Abstract
Arthritis pain affects millions of people worldwide yet we still have only a limited understanding of what makes our joints ache. This review examines the sensory innervation of diarthroidal joints and discusses the neurophysiological processes that lead to the generation of painful sensation. During inflammation, joint nerves become sensitized to mechanical stimuli through the actions of neuropeptides, eicosanoids, proteinase-activated receptors and ion channel ligands. The contribution of immunocytes to arthritis pain is also reviewed. Finally, the existence of an endogenous analgesic system in joints is considered and the reasons for its inability to control pain are postulated.
Introduction
According to a recent report released by the World Health Organization [1], musculoskeletal disorders are the most frequent cause of disability in the modern world, and the prevalence of these diseases is rising at an alarming rate. The most prominent reason for loss of joint mobility and function is chronic or episodic pain, which leads to psychological distress and impaired quality of life. Current therapies to help alleviate joint pain have limited effectiveness and certain drugs produce unwanted negative side effects, thereby precluding their long-term use. In short, millions of patients are suffering from the debilitating effects of joint pain for which there is no satisfactory treatment. One of the reasons for this lack of effective pain management is the paucity in our knowledge of what actually causes joint pain. We are only now starting to identify some of the mediators and mechanisms that cause joints to become painful, allowing us to develop future new targets that could better alleviate arthritis pain. This review summarizes what is known about the origin of joint pain by describing the neurobiological processes initiated in the joint that give rise to neural signals and that are ultimately decoded by the central nervous system into pain perception.
Joint innervation and nociception
Knee joints are richly innervated by sensory and sympathetic nerves [2,3]. Postganglionic sympathetic fibres terminate near articular blood vessels, where they regulate joint blood flow through varying degrees of vasoconstrictor tone. The primary function of sensory nerves is to detect and transmit mechanical information from the joint to the central nervous system. Large diameter myelinated nerve fibres encode and transmit proprioceptive signals, which can be interpreted as being either dynamic (movement sensations) or static (position sense). Pain-sensing nerve fibres are typically less than 5 μm in diameter and are either unmyelinated (type IV) or myelinated with an unmyelinated 'free' nerve ending (type III). These slowly conducting fibres typically have a high threshold and only respond to noxious mechanical stimuli, and as such are referred to as nociceptors [4]. In the rat and cat, 80% of all knee joint afferent nerve fibres are nociceptive [5-7], suggesting that joints are astutely designed to sense abnormal and potentially destructive movement.
Nociceptors are located throughout the joint, having been identified in the capsule, ligaments, menisci, periosteum and subchondral bone [8-13]. The most distal segment of type III and type IV afferents is devoid of a myelin sheath and perineurium, and it is believed that this is the sensory region of the nociceptive nerve. Transmission electron microscopy revealed an hour glass shape repeating pattern along the length of type III and type IV nerve terminals, and the multiple bulbous areas exhibit the characteristic features of receptive sites [14]. It is within these 'bead-like' structures on the terminals of 'free' nerve endings that joint pain originates.
The question of how a painful mechanical stimulus is converted into an electrical signal that can then be propagated along sensory nerves to the central nervous system is still unclear. The exposed nature of sensory 'free' nerve endings means that the axolemma of these fibres is probably subjected to significant stretch during joint movement. The recent identification of mechanogated ion channels on type III and type IV knee joint afferents by electrophysiological means provided the first insight into the physiological mechanisms responsible for mechanotransduction in joints [15]. The present theory is that movement of the joint generates shear stresses on the axolemma of the 'free' nerve endings, resulting in the opening of mechanogated ion channels. This leads to a depolarization of the nerve terminal and the generation of action potentials, which are subsequently transmitted to the central nervous system where they are decoded into mechanosensation. If a noxious movement is applied to the joint, the firing rate of the afferent nerve increases dramatically and the central nervous system interprets this nociceptive activity as pain [16-18].
Peripheral sensitization and joint inflammation
During inflammation, major plasticity changes occur in the peripheral and central nervous systems that lower the pain thresholds, giving rise to allodynia (pain in response to a normally innocuous stimulus) and hyperalgesia (heightened pain intensity in response to a normally painful stimulus). One means by which pain is generated in arthritic joints is via the stimulation of so-called 'silent nociceptors'. These afferent nerve fibres are quiescent in normal joints; however, following tissue injury or induction of inflammation these nociceptors become active and start to send nociceptive information to the central nervous system [18-20]. This supplementary input from the periphery by the 'silent nociceptors' is one of the contributing factors responsible for the generation of arthritis pain.
An additional process that initiates arthritis pain is peripheral sensitization wherein the activation threshold of joint nociceptors is reduced and afferent nerves become hyper-responsive to both normal and noxious types of movement [18-21]. The pioneering work of Coggeshall and coworkers [21] as well as Schaible and Schmidt [19,20,22] showed that chemical induction of an acute synovitis by intra-articular injection of kaolin and carrageenan reduced the activation threshold of type III and type IV knee joint afferents. The firing frequency of these mechanosensory nerves was dramatically enhanced during normal joint movements as well as during hyperextension and hyperflexion of the knee. It is believed that this augmentation in neuronal firing rate is interpreted by the central nervous system as joint pain and that this process is the neurophysiological basis for joint allodynia and hyperalgesia in these acutely inflamed joints. Decreased mechanical threshold and heightened afferent discharge rate have also been noted in adjuvant-induced chronic arthritis [23,24] as well as in an animal model of osteoarthritis [25]. Resting neuronal activity in the absence of any mechanical stimulation was also described in these arthritis models, which is consistent with an awakening of 'silent nociceptors'. This spontaneous firing of joint sensory nerves accounts for the resting joint pain commonly described by arthritis patients.
Factors contributing to joint peripheral sensitization
The evidence presented thus far clearly indicates that peripheral sensitization of joint afferents is the origin of arthritis pain. Hence, a greater understanding of the mechanisms and mediators responsible for the generation and maintenance of joint sensitization could lead to development of novel drug targets that could alleviate or even abolish arthritis pain. The factors that alter joint mechanosensitivity and promote nociception can be divided into two separate groups: mechanical factors and inflammatory mediators.
Mechanical factors involved in joint nociception
Diarthroidal joints are enveloped by a fibrous capsule that contains synovial fluid, the volume of which in normal human knee joints is between 1 and 4 ml. Following joint injury or during inflammation, synovial blood vessels become increasingly permeable to plasma proteins, which can leak out of the vasculature and accumulate in the intra-articular space. The subsequent shift in Starling forces promotes fluid exudation into the joint with subsequent oedema formation. Because the joint is an enclosed space, this effusion causes a dramatic increase in intra-articular pressure. In normal joints, intra-articular pressure is subatmospheric, ranging from -2 to -10 mmHg [26,27]; however, in rheumatoid arthritic knees synovial fluid volume can rise to 60 ml or more, with a concomitant increase in intra-articular pressure to approximately 20 mmHg supra-atmospheric [28]. A study in which a solution of dextrose and saline was infused into the knee joint revealed that intra-articular pressure rose more steeply in arthritic patients than in normal control individuals [28], probably due to a loss of capsular viscoelasticity and the occurrence of an invading pannus. As intra-articular pressure increased, the participants reported greater tightness around their knee and ultimately moderate pain was experienced. Animal studies [29,30] have shown that an elevation in intra-articular pressure results in burst firing of articular afferents, and the frequency of these neuronal discharges correlates with the level of pressure incurred. Thus, the increased intra-articular pressure associated with oedema formation in arthritic joints likely activates joint nociceptors, leading to pain.
Acute trauma and repetitive stress injuries are major causes of joint pain and disability. Acute joint trauma, such as sport-related injuries, typically involves damage to multiple soft tissues in the joint with varying degrees of damage. A large body of research has found that rupture of articular ligaments leads to joint instability and consequently abnormal loading patterns in the joint [31-34]. The relatively poor healing capacity of joint ligaments means that, over time, chronic instability results in focal erosion of the articulating surfaces, ultimately leading to joint degeneration and possibly osteoarthritis [35-40]. Inflammatory mediators released into the joint following trauma as well as the accumulation of cartilage degeneration products over time are probably the major contributors to peripheral sensitization in acute and repetitive joint injury, although the identity of these chemical agents is currently unknown. Altered joint biomechanics is also a likely candidate for initiating and maintaining joint pain; however, the processes that link loss of joint function and nociception have never been fully investigated. In one of the few reports on this matter, transection of the anterior cruciate ligament was found to cause increased electrical activity in the medial and posterior articular nerves in response to passive movement of the knee [41]. Again, it is unclear whether this heightened mechanosensitivity is due to local release of chemical sensitizers into the joint following surgery or whether abnormally high forces now act on the remaining uninjured articular tissues, leading to a rise in afferent firing rate. It is entirely feasible that both mechanical and chemical processes occur simultaneously in these unstable joints to generate pain, but further research is required to test this hypothesis.
Inflammatory mediators and peripheral sensitization
Following injury or pathogenic infection, joints typically exhibit a natural inflammatory response that mainly affects the synovium (synovitis). This process is necessary for the innate repair of damaged tissues, allowing the joint to recoup normal function. Inflammatory mediators released into the joint from such sources as nerves, immunocytes, synoviocytes, and vascular endothelium help to orchestrate these healing responses. These same inflammatory mediators also act on joint sensory nerves, leading to either excitation or sensitization. Indeed, local application of various compounds to normal joints elicits a frequency and burst profile of joint afferents that is similar to recordings made in arthritic knees. Identification of the inflammatory agents that evoke nociception is currently underway, and results from these studies will be of major therapeutic value in revealing novel targets that could inhibit peripheral sensitization and hence pain. The following is an overview of some of the better characterized inflammatory mediators that are associated with joint nociception.
Neuropeptides
Neuropeptides are a family of chemical mediators that are stored and released from the terminals of autonomic nerves and slowly conducting joint afferents. Local axon reflexes are responsible for the peripheral release of neuropeptides from sensory nerves, leading to neurogenic inflammation.
The inflammatory neuropeptides substance P (SP), calcitonin gene-related peptide (CGRP), and vasoactive intestinal peptide (VIP) have all been immunolocalized in joint tissues and their levels increase during arthritis [13,42-46]. Electrophysiological recording from knee joint primary afferents found that although local administration of SP had no direct effect on neuronal firing properties, it did cause peripheral sensitization of the nerves in response to normal and noxious joint movements [47]. Ionophoretic application of CGRP close to spinal cord neurones that have an input from knee joint afferents caused an increase in firing rate of these spinal, wide dynamic range neurones [48]. Furthermore, the hyper-responsiveness of these neurones following acute synovitis could be blocked by the selective antagonist CGRP8–37 [48], indicating that CGRP plays an important role in the central neurotransmission of painful mechanosensory information arising from the knee. The ability of CGRP to alter joint afferent activity peripherally has not yet been demonstrated. VIP is a 28-amino-acid neuropeptide that is contained in postganglionic sympathetic as well as capsaicin-sensitive sensory nerve fibres innervating the joint capsule [49-51]. Treatment of rat knee joints with exogenous VIP results in mechanonociceptive responses, as demonstrated by enhanced afferent firing frequency during joint rotation [25]. Animal behavioural studies confirmed that this elevation in sensory input to the central nervous system would translate into a pain response, because intra-articular injection of VIP causes a negative shift in hindlimb weight bearing as well as a reduction in hindpaw reaction thresholds to a tactile mechanical stimulus [52]. Interestingly, treatment of osteoarthritic knees with the VIP antagonist VIP6–28 reduced nociceptive and pain levels in these animals, highlighting the potential benefits in using this neuropeptide blocker to control arthritis pain [25,52].
A further sensory neuropeptide called nociceptin/orphanin FQ (N/OFQ) is also known to alter joint mechanosensitivity and modulate arthritis pain. N/OFQ is an opioid-like neuropeptide that has been immunolocalized in the peripheral and central nervous systems [53-55], where it controls central pain mechanisms [56-58]. In the knee joint, N/OFQ was found to have a dual effect on sensory nerve activity depending on dose of peptide, on level of mechanical manipulation of the knee, and on whether the joint was inflamed [59]. With normal rotation of control and acutely inflamed rat knees, N/OFQ had a sensitizing effect on joint afferents; however, high doses of N/OFQ desensitized joint mechanosensory nerves during hyper-rotation of inflamed knees. It was later found that the sensitizing effect of N/OFQ was due to the secondary release of SP into the joint because the selective NK1 receptor antagonist RP67580 blocked N/OFQ-mediated nociception [60]. The ability of N/OFQ to induce hyperalgesia and allodynia in the joint was recently demonstrated in experiments in which peripheral injection of N/OFQ produced a deficit in ipsilateral hindlimb weight bearing and increased von Frey hair mechano-sensitivity [61].
Taken together, these studies clearly show that the sensory neuropeptides SP, CGRP, VIP and N/OFQ are all involved in the generation and promotion of knee pain.
Eicosanoids
Eicosanoids are lipid membrane derived metabolites of arachidonic acid that include the prostaglandins, leukotrienes, lipoxins, thromboxanes and endocannabinoids. The most heavily studied eicosanoids with respect to joint inflammation and pain are the prostaglandins, which are extensively reviewed elsewhere [62-64]. Prostaglandins are formed via a complex enzymatic pathway in which arachidonic acid released from membrane phospholipids is oxygenated by cyclo-oxygenases to produce cyclic endoperoxide prosta-glandins. Tissue specific synthases and isomerases then transform these chemically unstable intermediates into the prostaglandins, thromboxanes and prostacyclins.
The pain field has generally focused on the activity of the cyclo-oxygenases, of which there are two isoforms: cyclo-oxygenase (COX)-1 and COX-2 (for review, see Smith and coworkers [65]). COX-1 is constitutively expressed in most cells, where its function is to maintain normal physiological processes in the tissue such as blood flow. Conversely, COX-2 is primarily upregulated during inflammatory situations by various inflammatory mediators such as cytokines [66], and it is therefore often referred to as the inducible isoform of the enzyme (although COX-2 is constitutively expressed in the central nervous system and kidney). In joints, COX-2 is not normally expressed but has been found to occur in significant amounts in the synovium, macrophages and endothelial cells of rheumatoid arthritis patients [67,68]. Because COX-2 is the predominant cyclo-oxygenase present at the site of inflammation, drugs that selectively inhibit COX-2 activity (the coxibs) were believed to have better therapeutic value than the nonselective nonsteroidal anti-inflammatory drugs (NSAIDs). It was initially thought that another advantage to coxib use was that it produced less gastrointestinal toxicity compared with traditional NSAIDs [69]. Although the anti-inflammatory and analgesic capacity of coxibs in arthritis is convincing, a number of these agents produce severely hazardous side effects such as myocardial infarction, hypertension and chronic renal failure. Clearly, a peripherally acting NSAID or intra-articular treatment with either selective and/or nonselective prostaglandin inhibitors could prove to be beneficial in treating joint pain while minimizing systemic side effects.
Peripheral intra-arterial injection of prostacyclin (prostaglandin [PG]I2), PGE1 and PGE2 have all been found to sensitize joint afferents in the rat and cat [70-72]. The sensitizing effect of these prostanoids was rapid in onset and led to an augmentation in afferent firing rate in response to mechanical as well as chemical stimuli. Furthermore, the sensitization of joint nociceptors by acute and chronic inflammation can be inhibited by the nonselective NSAIDs indomethacin and acetylsalicylic acid [73-75]. A recent study demonstrated that systemic administration of the COX-2 inhibitor meloxicam reduced pain evoked vocalization and joint favouring in adjuvant monoarthritic rats [76], although a direct antinociceptive effect of the drug on joint nociceptors was not definitively shown. Further study is necessary, therefore, to test the effectiveness of highly selective coxibs on joint nociception using animal models of arthritis.
The endocannabinoid anandamide is enzymatically synthesized from free arachidonic acid and ethanolamine [77]. Anandamide is a nonselective ligand that binds to both CB1 and CB2 cannabinoid G-protein-coupled receptors. CB1 receptors are mainly found on central and peripheral nerves, whereas CB2 receptors are associated with immunocytes [78-82]. The location of neuronal central and peripheral CB receptors indicates that activation of these receptors could modulate pain generation and perception [78,82-85]. In joints, high doses of anandamide actually caused excitation of polymodal sensory nerves, indicating a pro-nociceptive effect of the endocannabinoid [86], although the authors did suggest that low doses of anandamide could elicit an antinociceptive effect. An alternative explanation is the fact that anandamide acts on both CB receptor subtypes, and the net effect of the cannabinoid is an excitatory action. Experiments are currently underway to test the role of selective CB1 and CB2 agonists on joint mechanosensitivity to determine whether a differential response exists between these two receptor subtypes. An interesting aspect of the anandamide study was that its stimulatory effect on joint nociceptors was attained by activating the transient receptor potential (TRP) vanilloid channel 1 (TRPV1). This pathway was reaffirmed by joint blood flow experiments that showed that the vasomotor effects of a selective CB1 agonist in rat knees could be blocked by TRPV1 antagonism [87]. Zygmunt and coworkers [88] deduced that anandamide activation of TRPV1 channels on sensory nerves causes the secondary release of CGRP. It is possible, therefore, that the excitatory action of anandamide on joint afferents could be due to the secondary release of CGRP or other inflammatory neuropeptides into the joint.
Ion channel ligands
Multiple different types of ion channels exist on the terminals of nociceptors, and their activation either directly or via receptor coupling is necessary for nociceptive processing to occur. Opening of voltage-gated sodium channels permits depolarization of the afferent nerve terminal and propagation of action potentials towards the central nervous system. Sodium channels are typically blocked by the puffer fish poison tetrodotoxin (TTX); however, a significant population of sodium channels present on small diameter sensory neurones are resistant to TTX, and their function is to modulate nociceptive neurotransmission [89,90]. Chronic inflammation with concomitant persistence in nociceptive input has been shown to upregulate sodium channel expression and sodium channel currents in various tissues [91,92], including the temperomandibular joint [93]. Inflammatory mediators such as PGE2, adenosine and 5-hydroxytryptamine have all be shown to augment sodium channel kinetics and TTX-resistant sodium currents [94,95]. Thus, blockade of sodium channels on nociceptors may be a viable means of inhibiting pain. Indeed, treatment of adjuvant monoarthritic rat ankle joints with the sodium channel blockers mexilitine and crobenetine inhibited joint mechanical hyperalgesia and alleviated restrictions in animal mobility [96].
Calcium channels have also been implicated in pain processing (for review, see Yaksh [97]). Opening of voltage-gated calcium channels on primary afferent nerves leads to an increase in intracellular calcium concentration and consequently neurotransmitter release into the extraneuronal space. As is described above, a large number of these neuromediators can have a sensitizing effect on the sensory nerve and thereby promote nociception. In addition to the secondary release of algogenic agents from sensory nerve terminals, activation of voltage-gated calcium channels can directly have a positive effect on neuronal excitability and hence firing rate [97]. The role of calcium channels in joint pain is largely unexplored. In one of the few studies to address this issue, the anticonvulsant gabapentin, which binds to the α2δ subunit of calcium channels, was shown to reduce the mechanosensitivity of normal and acutely inflamed knee joints [98]. The full relevance of this finding to calcium channel neurobiology is uncertain.
In addition to voltage-gated cation channels, knee joints were recently found to possess mechanogated ion channels that are sensitive to changes in shear stress forces being applied to the neuronal membrane [15]. The forces generated by physical movement of a joint are transmitted throughout the organ where they are perceived by the articular innervation. The shear stress causes a conformational change in the mechanogated ion channels present on the nerve terminal, which leads to channel opening and consequently nerve depolarization. If movement becomes noxious, then greater forces are applied to the joint and the probability of mechanogated ion channel opening is increased and depolarization events become more frequent [15]. This enhanced activity is the molecular basis of joint pain.
Another superfamily of ion channels that has received a lot of attention recently are the TRP channels. Of particular interest in pain research are the TRPM (melanostatin) and the TRPV (vanilloid) channel subfamilies. The eighth member of the TRPM channels (TRPM8) is activated by cooling temperatures (22–26°C) as well as by agents such as menthol that produce a cool sensation [99,100]. It is thought that pharmacological activation of TRPM8 channels could elicit an anti-nociceptive effect in much the same way that applying ice packs to an injured joint can reduce pain sensation. Current research into this channel, however, has been hampered by the lack of efficacious and highly selective pharmacological tools. The use of heat to help control joint aches and pains has been appreciated for many years, but the molecular mechanism by which this is achieved has only recently been elucidated. The ion channel responsible for noxious thermosensation is TRPV1, which was first identified on rat sensory neurones by an expression-cloning approach [101]. In addition to being activated by temperatures above 43°C, TRPV1 is sensitive to protons, lipids, phorbols and cannabinoids. The CB1 agonist arachidonyl-2-chloroethylamide, for example, exerts its physiological effects in joints via a TRPV1-dependent pathway [87]. Unlike other TRP channels, several agonists and antagonists have been developed that are selective for TRPV1, including the blocker SB366791, which has been shown to be effective in joint tissues [102]. Electrophysiological studies have revealed that capsaicin (the hot spicy component of chilli peppers) sensitizes joint afferents probably by causing secondary release of inflammatory neuropeptides into the joint (unpublished observations). The joint subsequently becomes insensitive to further noxious mechanical stimuli, although the precise mechanism underlying this process is unknown.
Other chemical mediators
The preceding discussion has addressed the most commonly studied inflammatory mediators that are known to sensitize joint afferents, but it is far from exhaustive. Other chemical compounds that demonstrate peripheral sensitization in joints include bradykinin [103,104], histamine [105], 5-hydroxytryptamine [106], adenosine [107,108], and nitric oxide [109]. As the list of new potential targets continues to grow at a rapid rate, this exciting area of joint neurobiology will probably yield useful and beneficial pain control medicines that could act on one or a combination of these nociceptive pathways.
Neuroimmune pain pathways
The histological identification of synovial mast cells in close proximity to type III and type IV knee joint afferents [110,111], as well as the ability of neuromediators to stimulate leucocyte infiltration into joints [112,113] suggests an important involvement of immunocytes in neurogenic inflammation and pain. This concept is supported by the fact that mast cells and neutrophils can be activated by various sensory neuropeptides [114-123], resulting in explosive degranulation and subsequent release of inflammatory mediators into the local microenvironment. These immunocyte-derived factors can themselves cause joint inflammation and impart tissue hyperalgesia. For example, in acutely inflamed knees the vasomotor effect of N/OFQ is dependent on the presence of synovial mast cells and leucocytes [124], indicating a neuroimmune mode of action for this neuropeptide.
Another group of agents that recently have been found to activate mast cells leading to pain and inflammation are the serine proteinases. Proteinase levels are known to be augmented in patients with inflammatory joint disease [125-128], and it is believed that their enzymatic destruction of cartilage and other intra-articular tissues is a major contributing factor to the pathogenesis of rheumatoid arthritis. In addition to their classical proteolytic effects, proteinases were recently found to regulate cell signalling via specialized G-protein-coupled receptors. The unique characteristic of these proteinase-activated receptors (PARs) is the novel mechanism by which these receptors are triggered. Firstly, the proteinase hydrolyzes a specific arginine cleavage site located on the extracellular amino-terminus of the G-protein-coupled receptor, thereby exposing a new amino-terminal sequence. This modified amino-terminal sequence, while remaining tethered to the receptor, can now bind to a docking domain within the same receptor, leading to activation and cell signalling. Four PARs have thus far been identified (PAR1 to PAR4), and evidence is emerging that suggests that these receptors are involved in pain signalling [129,130]. In knee joint electrophysiology studies we found that administration of a PAR4-activating peptide can evoke spontaneous activity and sensitize joint afferents in response to mechanical manipulation of the knee (Figure 1). Inhibition of proteinase activity in diseased joints could have the dual benefit of reducing nociception as well as attenuating joint destruction through proteolysis. Thus, the PARs are an exciting new target for investigating joint pain modulation and for the potential development of disease-modifying drugs.
Figure 1.
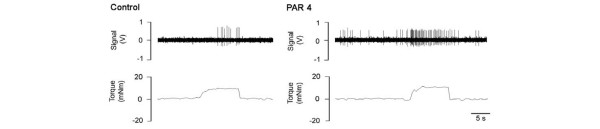
Specimen recording from a knee joint afferent fibre during rotation (torque) of the knee. Close intra-arterial injection of a PAR4 agonist caused spontaneous nerve activity as well as increased afferent firing rate during normal rotation compared with control. This PAR4 sensitization of the nerve would be decoded as joint pain by the central nervous system. PAR, proteinase-activated receptor.
Endogenous anti-nociceptive ligands
In an attempt to offset peripheral sensitization responses, it is becoming evident that joints also possess anti-nociceptive capabilities. The endogenous μ-opioid receptor ligand endomorphin-1 has been immunolocalized in capsaicin-sensitive nerves innervating rat synovial tissue [131,132], where it acts to reduce inflammation and inhibit nociception following an acute synovitis [24]. Interestingly, the anti-nociceptive capacity of endomorphin-1 was lost during chronic arthritis due to a reduction in μ-opioid receptor expression in the joint. This observation could begin to explain why the endogenous opioid system is unable to ameliorate arthritis pain. Other substances that are tonically released into the joint to offset inflammation-induced peripheral sensitization include galanin [133] and somatostatin [134]. These peptides have been shown to reduce nociceptor activity during noxious movement of normal knees as well as during normal rotation and hyper-rotation of acutely inflamed joints. Future research is required to characterize other endogenous anti-nociceptive mediators and to elucidate the reasons for their limited effectiveness in controlling arthritis pain.
Central processes in joint pain
Action potentials are transmitted along nociceptors from the knee to the central nervous system and enter the dorsum of the spinal cord predominantly in the lumbosacral region. Joint nociceptors terminate in the dorsal horn of the spinal cord, where they synapse with spinal neurones. These neurones constitute either spinal inter-neurones that aim to modulate sensory input, or ascending processes that transmit nociceptive information to the brain via the spinothalamic, spinomesencephalic, spinoreticular and spinocervical tracts. Neurophysiological processes at the intraspinal level can either intensify (central sensitization) or dampen (inhibition) the nociceptor signals before they reach the sensory cortex. As such, the intensity of the nociceptive information originating from joint primary afferents can undergo significant modification before leaving the spinal cord. The complex mechanisms and chemical mediators involved in these central processes are outside the scope of this review.
An initial attempt to determine the regions of the brain to which joint nerves project was recently reported in the rat. By measuring evoked potentials in the cerebral cortex in response to electrical stimulation the knee joint innervation, it was determined that joint afferents project to areas SI and SII of the somatosensory cortex [135]. By mechanisms that are not clearly understood, the brain interprets these high-intensity signals as joint pain. In addition to this cognitive aspect of arthritis pain, there is also an affective or emotional component to the disease. Patients who suffer from chronic arthritis pain exhibit clinical signs of depression and anxiety that appear to have a physiological basis [136]. In one of the few studies to try to discern the neurophysiological pathways responsible for the negative affect of arthritis pain, Neugebauer and Li [137] recorded from neurones located in the amygdala, an area of the brain that is synonymous with pain and emotion [138]. They found that noxious mechanical stimuli applied to acutely inflamed joints had an excitatory effect on the firing rate of neurones in the central nucleus of the amygdala. These data provide the first electrophysiological evidence that the amygdala is involved in transforming nociceptive information arising from arthritic joints into an emotional, painful experience.
Conclusion
Recent advances in molecular technology and the development of selective and efficacious pharmacological tools have enabled us to piece together the complex processes involved in the generation of arthritis pain. Nevertheless, as this review consistently reminds us, there are still very large gaps in our knowledge of what is occurring in the nociceptors to maintain this chronic pain state. For example, why is some arthritis pain episodic whereas other patients complain of chronic persistent joint pain? Why is there a disconnect between the degree of joint deterioration and the level of joint pain reported? As we get older, our peripheral nerves degenerate and as such some patients may be experiencing neuropathic pain rather than arthritis pain per se. Indeed, gabapentin (a drug commonly prescribed to relieve neuropathic pain) shows some promise in controlling arthritis pain [98]. Although analgesia could be achieved by intervening at different levels in the pain pathway, the possibility of reducing pain in the periphery is very appealing because drug doses can be titred to a lower level and there is less scope for negative systemic side effects. The fact that pain and inflammation are inherently linked indicates that interventions that relieve the symptoms of arthritis may also moderate the severity of the underlying disease. Carefully planned studies using multiple arthritis models and relevant methodological approaches are therefore imperative to further our understanding of the origin of joint pain.
Abbreviations
CGRP = calcitonin gene-related peptide; COX = cyclo-oxygenase; N/OFQ = nociceptin/orphanin FQ; NSAID = nonsteroidal anti-inflammatory drug; PAR = proteinase-activated receptor; PG = prostaglandin; SP = substance P; TRP = transient receptor potential; TTX = tetrodotoxin; VIP = vasoactive intestinal peptide.
Competing interests
The authors declare that they have no competing interests.
Note
This review is part of a series on Arthritis and pain edited by Jason McDougall. Other articles in this series can be found at http://arthritis-research.com/articles/review-series.asp?series=ar_pain.
Acknowledgments
Acknowledgements
The nerve recording shown in Figure 1 was produced by Dr Niklas Schuelert. JJ McDougall is the recipient of an Alberta Heritage Foundation for Medical Research Scholar award and is an Arthritis Society of Canada Investigator.
References
- The burden of musculoskeletal conditions at the start of the new millennium. World Health Organ Tech Rep Ser. 2003;919:i-x1–218. [PubMed] [Google Scholar]
- Samuel EP. The autonomic and somatic innervation of the articular capsule. Anat Rec. 1952;113:53–70. doi: 10.1002/ar.1091130104. [DOI] [PubMed] [Google Scholar]
- Skoglund S. Anatomical and physiological studies of knee joint innervation in the cat. Acta Physiol Scand. 1956. pp. 1–101. [PubMed]
- Sherrington C. The Integrative Action of the Nervous System. New York: Scribner; 1906. [Google Scholar]
- Langford LA. Unmyelinated axon ratios in cat motor, cutaneous and articular nerves. Neurosci Lett. 1983;40:19–22. doi: 10.1016/0304-3940(83)90085-X. [DOI] [PubMed] [Google Scholar]
- Langford LA, Schmidt RF. Afferent and efferent axons in the medial and posterior articular nerves of the cat. Anat Rec. 1983;206:71–78. doi: 10.1002/ar.1092060109. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Oqvist G, Brax L, Tuisku F. Anatomy of the rat knee joint and composition of a major articular nerve. Anat Rec. 1991;229:545–555. doi: 10.1002/ar.1092290415. [DOI] [PubMed] [Google Scholar]
- Marinozzi G, Ferrante F, Gaudio E, Ricci A, Amenta F. Intrinsic innervation of the rat knee joint articular capsule and ligaments. Acta Anat. 1991;141:8–14. doi: 10.1159/000147091. [DOI] [PubMed] [Google Scholar]
- Freeman MAR, Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. J Anat. 1967;101:505–532. [PMC free article] [PubMed] [Google Scholar]
- O'Connor BL. The mechanoreceptor innervation of the posterior attachments of the lateral meniscus of the dog knee joint. J Anat. 1984;138:15–26. [PMC free article] [PubMed] [Google Scholar]
- Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25:623–629. doi: 10.1016/S8756-3282(99)00215-X. [DOI] [PubMed] [Google Scholar]
- Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O'Leary P, Mantyh PW. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/S0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Bray RC, Sharkey KA. A morphological and immunohistochemical examination of nerves in normal and injured collateral ligaments of rat, rabbit and human knee joints. Anat Rec. 1997;248:29–39. doi: 10.1002/(SICI)1097-0185(199705)248:1<29::AID-AR4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Messlinger K, Neiss WF, Schmidt RF. Ultrastructural three-dimensional reconstruction of group III and group IV sensory nerve endings ('free nerve endings') in the knee joint capsule of the cat: evidence for multiple receptive sites. J Comp Neurol. 1990;292:103–116. doi: 10.1002/cne.902920107. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, McDougall JJ. Inhibitory effect of amiloride and gadolinium on fine afferent nerves in the rat knee: evidence of mechanogated ion channels in joints. Exp Brain Res. 2005;167:114–118. doi: 10.1007/s00221-005-0040-z. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Activation of groups III and IV sensory units in medial articular nerve by local mechanical stimulation of knee joint. J Neurophysiol. 1983;49:35–44. doi: 10.1152/jn.1983.49.1.35. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Responses of fine medial articular nerve afferents to passive movements of knee joints. J Neurophysiol. 1983;49:1118–1126. doi: 10.1152/jn.1983.49.5.1118. [DOI] [PubMed] [Google Scholar]
- Grigg P, Schaible HG, Schmidt RF. Mechanical sensitivity of group III and IV afferents from posterior articular nerve in normal and inflamed cat knee. J Neurophysiol. 1986;55:635–643. doi: 10.1152/jn.1986.55.4.635. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol. 1988;60:2180–2195. doi: 10.1152/jn.1988.60.6.2180. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol. 1985;54:1109–1122. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Hong KA, Langford LA, Schaible HG, Schmidt RF. Discharge characteristics of fine medial articular afferents at rest and during passive movements of inflamed knee joints. Brain Res. 1983;272:185–188. doi: 10.1016/0006-8993(83)90379-7. [DOI] [PubMed] [Google Scholar]
- Schaible H, Schmidt RF, Willis WD. Responses of spinal cord neurones to stimulation of articular afferent fibres in the cat. J Physiol. 1986;372:575–593. doi: 10.1113/jphysiol.1986.sp016026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbaud G, Iggo A, Tegner R. Sensory receptors in ankle joint capsules of normal and arthritic rats. Exp Brain Res. 1985;58:29–40. doi: 10.1007/BF00238950. [DOI] [PubMed] [Google Scholar]
- Li Z, Proud D, Zhang C, Wiehler S, McDougall JJ. Chronic arthritis downregulates peripheral mu-opioid receptor expression with concomitant loss of endomorphin-1 anti-nociception. Arthritis Rheum. 2005;52:3210–3219. doi: 10.1002/art.21359. [DOI] [PubMed] [Google Scholar]
- Schuelert N, McDougall JJ. Electrophysiological evidence that the vasoactive intestinal peptide receptor antagonist VIP(6–28) reduces nociception in an animal model of osteoarthritis. Osteoarthritis Cartilage. 2006;14:1155–1162. doi: 10.1016/j.joca.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Reeves B. Negative pressures in knee joints. Nature. 1966;212:1046. doi: 10.1038/2121046a0. [DOI] [Google Scholar]
- Levick JR. An investigation into the validity of subatmospheric pressure recordings from synovial fluid and their dependence on joint angle. J Physiol. 1979;289:55–67. doi: 10.1113/jphysiol.1979.sp012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayson MI, St Dixon AJ. Intra-articular pressure in rheumatoid arthritis of the knee. I. Pressure changes during passive joint distension. Ann Rheum Dis. 1970;29:261–265. doi: 10.1136/ard.29.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew BL, Dodt E. The deployment of sensory nerve endings at the knee joint of the cat. Acta Physiol Scand. 1953;28:8287–8296. doi: 10.1111/j.1748-1716.1953.tb00982.x. [DOI] [PubMed] [Google Scholar]
- Ferrell WR, Nade S, Newbold PJ. The interrelation of neural discharge, intra-articular pressure, and joint angle in the knee of the dog. J Physiol. 1986;373:353–365. doi: 10.1113/jphysiol.1986.sp016052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee: a biomechanical study. J Bone Joint Surg Am. 1980;62A:259–270. [PubMed] [Google Scholar]
- Gollehon DL, Torzilli PA, Warren RF. The role of the posterolateral and cruciate ligaments in the stability of the human knee. J Bone Joint Surg Am. 1987;69-A:233–242. [PubMed] [Google Scholar]
- Markolf KL, Kochan A, Amstutz HC. Measurement of knee stiffness and laxity in patients with documented absence of the anterior cruciate ligament. J Bone Joint Surg Am. 1984;66-A:242–253. [PubMed] [Google Scholar]
- McDougall JJ, Bray RC. Animal models of ligament repair. In: An YH, Friedman RD, editor. Animal Models in Orthopaedic Research. Boca Raton, Florida: CRC Press LLC; 1999. pp. 461–476. [Google Scholar]
- Marshall JL, Olsson S-E. Instability of the knee: a long term experimental study in dogs. J Bone Joint Surg Am. 1971;53A:1561–1570. [PubMed] [Google Scholar]
- Pond MJ, Nuki G. Experimentally-induced osteoarthritis in dog. Ann Rheum Dis. 1973;32:387–388. doi: 10.1136/ard.32.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen K. Osteoarthrosis following insufficiency of the cruciate ligaments in man: a clinical study. Acta Orthop Scand. 1977;48:520–526. doi: 10.3109/17453677708989742. [DOI] [PubMed] [Google Scholar]
- McDaniel WJ, Dameron TB. Untreated ruptures of the anterior cruciate ligament. J Bone Joint Surg Am. 1980;62-A:696–705. [PubMed] [Google Scholar]
- Brandt KD, Braunstein EM, Visco DM, O'Connor B, Heck D, Albrecht M. Anterior (cranial) cruciate ligament transection in the dog: a bona fide model of osteoarthritis, not merely of cartilage injury and repair. J Rheumatol. 1991;18:436–446. [PubMed] [Google Scholar]
- Brandt KD, Myers SL, Burr D, Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four weeks after transection of the anterior cruciate ligament. Arthritis Rheum. 1991;34:1560–1570. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- Gomez-Barrena E, Nunez A, Martinez-Moreno E, Valls J, Munuera L. Neural and muscular electric activity in the cat's knee. Changes when the anterior cruciate ligament is transected. Acta Orthop Scand. 1997;68:149–155. doi: 10.3109/17453679709003998. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Bjurholm A, Kreicbergs A, Schultzberg M. Neuropeptide Y, tyrosine hydroxylase, and vasoactive intestinal polypeptide-immunoreactive nerve fibres in the vertebral bodies, discs, dura mater, and spinal ligaments of the rat lumbar spine. Spine. 1993;18:268–273. doi: 10.1097/00007632-199302000-00016. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Bjurholm A, Schultzberg M, Theodorsson E, Kreicbergs A. Increased levels of substance P and calcitonin gene-related peptide in rat adjuvant arthritis. A combined immunohistochemical and radioimmunoassay analysis. Arthritis Rheum. 1995;38:699–709. doi: 10.1002/art.1780380519. [DOI] [PubMed] [Google Scholar]
- Buma P, Elmans L, Van Den Berg WB, Schrama LH. Neurovascular plasticity in the knee joint of an arthritic mouse model. Anat Rec. 2000;260:51–61. doi: 10.1002/1097-0185(20000901)260:1<51::AID-AR60>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Hanesch U, Heppelmann B, Schmidt RF. Substance P and calcitonin gene-related peptide immunoreactivity in primary afferent neurons of the cat's knee joint. Neuroscience. 1991;45:185–193. doi: 10.1016/0306-4522(91)90114-4. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Shahbazian Z, Hanesch U. Quantitative examination of calcitonin gene-related peptide immunoreactive nerve fibres in the cat knee joint. Anat Embryol (Berl) 1997;195:525–530. doi: 10.1007/s004290050072. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Pawlak M. Sensitisation of articular afferents in normal and inflamed knee joints by substance P in the rat. Neurosci Lett. 1997;223:97–100. doi: 10.1016/S0304-3940(97)13408-5. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Rumenapp P, Schaible HG. Calcitonin gene-related peptide is involved in the spinal processing of mechanosensory input from the rat's knee joint and in the generation and maintenance of hyperexcitability of dorsal horn-neurons during development of acute inflammation. Neuroscience. 1996;71:1095–1109. doi: 10.1016/0306-4522(95)00473-4. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Bjurholm A, Theodorsson E, Schultzberg M, Kreicbergs A. Neuropeptide Y- and vasoactive intestinal polypeptide-like immunoreactivity in adjuvant arthritis: effects of capsaicin treatment. Neuropeptides. 1995;29:33–43. doi: 10.1016/0143-4179(95)90054-3. [DOI] [PubMed] [Google Scholar]
- Abramovici A, Daizade I, Yosipovitch Z, Gibson SJ, Polak JM. The distribution of peptide-containing nerves in the synovia of the cat knee joint. Histol Histopathol. 1991;6:469–476. [PubMed] [Google Scholar]
- Catre MG, Salo PT. Quantitative analysis of the sympathetic innervation of the rat knee joint. J Anat. 1999;194:233–239. doi: 10.1046/j.1469-7580.1999.19420233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall JJ, Watkins L, Li Z. Vasoactive intestinal peptide (VIP) is a modulator of joint pain in a rat model of osteoarthritis. Pain. 2006;123:98–105. doi: 10.1016/j.pain.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999;406:503–547. doi: 10.1002/(SICI)1096-9861(19990419)406:4<503::AID-CNE7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Riedl M, Shuster S, Vulchanova L, Wang J, Loh HH, Elde R. Orphanin FQ/nociceptin-immunoreactive nerve fibers parallel those containing endogenous opioids in rat spinal cord. Neuroreport. 1996;7:1369–1372. doi: 10.1097/00001756-199605310-00007. [DOI] [PubMed] [Google Scholar]
- Schuligoi R, Amann R, Angelberger P, Peskar BA. Determination of nociceptin-like immunoreactivity in the rat dorsal spinal cord. Neurosci Lett. 1997;224:136–138. doi: 10.1016/S0304-3940(97)13453-X. [DOI] [PubMed] [Google Scholar]
- Civelli O, Nothacker HP, Reinscheid R. Reverse physiology: discovery of the novel neuropeptide, orphanin FQ/nociceptin. Crit Rev Neurobiol. 1998;12:163–176. doi: 10.1615/critrevneurobiol.v12.i3.10. [DOI] [PubMed] [Google Scholar]
- Darland T, Grandy DK. The orphanin FQ system: an emerging target for the management of pain? Brit J Anaesth. 1998;81:29–37. doi: 10.1093/bja/81.1.29. [DOI] [PubMed] [Google Scholar]
- Darland T, Heinricher MM, Grandy DK. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/S0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Pawlak M, Hanesch U, Schmidt RF. Peripheral modulation of rat knee joint afferent mechanosensitivity by nociceptin/orphanin FQ. Neurosci Lett. 2000;288:123–126. doi: 10.1016/S0304-3940(00)01211-8. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Hanesch U, Pawlak M, Schmidt RF. Participation of NK1 receptors in nociceptin-induced modulation of rat knee joint mechanosensitivity. Exp Brain Res. 2001;137:249–253. doi: 10.1007/s002210000650. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Larson SEM. Nociceptin/orphanin FQ evokes knee joint pain in rats via a mast cell independent mechanism. Neurosci Lett. 2006;398:135–138. doi: 10.1016/j.neulet.2005.12.066. [DOI] [PubMed] [Google Scholar]
- Kean WF, Buchanan WW. The use of NSAIDs in rheumatic disorders 2005: a global perspective. Inflammopharmacology. 2005;13:343–370. doi: 10.1163/156856005774415565. [DOI] [PubMed] [Google Scholar]
- Schaible H, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain. 1993;55:5–54. doi: 10.1016/0304-3959(93)90183-P. [DOI] [PubMed] [Google Scholar]
- McQueen DS. Inflammatory pain and the joint. In: Brain SD, Moore PK, editor. Pain and Neurogenic Inflammation. Basel, Switzerland: Birkhäuser Verlag; 1999. pp. 137–166. [Google Scholar]
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- Vane JR, Botting RM. Mechanism of action of anti-inflammatory drugs. Scand J Rheumatol Suppl. 1996;(102):9–21. doi: 10.3109/03009749609097226. [DOI] [PubMed] [Google Scholar]
- Crofford LJ, Wilder RL, Ristimaki AP, Sano H, Remmers EF, Epps HR, Hla T. Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester, and corticosteroids. J Clin Invest. 1994;93:1095–1101. doi: 10.1172/JCI117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle I, Klein T, Backman JT, Saal JG, Nusing RM, Fritz P. Expression of cyclooxygenase 1 and cyclooxygenase 2 in human synovial tissue: differential elevation of cyclooxygenase 2 in inflammatory joint diseases. Arthritis Rheum. 1998;41:122–129. doi: 10.1002/1529-0131(199801)41:1<122::AID-ART15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. New Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. 2 p following 1528. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Excitation and sensitization of fine articular afferents from cat's knee joint by prostaglandin E2. J Physiol. 1988;403:91–104. doi: 10.1113/jphysiol.1988.sp017240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepelmann K, Messlinger K, Schaible HG, Schmidt RF. Inflammatory mediators and nociception in the joint: excitation and sensitization of slowly conducting afferent fibers of cat's knee by prostaglandin I2. Neuroscience. 1992;50:237–247. doi: 10.1016/0306-4522(92)90395-I. [DOI] [PubMed] [Google Scholar]
- Birrell GJ, McQueen DS, Iggo A, Coleman RA, Grubb BD. PGI2-induced activation and sensitization of articular mechanonociceptors. Neurosci Lett. 1991;124:5–8. doi: 10.1016/0304-3940(91)90809-8. [DOI] [PubMed] [Google Scholar]
- Grubb BD, Birrell GJ, McQueen DS, Iggo A. The role of PGE2 in the sensitization of mechanoreceptors in normal and inflamed ankle joints of the rat. Exp Brain Res. 1991;84:383–392. doi: 10.1007/BF00231460. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Pfeffer A, Schaible HG, Schmidt RF. Effects of acetylsalicylic acid and indomethacin on single groups III and IV sensory units from acutely inflamed joints. Pain. 1986;26:337–351. doi: 10.1016/0304-3959(86)90062-X. [DOI] [PubMed] [Google Scholar]
- Guilbaud G, Iggo A. The effect of lysine acetylsalicylate on joint capsule mechanoreceptors in rats with polyarthritis. Exp Brain Res. 1985;61:164–168. doi: 10.1007/BF00235631. [DOI] [PubMed] [Google Scholar]
- Laird JM, Herrero JF, Garcia de la Rubia P, Cervero F. Analgesic activity of the novel COX-2 preferring NSAID, meloxicam in mono-arthritic rats: central and peripheral components. Inflamm Res. 1997;46:203–210. doi: 10.1007/s000110050174. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-G. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/S0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Griffin G, Fernando SR, Ross RA, McKay NG, Ashford ML, Shire D, Huffman JW, Yu S, Lainton JA, Pertwee RG. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur J Pharmacol. 1997;339:53–61. doi: 10.1016/S0014-2999(97)01336-8. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/S0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Rice AS, Farquhar-Smith WP, Nagy I. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot Essent Fatty Acids. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, Malan TP., Jr Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 2003;99:955–960. doi: 10.1097/00000542-200310000-00031. [DOI] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Egertova M, Bradbury EJ, McMahon SB, Rice AS, Elphick MR. Cannabinoid CB(1) receptor expression in rat spinal cord. Mol Cell Neurosci. 2000;15:510–521. doi: 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- Gauldie SD, McQueen DS, Pertwee R, Chessell IP. Anandamide activates peripheral nociceptors in normal and arthritic rat knee joints. Br J Pharmacol. 2001;132:617–621. doi: 10.1038/sj.bjp.0703890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CL, McDougall JJ. The cannabinomimetic arachidonyl-2-chloroethylamide (ACEA) acts on capsaicin-sensitive TRPV1 receptors but not cannabinoid receptors in rat joints. Br J Pharmacol. 2004;142:1361–1367. doi: 10.1038/sj.bjp.0705902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, et al. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol. 2003;550:739–752. doi: 10.1113/jphysiol.2003.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108:237–247. doi: 10.1016/j.pain.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Gould HJ, 3rd, England JD, Liu ZP, Levinson SR. Rapid sodium channel augmentation in response to inflammation induced by complete Freund's adjuvant. Brain Res. 1998;802:69–74. doi: 10.1016/S0006-8993(98)00568-X. [DOI] [PubMed] [Google Scholar]
- Flake NM, Gold MS. Inflammation alters sodium currents and excitability of temporomandibular joint afferents. Neurosci Lett. 2005;384:294–299. doi: 10.1016/j.neulet.2005.04.091. [DOI] [PubMed] [Google Scholar]
- England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol. 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird JM, Carter AJ, Grauert M, Cervero F. Analgesic activity of a novel use-dependent sodium channel blocker, crobenetine, in mono-arthritic rats. Br J Pharmacol. 2001;134:1742–1748. doi: 10.1038/sj.bjp.0704428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL. Calcium channels as therapeutic targets in neuropathic pain. J Pain. 2006;7:S13–S30. doi: 10.1016/j.jpain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Hanesch U, McDougall JJ, Pawlak M. Inhibitory effects of gabapentin on rat articular afferent mechanosensitivity. Reg Pep. 2000;89:63. [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/S0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Varga A, Nemeth J, Szabo A, McDougall JJ, Zhang C, Elekes K, Pinter E, Szolcsanyi J, Helyes Z. Effects of the novel TRPV1 receptor antagonist SB366791 in vitro and in vivo in the rat. Neurosci Lett. 2005;385:137–142. doi: 10.1016/j.neulet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Schaible HG, Schmidt RF. Sensitization of articular afferents to mechanical stimuli by bradykinin. Pflügers Arch. 1989;415:330–335. doi: 10.1007/BF00370884. [DOI] [PubMed] [Google Scholar]
- Kanaka R, Schaible HG, Schmidt RF. Activation of fine articular afferent units by bradykinin. Brain Res. 1985;327:81–90. doi: 10.1016/0006-8993(85)91501-X. [DOI] [PubMed] [Google Scholar]
- Herbert MK, Just H, Schmidt RF. Histamine excites groups III and IV afferents from the cat knee joint depending on their resting activity. Neurosci Lett. 2001;305:95–98. doi: 10.1016/S0304-3940(01)01817-1. [DOI] [PubMed] [Google Scholar]
- Birrell GJ, McQueen DS, Iggo A, Grubb BD. The effects of 5-HT on articular sensory receptors in normal and arthritic rats. Br J Pharmacol. 1990;101:715–721. doi: 10.1111/j.1476-5381.1990.tb14146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd E, McQueen DS, Chessell IP, Humphrey PP. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. Br J Pharmacol. 1998;125:341–346. doi: 10.1038/sj.bjp.0702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd E, McQueen DS, Chessell IP, Humphrey PP. Adenosine A1 receptor-mediated excitation of nociceptive afferents innervating the normal and arthritic rat knee joint. Br J Pharmacol. 1998;125:1267–1271. doi: 10.1038/sj.bjp.0702185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DC, Asghar AU, Marr CG, McQueen DS. Nitric oxide modulates articular sensory discharge and responsiveness to bradykinin in normal and arthritic rats in vivo. NeuroReport. 2001;12:121–125. doi: 10.1097/00001756-200101220-00032. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Messlinger K, Neiss WF, Schmidt RF. Fine sensory innervation of the knee joint capsule by group III and group IV nerve fibers in the cat. J Comp Neurol. 1995;351:415–428. doi: 10.1002/cne.903510308. [DOI] [PubMed] [Google Scholar]
- Hukkanen M, Grönblad M, Rees R, Konttinen YT, Gibson SJ, Hietanen J, Polak JM, Brewerton DA. Regional distribution of mast cells and peptide containing nerves in normal and adjuvant arthritic rat synovium. J Rheumatol. 1991;18:177–183. [PubMed] [Google Scholar]
- Bjerknes L, Coderre TJ, Green PG, Basbaum AI, Levine JD. Neutrophils contribute to sympathetic nerve terminal-dependent plasma extravasation in the knee joint of the rat. Neuroscience. 1991;43:679–685. doi: 10.1016/0306-4522(91)90326-J. [DOI] [PubMed] [Google Scholar]
- Lo EJ, Green PG, Miao FJ, Relchling DB, Levine JD. Bradykinin-induced neurogenic migration of neutrophils into the rat knee joint. NeuroReport. 1999;10:3821–3824. doi: 10.1097/00001756-199912160-00018. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Donnerer J, Petronijevic S, Saria A, Lembeck F. Release of histamine by neuropeptides from the perfused rat hindquarter. Naunyn Schmiedeberg's Arch Pharmacol. 1983;322:153–157. doi: 10.1007/BF00512389. [DOI] [PubMed] [Google Scholar]
- Shanahan F, Denburg JA, Fox J, Bienenstock J, Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985;135:1331–1337. [PubMed] [Google Scholar]
- Piotrowski W, Foreman JC. On the actions of substance P, somatostatin, and vasoactive intestinal polypeptide on rat peritoneal mast cells and in human skin. Naunyn Schmiedeberg's Arch Pharmacol. 1985;331:364–368. doi: 10.1007/BF00500821. [DOI] [PubMed] [Google Scholar]
- Nemeth J, Helyes Z, Oroszi G, Than M, Pinter E, Szolcsanyi J. Inhibition of nociceptin on sensory neuropeptide release and mast cell-mediated plasma extravasation in rats. Eur J Pharmacol. 1998;347:101–104. doi: 10.1016/S0014-2999(98)00216-7. [DOI] [PubMed] [Google Scholar]
- Foreman J, Jordan C. Histamine release and vascular changes induced by neuropeptides. Agents Actions. 1983;13:105–116. doi: 10.1007/BF01967311. [DOI] [PubMed] [Google Scholar]
- Johnson AR, Erdos EG. Release of histamine from mast cells by vasoactive peptides. Proc Soc Exp Biol Med. 1973;142:1252–1256. doi: 10.3181/00379727-142-37219. [DOI] [PubMed] [Google Scholar]
- Kimura T, Kitaichi K, Hiramatsu K, Yoshida M, Ito Y, Kume H, Yamaki K, Suzuki R, Takagi K. Intradermal application of nociceptin increases vascular permeability in rats: the possible involvement of histamine release from mast cells. Eur J Pharmacol. 2000;407:327–332. doi: 10.1016/S0014-2999(00)00746-9. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Fierro IM, Chiang N, Pouliot M. Cutting edge: nociceptin stimulates neutrophil chemotaxis and recruitment: inhibition by aspirin-triggered-15-epi-lipoxin A4. J Immunol. 2001;166:3650–3654. doi: 10.4049/jimmunol.166.6.3650. [DOI] [PubMed] [Google Scholar]
- Kidd BL. The expanding role of the nervous system in arthritis. EULAR Bull. 1993;4:134–137. [Google Scholar]
- Fiset ME, Gilbert C, Poubelle PE, Pouliot M. Human neutrophils as a source of nociceptin: a novel link between pain and inflammation. Biochemistry. 2003;42:10498–10505. doi: 10.1021/bi0300635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, McDougall JJ. Stimulation of sensory neuropeptide release by nociceptin/orphanin FQ leads to hyperaemia in acutely inflamed rat knees. Br J Pharmacol. 2006;148:938–946. doi: 10.1038/sj.bjp.0706804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery JP, Lisse JR. Preliminary study of the tryptase levels in the synovial fluid of patients with inflammatory arthritis. Ann Allergy. 1994;72:425–427. [PubMed] [Google Scholar]
- Nakano S, Ikata T, Kinoshita I, Kanematsu J, Yasuoka S. Characteristics of the protease activity in synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Clin Exp Rheumatol. 1999;17:161–170. [PubMed] [Google Scholar]
- Mican JM, Metcalf DD. Arthritis and mast cell activation. J Allergy Clin Immunol. 1990;86:677–683. doi: 10.1016/s0091-6749(05)80240-4. [DOI] [PubMed] [Google Scholar]
- de Paulis A, Marino I, Ciccarelli A, de Crescenzo G, Concardi M, Verga L, Arbustini E, Marone G. Human synovial mast cells. I. Ultrastructural in situ and in vitro immunologic characterization. Arthritis Rheum. 1996;39:1222–1233. doi: 10.1002/art.1780390723. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signalling and pain. Trends Pharmacol Sci. 2001;22:146–152. doi: 10.1016/S0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, Andrade-Gordon P, et al. Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway. Nat Med. 2001;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Barin AK, McDougall CM. Loss of vasomotor responsiveness to the m-opioid receptor ligand endomorphin-1 in adjuvant monoarthritic rat knee joints. Am J Physiol: Reg Integr Comp Physiol. 2004;286:R634–R641. doi: 10.1152/ajpregu.00464.2003. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Baker CL, Hermann PM. Attenuation of knee joint inflammation by peripherally administered endomorphin-1. J Mol Neurosci. 2004;22:125–137. doi: 10.1385/JMN:22:1-2:125. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Just S, Pawlak M. Galanin influences the mechanosensitivity of sensory endings in the rat knee joint. Eur J Neurosci. 2000;12:1567–1572. doi: 10.1046/j.1460-9568.2000.00045.x. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Pawlak M. Inhibitory effect of somatostatin on the mechanosensitivity of articular afferents in normal and inflamed knee joints of the rat. Pain. 1997;73:377–382. doi: 10.1016/S0304-3959(97)00124-3. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Pawlak M, Just S, Schmidt RF. Cortical projection of the rat knee joint innervation and its processing in the somatosensory areas SI and SII. Exp Brain Res. 2001;141:501–506. doi: 10.1007/s002210100888. [DOI] [PubMed] [Google Scholar]
- Huyser BA, Parker JC. Negative affect and pain in arthritis. Rheum Dis Clin North Am. 1999;25:105–121, vi. doi: 10.1016/S0889-857X(05)70057-0. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol. 2003;89:716–727. doi: 10.1152/jn.00799.2002. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]


