Abstract
A crucial determinant for the success of intensive insulin therapy in critically ill patients is the frequent and accurate measurement of blood glucose values with immediate feedback of results. In general, therefore, this is achieved by point-of-care testing, raising the question of the best way of monitoring blood glucose. Corstjens and coworkers, in the previous issue of Critical Care, demonstrate that, in spite of good correlation to "conventional" laboratory glucose assessment, absolute glucose levels may differ systematically. This commentary reviews the problems of glucose measurements arising from matrix effects, interferences and the use of different assays.
Intensive insulin therapy in critically ill patients
In the preceding issue of Critical Care Corstjens and coworkers [1] investigated three different methods of glucose measurements. While data about the beneficial effects of normoglycemia in critically ill patients are conflicting and inconsistent [2,3], there is no doubt about the importance of accurate glucose measurements to achieve glycemic control without increased risk of hypoglycemia. Similar to the results from the Diabetes Control and Complications Trial, which showed increasing frequency of hypoglycemia after tight glycemic control to reduce long-term complications [4], there is an increase of hypoglycemic episodes in critically ill patients when strict glycemic control is established [5,6].
Whole blood glucose: what are we actually measuring?
Although the measurement of glucose is one of the oldest established tests in the clinical chemistry laboratory, it is extremely complex and sometimes rather approximate due to the different fractions of the blood sample used [7,8]. Glucose measurement can be performed in whole blood, plasma and serum and these may be native or deproteinized, or hemolyzed in the case of capillary whole blood. Furthermore, blood may be arterial, capillary or venous in origin. Do all samples give the same result? The simple answer is no, and moreover, the difference may depend on nutritional state, perfusion, hematocrit or albumin blood concentrations.
Glucose is dissolved only in the aqueous part of the drawn sample and not in its entire volume (which contains other dissolved solids such as proteins). This is the major reason for differing glucose concentrations in plasma and whole blood samples. The protein content differs in blood cells (mostly red blood cells) and plasma (or serum, or hemolysate). The water content (Figure 1) of red blood cells is lower (because of a high concentration of hemoglobin) than that of an equal volume of plasma (which has a lower concentration of albumin and other plasma proteins). Even with the glucose concentration being the same in plasma water and red blood cell water, the concentration of glucose per unit volume of red blood cells is lower than that per unit volume of plasma. The concentration of glucose per unit volume of whole blood is in between that for plasma and red blood cells. As the water content of whole blood is the sum of plasma water and red blood cell water, glucose concentration will strongly depend on the hematocrit of the sample (Figure 1). With no changes in the protein concentration of plasma or red blood cells, a change in hematocrit from 0.4 to 0.7 will change the plasma/whole blood ratio for glucose from 1.10 to 1.38, an error of 26%.
Figure 1.
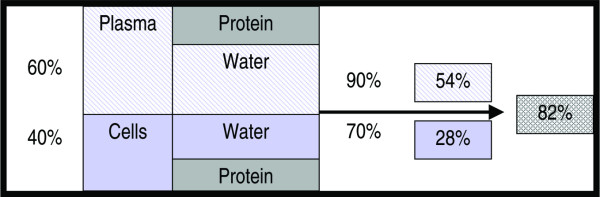
Glucose concentration: water content expressed as percent volume. With a hematocrit of 0.4 and a water content for the fraction 'Cells' of approximately 70%, the total water content will be 28% of total volume (whole blood). From the 60% plasma volume, 90% will be water, thus giving a water content of 54% for the plasma portion of whole blood. The total water content of whole blood then is 82% (54% + 28%). For a hematocrit of 0.4 the plasma/whole blood ratio of water content is 0.9/0.82 = 1.10, which reflects the 10% higher glucose values in plasma compared to whole blood.
Blood glucose strips retain red blood cells through a filtering process and measure glucose content in plasma in their reaction zone. This is yet another way by which hematocrit can influence the results: whole blood samples with differing amounts of red blood cells alter flow and volume of plasma entering the reaction zone. Even the Yellow Springs Instruments' Blood Glucose Analyzer, which is often used as a reference method, such as in the study by Corstjens and colleagues [1], yields glucose results dependent on hematocrit when whole blood samples are used. This is due to the fact that the instrument performs a 25-fold sample dilution before analysis. In theory, the only systems that should not be affected by hematocrit are instruments using direct-reading electrodes without sample dilution, such as those used in blood gas analysis [9].
Calibration
Next to the composition of the blood sample, calibration of the instrument is equally important and may often add to unknown errors. Depending on the glucose standard and reference method used for calibration, the same instrument will give varying results, the most obvious being the calibration for whole blood and plasma. And just what is the standard reference method for glucose [10], and should there not be a reference method for each sample type – whole blood, plasma and hemolysate?
Evaluation of instruments
The methodology of glucose analysis in routine clinical use is nowadays based on either chromogenic or electrochemical reactions of the three enzymes glucose oxidase, dehydrogenase and hexokinase. This gives rise to method-based specific interferences, such as the blood oxygen tension dependency of glucose oxidase, which is a major issue in intensive care unit patients. Critically ill patients may have very low hematocrits, high or low arterial of venous oxygen tensions and may present extreme acid-base abnormalities, all of which have to be evaluated for every glucose analyzer under consideration [11,12]. Special attention must be paid to specific interference from intensive care unit typical medication [13]. Precision and accuracy have to be determined using standardized protocols and care has to be taken to choose the suitable reference method [10], which may not be the one available in the central laboratory where the study is being performed. Instruments such as the Continuous Glucose Monitoring System (CGMS System Gold, Medtronic Minimed) used in the study of Corstjens and colleagues seem to find their place in the monitoring of diabetic patients [14] but still need further evaluation of their clinical utility in critically ill patients.
Conclusion
At the end of their discussion Corstjens and colleagues state the following: "our study has too few patients and therefore too little data points under extreme conditions of pH, temperature, electrolyte disturbances and hypoglycaemia to make statements about reliability of the specific analyzers under these circumstances." But this is exactly what is needed to be done – otherwise we might never get an evidence-based answer about benefit versus potential harm of intensive insulin therapy in critically ill patients.
Competing interests
The authors declare that they have no competing interests.
See related research by Ligtenberg et al., http://ccforum.com/content/10/5/R135
References
- Corstjens AM, Ligtenberg JJM, Van der horst ICC, Spanjersberg R, Lind JSW, Tulleken JE, Meertens JHJM, Zijlstra JG. Accuracy and feasibility of point-of-care and continuous blood glucose analyzing in critically ill ICU patients. Crit Care. 2006;10:R135. doi: 10.1186/cc5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46:271–286. [PubMed] [Google Scholar]
- Van den Berghe G, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, Bouillon R, Schetz M. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55:3151–3159. doi: 10.2337/db06-0855. [DOI] [PubMed] [Google Scholar]
- Kanji S, Singh A, Tierney M, Meggison H, McIntyre L, Hebert PC. Standardization of intravenous insulin therapy improves the efficiency and safety of blood glucose control in critically ill adults. Intensive Care Med. 2004;30:804–810. doi: 10.1007/s00134-004-2252-2. [DOI] [PubMed] [Google Scholar]
- Wiener K. Whole blood glucose: what are we actually measuring? Ann Clin Biochem. 1995;32:1–8. doi: 10.1177/000456329503200101. [DOI] [PubMed] [Google Scholar]
- Burnett RW, D'Orazio P, Fogh-Andersen N, Kuwa K, Kulpmann WR, Larsson L, Lewnstam A, Maas AH, Mager G, Spichiger-Keller U, Scientific Division, Working Group on Selective Electrodes IFCC recommendation on reporting results for blood glucose. Clin Chim Acta. 2001;307:205–209. doi: 10.1016/S0009-8981(01)00431-4. [DOI] [PubMed] [Google Scholar]
- Fogh-Andersen N, D'Orazio P. Proposal for standardizing direct-reading biosensors for blood glucose. Clin Chem. 1998;44:655–659. [PubMed] [Google Scholar]
- Hannestad U, Lundblad A. Accurate and precise isotope dilution mass spectrometry method for determining glucose in whole blood. Clin Chem. 1997;43:794–800. [PubMed] [Google Scholar]
- Tang Z, Louie RF, Lee JH, Lee DM, Miller EE, Kost GJ. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29:1062–1070. doi: 10.1097/00003246-200105000-00038. [DOI] [PubMed] [Google Scholar]
- Tang Z, Du X, Louie RF, Kost GJ. Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Arch Pathol Lab Med. 2000;124:577–582. doi: 10.5858/2000-124-0577-EOPOGM. [DOI] [PubMed] [Google Scholar]
- Tang Z, Du X, Louie RF, Kost GJ. Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am J Clin Pathol. 2000;113:75–86. doi: 10.1309/QAW1-X5XW-BVRQ-5LKQ. [DOI] [PubMed] [Google Scholar]
- Lodwig V, Heinemann L, Glucose Monitoring Study Group Continuous glucose monitoring with glucose sensors: calibration and assessment criteria. Diabetes Technol Ther. 2003;5:572–586. doi: 10.1089/152091503322250596. [DOI] [PubMed] [Google Scholar]


