Abstract
Epicardial catheter ablation is increasingly important in the treatment of ventricular arrhythmias. Collateral damage to adjacent structures like the phrenic nerve is an important concern with epicardial ablation. This report describes the use of a novel method to prevent phrenic nerve injury during epicardial ablation of ventricular tachycardia.
Keywords: catheter ablation, ventricular tachycardia, phrenic nerve injury
Percutaneous epicardial catheter ablation was pioneered by Sosa and colleagues for the treatment of ventricular tachycardia in patients with Chagas disease, wherein the reentrant circuits are often epicardial1. The technique has also been used for post-infarction ventricular tachycardias related to epicardial scars2-4 as well as epicardial accessory pathways as an alternative to cardiac surgery5. It is likely that epicardial ablation will continue to have a role in the treatment of cardiac arrhythmias6-8.
During epicardial ablation, in contrast to endocardial ablation, the lack of intervening myocardium to shield adjacent structures increases the risk of collateral thermal damage to lung, coronary arteries, and the phrenic nerve. Several innovative methods have been devised and tested in both animals and humans to avoid damage to epicardial coronary arteries9, 10. Although pace mapping can identify sites close to the phrenic nerve to avoid ablation, no specific approaches have been described to prevent phrenic nerve injury during catheter ablation. We present a novel method for protecting the phrenic nerve during epicardial catheter ablation by using a balloon catheter in the pericardial space to mechanically separate the left phrenic nerve from the ablation catheter. This technique was successful in protecting the left phrenic nerve from injury.
CASE REPORT
A 78-year-old man with a history of ischemic cardiomyopathy was transferred from an outside hospital due to repetitive drug-refractory monomorphic ventricular tachycardia resulting in multiple device shocks (delivered by a cardiac resynchronization defibrillator). During a prior attempt at endocardial ablation (at the referring center), the tachycardia was mapped to the lateral wall of the left ventricle, but could not be ablated. Twelve-lead electrocardiogram of the clinical tachycardia, induced in the ablation lab, is shown in figure 1.
Figure 1.
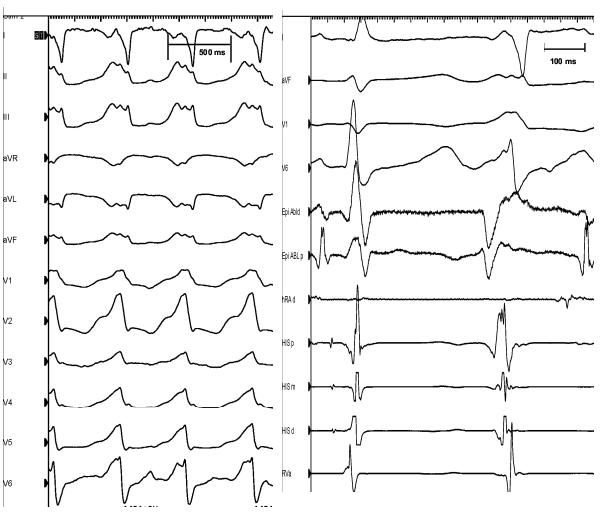
Left panel: Surface 12-lead electrocardiogram of ventricular tachycardia (cycle length= 485 ms) observed during the ablation procedure (that was identical to the clinical tachycardia). Right panel: Intracardiac tracing of PVC’s mapped during ablation. HRAd: right atrial catheter, His px; His bundle proximal, His md: His bundle mid, His ds: His bundle distal, Rva: RV apex, Epi Abld: distal pole of epicardial ablation catheter, Epi Abl p: proximal pole of the epicardial ablation catheter
Endocardial mapping was performed with an electroanatomic ablation catheter (Biosense Webster) in the right ventricular outflow tract, proximal aorta, and left ventricular outflow tract via the transaortic approach. Ventricular premature beats and sustained monomorphic ventricular tachycardia beats were mapped. This demonstrated earliest activation at the posterior-superior aspect of the left ventricle. Under general anesthesia pericardial access was obtained via the subxiphoid approach with a Tuohy needle. An internal irrigated ablation catheter (Boston Scientific) was advanced into the pericardial space for mapping of epicardial sites (Figure 2).
Figure 2.
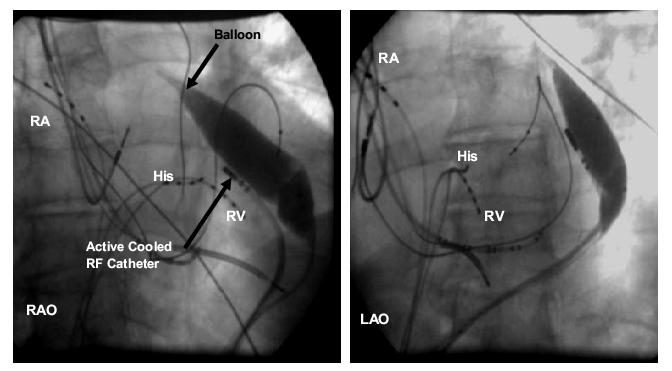
Right anterior oblique (RAO) and left anterior oblique (LAO) fluoroscopic images showing balloon inflated to increase separation between the ablation catheter and the left phrenic nerve. When the balloon was deflated, there was consistent phrenic nerve capture with high-output pacing from the site of earliest activation. After inflation, phrenic nerve capture was no longer observed. RA= right atrial catheter, His= His bundle catheter, RV= right ventricular catheter
Activation and pace mapping localized the source of ventricular premature beats and induced ventricular tachycardia to the LV epicardium. At the site of earliest activation, high-output unipolar and bipolar pacing resulted in phrenic nerve capture (movie clip 1), and RF delivery could not be performed due to the risk of phrenic nerve injury. At this point in the procedure, a second pericardial wire was placed and an 18-mm by 4-cm balloon dilation catheter (Meditech, Boston Scientific) was advanced into the pericardial space and manipulated into position near the ablation catheter. Inflation of this balloon physically moved the heart away from the phrenic nerve and repeat high-output pacing showed absence of phrenic nerve capture (movie clip 2). Coronary angiography showed sufficient distance between ablation sites and course of the coronary arteries (Figure 3). The cooled-tip ablation catheter was then used to ablate at that site while the balloon was kept inflated. Programmed stimulation demonstrated lack of VT inducibility after the ablation. Normal function of the left hemidiaphragm was verified by fluoroscopy. The temperature was set to 40 degrees centigrade, power was set to 30 Watts and each RF application was 60 seconds in duration. The impedance with the balloon inflated was about 170-180 and the impedance reduced to about 140 during RF application. There was a reduction in the local EGM amplitude and after 3 RF applications; there was no recordable EGM in the targeted region. Post-ablation there were no PVC’s and the tachycardia was not inducible. The balloon catheter did not demonstrate any evidence of thermal damage when it was visually inspected after the ablation. The patient has been arrhythmia free at 10 months of follow-up.
Figure 3.
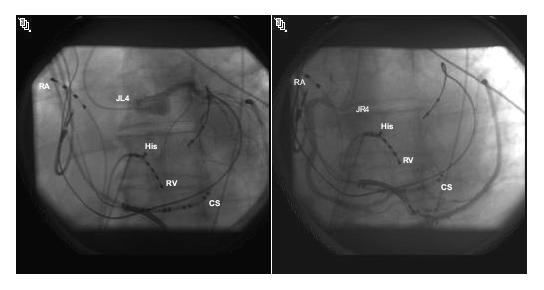
Contrast injection of the left (A) and right (B) coronary arteries shows course of epicardial coronary arteries prior to injection, to ensure adequate distance from ablation sites. RA=right atrial catheter, His=His bundle catheter, RV=right ventricular catheter, CS=coronary sinus catheter, JL4=Judkins left coronary catheter, JR4=Judkins right coronary catheter
DISCUSSION
Phrenic nerve injury is a recognized complication of catheter ablation procedures; it has been reported following ablation of atrial arrhythmias as well as ventricular tachycardia11, 12. The left phrenic nerve descends behind the innominate vein, passes near the aortic arch, pulmonary trunk, and left atrial appendage, then courses along the lateral border of the LV before inserting in the diaphragm13. Pacing can be used to identify areas in close proximity to the phrenic nerve, and electroanatomic maps can incorporate tags at these sites, to help the operator avoid ablation near the phrenic nerve6. Ablation in regions close to the phrenic nerve is sometimes necessary during ablation of ventricular tachycardia, but is avoided when phrenic nerve capture is demonstrated by pacing from the ablation catheter (see figure 3, ref 6). However, apart from limiting ablation sites, there has been no previously described method for reducing the risk of phrenic nerve injury during ablation. This case illustrates the use of a novel technique for preventing damage to nearby structures during radiofrequency catheter ablation. The technique we describe could also be used for ablation along the lateral right atrium to prevent damage to the right phrenic nerve. A procedural strategy for right atrial tachycardias would include inflation of the pericardial balloon to move the heart away from the right phrenic nerve while radiofrequency energy is delivered from the endocardial aspect. Although this makes intuitive sense, it is likely that there may be hemodynamic consequences to such an approach, as the RA will be compressed. Indeed, we observed subtle LV compression (albeit without hemodynamic consequence) in our case (comparing movie clips 1 and 2).
Several limitations of this technique should also be considered. First, the balloon catheter is not steerable, and it may be impossible in some cases to position the balloon so that phrenic nerve capture is avoided. Also, the presence of an inflated balloon in the pericardial space may displace the ablation catheter, or interfere with attempts to reposition it, making the ablation more difficult. There is potential for a sub-optimal outcome due to catheter trauma-induced termination of arrhythmia. Finally, our description here is based on a single successful case; understanding the general utility of this technique will require investigation in a larger number of patients.
Supplementary Material
Footnotes
Support: Supported by the AHA (National Affiliate (0430287N) and NIH RO1-HL084261 grants to KS
REFERENCES
- 1.Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996 Jun;7(6):531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 2.Cesario DA, Vaseghi M, Boyle NG, Fishbein MC, Valderrabano M, Narasimhan C, Wiener I, Shivkumar K. Value of high-density endocardial and epicardial mapping for catheter ablation of hemodynamically unstable ventricular tachycardia. Heart Rhythm. 2006 Jan;3(1):1–10. doi: 10.1016/j.hrthm.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Brugada J, Berruezo A, Cuesta A, Osca J, Chueca E, Fosch X, Wayar L, Mont L. Nonsurgical transthoracic epicardial radiofrequency ablation: an alternative in incessant ventricular tachycardia. J Am Coll Cardiol. 2003 Jun 4;41(11):2036–2043. doi: 10.1016/s0735-1097(03)00398-x. [DOI] [PubMed] [Google Scholar]
- 4.Sosa E, Scanavacca M, D’Avila A, Piccioni J, Sanchez O, Velarde JL, Silva M, Reolao B. Endocardial and epicardial ablation guided by nonsurgical transthoracic epicardial mapping to treat recurrent ventricular tachycardia. J Cardiovasc Electrophysiol. 1998 Mar;9(3):229–239. doi: 10.1111/j.1540-8167.1998.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 5.Valderrabano M, Cesario DA, Ji S, Shannon K, Wiener I, Swerdlow CD, Oral H, Morady F, Shivkumar K. Percutaneous epicardial mapping during ablation of difficult accessory pathways as an alternative to cardiac surgery. Heart Rhythm. 2004 Sep;1(3):311–316. doi: 10.1016/j.hrthm.2004.03.073. [DOI] [PubMed] [Google Scholar]
- 6.Soejima K, Stevenson WG, Sapp JL, Selwyn AP, Couper G, Epstein LM. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004 May 19;43(10):1834–1842. doi: 10.1016/j.jacc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Cole CR, Marrouche NF, Natale A. Evaluation and management of ventricular outflow tract tachycardias. Card Electrophysiol Rev. 2002 Dec;6(4):442–447. doi: 10.1023/a:1021148711621. [DOI] [PubMed] [Google Scholar]
- 8.Wood MA. Percutaneous pericardial instrumentation in the electrophysiology laboratory: a case of need. Heart Rhythm. 2006 Jan;3(1):11–12. doi: 10.1016/j.hrthm.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Thyer IA, Kovoor P, Barry MA, Pouliopoulos J, Ross DL, Thiagalingam A. Protection of the coronary arteries during epicardial radiofrequency ablation with intracoronary chilled saline irrigation: assessment in an in vitro model. J Cardiovasc Electrophysiol. 2006 May;17(5):544–549. doi: 10.1111/j.1540-8167.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson WG, Soejima K. Inside or out? Another option for incessant ventricular tachycardia. J Am Coll Cardiol. 2003 Jun 4;41(11):2044–2045. doi: 10.1016/s0735-1097(03)00404-2. [DOI] [PubMed] [Google Scholar]
- 11.Durante-Mangoni E, Del Vecchio D, Ruggiero G. Right diaphragm paralysis following cardiac radiofrequency catheter ablation for inappropriate sinus tachycardia. Pacing Clin Electrophysiol. 2003 Mar;26(3):783–784. doi: 10.1046/j.1460-9592.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee BK, Choi KJ, Kim J, Rhee KS, Nam GB, Kim YH. Right phrenic nerve injury following electrical disconnection of the right superior pulmonary vein. Pacing Clin Electrophysiol. 2004 Oct;27(10):1444–1446. doi: 10.1111/j.1540-8159.2004.00652.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Quintana D, Cabrera JA, Climent V, Farre J, Weiglein A, Ho SY. How close are the phrenic nerves to cardiac structures? Implications for cardiac interventionalists. J Cardiovasc Electrophysiol. 2005 Mar;16(3):309–313. doi: 10.1046/j.1540-8167.2005.40759.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


