Abstract
Introduction
Diffuse alveolar hemorrhage (DAH) is a serious pulmonary complication seen in patients with autoimmune disorders and patients treated with chemotherapy or after hematopoietic stem cell transplantation. The clinical management of DAH is complex and the condition has a high mortality rate. Tissue factor is expressed in the lung alveoli during inflammation and therefore pulmonary administration of human recombinant activated factor VIIa (rFVIIa) could be a rational treatment option.
Methods
Six patients with acute, bronchoscopically confirmed DAH from a single intensive care unit university hospital center were included in the study of acute DAH in critically ill patients. The patients were treated with intrapulmonary administration of 50 μg/kg rFVIIa in 50 ml of sodium chloride by bronchoalveolar lavage (BAL) with 25 ml in each of the main bronchi, which was repeated after 24 hours in case of treatment failure.
Results
An excellent response, defined as complete and sustained hemostasis after a single dose of rFVIIa, was seen in three patients. A good response, meaning that sustained hemostasis was achieved by a repeated rFVIIa administration, was seen in the remaining three patients. In one of these patients, the BAL treatment was repeated twice; in another patient, the second dose of rFVIIa was administered by nebulizer after extubation after the initial BAL. The hemostatic effect was statistically significant (p = 0.031). The oxygenation capacity, as reflected by the PaO2/FiO2 (arterial oxygen pressure/inspiratory fractional oxygen content) ratio, increased significantly (p = 0.024) in all six patients following the local rFVIIa therapy.
Conclusion
Symptomatic therapy of DAH after intrapulmonary administration of one or more doses of rFVIIa was found to have a good to excellent hemostatic effect in six consecutive patients with DAH. The intrapulmonary administration of rFVIIa seemed to have a high benefit-to-risk ratio. Larger series should confirm the safety of this approach.
Introduction
Diffuse alveolar hemorrhage (DAH) is a serious pulmonary complication of mostly unknown etiology and pathogenesis, although injury to alveolar capillary endothelium and alveolar inflammation, resulting in the release of inflammatory cytokines, have been implicated [1,2]. The disease is seen after hematopoietic stem cell transplantation (HSCT), after chemotherapy, and in patients with autoimmune disorders [3]. The extensive pulmonary inflammation leads to abundant intra-alveolar expression of tissue factor (TF), resulting in a several-fold increase in molecular markers of thrombin generation in bronchoalveolar lavage (BAL) fluid [4]. Effective local hemostatic strategies are lacking, and mortality rates exceed 50% in those who require mechanical ventilator support [5]. We hypothesized that local administration of human recombinant activated factor VIIa (rFVIIa) might be an effective treatment option.
Materials and methods
Six consecutive patients with pulmonary bleeding, of whom four had an inspiratory fractional oxygen content (FiO2) demand of 1.0, not responding to conventional therapy were studied. At our institution, treatment of pulmonary bleeding includes transfusion of fresh frozen plasma (FFP) and platelet concentrate (PC) to normalize systemic coagulation ability, endotracheal and intravenous (i.v.) administration of tranexamic acid, and if no hemostatic effect is obtained, this is followed by continuous aprotinin i.v. infusion. The diagnosis of DAH was confirmed bronchoscopically by identification of ongoing bleeding at the bronchial segmental level before treatment with rFVIIa. The dose was approximately 50 μg/kg dissolved in 50 ml of saline distributed evenly in the right and left main bronchi. In one non-intubated patient, rFVIIa was administered as an inhalant at a dose of 50 μg/kg via a jet-driven nebulizer.
Treatment efficacy of rFVIIa was graded as an excellent, good, or poor response. An 'excellent' response was defined as a complete and sustained hemostasis after a single treatment with rFVIIa. The response was graded as 'good' when repeated intrapulmonary administration of rFVIIa was required to obtain hemostasis. A 'poor' response was characterized by the lack of any effect by rFVIIa on bleeding. The hemostatic effect and the oxygenation capacity were statistically analyzed using the non-parametric tests, McNemar's test and Wilcoxon signed paired rank test, respectively. A waiver of informed consent was obtained from the Institutional Review Board.
Results
Patients
Patient 1
A 46-year-old male with chronic lymphocytic leukemia underwent allogenic non-myeloablative stem cell transplantation (HSCT). The post-transplant course was complicated by severe graft-versus-host disease (GvHD) of the skin, thrombocytopenia, and systemic cytomegalovirus (CMV) infection, causing treatment-induced renal failure that necessitated hemodialysis (Table 1).
Table 1.
Comparison of underlying disease, the effect of intrapulmonary rFVIIa therapy, and survival of DAH
| Patient | Gender | Diagnosis | Pathogenesis of DAH | rFVIIa doses via BAL | Evaluation of rFVIIa effecta | Survival or cause of death |
| 1 | Male | Allo-HSCTb (CLL) | CMV, GvHD | 3 | Good | Septic shock and cardiorespiratory failure |
| 2 | Male | Neurosarcoidosis | Unknown | 1 | Excellent | Septic shock |
| 3 | Male | AML | Unknown | 1 | Excellent | Survived |
| 4 | Female | Wegener's granulomatosis | Unknown | 1c | Good | Survived |
| 5 | Female | AIDS | Unknown | 2 | Good | Septic shock and respiratory failure |
| 6 | Male | Allo-HSCT (AML) | Unknown | 1 | Excellent | Survived |
aThe hemostatic effect was statistically significant (p = 0.031, McNemar's test). bNon-myeloablative allogeneic stem cell transplantation. cOne rFVIIa dose via BAL and, three days later when not intubated, subsequent three consecutive doses of rFVIIa via jet nebulizer. AML, acute myeloid leukemia; BAL, bronchoalveolar lavage; CLL, chronic lymphatic leukemia; CMV, cytomegalovirus; DAH, diffuse alveolar hemorrhage; GvHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; rFVIIa, human recombinant activated factor VII.
Nine months after HSCT, he was admitted to the intensive care unit (ICU) with septic shock, which was treated with empiric antibiotics, vasoactive medication, and mechanical ventilation. Seven days after ICU admission, the FiO2 requirement suddenly increased to 1.0 and fresh blood was coming from the endotracheal tube. The platelet count was 10 × 109 per liter and the activated partial thromboplastin time (APTT) was 40 seconds. Despite platelet transfusion to a platelet count of more than 100 × 109 per liter and FFP administration to an APTT of less than 35 seconds (in addition to the standard treatment as described in Materials and methods), the bleeding increased in severity. rFVIIa was administered intravenously in doses of up to 120 μg/kg without hemostatic effect. Bronchoscopy revealed fresh bleeding from both lungs. rFVIIa (50 μg/kg) dissolved in saline was administered by BAL into the left and right main bronchi simultaneously with the systemic i.v. administration of 50 μg/kg rFVIIa. This resulted in an immediate cessation of the bleeding from the endotracheal tube. The arterial oxygen pressure (PaO2)/FiO2 ratio increased the subsequent day. The hemostatic effect lasted for approximately 36 hours, after which bleeding recurred. The treatment was repeated twice also, and bleeding ceased for more than 24 hours. Hereafter, the patient received rFVIIa (50 μg/kg) by BAL alone twice, and bleeding ceased for 24 to 36 hours after each administration. Complete hemostasis was obtained after the third BAL administration of rFVIIa and lasted for three months (Table 2). The patient died three months after the first rFVIIa treatment, due to respiratory and circulatory failure secondary to septic shock without evidence of active pulmonary bleeding. Postmortem examination revealed no signs of acute respiratory distress syndrome (ARDS) in the alveoli or thromboembolic complications.
Table 2.
Comparison of hemostatic variables and oxygenation capacity before and after rFVIIa in patients with DAH
| Timing | 24 hours prior to rFVIIa | 24 hours after rFVIIaa | |||||||||||
| Patient number | 1 | 2 | 3 | 4 | 5 | 6 | 1b | 2 | 3 | 4 | 5 | 6 | |
| Coagulation | Platelet count (109 per liter) | 110 | 88 | 112 | 105 | 99 | 90 | 100 | 80 | 106 | 105 | 102 | 69 |
| APTT (seconds) | 31 | 36 | 32 | 33 | 41 | 27 | 33 | 38 | 32 | 33 | 38 | 27 | |
| Transfusion (units) | RBCs | 4 | 3 | 2 | 4 | 3 | 4 | 1 | 0 | 0 | 4 | 1 | 2 |
| FFP | 4 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | |
| PC | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | |
| Oxygenation capacity | PaO2/FiO2 ratio (mm Hg) | 67 | 62 | 88 | 64 | 187 | 100 | 176 | 152 | 132 | 313 | 213 | 345 |
aThe increase in oxygenation capacity was significant (p = 0.024, Wilcoxon signed paired rank test). bThere was a steady increase in oxygenation capacity subsequent to rFVIIa therapy (that is, the PaO2/FiO2 ratio was 176 mm Hg after one day, 137 mm Hg after one month, 136 mm Hg after two months, and finally 252 mm Hg after three months). APTT, activated partial thromboplastin time; DAH, diffuse alveolar hemorrhage; FFP, fresh frozen plasma; PaO2/FiO2, arterial oxygen pressure/inspiratory fractional oxygen content; PC, platelet concentrate; RBC, red blood cell; rFVIIa, human recombinant activated factor VII.
Patient 2
A 63-year-old male with progressive neurosarcoidosis was treated with infusion of methotrexate. After the second treatment, he developed pancytopenia and septic shock and was transferred to the ICU, where he was intubated and mechanically ventilated. Fresh blood was observed from the tracheal tube, and chest x-ray showed interstitial diffuse alveolar infiltrates indicating pulmonary bleeding. Standard treatment was instituted, resulting in an increase in platelet count from 18 × 109 per liter to 88 × 109 per liter with a reduction in APTT from 45 to 36 seconds (Table 2). Multiple organ failure developed and increasing amounts of fresh blood were observed from the tube while the FiO2 requirement increased from 0.7 to 1.0. A single dose (20 μg) of i.v. desmopressin did not improve hemostasis. Bronchoscopy revealed ongoing bleeding from the distal bronchial tree bilaterally. A 50 μg/kg dose of rFVIIa was administered via BAL, resulting in immediate cessation of the pulmonary bleeding. Four hours after rFVIIa administration, FiO2 could be reduced to 0.8 and was further reduced to 0.6 the following morning (Table 2). Despite continued improvement in pulmonary function, increasing circulatory instability secondary to septic shock became evident and the patient died four days after rFVIIa treatment. No pulmonary bleeding or thromboembolic complications were found after the intrapulmonary administration of rFVIIa.
Patient 3
A 44-year-old male with acute myeloid leukemia (AML) developed high fever and hemoptysis 14 days after induction of a combination of chemotherapy comprising cytarabine, amsacrine, and etoposide. Chest x-ray showed bilateral infiltrations, and BAL revealed Stenotrophomonas maltophilia. The patient was admitted to the ICU, where mechanical ventilation was instituted with an FiO2 of 1.0, and was started with inotropic support and antimicrobial therapy (i.v. ceftazidime and inhalation of colistin). Pulmonary bleeding increased despite standard treatment (Table 2). Bronchoscopy revealed ongoing bleeding from the distal bronchial tree bilaterally, and rFVIIa (50 μg/kg) was administered by BAL. The bleeding ceased and the FiO2 was decreased to 0.45 over the next 24 hours. Over the next 12 days, the situation stabilized and improvement of the pulmonary and the circulatory functions was observed and the patient could be weaned off of the ventilator and discharged from the ICU. No pulmonary bleeding or thromboembolic complications were observed after the intrapulmonary administration of rFVIIa.
Patient 4
A 34-year-old woman was suspected of having Wegener's granulomatosis on the basis of eosinophilia, positive anti-neutrophil cytoplasmatic antibodies, hematuria, and proteinuria. Treatment with systemic corticosteroids was instituted, but due to respiratory distress and the presence of hemoptysis, the patient was admitted to the ICU. Chest x-ray showed interstitial subtle bilateral alveolar infiltrates indicating pulmonary bleeding. The patient was intubated and mechanically ventilated with an FiO2 of 1.0, and systemic antibiotic treatment was initiated in conjunction with pulse treatment with methylprednisone (1,000 mg intravenously every day for three days and then 40 mg every day). Treatment including continuous aprotinin infusion was instituted. The pulmonary bleeding ceased, FiO2 demand was reduced to 0.35, and antifibrinolytic treatment was discontinued. Twelve hours after discontinuation of aprotinin, fresh pulmonary bleeding again became apparent, together with an increase in FiO2 requirements to 1.0. The bleeding was refractory to standard treatment, including aprotinin. Bronchoscopy revealed ongoing bleeding at the segmental level, and rFVIIa (50 μg/kg) was administered by BAL, leading to immediate cessation of the bleeding; FiO2 was reduced to 0.3 at six hours after rFVIIa treatment, and the patient was extubated the following morning.
A biopsy from the skin showed perivascular eosinophilic infiltration indicating Churg-Strauss vasculitis, and the patient responded to the corticosteroid therapy treatment with regression of paresis and normalization of urine tests. Three days after extubation, bleeding from the lungs reoccurred together with an increase in O2 demand. To avoid re-intubation, rFVIIa was administered through a jet nebulizer with a prompt hemostatic effect. The aerosolized rFVIIa was repeated twice over the following 12 hours. A sustained hemostasis and a decrease in O2 requirements from 15 to 4 liters/minute were obtained. The further clinical course was uneventful, and the patient was discharged from the ICU three days later.
Patient 5
A 44-year-old HIV-positive female with chronic hemodialysis requirement, severe critical illness polyneuropathy, and enterocolitis due to Clostridium difficile infection, for which she received i.v. vancomycin, underwent surgery due to gastrointestinal bleeding. Postoperatively, she was transferred to the ICU, receiving mechanical ventilator support, and developed ventilator-associated pneumonia due to Pseudomonas aeruginosa, which was treated successfully with broad-spectrum antibiotics. In addition, a systemic CMV infection developed for which she was treated with Foscarnet, leading to stabilization over the following weeks. At 51 days after ICU admission, however, fresh bleeding occurred from the tracheotomy but did not respond to standard treatment as described previously. BAL revealed localized bleeding at the segmental level bilaterally, and rFVIIa (50 μg/kg) dissolved in 50 ml of sodium chloride was administered with 25 ml in each main bronchus. The pulmonary bleeding ceased but reappeared within 24 hours after the treatment, and rFVIIa administration by BAL was repeated. Hereafter, the bleeding ceased to occur. The patient expired due to infection and respiratory insufficiency 115 days after rFVIIa treatment, without any signs of thromboembolic complications.
Patient 6
A 63-year-old male with AML underwent non-myeloablative stem cell transplantation. The post-transplant course was complicated by GvHD of the skin and temporary poor graft function with pancytopenia. Six months post-transplant, the patient was transferred to the ICU due to respiratory insufficiency secondary to pulmonary infection. The patient was intubated and mechanically ventilated with an FiO2 of 0.45. Diagnostic BAL showed fresh blood at segmental levels bilaterally, but the focus of bleeding could not be identified. The platelet count was 35 × 109 per liter and APTT was 40 seconds, for which the patient received FFP to achieve an APTT of less than 30 seconds and PCs to achieve a platelet count of more than 80 × 109 per liter but without significant effect on the bleeding. The patient received empirical broad-spectrum antibiotics and antimycotics, resulting in a decrease of the C-reactive protein over the next five days. Twelve days after ICU admission, the pulmonary bleeding increased and the FiO2 demand was increased to 0.6. The patient had a normal TEG (thrombelastografic in vitro coagulation) profile, a platelet count of 80 × 109 per liter, and an APTT of 27 seconds, indicating a localized coagulopathy. Due to further increase in bleeding and FiO2 demand, a diagnostic BAL was performed, showing fresh bleeding bilaterally at segmental levels; rFVIIa at a dose of 50 μg/kg dissolved in 50 ml of saline was administered, resulting in immediate cessation of the pulmonary bleeding. The FiO2 was reduced to 0.35 within the next eight hours, and the patient was extubated the following morning. Three days after treatment with local pulmonary rFVIIa, the patient was discharged from the ICU without further bleeding episodes.
Discussion
DAH is a clinical syndrome with acute onset of alveolar infiltrates and hypoxemia, yielding progressively diffuse alveolar bleeding. Clinical features include dyspnea, cough, hemoptysis, abnormal chest x-ray with bilateral alveolar infiltrates, and hypoxia usually accompanied with fever [6,7]. The treatment of DAH is empiric in as much as the condition is a life-threatening medical emergency with no specific or proven effective therapy. Treatment with high-dose steroids may be beneficial when given early [7], but overall mortality remains high; plasmapheresis has been advocated, but there is no evidence that this intervention is successful in the treatment of ongoing low-volume critical bleeding [8].
Here, we report a series of six patients of DAH verified by bronchoscopy in mechanically ventilated patients. There was no or insufficient hemostatic effect of standard therapy including transfusion of FFP and PCs, i.v. infusion of aprotinin, and tranexamic acid intravenously or via the endotracheal route (Table 1).
rFVIIa is an approved agent for the i.v. treatment of bleeding episodes in patients with hemophilia A or B with inhibiting antibodies toward factor VIII or factor IX, respectively. Factor VII initiates clot formation by its interaction with TF [9,10]. The FVIIa-TF complex activates factor X. In high doses (80 to 100 μg/kg), however, activated FVII also activates factor X in the absence of TF, probably by activation of factor X bound to the surface of activated platelets. Activated factor X activates prothrombin to thrombin, which in turn converts fibrinogen to fibrin [10].
High and repeated i.v. doses of rFVIIa have been reported to have some hemostatic effect in patients with DAH [11-13]. As described in this report, however, i.v. administration of a very high dose of rFVIIa did not induce hemostasis in our first patient with DAH. This led us to explore the effect of local pulmonary administration of rFVIIa, and the efficacy of this treatment was demonstrated in six consecutive patients with DAH of different etiologies. The rFVIIa was administered at a dose of approximately 50 μg/kg via BAL in six patients and as a nebulized aerosol on one occasion. The intervention with local intrapulmonary rFVIIa had a significant hemostatic effect (p = 0.031). To our knowledge, this is the first time an effective treatment of DAH using symptomatic treatment with local intrapulmonary rFVIIa has been reported.
Clinical observation supports the hypothesis that pulmonary hemostasis can be induced more effectively from the alveolar side in DAH and than from the endothelial side. This viewpoint is supported by the fact that alveolar TF is demonstrated in high concentrations in inflammatory pulmonary conditions like ARDS [14], pneumonia [15], and after lipopolysaccharide challenge locally in the alveoli [16]. The mode of action of the observed alveolar hemostasis is most likely primarily explained by the TF-dependent pathway, where alveolar TF is expressed during the inflammatory phase of DAH. On the other hand, TF pathway inhibitor (TFPI) is a strong inhibitor of the local activation of factor X to Xa by the FVIIa-TF complex. In acute lung injury, TFPI produced by alveolar macrophages may be increased 20-fold [17]. Our observations indicate that intrapulmonary administration of FVIIa overrides the anticoagulant effect of TFPI (Figure 1).
Figure 1.
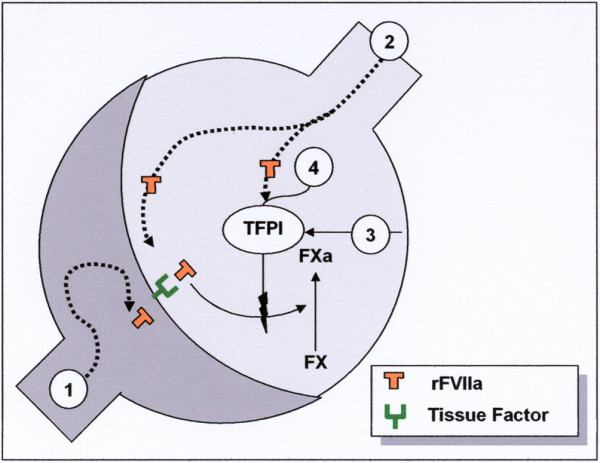
Rationale for the local mode of action of intra-alveolar human recombinant activated factor VII (rFVIIa) in diffuse alveolar hemorrhage (DAH). Intravenous rFVIIa does not reach the alveoli in a sufficient concentration (1) in contrast to the airway route (2). Alveolar tissue factor (TF)-FVIIa complex activates coagulation factor IX and X. TF and TF pathway inhibitor (TFPI) are constitutively expressed in the airspace, secondary to inflammation induced in DAH (3). TFPI counteracts the activation effect of the FVIIa-TF complex. Alveolar rFVIIa in high concentration counteracts the TFPI anticoagulation (4).
A safety issue of the local rFVIIa treatment, however, is the possible risk of inducing widespread alveolar fibrin deposition (that is, hyaline membrane formation), which is a hallmark of ARDS. There were, however, no signs of developing ARDS in the six treated patients because the oxygenation capacity, as reflected by the PaO2/FiO2 ratio, increased significantly in the six patients after the pulmonary rFVIIa administration (Figure 2). The benefit, the hemostatic effect, was excellent in three patients and good in three patients (that is, a statistically significant treatment effect was observed in all patients) (Table 2). The benefit-to-risk ratio of local rFVIIa treatment in DAH therefore seems to be high.
Figure 2.
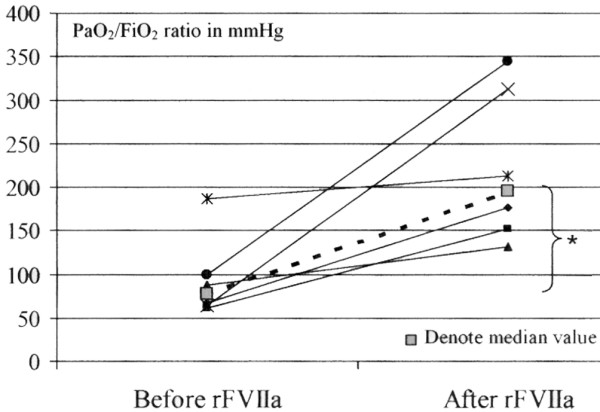
Oxygenation capacity before and after local pulmonary human recombinant activated factor VII (rFVIIa) therapy. A significant improvement in PaO2/FiO2 (arterial oxygen pressure/inspiratory fractional oxygen content) ratio was observed after the hemostatic treatment (*p = 0.024, Wilcoxon signed paired rank test).
Conclusion
A new symptomatic therapy, involving intrapulmonary administration of rFVIIa, to stop the life-threatening critical bleeding in DAH is documented in six consecutive patients with DAH. It seems that pulmonary hemostasis occurs most likely from the alveolar side in DAH and to a much lesser extent from the lung vascular endothelial side, a viewpoint that is supported by the clinical observation of the patients with DAH and by the well-described pathophysiology of the lung as a hemostatic organ, with TF-dependent and TF-independent modes of action. But irrespective of the mode of action, FVIIa has a potentially high benefit-to-risk ratio when administered via the local intrapulmonary route. These findings warrant further exploration of the local pulmonary effect of rFVIIa and the safety of this novel treatment strategy in patients with DAH.
Key messages
• DAH has a high mortality and no documented specific intervention.
• Symptomatic therapy with local intrapulmonary therapy with one or more doses of recombinant FVIIa was found to have a good to excellent hemostatic effect in patients with DAH.
• No adverse effects could be demonstrated.
• The treatment with rFVIIa seems to have a high benefit-to-risk ratio in DAH.
Abbreviations
AML = acute myeloid leukemia; APTT = activated partial thromboplastin time; ARDS = acute respiratory distress syndrome; BAL = bronchoalveolar lavage; CMV = cytomegalovirus; DAH = diffuse alveolar hemorrhage; FFP = fresh frozen plasma; FiO2 = inspiratory fractional oxygen content; GvHD = graft-versus-host disease; HSCT = hematopoietic stem cell transplantation; ICU = intensive care unit; i.v. = intravenous; PaO2 = arterial oxygen pressure; PC = platelet concentrate; rFVIIa = human recombinant activated factor VII; TF = tissue factor; TFPI = tissue factor pathway inhibitor.
Competing interests
LH has shares in the pharmacompany Pharmaorigin, Copenhagen, Denmark, which is holding a patent related to the local pulmonary treatment with rFVIIa, but has not received reimbursements, fees, funding, or salary from any organization relating to the content or the preparation of the manuscript. LH declares that he has no other competing interests. JDN, ML, HS, and PIJ declare that they have no competing financial interests related to the preparation or the content of the manuscript.
Authors' contributions
LH and PIJ developed the study design and coordinated its implementation. JDN, HS, and ML participated in the interpretation and discussion of results and drafted and revised the manuscript. LH and PIJ were responsible for patient recruitment as well as data collection. LH carried out the statistical analysis. All authors read and approved the final manuscript.
Contributor Information
Lars Heslet, Email: heslet@rh.dk.
Jorn Dalsgaard Nielsen, Email: jdn@dadlnet.dk.
Marcel Levi, Email: m.m.levi@amc.uva.nl.
Henrik Sengeløv, Email: h.sengeloev@rh.dk.
Pär I Johansson, Email: rh08061@rh.dk.
References
- Huaringa AJ, Leyva FJ, Giralt SA, Blanco J, Signes-Costa J, Velarde H, Champlin RE. Outcome of bone marrow transplantation patients requiring mechanical ventilation. Crit Care Med. 2000;28:1014–1017. doi: 10.1097/00003246-200004000-00017. [DOI] [PubMed] [Google Scholar]
- Agusti C, Ramirez J, Picado C, Xaubet A, Carreras E, Ballester E, Torres A, Battochia C, Rodriguez-Roisin R. Diffuse alveolar hemorrhage in allogeneic bone marrow transplantation. A postmortem study. Am J Respir Crit Care Med. 1995;151:1006–1110. doi: 10.1164/ajrccm/151.4.1006. [DOI] [PubMed] [Google Scholar]
- Lewis ID, Defor T, Weisdorf DJ. Increasing incidence of diffuse alveolar hemorrhage following allogeneic bone marrow transplantation: cryptic etiology and uncertain therapy. Bone Marrow Transplant. 2000;26:539–543. doi: 10.1038/sj.bmt.1702546. [DOI] [PubMed] [Google Scholar]
- Levi M, van Der Poll T, ten Cate H, Kuipers B, Biemond BJ, Jansen HM, ten Cate JW. Differential effects of anti-cytokine treatment on bronchoalveolar hemostasis in endotoxemic chimpanzees. Am J Respir Crit Care Med. 1998;158:92–98. doi: 10.1164/ajrccm.158.1.9709007. [DOI] [PubMed] [Google Scholar]
- Huaringa AJ, Leyva FJ, Signes-Costa J, Morice RC, Raad I, Darwish AA, Champlin RE. Bronchoalveolar lavage in the diagnosis of pulmonary complications of bone marrow transplantation patients. Bone Marrow Transplant. 2000;25:975–980. doi: 10.1038/sj.bmt.1702335. [DOI] [PubMed] [Google Scholar]
- Weisdorf DJ. Diffuse alveolar hemorrhage: an evolving problem? Leukemia. 2003;17:1049–1050. doi: 10.1038/sj.leu.2402921. [DOI] [PubMed] [Google Scholar]
- Raptis A, Mavroudis D, Suffredini A, Molldrem J, Rhee FV, Childs R, Phang S, Barrett A. High-dose corticosteroid therapy for diffuse alveolar hemorrhage in alloeneic bone marrow stem cell transplant recipients. Bone Marrow Transplant. 1999;24:879–883. doi: 10.1038/sj.bmt.1701995. [DOI] [PubMed] [Google Scholar]
- Speck U. Diffuse alveolar hemorrhage syndromes. Curr Opin Rheumatol. 2001;13:12–17. doi: 10.1097/00002281-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Lisman T, de Groot PH. Mechanism of action of recombinant factor VIIa. J Thromb Haemost. 2003;1:1138–1139. doi: 10.1046/j.1538-7836.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- Hedner U. Recombinant factor VIIa (NovoSeven) as a hemostatic agent. Dis Mon. 2003;49:39–48. doi: 10.1053/shem.2001.29507b. [DOI] [PubMed] [Google Scholar]
- Pastores SM, Papadopoulos E, Voigt L, Halpern NA. Diffuse alveolar hemorrhage after allogeneic hematopoietic stem-cell transplantation: treatment with recombinant factor VIIa. Chest. 2003;124:2400–2403. doi: 10.1378/chest.124.6.2400. [DOI] [PubMed] [Google Scholar]
- Hicks K, Peng D, Gajewski JL. Treatment of diffuse alveolar hemorrhage after allogeneic bone marrow transplant with recombinant factor VIIa. Bone Marrow Transplant. 2002;30:975–978. doi: 10.1038/sj.bmt.1703731. [DOI] [PubMed] [Google Scholar]
- Henke D, Falk RJ, Gabriel DA. Successful treatment of diffuse alveolar hemorrhage with activated Factor VII. Ann Intern Med. 2004;140:493–494. doi: 10.7326/0003-4819-140-6-200403160-00033. [DOI] [PubMed] [Google Scholar]
- Schultz MJ, Haitsma JJ, Zhang H, Slutsky A. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia. Crit Care Med. 2006;34:871–877. [PubMed] [Google Scholar]
- Schultz MJ, Millo J, Levi M, Hack CE, Weverling GJ, Garrard CS, van der Poll T. Local activation of coagulation and inhibition of fibrinolysis in the lung during ventilator associated pneumonia. Thorax. 2004;59:130–135. doi: 10.1136/thorax.2003.013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M, Schultz MJ, Rijneveld AJ, van der Poll T. Bronchoalveolar coagulation and fibrinolysis in endotoxemia and pneumonia. Crit Care Med. 2003;31:S238–S242. doi: 10.1097/01.CCM.0000057849.53689.65. [DOI] [PubMed] [Google Scholar]
- Sabharwal AK, Bajaj SP, Ameri A, Tricomi SM, Hyers TM, Dahms TE, Taylor FB, Jr, Bajaj MS. Tissue factor pathway inhibitor and von Willebrand factor antigen levels in adult respiratory distress syndrome and in a primate model of sepsis. Am J Respir Crit Care Med. 1995;151:758–767. doi: 10.1164/ajrccm/151.3_Pt_1.758. [DOI] [PubMed] [Google Scholar]


