Abstract
Introduction
Apoptosis of neutrophils (polymorphonuclear neutrophils [PMNs]) may limit inflammatory injury in sepsis and acute respiratory distress syndrome (ARDS), but the relationship between the severity of sepsis and extent of PMN apoptosis and the effect of superimposed ARDS is unknown. The objective of this study was to correlate neutrophil apoptosis with the severity of sepsis and sepsis-induced ARDS.
Methods
A prospective cohort study was conducted in intensive care units of three tertiary hospitals in Porto Alegre, southern Brazil. Fifty-seven patients with sepsis (uncomplicated sepsis, septic shock, and sepsis-induced ARDS) and 64 controls were enrolled. Venous peripheral blood was collected from patients with sepsis within 24 hours of diagnosis. All surgical groups, including controls, had their blood drawn 24 hours after surgery. Control patients on mechanical ventilation had blood collected within 24 hours of initiation of mechanical ventilation. Healthy controls were blood donors. Neutrophils were isolated, and incubated ex vivo, and apoptosis was determined by light microscopy on cytospun preparations. The differences among groups were assessed by analysis of variance with Tukeys.
Results
In medical patients, the mean percentage of neutrophil apoptosis (± standard error of the mean [SEM]) was lower in sepsis-induced ARDS (28% ± 3.3%; n = 9) when compared with uncomplicated sepsis (57% ± 3.2%; n = 8; p < 0.001), mechanical ventilation without infection, sepsis, or ARDS (53% ± 3.0%; n = 11; p < 0.001) and healthy controls (69% ± 1.1%; n = 33; p < 0.001) but did not differ from septic shock (38% ± 3.7%; n = 12; p = 0.13). In surgical patients with sepsis, the percentage of neutrophil apoptosis was lower for all groups when compared with surgical controls (52% ± 3.6%; n = 11; p < 0.001).
Conclusion
In medical patients with sepsis, neutrophil apoptosis is inversely proportional to the severity of sepsis and thus may be a marker of the severity of sepsis in this population.
Introduction
Sepsis is a leading cause of death in intensive care unit (ICU) patients [1], with an estimated incidence of 700,000 cases per year in the United States resulting in more than 200,000 deaths annually [2,3]. Acute respiratory distress syndrome (ARDS) is a frequent complication of sepsis [4-6]. The mortality rate of ARDS remains high, ranging between 20% and 60% [4,7-13]. Leucocytes, including neutrophils and macrophages, are believed to contribute to inflammatory tissue injury in sepsis and ARDS. It is hypothesised that unrestrained release of leucocyte-derived cytotoxic products contributes to injury of lungs and other organs [14-16]. A better understanding of the pathophysiology of sepsis and ARDS is essential for the treatment or prevention of these devastating conditions.
Apoptosis is involved in removal of senescent cells and is thought to be essential for the non-injurious resolution of inflammation [17-27]. The role of apoptosis in the pathophysiology of sepsis and multiple organ dysfunction syndrome (MODS) has been the focus of recent studies. There is evidence of an association between apoptosis and outcomes of patients with MODS [15,20,22,23,25,28]. Recent studies suggest that neutrophil apoptosis is decreased in systemic inflammatory response syndrome (SIRS) [28,29], sepsis [30-37], and ARDS [12,14,16,26,38-40]. The increased life span of neutrophils may be associated with increased tissue injury in these syndromes [12,14-16,20,22,29]. Currently, information on the relationship between neutrophil apoptosis and the severity of sepsis and sepsis-induced ARDS is incomplete [22,23,32-35,41]. Accordingly, the objective of the current study was to determine whether neutrophil apoptosis correlates with the severity of sepsis and sepsis-induced ARDS.
Materials and methods
Patient selection and study protocol
A prospective cohort study enrolled patients at three tertiary teaching hospitals in Porto Alegre city, southern Brazil, from January 2000 to December 2004. Patients were included in the study if they met criteria for sepsis and ARDS.
Sepsis
Sepsis and its subsets were defined according to the Consensus Conference of the American College of Chest Physicians and the Society of Critical Care Medicine [1]. Sepsis, a systemic inflammatory response secondary to infection, was defined by two or more of the following criteria: (a) body temperature greater than 38°C or less than 36°C, (b) heart rate greater than 90 beats per minute, (c) respiratory rate greater than 20 breaths per minute or a PaCO2 (arterial partial pressure of carbon dioxide) less than 32 mm Hg, and (d) leucocytes greater than 12,000 cells per cubic millimetre, less than 4,000 cells per cubic millimetre, or greater than 10% bands. Septic shock was defined as sepsis-induced hypotension, despite adequate fluid resuscitation, along with the presence of hypoperfusion abnormalities or organ dysfunction. In our study, the term 'uncomplicated sepsis' was used for patients with sepsis according to the Consensus criteria instead of the more frequently used, but ambiguous, term 'sepsis.'
ARDS
ARDS was defined according to criteria of the 1994 American-European Consensus Conference on ARDS [42]. These included acute hypoxemia, ratio of PaO2 (arterial partial pressure of oxygen) to FiO2 (fraction of inspired oxygen) of 200 mm Hg or less, bilateral infiltrates on chest x-ray, pulmonary artery wedge pressure less than or equal to 18 mm Hg, or no clinical evidence of left atrial hypertension.
Control groups
1. Healthy controls were healthy blood donors (more than 18 years old) at the Hospital de Clínicas de Porto Alegre.
2. Surgical controls were patients submitted for elective surgery who had no evidence of infection, sepsis, or ARDS. Studies suggest that surgery itself has an influence on neutrophil apoptosis [43-46].
3. The mechanical ventilation (MV) group consisted of patients submitted to MV but without evidence of infection, sepsis, or ARDS. The objective was to verify whether the MV itself influenced neutrophil apoptosis. All patients of this group were on MV for a period of 24 hours.
Exclusion criteria
Exclusion criteria were congestive heart failure, ARDS secondary to factors other than sepsis (for example, pancreatitis, burns, and multiple trauma), interstitial lung disease, use of immunosuppressive drugs (for example, corticosteroids), AIDS, malignancies, chronic inflammatory diseases (for example, rheumatoid arthritis), and transfusion of blood or blood products within the preceding 24 hours.
Ethical issues
The study was approved by the hospitals' ethics committees, and informed consent was obtained from the patient or a surrogate and from the healthy volunteers.
Sample and data collection
The venous blood sampling of medical patients was performed within 24 hours of diagnosis of sepsis and its subsets, ARDS, and for patients on MV. All surgical groups, including controls, had their blood drawn 24 hours after surgery. For healthy controls, a blood sample was obtained at the time of blood donation. The investigators followed each patient admitted to the ICU to identify patients who fulfilled the entry criteria. For each patient, a data record was completed and stored in a data bank.
Study variables
Outcome variables
The primary outcome variable was mean percentage of neutrophil apoptosis.
Independent variables
Independent variables were age, gender, medical/surgical patient status, Acute Physiology and Chronic Health Disease Classification System II (APACHE II) score, total maximum sequential organ failure assessment (SOFA) score, organ system failure based on the SOFA score, and 28-day mortality from the time of entry into the study. If the patient was discharged from the hospital, mortality was assessed by telephone or mail.
Study procedures
Neutrophil isolation
Human neutrophils (more than 98% pure) were isolated from whole blood using dextran sedimentation and discontinuous plasma-Percoll (Amersham Biosciences AB, now part of GE Healthcare, Little Chalfont, Buckinghamshire, UK) gradients as described previously [47]. The separation procedure required two hours, and the cells were used immediately after isolation for the experiments described. The functional integrity and non-activated state of isolated neutrophils have been validated in previous reports [47,48]. Neutrophil viability was greater than 97% using Trypan blue exclusion.
Neutrophil apoptosis
After isolation, neutrophils were washed twice and resuspended at a density of 1 × 106 cells per millilitre in RPMI 1640 with 10% foetal bovine serum, L-glutamine (2 mM), penicillin (100 mg/ml), and streptomycin (100 μg/ml) (Gibco, now part of Invitrogen Corporation, Carlsbad, CA, USA). Cells were then incubated at 37°C in a 5% CO2 atmosphere for 24 hours in polypropylene tubes to prevent adherence. Cell viability assessed by Trypan blue exclusion exceeded 97%. After 24 hours, neutrophils were sedimented by cytocentrifugation on a glass microscope slide as described below.
Quantification of neutrophil apoptosis
Neutrophil apoptosis was assessed by light microscopy (×200) analysis of cytospun cells stained with Wright's Giemsa method and identification of nuclear changes (condensation of chromatin and simplification of nuclear structure) characteristic of apoptosis [17,49,50]. Two blinded investigators assessed the percentage of neutrophil apoptosis on cytospun preparations by analysing 500 cells per slide each. The analysis was performed on two different slides from the same patient. Data were reported as the percentage of apoptotic cells. The percentage was obtained by using the mean value obtained by the two investigators.
To validate the light microscopic method of assessment of neutrophil apoptosis, we used a second independent method in healthy donors, annexin V binding with quantification by flow cytometry [51]. In brief, neutrophils (1 × 106) were washed with ice-cold phosphate-buffered saline (PBS) and then incubated with fluorescein isothiocyanate (FITC)-conjugated annexin V (R&D Systems, Inc., Minneapolis, MN, USA) in the presence of propidium iodide (PI) for 30 minutes at 4°C. Cells were washed, resuspended in PBS, and analysed by flow cytometry (FACStar; Becton Dickinson, Mountain View, CA, USA). Cells that were FITC-positive and PI-negative were considered to be apoptotic. The extent of neutrophil apoptosis was compared with the percentage of neutrophil apoptosis determined by nuclear morphology and light microscopy (linear regression slope 0.87 R2 = 0.968, n = 6). These results confirm the validity of Wright's Giemsa staining to assess apoptosis.
Sample size
The sample size was calculated using data from the study patients because there was no information in the literature to help sample size estimation. The study power for the study comparisons was 90%.
Data quality control
A database coordinator was responsible for monitoring all data collection and entry. All data were checked for any inconsistencies. A random sample of 20% of the records was selected and compared with the original data-collection forms to detect any data-entry errors.
Statistical analysis
A stratified analysis was performed considering the status of medical or surgical patients. For each strata, the percentage of neutrophil apoptosis measured in the different groups was compared using one-way analysis of variance (ANOVA), considering that the study variables were normally distributed and that the variances were equal. All comparisons with a p value less than 0.05 were considered statistically significant. A post hoc Tukey test was used. Continuous variables, other than the percentage of neutrophil apoptosis, were also compared using ANOVA and the post hoc Tukey tests. For continuous variables comparing two groups, the Student t test was used. Categorical variables were compared using the χ2 test. Correlation analysis (Pearson) was performed between the main outcome of neutrophil apoptosis and other continuous variables, including age and APACHE II and SOFA scores, stratified for medical and surgical status. All analyses were performed using the Statistical Package for Social Sciences, version 12 (SPSS Inc., Chicago, IL, USA).
Results
A total of 57 patients and 64 controls were included in the study (see Table 1 for population characteristics). A detailed description of the diagnoses, sites of infection, microbiology, and sources of materials for culture from all patients is included in Table 2 (medical patients) and Table 3 (surgical patients).
Table 1.
Characteristics of the study population according to group allocation
| Variables | Uncomplicated sepsis (n = 16) | Septic shock (n = 23) | Sepsis-induced ARDS (n = 18) | Mechanical ventilation (n = 20) | Controls (n = 44) |
P valuea |
| Age (years, mean ± SEM) | 57 ± 3.3 | 57 ± 4.5 | 46 ± 4.4 | 54 ± 3.5 | 43 ± 1.8 | 0.002 |
| Male/Female (percentage) | 62.5/37.5 | 52.2/47.8 | 50/50 | 55/45 | 50/50 | 0.93 |
| Medical/Surgical (percentage) | 50/50 | 52.2/47.8 | 50/50 | 55/45 | 75/25 | - |
aAnalysis of variance or χ2 test. ARDS, acute respiratory distress syndrome; SEM, standard error of the mean.
Table 2.
Detailed description of the medical patients
| Patient | Group | ||||
| Uncomplicated sepsis (n = 8) | |||||
| Diagnosis | Site of infection | Microorganism | Material | ||
| 1 | Pneumonia/COPD | Respiratory | Not identified | Sputum/Blood | |
| 2 | Pneumonia/COPD | Respiratory | Staphylococcus aureus | Blood | |
| 3 | Pneumonia/Stroke | Respiratory | Not identified | Sputum/Blood | |
| 4 | Pneumonia/Guillain-Barre syndrome | Respiratory | Enterobacter sp | Sputum | |
| 5 | Pneumonia/Subarachnoid hemorrhage | Respiratory | Staphylococcus sp | Blood | |
| 6 | Pneumonia/DM/Pickwick syndrome | Respiratory | Not identified | Sputum/Blood | |
| 7 | Pneumonia/Head trauma | Respiratory | Pseudomonas aeruginosa | Sputum | |
| 8 | Pneumonia/Intracerebral hemorrhage | Respiratory | S. aureus | Sputum | |
| Septic shock (n = 12) | |||||
| Diagnosis | Site of infection | Microorganism | Material | ||
| 1 | Pneumonia/COPD | Respiratory | S. aureus | Blood | |
| 2 | Pneumonia | Respiratory | S. aureus | Sputum | |
| 3 | Pneumonia/COPD | Respiratory | S. aureus | Blood | |
| 4 | Pneumonia/COPD | Respiratory | P. aeruginosa/Haemophilus influenzae | Sputum | |
| 5 | Pneumonia/UTI | Respiratory/Urinary | Not identified/Klebsiella pneumoniae | Sputum/Urine | |
| 6 | Pneumonia/UTI/DM | Respiratory/Urinary | Not identified/Candida sp | Sputum/Blood and urine | |
| 7 | UTI/SBP/Cirrhosis | Abdominal/Urinary | S. aureus and Streptococcus viridans/Enterococcus faecium, S. viridans, and Escherichia coli | Ascites/Urine | |
| 8 | Pneumonia | Respiratory | Not identified | Sputum/Blood | |
| 9 | Pneumonia/COPD | Respiratory | Not identified | Sputum/Blood | |
| 10 | Meningitis | CNS | Neisseria meningitis | Liquor/Blood | |
| 11 | UTI/Lyell syndrome | Urinary/Skin | Enterococcus sp/Acinetobacter sp | Urine/Skin secretion | |
| 12 | Pneumonia | Respiratory | S. aureus | Blood | |
| Sepsis-induced ARDS (n = 9) | |||||
| Diagnosis | Site of infection | Microorganism | Material | ||
| 1 | Pneumonia/Leptospirosis | Respiratory | Enterobacter sp | Sputum | |
| 2 | Pneumonia/Suicide attempt (glicosate ingestion) | Respiratory | P. aeruginosa | Sputum | |
| 3 | Pneumonia/UTI/Diarrhea | Respiratory, Urinary, and Intestinal | Not identified/K. pneumoniae/E. coli OH157 | Sputum/Urine/Feces | |
| 4 | Pneumonia/UTI/DM | Respiratory/Urinary | Streptococcus agalactiae, S. aureus/Staphylococcus sp, E. coli | Blood/Urine | |
| 5 | Pneumonia | Respiratory | Haemophilus sp | Sputum | |
| 6 | Septic arthritis | Joint | S. aureus | Sinovial liquid | |
| 7 | Pneumonia | Respiratory | Not identified | Sputum/Blood | |
| 8 | Pneumonia | Respiratory | E. coli, Moraxella sp | Sputum | |
| 9 | Pneumonia/COPD | Respiratory | Not identified | Sputum/Blood | |
| Mechanical ventilation (n = 11) | |||||
| Diagnosis | |||||
| 1 | Anaphylaxis (anaesthesia) | ||||
| 2 | Head trauma | ||||
| 3 | Spinal cord trauma | ||||
| 4 | Subarachnoid hemorrhage | ||||
| 5 | Subarachnoid hemorrhage | ||||
| 6 | Intracerebral hemorrhage | ||||
| 7 | Anaphylaxis (anaesthesia) | ||||
| 8 | Guillain-Barre syndrome | ||||
| 9 | Intracerebral hemorrhage | ||||
| 10 | Anaphylaxis (anaesthesia) | ||||
| 11 | Epilepsy | ||||
ARDS, acute respiratory distress syndrome; CNS, central nervous system; COPD, chronic obstructive respiratory disease; DM, diabetes mellitus; SBP, spontaneous bacterial peritonitis; UTI, urinary tract infection.
Table 3.
Detailed description of the surgical patients
| Patient | Group | ||||
| Uncomplicated sepsis (n = 8) | |||||
| Diagnosis | Site of infection | Microorganism | Material | Surgery | |
| 1 | Pneumonia/Intracerebral haemorrhage | Respiratory | Pseudomonas aeruginosa | Sputum | Craniotomy |
| 2 | Pneumonia/COPD | Respiratory | P. aeruginosa | Sputum | Aortic-femoral bypass |
| 3 | Pneumonia/Perforated ulcer | Abdominal | Candida albicans | Ascites | Laparatomy |
| 4 | Cholangitis/UTI | Abdominal/Urinary | Enterococcus sp/Escherichia coli | Blood/Urine | Exploratory laparotomy |
| 5 | Pneumonia/Peritonitis/Colonic perforation due to colonoscopy | Respiratory/Abdominal | Enterobacter sp/Not identified | Sputum/Blood | Exploratory laparotomy |
| 6 | Cholecystitis | Abdominal | Enterococcus sp | Blood | Cholecystectomy |
| 7 | Pneumonia/Intracerebral haemorrhage | Respiratory | Enterobacter sp, Haemophilus sp, and Staphylococcus aureus | Sputum | Craniotomy |
| 8 | Pneumonia/Stroke/UTI/Celulitis | Respiratory/Urinary/Skin | Not identified/Enterobacter sp/S. aureus | Sputum/Urine/Skin secretion | Abdominal aortic aneurysm repair |
| Septic shock (n = 11) | |||||
| Diagnosis | Site of infection | Microorganism | Material | Surgery | |
| 1 | Pneumonia | Respiratory/Catheter | P. aeruginosa/S. aureus | Sputum/Catheter | Carotid aneurysm repair |
| 2 | Diverticulitis/UTI | Abdominal/Urinary | Not identified/Candida sp | Blood/Urine | Small bowel resection with anastomosis |
| 3 | Perforated peptic ulcer/Cirrhosis/Alcohol abuse | Abdominal | S. aureus | Blood/Ascites | Laparatomy |
| 4 | Septic arthritis (Hip) | Joint | Streptococcus agalactiae | Joint fluid | Surgical drainage |
| 5 | Pneumonia/Head trauma (subdural haematoma) | Respiratory | Not identified | Sputum/Blood | Craniotomy |
| 6 | Pyelonephritis/Nephrolitiasis/Neurogenic bladder | Urinary | Staphylococcus sp/P. aeruginosa | Blood/Urine | Nephrectomy and abscess drainage |
| 7 | Perforated peptic ulcer | Abdominal | S. aureus, Streptococcus viridans, and Enterobacter sp | Ascites | Exploratory laparotomy |
| 8 | Pneumonia/Endocarditis/Intracerebral haemorrhage | Respiratory/Heart | Pseudomonas sp/Not identified | Sputum/Blood | Craniotomy |
| 9 | Pneumonia/COPD/UTI Oesophageal laceration | Respiratory/Urinary | S. aureus and Acinetobacter sp/E. coli | Sputum/Urine | Oesophageal laceration repair |
| 10 | Cholangitis | Abdominal | E. coli | Blood | Exploratory laparotomy |
| 11 | Peritonitis/Perforated peptic ulcer | Abdominal | Enterococcus sp, Candida sp, and S. aureus | Ascites | Laparotomy |
| Sepsis-induced ARDS (n = 9) | |||||
| Diagnosis | Site of infection | Microorganism | Material | Surgery | |
| 1 | Cholangitis | Abdominal | Klebsiella pneumoniae | Ascites | Hepatic artery aneurysm ligation |
| 2 | Diverticulitis | Abdominal | E. coli | Blood | Small bowel resection with anastomosis |
| 3 | Pneumonia | Respiratory | P. aeruginosa | Sputum | Pleurostomy closure |
| 4 | Pneumonia | Respiratory | Staphylococcus coag neg | Blood | C-section |
| 5 | Septic arthritis | Hip joint | Staphylococcus haemolyticus | Blood | Hip drainage |
| 6 | UTI/Intestinal fistula | Urinary | Candida sp and Enterococcus sp | Blood and urine | Intestinal fistula closure |
| 7 | Pneumonia/UTI/Peripheral vascular disease | Respiratory/Urinary/Skin | Not identified/Candida sp/S. aureus | Sputum/Urine/Skin secretion | Above-knee amputation |
| 8 | Septic arthritis | Hip joint | Stenotroptomonas maltophilia and Staphylococcus coag neg/S. agalactiae | Blood and joint fluid | Hip drainage |
| 9 | Cholecystitis | Abdominal/Catheter | E. coli/S. aureus | Blood/Catheter | Cholecystectomy |
| Mechanical ventilation (n = 9) | |||||
| Diagnosis | Surgery | ||||
| 1 | Head trauma (subdural haematoma) | Craniotomy | |||
| 2 | Intracerebral haemorrhage | Craniotomy | |||
| 3 | Head trauma (subdural haematoma) | Craniotomy | |||
| 4 | Head trauma (subdural haematoma) | Craniotomy | |||
| 5 | Head trauma (epidural haematoma) | Craniotomy | |||
| 6 | Uterine leyomioma | Hysterectomy | |||
| 7 | Abdominal trauma | Exploratory laparotomy | |||
| 8 | Head trauma (subdural haematoma) | Craniotomy | |||
| 9 | Intracerebral haemorrhage | Craniotomy | |||
| Controls (n = 11) | |||||
| Surgery | |||||
| 1 | Humeral prostheses | ||||
| 2 | Inguinal hernia repair | ||||
| 3 | Septoplasty | ||||
| 4 | Inguinal hernia repair | ||||
| 5 | Arthrodesis (tibia-tarsus) | ||||
| 6 | Tibial osteosynthesis | ||||
| 7 | Septoplasty | ||||
| 8 | Diaphragmatic hernia repair and laparoscopic fundoplication | ||||
| 9 | Iliofemoral bypass | ||||
| 10 | Incisional hernia repair | ||||
| 11 | Septoplasty | ||||
ARDS, acute respiratory distress syndrome; COPD, chronic obstructive respiratory disease; UTI, urinary tract infection.
The comparison of the percentage of neutrophil apoptosis was significantly different among all groups (p < 0.001; ANOVA). A stratified analysis was performed considering surgical/medical status. The mean percentage of neutrophil apoptosis (± standard error of the mean [SEM]) was significantly lower in the surgical controls (52% ± 3.6%) when compared with healthy controls (69% ± 1.1%; p = 0.001; Student t test).
In medical patients, a significant difference was observed in the age variable (Table 4). The control group was younger than the MV group (p = 0.02; Tukey test). A Pearson correlation test showed a weak and negative correlation (p = 0.35) between age and neutrophil apoptosis, suggesting that age did not have a major effect on the percentage of neutrophil apoptosis in this study (data not shown).
Table 4.
Characteristics of the medical patients
| Uncomplicated sepsis (n = 8) |
Septic shock (n = 12) |
Sepsis-induced ARDS (n = 9) |
Mechanical ventilation (n = 11) |
Controls (n = 33) |
P value | |
| Age (years, mean ± SEM) | 50.8 ± 4.9 | 56 ± 5.6 | 43.2 ± 5.8 | 57.5 ± 4.9 | 37.1 ± 1.7 | <0.008a |
| Male/Female (percentage) | 50.0/50.0 | 58.3/41.7 | 44.4/55.6 | 36.4/63.6 | 48.5/51.5 | 0.7b |
| APACHE II score (percentage) | 13.6 ± 2.2 | 21 ± 2.2 | 21.5 ± 1.2 | - | - | 0.1 |
| Maximum SOFA score (percentage) | 4.7 ± 0.7 | 9.9 ± 1.0 | 12 ± 0.8 | - | - | <0.001a |
| Organ dysfunction (percentage) | ||||||
| 0 | 50 | 25 | 0 | - | - | - |
| 1 | 0 | 16.7 | 77.8 | - | - | - |
| 2 | 25 | 41.7 | 0 | - | - | - |
| 3 | 25 | 16.7 | 22.2 | - | - | - |
| Organ failure (percentage) | ||||||
| 0 | 25 | 0 | 0 | - | - | - |
| 1 | 62.5 | 25 | 0 | - | - | - |
| 2 | 12.5 | 41.7 | 55.6 | - | - | - |
| 3 | 0 | 33.3 | 33.3 | - | - | - |
| 4 | 0 | 0 | 11.1 | - | - | - |
| Mortality in 28 days (percentage) | 37.5 | 75 | 77.8 | - | - | - |
| Neutrophil apoptosis (mean percentage ± SEM) | 57 ± 3.2 | 38 ± 3.7 | 28 ± 3.3 | 53 ± 3.0 | 69 ± 1.1 | <0.001a |
aP value from the comparisons using analysis-of-variance test; bp value from the comparisons using χ2 test. APACHE II, Acute Physiology and Chronic Health Disease Classification System II; ARDS, acute respiratory distress syndrome; SEM, standard error of the mean; SOFA, sequential organ failure assessment.
Neutrophil apoptosis differed significantly among the groups of medical patients. Figure 1 shows images of neutrophil apoptosis in Wright's Giemsa-stained slides obtained from a healthy control (a) and from a patient with ARDS (b). The percentage of neutrophil apoptosis (± SEM) was lower in ARDS (28% ± 3.3%; n = 9) compared with uncomplicated sepsis (57% ± 3.2%; n = 8; p < 0.001), MV (53% ± 3.0%; n = 11; p < 0.001), and with healthy controls (69% ± 1.1%; n = 33; p < 0.001). However, it did not differ from septic shock (38% ± 3.7%; n = 12; p = 0.13) (Tukey test; Figure 2). In the septic shock group, the mean percentage of neutrophil apoptosis was significantly lower than in uncomplicated sepsis, MV, and healthy controls (p < 0.001; Tukey test). The mean percentage of neutrophil apoptosis was significantly lower in patients with uncomplicated sepsis (p = 0.02; Tukey test) and in the MV group (p < 0.001; Tukey test) compared with healthy controls. There was no difference in the mean percentage of neutrophil apoptosis between the uncomplicated sepsis and the MV groups (p = 0.8; Tukey test). These observations suggest that in medical patients, the severity of sepsis is inversely proportional to the mean percentage of neutrophil apoptosis (Figure 2).
Figure 1.
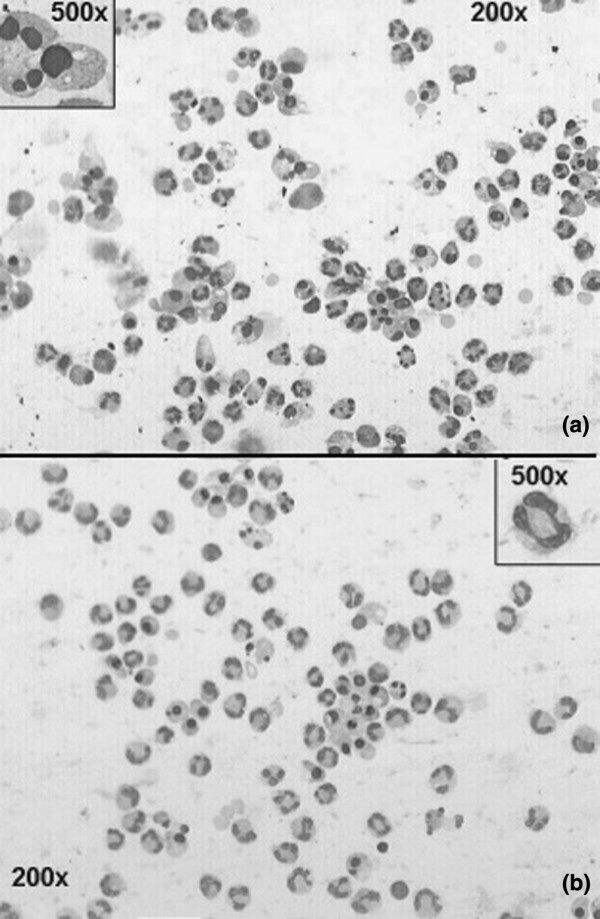
Apoptosis of neutrophils in a healthy donor and in a patient with sepsis-induced acute respiratory distress syndrome (ARDS). (a) Apoptosis of neutrophils in a healthy donor. Wright's Giemsa staining of cytocentrifuge smear shows predominance of cells in apoptosis. Inset shows morphological detail of an apoptotic cell, with loss of chromatin fine granularity (condensation) and karyorrhexis. (b) Apoptosis of neutrophils in a patient with sepsis-induced ARDS. Wright's Giemsa staining of cytocentrifuge smear shows predominance of normal-looking cells. Inset shows morphological detail of a normal cell, with fine granularity of chromatin and normal lobulated nucleus. Magnifications ×200 (insets ×500).
Figure 2.
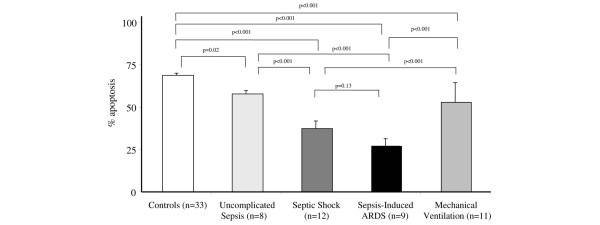
Mean percentage of neutrophil apoptosis in medical patients. There was a statistically significant difference among the groups (p < 0.001; analysis of variance). The differences between individual groups as determined by a post hoc Tukey test are illustrated. ARDS, acute respiratory distress syndrome.
Variables such as 28-day mortality and APACHE II and SOFA scores were also analysed in the medical groups (Table 4). Twenty-eight-day mortality was higher in the ARDS and septic shock groups when compared with the group with uncomplicated sepsis (Table 4). ARDS and septic shock groups had a higher mean SOFA score when compared with the other groups (p < 0.001; Tukey test) (Table 4). However, no statistical difference was observed between the ARDS and septic shock groups (p = 0.3; Tukey test).
Detailed data regarding number of organ dysfunctions/failures, according to SOFA score, are summarised in Table 4. Many patients with uncomplicated sepsis developed organ failure after blood sampling and during their hospitalisation in the ICU.
In surgical patients, the mean percentage of neutrophil apoptosis in all groups (uncomplicated sepsis [p = 0.04], septic shock [p = 0.04], ARDS [p < 0.002], and MV [p = 0.007] groups [Tukey test]) was significantly lower than in controls (Figure 3). No statistical difference was found among the mean percentage of neutrophil apoptosis of uncomplicated sepsis, septic shock, ARDS, and MV groups. Other variables were also analysed in surgical groups (Table 5).
Figure 3.
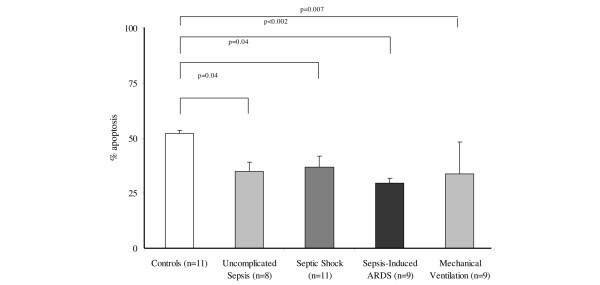
Mean percentage of neutrophil apoptosis in surgical patients. There were statistically significant differences among the groups (p < 0.001; analysis of variance). Post hoc Tukey test results are illustrated. ARDS, acute respiratory distress syndrome.
Table 5.
Characteristics of the surgical patients
| Uncomplicated sepsis (n = 8) | Septic shock (n = 11) | Sepsis-induced ARDS (n = 9) | Mechanical ventilation (n = 9) | Controls (n = 11) | P value | |
| Age (years, mean ± SEM) | 64.0 ± 3.3 | 58 ± 7.4 | 49.5 ± 6.9 | 51 ± 5 | 45.9 ± 5.5 | 0.1 |
| Male/Female (percentage) | 75/25 | 45.5/54.5 | 55.6/44.4 | 77.8/22.2 | 54.5/45.5 | 0.5a |
| APACHE II score (percentage) | 15.3 ± 1.8 | 21.1 ± 1.9 | 21.7 ± 3.9 | - | - | 0.1 |
| Maximum SOFA score (percentage) | 6.8 ± 1.1 | 10 ± 1.3 | 12.2 ± 0.8 | - | - | <0.001b |
| Organ dysfunction (percentage) | ||||||
| 0 | 0 | 9.1 | 11.1 | - | - | - |
| 1 | 50 | 18.2 | 44.4 | - | - | - |
| 2 | 37.5 | 36.4 | 22.2 | - | - | - |
| 3 | 12.5 | 18.2 | 22.2 | - | - | - |
| 4 | 0 | 18.2 | 0 | |||
| Organ failure (percentage) | ||||||
| 0 | 12.5 | 9.1 | 0 | - | - | - |
| 1 | 62.5 | 36.4 | 11.1 | - | - | - |
| 2 | 25 | 36.4 | 66.7 | - | - | - |
| 3 | 0 | 18.2 | 22.2 | - | - | - |
| Mortality in 28 days (percentage) | 62.5 | 45.5 | 66.7 | - | - | - |
| Neutrophil apoptosis (mean percentage ± SEM) | 35 ± 3.2 | 36 ± 5.2 | 29 ± 2.1 | 32 ± 3.9 | 52 ± 3.6 | <0.001b |
aP value from the comparisons using χ2 test; bp value from the comparisons using analysis-of-variance test. APACHE II, Acute Physiology and Chronic Health Disease Classification System II; ARDS, acute respiratory distress syndrome; SEM, standard error of the mean; SOFA, sequential organ failure assessment.
We attempted to perform a subgroup analysis based on the different degrees of severity of sepsis in medical and surgical patients to ascertain whether there was an association between neutrophil apoptosis and mortality. This was not successful, probably due to the small sample size studied. A moderate and negative correlation between the mean SOFA score and the percentage of neutrophil apoptosis in medical patients was observed (R = -0.56; p < 0.001), indicating that the lower the mean percentage of apoptosis, the higher the mean SOFA score. However, in surgical patients, this correlation was weak and not statistically significant.
Discussion
The primary observation of the current study is that the extent of neutrophil apoptosis correlates inversely with the severity of sepsis and sepsis-induced ARDS in medical patients. Neutrophils from medical patients with uncomplicated sepsis, septic shock, and ARDS displayed lower degrees of apoptosis as compared with controls. Furthermore, we observed a progressive decrease in neutrophil apoptosis as the severity of sepsis increased. This is the first study to correlate the extent of apoptosis of peripheral blood neutrophils with the severity of sepsis and ARDS.
Our study confirms and extends the previous reports of decreased neutrophil apoptosis in patients with sepsis and ARDS with or without sepsis [30-33,35,36,38-40]. One study reported that neutrophil apoptosis was decreased in patients with sepsis compared with healthy controls [33]. However, that study combined patients with different degrees of severity of sepsis into one large group (labelled 'sepsis') that was compared with healthy controls but did not correlate the extent of neutrophil apoptosis with the severity of sepsis. Other studies that examined apoptosis of circulating neutrophils from septic patients assessed only one level of severity of sepsis (for example, only severe sepsis [30-32] or MODS [35]). Another study examined the rates of apoptosis of neutrophils in bronchoalveolar lavage fluid (BALF) of septic patients and demonstrated decreased apoptosis when all cells (including neutrophils) from the BALF were analysed ex vivo [36].
In patients with ARDS, our study is in agreement with previous studies that have demonstrated decreased neutrophil apoptosis in patients with ARDS, including those with sepsis-induced ARDS [38-40]. Several studies have documented that BALF recovered from patients during the early stages of both septic and non-septic ARDS is able to prolong the life span of neutrophils incubated ex vivo and that this effect may be ascribable to elevated levels of cytokines such as granulocyte-colony stimulating factor, granulocyte macrophage-colony stimulating factor (GM-CSF), and interleukin (IL)-2 [38-40]. Interestingly, Matute-Bello and colleagues [39] reported that higher GM-CSF levels in BALF correlated with survival in patients with ARDS. The authors suggested that this effect may not be related to modulation of neutrophil apoptosis but rather due to effects on other cells such as alveolar macrophages and epithelial cells. Lesur and colleagues [40] also demonstrated that exposure of normal blood neutrophils to BALF from patients with ARDS delayed apoptosis in vitro. In general, these results are in agreement with our observations and indicate that modulation of apoptosis of neutrophils and other lung cells is an early phenomenon in the inflammatory milieu of the lung in sepsis. It is noteworthy that our study is the first to evaluate apoptosis of peripheral blood neutrophils specifically from patients with sepsis-induced ARDS.
The mechanisms responsible for the decreased neutrophil apoptosis in sepsis and ARDS are incompletely understood. One potential mechanism involves activation of nuclear factor-κB with a concomitant reduction of the activity of caspases 3 and 9, and maintenance of mitochondrial transmembrane potential [33]. Other possible mechanisms involve modulation of Mcl-1 (myeloid cell leukaemia-1) [32], PBEF (pre-B cell colony-enhancing factor) [35], and p38 mitogen-activated protein kinase (p38 MAPK) [41] signalling pathways.
The current study was stratified (medical/surgical status) because previous studies have suggested that surgery per se may influence neutrophil apoptosis [43-46]. Additionally, because MV has been shown to affect apoptosis in other cell types [52-57], we included a control group of patients (medical and surgical) submitted to MV but who had no history of infection, sepsis, or ARDS.
We observed that the extent of neutrophil apoptosis was significantly lower in the surgical controls when compared with medical controls, an effect that has been reported by others [43-45]. Indeed, we observed a decrease in neutrophil apoptosis in all surgical groups. However, there was no statistical difference between these groups. Therefore, the correlation between neutrophil apoptosis and the severity of sepsis observed in medical patients was not observed in the surgical groups. There are several factors that might account for the decreased neutrophil apoptosis in surgical patients, including effects of anaesthesia and of the localised tissue trauma related to the surgical procedure with release of cytokines such as IL-6 [43] and IL-8 [45]. In this regard, a recent study [58] examined the effects of surgery on Fas-induced neutrophil apoptosis and reported that the anti-apoptotic action of plasma was not affected by the addition of neutralising antibodies to GM-CSF, IL-6, or IL-8, indicating that these cytokines are not a dominant factor mediating the anti-apoptotic effects on Fas-induced apoptosis in surgical patients. However, the anti-apoptotic effect of plasma was attenuated by pharmacological inhibitors of either PI3 kinase or extracellular signal-regulated kinase (ERK), but not by a p38 MAPK inhibitor, implicating PI3 kinase and ERK in the signalling pathway mediating the anti-apoptotic effect of plasma under the conditions described above. Another study demonstrated a decrease in apoptosis of exudative neutrophils obtained from peritoneal fluid from patients with recent gastrointestinal surgery [44]. In contrast, a recent report describes enhanced apoptosis of peripheral blood neutrophils of patients undergoing elective surgery under general anaesthesia [46]. Taken together, alterations in neutrophil function which occur in the post-operative period may predispose to untoward outcomes via modulation of the complex inflammatory response to surgery.
Previous studies support the concept that injurious modes of MV per se may result in release of inflammatory mediators that lead to inflammatory lung injury [52,53,59,60]. In support of this notion, we observed that neutrophil apoptosis was diminished in the group of patients subject to MV but without evidence of infection, sepsis, or ARDS. However, our results also indicate that MV per se did not account for the low percentage of neutrophil apoptosis observed in the group of patients with more complicated sepsis. The effect of MV extends beyond the lungs to other organs and has been termed 'biotrauma' [54,55]. Imai and collaborators [52] documented effects of MV on epithelial cell apoptosis in the lung as well as in the kidneys and small intestine, the former accompanied by biochemical evidence of organ dysfunction. A previous study from our group demonstrated that BALF obtained from ARDS patients ventilated with injurious MV activated neutrophil oxidant production and release of elastase, effects that correlated to the degree of lung injury and systemic inflammatory response and to multiple organ failure [61]. Although the effect of BALF on neutrophil apoptosis was not assessed in this study, we predict that it would decrease apoptosis. The 'biotrauma' hypothesis is supported by evidence from experimental models, including humans [59], animals [62], isolated lung [54], and stressed cell systems [63].
We observed that the mortality rate was higher in medical patients with ARDS, followed by septic shock, when compared with the uncomplicated sepsis group. To understand the significance of these mortality rates, we used instruments such as the total maximum SOFA score to quantify the severity of illness. From a correlation test evaluating the association between the mean percentage of neutrophil apoptosis and the mean SOFA score, two correlations merit further consideration: (a) the correlation between the severity of sepsis and the percentage of neutrophil apoptosis and (b) the association among the severity of sepsis, percentage of neutrophil apoptosis, and mortality. The correlation analysis suggests an inverse association between disease severity and the percentage of neutrophil apoptosis. Because the mean SOFA score correlates with mortality [64,65], our findings suggest that there is an association between the severity of sepsis, the extent of neutrophil apoptosis, and mortality.
We did not observe an association between neutrophil apoptosis and mortality in the current study. One limitation in this regard is that the sample size was not sufficient to assess such an association. However, the observed results of the mean percentage of neutrophil apoptosis, the mean SOFA score, and the mortality rates suggest that the higher the mortality rate (and disease severity), the lower the percentage of neutrophil apoptosis. In future studies with a larger sample size, it will be important to evaluate whether the percentage of neutrophil apoptosis is associated with mortality within the different degrees of severity of sepsis.
Study limitations
The decrease in the percentage of neutrophil apoptosis may not be specific for sepsis and ARDS. In fact, it appears that any event resulting in SIRS (such as sepsis) has the potential to affect the immune system, including neutrophil survival and function. However, the patients included in our study, including the controls groups, were carefully selected to allow us to study the specific correlation between neutrophil apoptosis and sepsis. Our results demonstrate that in medical patients with sepsis, neutrophil apoptosis is inversely proportional to the severity of sepsis. The correlations of neutrophil apoptosis with other causes of SIRS, if any, require further study.
The observations in the current study represent an important first step to a better understanding of the influence of sepsis on neutrophil apoptosis by defining the clinical associations of differing degrees of neutrophil apoptosis in this milieu. However, the observational design of the current study did not allow us to explore the possible mechanism(s) such as the role of specific receptors and intracellular signalling pathways in modulation of neutrophil apoptosis during sepsis. Further studies will be important to address these issues and will provide important information on the signal transduction pathways modulating neutrophil apoptosis during sepsis.
Conclusion
We observed that in medical patients with sepsis, neutrophil apoptosis is inversely proportional to the severity of this syndrome, including ARDS. In surgical patients, the mean percentage of neutrophil apoptosis for all sepsis groups was significantly lower than in the control group, but was not proportional to the severity of sepsis. These observations suggest that in medical patients, neutrophil apoptosis may be a marker of the severity of sepsis. We speculate that an influx of long-lived neutrophils may contribute to enhanced inflammatory injury to the lungs and other organs. The identification of specific mechanisms of neutrophil apoptosis in sepsis, including sepsis-induced ARDS, may lead to new strategies to improve the survival of those patients and patients with other inflammatory disorders in which neutrophils have been directly implicated.
Key messages
• In medical patients with sepsis, neutrophil apoptosis is inversely proportional to the severity of sepsis.
• In surgical patients with sepsis, the rate of apoptosis was lower than in controls but was not proportional to the severity of sepsis.
• These observations suggest that in medical patients, neutrophil apoptosis may be a marker of the severity of sepsis.
Abbreviations
ANOVA = analysis of variance; APACHE II = Acute Physiology and Chronic Health Disease Classification System II; ARDS = acute respiratory distress syndrome; BALF = bronchoalveolar lavage fluid; ERK = extracellular signal-regulated kinase; FITC = fluorescein isothiocyanate; GM-CSF = granulocyte macrophage-colony stimulating factor; ICU = intensive care unit; IL = interleukin; MODS = multiple organ dysfunction syndrome; MV = mechanical ventilation; p38 MAPK = p38 mitogen-activated protein kinase; PBS = phosphate-buffered saline; PI = propidium iodide; SEM = standard error of the mean; SIRS = systemic inflammatory response syndrome; SOFA = sequential organ failure assessment.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LF participated in the design, coordination, data collection, and analysis of the study and helped to draft the manuscript. LFF performed the study and helped to draft the manuscript. MCB participated in the study design, performed the statistical analysis, and helped to draft the manuscript. ARM, EMRF, RML, PP, RMM, and EV participated in the acquisition of the data for the study. JCP helped in neutrophil apoptosis assessment and digital imaging of Wright's Giemsa slides. GPD participated in the study design and development and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Dr. Vinícius Duval da Silva for his contribution in digital imaging of Wright's Giemsa slides and Ada Regina Schenini Diehl for her orientation on cytopathology techniques. LF and MCB are recipients of a Research Award from the National Council of Scientific and Technological Development (CNPq). GPD holds a Tier 1 Canada Research Chair. This study received funding from Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS), Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE/HCPA), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Canadian Institutes of Health Research.
Contributor Information
Léa Fialkow, Email: lfialkow@terra.com.br.
Luciano Fochesatto Filho, Email: fochesattomed@yahoo.com.br.
Mary C Bozzetti, Email: mcb@famed.ufrgs.br.
Adriana R Milani, Email: adriana_milani@yahoo.com.br.
Edison M Rodrigues Filho, Email: vitangel@terra.com.br.
Roberta M Ladniuk, Email: robyladniuk@gmail.com.
Paula Pierozan, Email: paulapierozan@hotmail.com.
Rafaela M de Moura, Email: rafinha_farma@hotmail.com.
João C Prolla, Email: jcprolla@yahoo.com.
Eric Vachon, Email: eric.vachon@utoronto.ca.
Gregory P Downey, Email: gregory.downey@utoronto.ca.
References
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 trough 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- Udobi KE, Childs ED, Touijer K. Acute respiratory distress syndrome. Am Fam Phys. 2003;67:315–322. [PubMed] [Google Scholar]
- Dreyfuss D, Ricard JD. Acute lung injury and bacterial infection. Clin Chest Med. 2005;26:105–112. doi: 10.1016/j.ccm.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Amato MBP, Barbas CSV, Medeiros DM, Magaldi RB, Schettino GPP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, Mazer D, McLean RF, Rogovein TS, Schouten D, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med. 1998;338:355–361. doi: 10.1056/NEJM199802053380603. [DOI] [PubMed] [Google Scholar]
- Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondéjar E, Clémenti E, Mancebo J, Factor P, Matamis D, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The multicenter trial group on tidal volume reduction in ARDS. Am J Respir Crit Care Med. 1998;158:1831–1838. doi: 10.1164/ajrccm.158.6.9801044. [DOI] [PubMed] [Google Scholar]
- Brower RG, Shanholtz CB, Fessler HE, Shade DM, White P, Jr, Wiener CM, Teeter JG, Dodd-o JM, Almog Y, Piantadosi S. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med. 1999;27:1492–1498. doi: 10.1097/00003246-199908000-00015. [DOI] [PubMed] [Google Scholar]
- The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. Clinical progress: the acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Eichacker PQ, Gerstenberger EP, Banks SM, Cui X, Natanson C. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials testing low tidal volumes. Am J Respir Crit Care Med. 2002;166:1510–1514. doi: 10.1164/rccm.200208-956OC. [DOI] [PubMed] [Google Scholar]
- Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–148. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31(4 Suppl):S195–9. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J, Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Seminars Cell Biol. 1995;6:385–393. doi: 10.1016/S1043-4682(05)80009-1. [DOI] [PubMed] [Google Scholar]
- Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med. 1999;160:S5–S11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- Mahidhara R, Billiar TR. Apoptosis in sepsis. Crit Care Med. 2000;28:N105–N113. doi: 10.1097/00003246-200004001-00013. [DOI] [PubMed] [Google Scholar]
- Giles KM, Hart SP, Haslett C, Rossi AG, Dransfield I. An appetite for apoptotic cells? Controversies and challenges. Br J Haematol. 2000;109:1–12. doi: 10.1046/j.1365-2141.2000.01805.x. [DOI] [PubMed] [Google Scholar]
- Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J. 2001;15:879–892. doi: 10.1096/fj.00-058rev. [DOI] [PubMed] [Google Scholar]
- Power C, Fanning N, Redmond HP. Cellular apoptosis and organ injury in sepsis: a review. Shock. 2002;18:197–211. doi: 10.1097/00024382-200209000-00001. [DOI] [PubMed] [Google Scholar]
- Martin TR, Nakamura M, Matute-Bello G. The role of apoptosis in acute lung injury. Crit Care Med. 2003;31(4 Suppl):S184–S188. doi: 10.1097/01.CCM.0000057841.33876.B1. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo R-F, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI200319523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maianski NA, Maianski AN, Kuijpers TW, Roos D. Apoptosis of neutrophils. Acta Haematol. 2004;111:56–66. doi: 10.1159/000074486. [DOI] [PubMed] [Google Scholar]
- Steven HW. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 1998;279:300–307. doi: 10.1001/jama.279.4.300. [DOI] [PubMed] [Google Scholar]
- Papathanassoglou EDE, Moynihan JA, McDermott MP, Ackerman MH. Expression of Fas (CD95) and Fas ligand on peripheral blood mononuclear cells in critical illness and association with multiorgan dysfunction severity and survival. Crit Care Med. 2001;29:709–718. doi: 10.1097/00003246-200104000-00002. [DOI] [PubMed] [Google Scholar]
- Jimenez MF, Watson WG, Parodo J, Evans D, Foster D, Steinberg M, Rotstein OD, Marshall JC. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch Surg. 1997;132:1263–1270. doi: 10.1001/archsurg.1997.01430360009002. [DOI] [PubMed] [Google Scholar]
- Keel M, Ungethüm U, Steckholzer U, Niederer E, Hartung T, Trentz O, Ertel W. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90:3356–3363. [PubMed] [Google Scholar]
- Ertel W, Keel M, Infanger M, Ungethüm U, Steckholzer U, Trentz O. Circulating mediators in serum of injured patients with septic complications inhibit neutrophil apoptosis through up-regulation of protein-tyrosine phosphorylation. J Trauma. 1998;44:767–776. doi: 10.1097/00005373-199805000-00005. [DOI] [PubMed] [Google Scholar]
- Härter L, Mica L, Stocker R, Trentz O, Keel M. Mcl-1 correlates with reduced apoptosis in neutrophils from patients with sepsis. J Am Coll Surg. 2003;197:964–973. doi: 10.1016/j.jamcollsurg.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Taneja R, Parodo J, Jia SH, Kapus A, Rotstein OD, Marshall JC. Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential and reduced caspase-9 activity. Crit Care Med. 2004;32:1460–1469. doi: 10.1097/01.CCM.0000129975.26905.77. [DOI] [PubMed] [Google Scholar]
- Sayeed MM. Delay of neutrophil apoptosis can exacerbate inflammation in sepsis patients: cellular mechanisms. Crit Care Med. 2004;32:1604–1606. doi: 10.1097/01.CCM.0000130997.85379.0F. [DOI] [PubMed] [Google Scholar]
- Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, Marshall JC. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–1327. doi: 10.1172/JCI200419930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liacos C, Katsaragakis S, Konstadoulakis MM, Messaris EG, Papanicolaou M, Georgiadis GG, Menenakos E, Vasiliadi-Chioti A, Androulakis G. Apoptosis in cells of bronchoalveolar lavage: a cellular reaction in patients who die with sepsis and respiratory failure. Crit Care Med. 2001;29:2310–2317. doi: 10.1097/00003246-200112000-00013. [DOI] [PubMed] [Google Scholar]
- Wesche DE, Lomas-Neira JL, Perl M, Shung C-S, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78:325–337. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Liles WC, Radella F, II, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156:1969–1977. doi: 10.1164/ajrccm.156.6.96-12081. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Liles WC, Radella F, II, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med. 2000;28:1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- Lesur O, Kokis A, Hermans C, Fülöp T, Bernard A, Lane D. Interleukin-2 involvement in early acute respiratory distress syndrome: relationship with polymorphonuclear neutrophil apoptosis and patient survival. Crit Care Med. 2000;28:3814–3822. doi: 10.1097/00003246-200012000-00010. [DOI] [PubMed] [Google Scholar]
- Sheth K, Friel J, Nolan B, Bankey P. Inhibition of p38 mitogen activated protein kinase increases lipopolysaccharide induced inhibition of apoptosis in neutrophils by activating extracellular signal-regulated kinase. Surgery. 2001;130:242–248. doi: 10.1067/msy.2001.115902. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R, et al. The American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- Fanning NF, Porter J, Shorten GD, Kirwan WO, Bouchier-Hayes D, Cotter TG, Redmond HP. Inhibition of neutrophil apoptosis after elective surgery. Surgery. 1999;126:527–534. [PubMed] [Google Scholar]
- Matsuda T, Saito H, Fukatsu K, Han I, Inoue T, Furukawa S, Ikeda S, Hidemura A. Cytokine-modulated inhibition of neutrophil apoptosis at local site augments exudative neutrophil functions and reflects inflammatory response after surgery. Surgery. 2001;129:76–85. doi: 10.1067/msy.2001.109060. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Iwase M, Miyaoka K, Kondo G, Watanabe H, Ohashi M, Nagumo M. Modulation of neutrophil apoptosis in plasma of patients after orthognathic surgery. J Surg Res. 2006;130:110–118. doi: 10.1016/j.jss.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Delogu G, Moretti S, Famularo G, Antonucci A, Signore L, Marcellini S, Lo Bosco L, De Simone C. Circulating neutrophils exhibit enhanced apoptosis associated with mitochondrial dysfunctions after surgery under general anaesthesia. Acta Anaesthesiol Scand. 2001;45:87–94. doi: 10.1034/j.1399-6576.2001.450114.x. [DOI] [PubMed] [Google Scholar]
- Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Jr, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- Downey GP, Chan CK, Lea P, Takai A, Grinstein S. Phorbol ester-induced actin assembly in neutrophils: role of protein kinase C. J Cell Biol. 1992;116:695–706. doi: 10.1083/jcb.116.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SL, Henson JE, Henson PM. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982;156:430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. IL-10 enhances resolution of pulmonary inflammation in vivo by promoting apoptosis of neutrophils. Am J Physiol. 1996;271(Lung Cell Mol Physiol 15):L566–L571. doi: 10.1152/ajplung.1996.271.4.L566. [DOI] [PubMed] [Google Scholar]
- Brown S, Bailey K, Savill J. Actin is cleaved during constitutive apoptosis. Biochem J. 1997;323:233–237. doi: 10.1042/bj3230233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Parodo J, Kajikawa O, Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- Plötz FB, Slutsky AS, van Vught AJ, Heijnen CJ. Ventilator-induced lung injury and multiple system organ failure: a critical review of facts and hypotheses. Intensive Care Med. 2004;30:1865–1872. doi: 10.1007/s00134-004-2363-9. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky AS, Tremblay LN. Multiple system organ failure: is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157:1721–1725. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care. 2005;11:82–86. doi: 10.1097/00075198-200502000-00013. [DOI] [PubMed] [Google Scholar]
- Kuiper JW, Groeneveld ABJ, Slutsky AS, Plötz FB. Mechanical ventilation and acute renal failure. Crit Care Med. 2005;33:1408–1415. doi: 10.1097/01.CCM.0000165808.30416.EF. [DOI] [PubMed] [Google Scholar]
- Iwase M, Kondo G, Watanabe H, Takaoka S, Uchida M, Ohashi M, Nagumo M. Regulation of Fas-mediated apoptosis in neutrophils after surgery-induced acute inflammation. J Surg Res. 2006;134:114–123. doi: 10.1016/j.jss.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- dos Santos DD, Slutsky AS. Mechanotransduction, ventilator-induced lung injury and multiple organ dysfunction syndrome. Intensive Care Med. 2000;26:638–642. doi: 10.1007/s001340051217. [DOI] [PubMed] [Google Scholar]
- Zang H, Downey GP, Suter PM, Slutsky AS, Ranieri VM. Conventional mechanical ventilation is associated with bronchoalveolar lavage-induced activation of polymorphonuclear leukocytes: a possible mechanism to explain the systemic consequences of ventilator-induced lung injury in patients with ARDS. Anesthesiology. 2002;97:1426–1433. doi: 10.1097/00000542-200212000-00014. [DOI] [PubMed] [Google Scholar]
- Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:109–116. doi: 10.1164/ajrccm.160.1.9803046. [DOI] [PubMed] [Google Scholar]
- Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol. 1999;227(1 Pt 1):L167–73. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- Moreno R, Vincent JL, Matos R, Mendonça A, Cantraine F, Thijs L, Takala J, Sprung C, Antonelli M, Bruining H, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicenter study. Intensive Care Med. 1999;25:686–696. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- Ferreira FL, Bota DP, Bross A, Mélot C, Vincent J-L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]


