Abstract
The definition of what constitutes a 'normal' adrenal response to critical illness is unclear. Consequently, published studies have used a variety of biochemical criteria to define 'adrenal insufficiency'. These criteria have been based on the baseline cortisol level or the increment in cortisol following corticotropin administration. However, in critically ill patients there are a number of confounding factors that make interpretation of these tests difficult. Furthermore, in those patients who are most likely to benefit from treatment with low-dose glucocorticoids, there is no evidence that treatment should be based on adrenal function testing. In those patients in whom the diagnosis of adrenal insufficiency may be important, this diagnosis may best be made based on the free cortisol level or the total cortisol level stratified by serum albumin.
The definition of what constitutes a 'normal' adrenal response to critical illness is unclear [1]. Consequently, published studies have used a variety of biochemical criteria to define abnormalities in adrenal function during critical illness. These criteria have been based on the 'stress' (baseline) cortisol level or the increment in cortisol (delta cortisol) following administration of 250 μg corticotropin. However, in critically ill patients there are a number of confounding factors that make interpretation of these tests difficult. Most importantly, the commercially available assays for serum cortisol determine the total (free plus protein-bound fractions) hormone concentrations. In healthy individuals more than 90% of circulating cortisol is bound to corticosteroid-binding globulin (CBG), with less than 10% in the free, biologically active form. In critical illness CBG levels fall by approximately 50%, with marked interindividual variation. Furthermore, as CBG binding sites becomes saturated the percentage of free cortisol increases. Hence, in critically ill patients the total cortisol may not reflect the biologically free (unbound) cortisol.
In a cohort of critically ill patients, Hamrahian and colleagues [2] demonstrated that patients who were hypoproteinemic (serum albumin <2.5 g/dl) had significantly lower total baseline and stimulated cortisol levels as compared with patients who had a serum albumin above 2.5 g/dl, but the free baseline and free stimulated cortisol concentrations were similar. The importance of serum albumin (a surrogate marker of CBG levels) when interpreting total serum cortisol concentrations is elegantly demonstrated by the study conducted by Salgado and colleagues [1], which appeared in the previous issue of Critical Care. Those investigators performed a low-dose (1 μg) and high-dose (249 μg) corticotropin stimulation test in 102 patients in septic shock. The total baseline and stimulated cortisol levels were significantly lower in patients with a serum albumin below 2.5 g/dl than in those with a serum albumin above 2.5 g/dl. However, unlike the study by Hamrahian and colleagues [2], the delta cortisol was similar between groups.
The specificity, sensitivity, and performance of the commercially available assays are not uniform [3]. This further complicates the interpretation of the serum cortisol level. It is speculated that the variation in assay characteristics might be even more significant in critically ill patients, especially those with septic shock. The presence of interfering heterophile antibodies may account for this observation [4]. The most specific assay employs the use of mass spectrometry, but this test is not commonly available. To complicate matters further, patients may develop critical illness related corticosteroid insufficiency (CIRCI), despite 'adequate' free levels of cortisol, due to tissue resistance. Tissue resistance to cortisol may occur as a result of abnormalities in the glucocorticoid receptor or increased tissue conversion of cortisol to cortisone.
In those patients who are most likely to benefit from 'low-dose' glucocorticoids (those patients with severe sepsis, septic shock, and acute respiratory distress syndrome [ARDS]), it is not clear whether treatment should be based on the results of adrenal function testing. Confalonieri and colleagues [5] randomized 48 patients with severe community-acquired pneumonia to receive low-dose hydrocortisone or placebo. Similarly, Meduri and coworkers [6] randomized 91 patients with early ARDS to receive low-dose methylprednisolone or placebo. In both of these studies patient outcome was improved in the steroid-treated group, independent of adrenal function testing. Five randomized, placebo-controlled studies have evaluated low-dose hydrocortisone (200–300 mg/day) in patients with septic shock [7-11]. A meta-analysis of these studies demonstrated more rapid shock reversal and a survival benefit from corticosteroids [12]. The benefit in terms of shock reversal was seen in both corticotropin responders (delta cortisol >9 mg/dl) and nonresponders (delta cortisol <9 mg/dl; Figure 1). In the study by Salgado and colleagues [1] a baseline cortisol of 23.6 mg/dl was the best discriminator of hemodynamic response to corticosteroid treatment. This is remarkably similar to the threshold of 23.7 μg/dl that we previously reported [13]. This finding suggests that patients with septic shock, and perhaps those with early ARDS and severe community-acquired pneumonia, should be treated with low-dose corticosteroids independent of adrenal function testing. It is, however, unclear at this time whether patients with high serum cortisol levels (>25 μg/dl) will benefit from treatment with corticosteroids.
Figure 1.
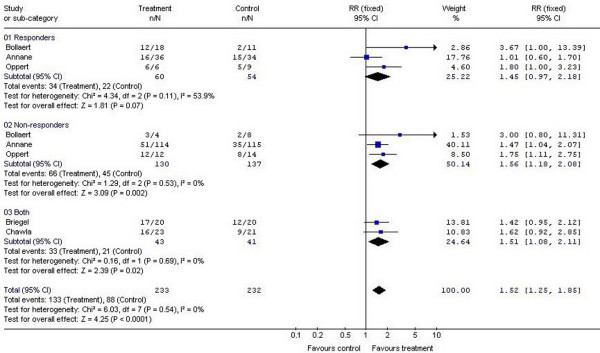
Meta-analysis. Summarized is a meta-analysis of the effect of treatment with low-dose hydrocortisone on shock reversal at day 7 in patients with septic shock grouped by response to cosyntropin.
Although the diagnosis of 'adrenal insufficiency' may not be clinically relevant in most critically ill patients, there may be groups of patients in whom this diagnosis may be important. This would include patients with adrenal hemorrhage/infarction, as well as patients with liver disease, head injury, pancreatitis, and burns, among others. At this time the diagnosis of adrenal insufficiency in these patients may best be made based on the free cortisol level or the total cortisol level stratified by serum albumin (Table 1) [14,15]. Because these criteria are based on limited data, it is likely that these diagnostic thresholds will be refined with time. However, it is important to stress that the diagnosis of adrenal insufficiency in critically ill patients should not be made on the basis of laboratory criteria alone.
Table 1.
Diagnostic criteria for adrenal insufficiency
| Albumin >2.5 g/dl | Albumin <2.5 g/dl | |
| Total cortisol (μg/dl [nmol/l]) | ||
| Baseline | 15 (410) | 10 (275) |
| Stimulated | 20 (550) | 15 (410) |
| Free cortisol (μg/dl [nmol/l]) | ||
| Baseline | 1.8 (50) | 1.8 (50) |
| Stimulated | 3.0 (85) | 3.0 (85) |
Abbreviations
ARDS = acute respiratory distress syndrome; CBG = corticosteroid-binding globulin.
Competing interests
The authors declare that they have no competing interests.
See related research by Salgado et al., http://ccforum.com/content/10/5/R149
References
- Salgado DR, Verdeal JC, Rocco JR. Adrenal function testing in patients with septic shock. Crit Care. 2006;10:R149. doi: 10.1186/cc5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrahian AH, Oseni TS, Arafah BM. Measurement of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629–1638. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- Vogeser M, Briegel J, Jacob K. Determination of serum cortisol by isotope-dilution liquid-chromatography electrospray ionization tandem mass spectrometry with online extraction. Clin Chem Lab Med. 2001;39:944–947. doi: 10.1515/CCLM.2001.151. [DOI] [PubMed] [Google Scholar]
- Bolland MJ, Chiu WW, Davidson JS, Croxson MS. Heterophile antibodies may cause falsely lowered serum cortisol values. J Endocrinol Invest. 2005;28:643–645. doi: 10.1007/BF03347264. [DOI] [PubMed] [Google Scholar]
- Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, Della Porta R, Giorgio C, Blasi F, Umberger R, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson M, Umberger R. Methyprednisolone infusion in patients with early acute respiratory distress syndrome (ARDS) significantly improves lung function: results of a randomized controlled trial (RCT) [abstract] Chest. 2006;128:129S. [Google Scholar]
- Chawala K, Kupfer Y, Tessler S. Hydrocortisone reverses refractory septic shock [abstract] Crit Care Med. 1999;(Suppl):A33. doi: 10.1097/00003246-199901001-00022. [DOI] [Google Scholar]
- Oppert M, Schindler R, Husung C, Offermann K, Graf KJ, Boenisch O, Barckow D, Frei U, Eckardt KU. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005;33:2457–2464. doi: 10.1097/01.CCM.0000186370.78639.23. [DOI] [PubMed] [Google Scholar]
- Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26:645–650. doi: 10.1097/00003246-199804000-00010. [DOI] [PubMed] [Google Scholar]
- Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, Hemmer B, Hummel T, Lenhart A, Heyduck M, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999;27:723–732. doi: 10.1097/00003246-199904000-00025. [DOI] [PubMed] [Google Scholar]
- Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. BMJ. 2004;329:480–489. doi: 10.1136/bmj.38181.482222.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003;31:141–145. doi: 10.1097/00003246-200301000-00022. [DOI] [PubMed] [Google Scholar]
- Arafah BM. Hypothalamic-pituitary adrenal function during critical illness: Limitations of current assessment methods. J Clin Endocrinol Metab. 2006;91:3725–3745. doi: 10.1210/jc.2006-0674. [DOI] [PubMed] [Google Scholar]
- Ho JT, Al-Musalhi H, Chapman MJ, Quach T, Thomas PD, Bagley CJ, Lewis JG, Torpy DJ. Septic shock and sepsis: A comparison of total and free plasma cortisol levels. J Clin Endocrinol Metab. 2006;91:105–114. doi: 10.1210/jc.2005-0265. [DOI] [PubMed] [Google Scholar]


