grant P60 DK20572, and a University of Michigan Cardiovascular Center McKay Research Grant. M.D.B. is supported by American Heart Association Predoctoral Fellowship 0515560Z.
Abbreviation
- NEM
N-ethylmaleimide.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Nerbonne JM, Kass RS. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 2.Nelson MT, Quayle JM. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 3.Jackson WF. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackerman MJ. Nat Med. 2004;10:463–464. doi: 10.1038/nm0504-463. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney M, Yuan JX. Respir Res. 2000;1:40–48. doi: 10.1186/rr11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A, Terzic A. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 7.Jan LY, Jan YN. J Physiol. 1997;505:267–282. doi: 10.1111/j.1469-7793.1997.267bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long SB, Campbell EB, Mackinnon R. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 9.Overturf KE, Russell SN, Carl A, Vogalis F, Hart PJ, Hume JR, Sanders KM, Horowitz B. Am J Physiol. 1994;267:C1231–C1238. doi: 10.1152/ajpcell.1994.267.5.C1231. [DOI] [PubMed] [Google Scholar]
- 10.Nattel S, Yue L, Wang Z. Cell Physiol Biochem. 1999;9:217–226. doi: 10.1159/000016318. [DOI] [PubMed] [Google Scholar]
- 11.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes TC, Fadool DA, Ren R, Levitan IB. Science. 1996;274:2089–2091. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- 13.Kwak YG, Hu N, Wei J, George AL, Jr, Grobaski TD, Tamkun MM, Murray KT. J Biol Chem. 1999;274:13928–13932. doi: 10.1074/jbc.274.20.13928. [DOI] [PubMed] [Google Scholar]
- 14.Kwak YG, Navarro-Polanco RA, Grobaski T, Gallagher DJ, Tamkun MM. J Biol Chem. 1999;274:25355–25361. doi: 10.1074/jbc.274.36.25355. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Takimoto K, Levitan ES. J Biol Chem. 2000;275:11597–11602. doi: 10.1074/jbc.275.16.11597. [DOI] [PubMed] [Google Scholar]
- 16.Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. Cell. 2005;121:37–47. doi: 10.1016/j.cell.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Johnson ES. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 18.Desterro JM, Thomson J, Hay RT. FEBS Lett. 1997;417:297–300. doi: 10.1016/s0014-5793(97)01305-7. [DOI] [PubMed] [Google Scholar]
- 19.Kirsh O, Seeler JS, Pichler A, Gast A, Muller S, Miska E, Mathieu M, Harel-Bellan A, Kouzarides T, Melchior F, Dejean A. EMBO J. 2002;21:2682–2691. doi: 10.1093/emboj/21.11.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotaja N, Karvonen U, Janne OA, Palvimo JJ. Mol Cell Biol. 2002;22:5222–5234. doi: 10.1128/MCB.22.14.5222-5234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian L, Benson MD, Iniguez-Lluhi JA. J Biol Chem. 2003;278:9134–9141. doi: 10.1074/jbc.M210440200. [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, Blobel G. Proc Natl Acad Sci USA. 2005;102:4777–4782. doi: 10.1073/pnas.0500537102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh ET, Gong L, Kamitani T. Gene. 2000;248:1–14. doi: 10.1016/s0378-1119(00)00139-6. [DOI] [PubMed] [Google Scholar]
- 24.Sampson DA, Wang M, Matunis MJ. J Biol Chem. 2001;276:21664–21669. doi: 10.1074/jbc.M100006200. [DOI] [PubMed] [Google Scholar]
- 25.Iniguez-Lluhi JA, Pearce D. Mol Cell Biol. 2000;20:6040–6050. doi: 10.1128/mcb.20.16.6040-6050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poukka H, Karvonen U, Janne OA, Palvimo JJ. Proc Natl Acad Sci USA. 2000;97:14145–14150. doi: 10.1073/pnas.97.26.14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmstrom S, Van Antwerp ME, Iniguez-Lluhi JA. Proc Natl Acad Sci USA. 2003;100:15758–15763. doi: 10.1073/pnas.2136933100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chupreta S, Holmstrom S, Subramanian L, Iniguez-Lluhi JA. Mol Cell Biol. 2005;25:4272–4282. doi: 10.1128/MCB.25.10.4272-4282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song J, Durrin LK, Wilkinson TA, Krontiris TG, Chen Y. Proc Natl Acad Sci USA. 2004;101:14373–14378. doi: 10.1073/pnas.0403498101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giorgino F, de Robertis O, Laviola L, Montrone C, Perrini S, McCowen KC, Smith RJ. Proc Natl Acad Sci USA. 2000;97:1125–1130. doi: 10.1073/pnas.97.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang Z, El Far O, Betz H, Scheschonka A. J Biol Chem. 2005;280:38153–38159. doi: 10.1074/jbc.M508168200. [DOI] [PubMed] [Google Scholar]
- 32.Johnson ES, Blobel G. J Cell Biol. 1999;147:981–994. doi: 10.1083/jcb.147.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuryshev YA, Gudz TI, Brown AM, Wible BA. Am J Physiol. 2000;278:C931–C941. doi: 10.1152/ajpcell.2000.278.5.C931. [DOI] [PubMed] [Google Scholar]
- 34.Kuryshev YA, Wible BA, Gudz TI, Ramirez AN, Brown AM. Am J Physiol. 2001;281:C290–C299. doi: 10.1152/ajpcell.2001.281.1.C290. [DOI] [PubMed] [Google Scholar]
- 35.Wible BA, Yang Q, Kuryshev YA, Accili EA, Brown AM. J Biol Chem. 1998;273:11745–11751. doi: 10.1074/jbc.273.19.11745. [DOI] [PubMed] [Google Scholar]
- 36.Wible BA, Wang L, Kuryshev YA, Basu A, Haldar S, Brown AM. J Biol Chem. 2002;277:17852–17862. doi: 10.1074/jbc.M201689200. [DOI] [PubMed] [Google Scholar]
- 37.Li SJ, Hochstrasser M. Nature. 1999;398:246–251. doi: 10.1038/18457. [DOI] [PubMed] [Google Scholar]
- 38.Hang J, Dasso M. J Biol Chem. 2002;277:19961–19966. doi: 10.1074/jbc.M201799200. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Saitoh H, Matunis MJ. Mol Cell Biol. 2002;22:6498–6508. doi: 10.1128/MCB.22.18.6498-6508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lalioti VS, Vergarajauregui S, Pulido D, Sandoval IV. J Biol Chem. 2002;277:19783–19791. doi: 10.1074/jbc.M110294200. [DOI] [PubMed] [Google Scholar]
- 41.Matunis MJ, Coutavas E, Blobel G. J Cell Biol. 1996;135:1457–1470. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palacios S, Perez LH, Welsch S, Schleich S, Chmielarska K, Melchior F, Locker JK. Mol Biol Cell. 2005;16:2822–2835. doi: 10.1091/mbc.E04-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurata HT, Fedida D. Prog Biophys Mol Biol. 2006;92:185–208. doi: 10.1016/j.pbiomolbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Gulbis JM, Zhou M, Mann S, MacKinnon R. Science. 2000;289:123–127. doi: 10.1126/science.289.5476.123. [DOI] [PubMed] [Google Scholar]
- 45.Girdwood D, Bumpass D, Vaughan OA, Thain A, Anderson LA, Snowden AW, Garcia-Wilson E, Perkins ND, Hay RT. Mol Cell. 2003;11:1043–1054. doi: 10.1016/s1097-2765(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 46.Minty A, Dumont X, Kaghad M, Caput D. J Biol Chem. 2000;275:36316–36323. doi: 10.1074/jbc.M004293200. [DOI] [PubMed] [Google Scholar]
- 47.Hecker CM, Rabiller M, Haglund K, Bayer P, Dikic I. J Biol Chem. 2006;281:16117–16127. doi: 10.1074/jbc.M512757200. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt D, Muller S. Proc Natl Acad Sci USA. 2002;99:2872–2877. doi: 10.1073/pnas.052559499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bossis G, Melchior F. Mol Cell. 2006;21:349–357. doi: 10.1016/j.molcel.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 50.Archer SL, Michelakis ED, Thebaud B, Bonnet S, Moudgil R, Wu XC, Weir EK. Novartis Found Symp. 2006;272:157–171. and discussion (2006) 272:171–175, 214–217. [PubMed] [Google Scholar]
- 51.Hulme JT, Coppock EA, Felipe A, Martens JR, Tamkun MM. Circ Res. 1999;85:489–497. doi: 10.1161/01.res.85.6.489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.