Abstract
In humans, mutations in the genes encoding components of the dystrophin–glycoprotein complex cause muscular dystrophy. Specifically, primary mutations in the genes encoding α-, β-, γ-, and δ-sarcoglycan have been identified in humans with limb-girdle muscular dystrophy. Mice lacking γ-sarcoglycan develop progressive muscular dystrophy similar to human muscular dystrophy. Without γ-sarcoglycan, β- and δ-sarcoglycan are unstable at the muscle membrane and α-sarcoglycan is severely reduced. The expression and localization of dystrophin, dystroglycan, and laminin-α2, a mechanical link between the actin cytoskeleton and the extracellular matrix, appears unaffected by the loss of sarcoglycan. We assessed the functional integrity of this mechanical link and found that isolated muscles lacking γ-sarcoglycan showed normal resistance to mechanical strain induced by eccentric muscle contraction. Sarcoglycan-deficient muscles also showed normal peak isometric and tetanic force generation. Furthermore, there was no evidence for contraction-induced injury in mice lacking γ-sarcoglycan that were subjected to an extended, rigorous exercise regimen. These data demonstrate that mechanical weakness and contraction-induced muscle injury are not required for muscle degeneration and the dystrophic process. Thus, a nonmechanical mechanism, perhaps involving some unknown signaling function, likely is responsible for muscular dystrophy where sarcoglycan is deficient.
The dystrophin–glycoprotein complex (DGC) is a multimeric assembly of both transmembrane- and membrane-associated proteins found in both skeletal and cardiac muscle (1–4). Molecular and biochemical analyses have demonstrated that the DGC is composed of the following components: dystrophin, an elongated cytoskeletal protein that binds actin (5–7); sarcoglycan, a multisubunit transmembrane glycoprotein (8–10); dystroglycan, a laminin receptor that also binds dystrophin (11, 12); the syntrophins, mammalian homologues of the Torpedo 58-kDa postsynaptic protein (13–15); and dystrobrevin, a dystrophin-related, dystrophin-associated protein (16–21). Mutations in the dystrophin gene result in Duchenne/Becker muscular dystrophy (DMD/BMD), a common X-linked disorder (5, 6). The mdx mouse is a spontaneously arising mutant that lacks full-length dystrophin and serves as a model for DMD (22).
In muscle, there are at least four sarcoglycan subunits, α, β, γ, and δ, and mutations in any of these four can result in autosomal recessive muscular dystrophy (23–27). A more widely distributed fifth sarcoglycan, ɛ-sarcoglycan, recently has been identified (28, 29), suggesting that sarcoglycan complexes may also function in tissues other than muscle. Sarcoglycan has a primary structure that includes an extracellular epidermal growth factor-like motif and is suggestive of a cell surface receptor (30); its exact role is unknown.
Dystrophin binds actin at its amino terminus and along its rod domain (31–33). In the cytoplasm, the carboxyl terminus of dystrophin interacts with dystroglycan, a transmembrane protein that associates with the extracellular matrix (ECM) protein laminin (12, 34). In skeletal muscle myotubes, this link is thought to be critical for mechanical integrity and resistance to hypoosmotic shock (35–37). The absence of dystrophin may lead to disruptions of the muscle plasma membrane during repeated cycles of contraction and relaxation, resulting in muscle degeneration and muscular dystrophy (38, 39). Elevations in intramyocyte calcium levels coupled with the appearance of muscle enzymes in the serum of DMD patients and mdx mice are consistent with such a defect in the sarcolemma (38, 40–42). However, other mechanisms may be responsible for both muscle enzyme release and calcium entry into the dystrophic myofiber. Moreover, eccentric contractions cause a significant increase in mechanically induced sarcolemmal damage in isolated mdx muscles (43–45). In mdx muscle there is also a significant linear relationship between peak force and the proportion of damaged fibers, suggesting that a mechanical defect results from the absence of dystrophin and arguing that this mechanical defect causes muscular dystrophy (45).
γ-Sarcoglycan is a 35-kDa dystrophin-associated protein, and mutations in γ-sarcoglycan are associated with the human disease SCARMD (severe childhood autosomal recessive muscular dystrophy), also known as limb-girdle muscular dystrophy type 2C (LGMD-2C). Mice lacking γ-sarcoglycan were generated by using homologous recombination in embryonic stem cells by targeting exon 2 of the murine γ-sarcoglycan gene to create a null allele. Like LGMD patients, mice lacking γ-sarcoglycan (gsg−/−) showed pronounced skeletal and cardiac muscle degeneration with reduced survival. In addition to the loss of γ-sarcoglycan, muscle from gsg−/− animals showed reduced levels of β- and δ-sarcoglycan but exhibited normal staining patterns for dystrophin, dystroglycan, and laminin-α2. Thus, sarcoglycan loss was sufficient to induce muscular dystrophy in the presence of an apparently intact dystrophin–dystroglycan–laminin axis.
To determine whether mechanical fragility was a consequence of the loss of an intact sarcoglycan complex, we performed mechanical measurements on isolated muscles from gsg−/− animals and found normal muscle mechanics. To confirm the physiologic significance of our findings, we strenuously exercised gsg−/− animals for a prolonged period and found no evidence for contraction-induced injury or accelerated disease progression. These data are consistent with a nonmechanical defect producing myofiber degeneration and muscular dystrophy. Because sarcoglycan loss is also a feature of DMD, this same nonmechanical defect may be contributing to pathology in dystrophin-deficient muscular dystrophy. Furthermore, these data reveal that the DGC is a multifunctional complex and define an independent role for γ-sarcoglycan in muscle survival.
MATERIALS AND METHODS
Animals.
All mice were housed and treated in accordance with standards set by the University of Chicago Institutional Animal Care and Use Committee and the University of Pennsylvania Animal Care and Use Committee. Genotypes were determined by PCR as described previously (46). Age-matched wild-type 129SvJ animals were used as controls (The Jackson Laboratory). Animals used for study were anesthetized and killed by cervical dislocation. Serum creatine kinase levels were determined from tail vein sampling collected in a Microtainer Serum Separator tube (Becton Dickinson) and analyzed by using a Vitros DT60II discrete chemistry analyzer (Ortho Clinical Products, Raritan, NJ).
Muscle Preparation and Mechanics.
Intact extensor digitorum longus (EDL) muscles were dissected from normal (gsg+/+) and homozygous mutant (gsg−/−) animals at 4–5 weeks of age or at 13–16 weeks of age as described previously (45). For contraction experiments, muscles were immersed in a physiologic solution containing the low-molecular-weight dye Procion orange (0.2% wt/vol; Sigma) in Ringer’s solution buffered to pH 7.4 with Hepes. The bath was oxygenated continuously with a mixture of 95% O2 and 5% CO2 and maintained at 25°C. Stimulation was performed by using two platinum plate electrodes positioned alongside the body of the EDL. The EDL was adjusted to that length at which maximum twitch force was obtained (Lo). Muscle fiber length (Lo) was measured by using fine calipers. Cross-sectional area was determined by dividing the muscle mass (mg) by the fiber length (mm) divided by the density of muscle (1.06 g/cm2) as described previously (47). The eccentric contraction (ECC) regimen consisted of five (fused tetani) maximal stimulation periods (frequency of 80 Hz) of 700-ms duration during which the muscle was lengthened by 10% Lo over the final 200 ms. Lengthening occurred at a rate of 0.5 Lo/s. A 4-min recovery period was allowed after each maximal stimulation during which the muscle was maintained at Lo. Following the final stimulation, each muscle remained in the oxygenated Ringer’s solution with Procion orange for 15 min. Muscles then were rinsed in oxygenated Ringer’s, dried, and weighed. After weighing, muscles were embedded in OCT compound (TissueTek, Sakura Finetek USA, Torrance, CA), rapidly frozen in liquid nitrogen-cooled isopentane, and stored at −80°C for further analysis. Differences between samples from normal and gsg−/− muscle were assessed by using Student’s two-tailed t test for independent samples.
Assessment of Sarcolemmal Damage.
Muscles subjected to the ECC protocol were sectioned on a cryostat (Leica) at midlength, viewed under the microscope (Leica), and photographed. From the photographs, the percentage of dye-positive fibers was determined from counting multiple sections. The edges of each section were excluded to avoid fibers potentially damaged in the dissection or in muscle handling. Differences between groups were assessed by using Student’s two-tailed t test for independent samples.
Exercise Regimen.
Age-matched, 10- to 12-week-old normal and homozygous mutant (gsg−/−) mice were selected. All mice used in the exercise protocol were selected based on their initial ability to swim for 10 min. Two mutant animals initially selected failed to meet this criterion. The swimming regimen initiated with two 10-min sessions per day separated by a minimum of 4 hr. Length of swim was increased daily by 5 min per session until a final duration of two 1-hr sessions per day was achieved. The length of the conditioning period was 10 days. Animals were exercised 6 days per week, and the protocol was continued for 7 weeks including the 10-day conditioning period. One homozygous mutant (gsg−/−) mouse was removed from the protocol after week 3 as a result of a cutaneous infection that failed to heal after treatment with oral antibiotics. Animals were observed for 15 min before and after swimming for behavior and activity level. Animals were weighed at the end of each week. Blood was collected on the second day after the swimming protocol was completed, and serum creatine kinase levels were measured as described above.
Histology.
Animals were sacrificed 2 days after the completion of the 7-week swimming protocol. Femoral quadriceps, triceps bracheii, gastrocnemius, and heart were dissected from surrounding tissues, frozen in liquid nitrogen-cooled isopentane, and stored at −80°C for further analysis. Frozen sections from each tissue were cut at midlength at −20°C by using a cryostat (Leica), and histology was performed as described previously (46). A scale from 0 to 3 was used to score the percentage of the section displaying fibrosis, adipose tissue infiltration, and degeneration. Scores were assigned as follows: no degeneration, fibrosis or adipose tissue was scored as 0; less than 10% of the section involved was scored as 1; 10–25% was scored as 2; greater than 25% was scored as 3. Each parameter was scored independently by two blinded reviewers. Two cross-sections from each muscle group were scored, and each pair of sections was separated by at least 2 mm of longitudinal distance within the muscle. The mean of these four scores was determined for each parameter to arrive at a score for the muscle in question. The combined mean and standard error of each parameter was determined for the four muscles studied in each class (exercised and no exercise). Differences between each group were assessed by using Student’s two-tailed t test for independent samples.
RESULTS
Mechanical Properties of Sarcoglycan-Deficient Muscle. To ascertain whether sarcoglycan is critical for normal muscle force generation and resistance to contraction-induced injury, we examined the mechanical properties of gsg−/− skeletal muscle at 4–5 weeks of age (young) and at 13–16 weeks of age (mature). The peak isometric force generated by the EDL muscle of both young and mature gsg−/− animals was similar to normal controls. Additionally, the peak tetanic force normalized for cross-sectional area also was similar in both normal and gsg−/− animals of both ages (Table 1). The slight decrease in the absolute magnitude of each of these parameters was not statistically significant and was consistent with the decreased physical activity seen in gsg−/− animals (46).
Table 1.
Mechanical properties of normal, gsg−/−, and mdx EDL
| Parameter | Genotype
|
|||
|---|---|---|---|---|
| Mature normal | Young gsg−/− | Mature gsg−/− | Mature mdx | |
| Lo, mm | 10.9 ± 0.2 | 8.9 ± 0.1 | 11.6 ± 0.1 | 11.5 ± 0.1 |
| EDL weight, mg | 9.4 ± 0.1 | 4.7 ± 0.3 | 9.4 ± 0.4 | 15.0 ± 0.4 |
| EDL CSA, mm2 | 1.8 ± 0.0 | 1.1 ± 0.1 | 1.7 ± 0.1 | 2.7 ± 0.1 |
| Twitch force, mN | 123.3 ± 9.3 | 49.6 ± 5.5 | 136.5 ± 7.3 | 117.1 ± 10.5 |
| Tetanic force, mN | 349.3 ± 11.2 | 150.5 ± 11.9 | 334.8 ± 9.5 | 355.3 ± 15.8 |
| Tetanic force CSA, mN/mm2 | 19.3 ± 0.6 | 13.8 ± 1.1 | 19.9 ± 1.0 | 13.1 ± 0.7 |
Results are presented as mean ± SEM. CSA, cross-sectional area.
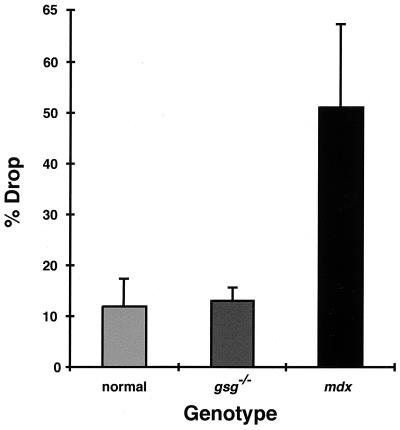
An ECC protocol was used to assess the degree to which muscle lacking γ-sarcoglycan was susceptible to contraction-induced injury. During a fused tetanic contraction, muscles were lengthened by 10% Lo, placing significant strain on the sarcolemma. This ECC protocol was repeated five times with 4 min of rest interposed between each ECC. In normal muscle, the percent drop in tetanic force between the first and fifth contraction reflects both fatigue and fiber loss because of mechanical overload. Strikingly, gsg−/− and normal EDLs showed a similar percent drop during the five ECC protocol, unlike what is seen in muscles from the mdx mouse (Fig. 1). Therefore, the absence of γ-sarcoglycan did not confer an increased susceptibility to fatigue or to contraction-induced injury.
Figure 1.
ECC and sarcolemmal damage in normal, gsg−/−, and mdx animals. The mean and SD of the percent drop in tetanic force generation between the first and fifth ECC is shown for mature normal (n = 4), gsg−/− (n = 9), and mdx (n = 7) EDLs. There is no difference in percent drop between the normal and gsg−/−, in contrast to the mdx muscles.
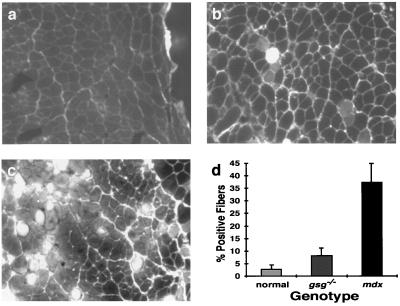
To detect sarcolemmal disruptions, eccentric contractions were performed in the presence of the low-molecular-weight dye Procion orange (Mr = 631). Intact muscle is impermeable to Procion orange, and dye uptake is a sensitive measure of changes in sarcolemmal integrity (48, 49). Both normal and gsg−/− animals of each age group suffered a similar degree of sarcolemmal breakdown over the course of the ECC protocol, which again differs from what is seen in mdx muscle (Fig. 2 a– c). In gsg−/− mice, the percentage of fibers that became permeable to dye is similar to normal, age-matched controls. In contrast, mdx muscles showed a dramatic increase in the percentage of fibers that became permeable to dye as a result of eccentric contraction (Fig. 2d). These data suggest that sarcoglycan is not necessary for normal resistance to the stresses placed on the membrane during ECC and proper maintenance of sarcolemma integrity.
Figure 2.
Eccentric contraction-induced Procion orange uptake in normal (n = 5), gsg−/− (n = 4), and mdx (n = 6) EDL. Representative sections from normal (a), gsg−/− (b), and mdx EDL muscles (c) after the five ECC protocol are shown. (d) The percentage of fibers showing dye uptake (±SD), indicative of sarcolemmal rupture, is similar between normal and gsg−/− EDL muscles. The mdx EDL shows a substantial increase in dye uptake after ECC indicative of sarcolemmal rupture.
Exercise in γ-Sarcoglycan-Deficient Mice. To test the physiologic significance of these findings, γ-sarcoglycan-deficient and normal age-matched animals were exercised for a 7-week period. Mice completed a swimming protocol that consisted of two 1-hr sessions per day, 6 days per week. Animals that were unable to complete a session were removed from the water. There was no difference in the percentage of unfinished sessions (6% for normal and 7% for gsg−/−) or the percentage of the total time omitted between normal and gsg−/− animals (1.6% and 1.9%, respectively). In the absence of exercise, gsg−/− mice display reduced activity (46). After the exercise protocol, the baseline activity level of gsg−/− mice increased so that they were indistinguishable from normal mice. Despite significant muscle involvement, gsg−/− mice tolerated the swimming protocol well; no gsg−/− mice died during swimming. Additionally, the baseline activity level of gsg−/− was increased markedly in exercised vs. nonexercised gsg−/− mice.
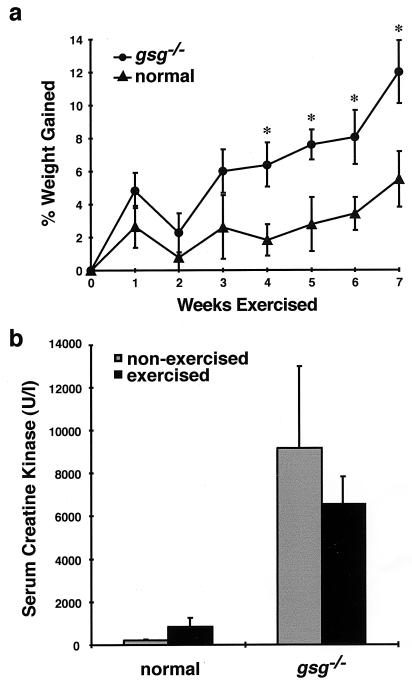
Animals were weighed before the protocol began and at the end of each week of swimming. The percentage of the starting weight that had been gained by the end of each week was calculated. Homozygous mutant (gsg−/−) mice began the protocol at a lower average weight than controls. The average weight for each group was 26.92 ± 1.93 g and 29.03 ± 1.90 g, respectively. Both groups of animals gained weight over the course of the protocol, but gsg−/− animals gained weight at a significantly greater rate than controls (P < 0.05 for weeks 4–7; Fig. 3a). After 7 weeks, mutant and normal mice were of nearly identical weights (30.04 ± 3.36 g and 30.63 ± 4.84 g, respectively). Moreover, serum creatine kinase levels, an indication of muscle degeneration, were not significantly elevated in gsg−/− mice that completed the swimming protocol compared with gsg−/− mice that had not swum (Fig. 3b). Based on these data, strenuous exercise did not worsen the degree of muscle disease in gsg−/− mice.
Figure 3.
The effect of exercise on weight gain (a) and serum creatine kinase levels (b) in γ-sarcoglycan-deficient muscular dystrophy. (a) gsg−/− animals were weighed at the end of each week of exercise. The percent increase in weight compared with the starting weight for each genotype was calculated. The mean ± SEM is shown. gsg−/− animals gained significantly more weight than normal controls (∗, P < 0.05 for weeks 4–7) over the course of the protocol. (b) Serum creatine kinase levels were measured 2 days after completion of the 7-week swimming protocol. The mean ± SEM is shown. Animals lacking γ-sarcoglycan failed to show an increase in creatine kinase that was consistent with a change in the overall amount of degeneration occurring in skeletal muscle.
Histopathology in Sarcoglycan-Deficient Muscle Is Unchanged by Exercise.
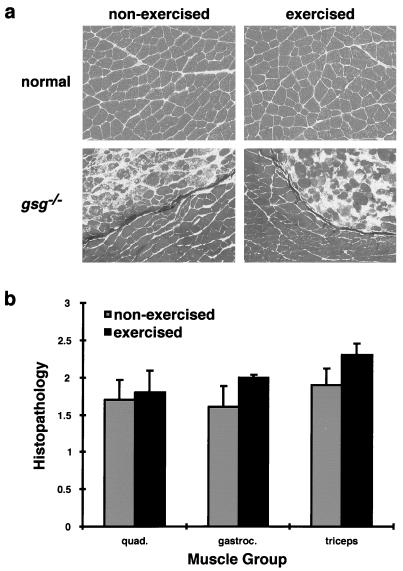
To ascertain the effect of exercise on sarcoglycan-deficient muscular dystrophy, mice from the swimming protocol were sacrificed 2 days after completion. Age-matched control animals also were sacrificed for comparison. Femoral quadriceps, triceps bracheii, and gastrocnemius were dissected and analyzed for histopathology. Normal mice showed no evidence of muscle degeneration as a result of exercise. gsg−/− animals that underwent 7 weeks of strenuous exercise did not show worsened histopathology compared with age-matched gsg−/− mice that did not undergo the exercise protocol (Fig. 4a). The degree of fibrosis, adipose tissue infiltration, and overt degeneration was quantified and did not differ significantly between exercised and nonexercised controls (Fig. 4b). Taken together, these data confirmed that mechanical stress in the animal, like eccentric contractions in the isolated muscle, did not induce sarcolemmal rupture and muscle degeneration in mice lacking γ-sarcoglycan.
Figure 4.
The effect of exercise on histopathology in normal and gsg−/− animals. (a) Representative sections of normal and gsg−/− quadriceps taken from age-matched animals that did (exercised, Right) and did not (non-exercised, Left) undergo the swimming protocol. Normal muscle showed no evidence of exercise-induced degeneration. gsg−/− muscles also showed no increase in histopathology as a result of exercise. (b) Quantitation of histopathology in age-matched exercised and nonexercised gsg−/− muscles. Histologic sections from femoral quadriceps (quad.), gastrocnemius (gastroc.), and triceps bracheii (triceps) were scored (blinded) for the presence of adipose tissue, degeneration, and fibrosis on a scale of 0–3. The mean ± SEM is shown. There was no significant difference in histopathology between exercised and nonexercised gsg−/− animals (P values of 0.80, 0.24, and 0.23, respectively).
DISCUSSION
The role of mechanical or contraction-induced injury in muscular dystrophy is unclear (39, 50, 51). Mechanical and exercise analyses of skeletal muscle lacking dystrophin have suggested that a mechanical deficiency results from dystrophin loss (43–45, 52–54). We have reported previously that mdx EDL and diaphragm muscles are more susceptible to mechanical injury than normal controls (45). These results support the idea that dystrophin protects the sarcolemma from the stress generated during muscle contraction and represent a functional correlate of the intact dystrophin–dystroglycan–laminin complex.
Mice lacking γ-sarcoglycan were reported previously (46) as were mice deficient for α-sarcoglycan (55). Mice lacking γ-sarcoglycan have a severe, progressive skeletal muscle degeneration and reduced survival (46). Immunohistochemical studies of gsg−/− muscle suggested that the dystrophin–dystroglycan–laminin axis was intact and that muscular dystrophy occurs independent of dystrophin. The absence of a mechanical deficit in muscle lacking γ-sarcoglycan suggests that the dystrophin–dystroglycan–laminin link is not only present, but that it appears to be functional in the absence of γ-sarcoglycan.
Eccentric contraction studies of isolated EDL muscles from mice lacking γ-sarcoglycan showed normal isometric and tetanic force generation. Furthermore, gsg−/− EDL failed to show the increase in contraction-induced injury we have shown previously to be a feature of the mdx EDL (45). Thus, at the level of an isolated muscle, the absence of γ-sarcoglycan did not result in a molecular defect that was sufficient to confer increased mechanical weakness on muscle. Like eccentric contraction, exercise also failed to increase the proportion of damaged myocytes in gsg−/− mice and confirmed the physiologic relevance of our findings in the isolated EDL. Interestingly, mice lacking α-sarcoglycan appear to have a functional defect in force production in the EDL muscle. The apparent mechanical difference between mice lacking γ-sarcoglycan and those lacking α-sarcoglycan may be explained, in part, by the observation that α-sarcoglycan is not completely absent in muscle lacking γ-sarcoglycan. The partial retention of α-sarcoglycan, 10–30% of normal levels, has been observed in mice lacking γ-sarcoglycan and humans who carry mutations in the γ-sarcoglycan gene (46, 56). It is possible that residual α-sarcoglycan protects against the development of a mechanical deficit. However, the phenotype in mice lacking γ-sarcoglycan is severe, suggesting that a nonmechanical mechanism is sufficient for the development of muscular dystrophy.
Mice lacking γ-sarcoglycan that had been exercised strenuously showed no significant increase in histopathology or serum creatine kinase levels. Such an increase would have been expected if an increased rate of cellular degeneration was caused by mechanical strain. In fact, gsg−/− mice gained a significantly larger amount of weight compared with controls and increased their basal activity level. Whether exercise improves survival remains unknown. However, these findings demonstrate that contraction-induced injury is not required for progressive muscle degeneration.
It has been thought previously that creatine kinase release occurs as a consequence of mechanical injury and tearing of muscle. Baseline serum creatine kinase levels are increased in mice lacking γ-sarcoglycan (46), but the data presented here suggest that creatine kinase release occurs by a mechanism other than mechanical injury. The phenomenon of creatine kinase increase with exercise is, in fact, noted in normal muscle, where normal mice show an approximate 5-fold increase in creatine kinase release with exercise (Fig. 3b). It is possible that creatine kinase release from normal muscle after exercise occurs by a mechanism other than membrane tearing. Serum creatine kinase elevation is present early in life in both mice and humans with LGMD and increases as muscle mass increases. Thus, serum creatine kinase levels are influenced by total muscle mass, microcirculation, and myofiber death. Mice lacking γ-sarcoglycan were shown previously to take up extensive amounts of the vital stain Evans Blue Dye (EBD) in the absence of exercise (46). Electron micrographs of gsg−/− muscle do not show any visible membrane defects (46). Thus, the phenomenon of creatine kinase release and EBD uptake across the muscle membrane may occur by an alternative means. Procion orange uptake, on the other hand, appears to require a mechanical deficit to gain entry into the myofiber because it occurs in mdx muscle only after contraction.
Dystrophin and sarcoglycan deficiency appear to result in different mechanical consequences and may represent alternative mechanisms leading to muscle degeneration (46, 57). However, dystrophin mutations also produce a secondary reduction of the sarcoglycan subunits; therefore, sarcoglycan deficiency is a feature seen in both DMD and in the mdx mouse. Thus, a nonmechanical mechanism may contribute to the phenotype in dystrophin deficiency. In DMD and the mdx mouse, there is clearly a mechanical defect, but our data indicate that muscular dystrophy occurs in its absence and that this mechanical defect may not be necessary for muscle degeneration. In mdx mice, overexpression of the dystrophin carboxyl terminus, DP71, appears to restore dystroglycan and α-sarcoglycan (adhalin or 50DAG), yet these mice still develop muscular dystrophy. These data support that mechanical defects may be sufficient to produce the dystrophic process. We hypothesize that both mechanical and nonmechanical defects are present in dystrophin deficiency and that the nonmechanical defect is sufficient to produce the dystrophic process in γ-sarcoglycan deficiency.
Alternative hypotheses to the mechanical defect as a cause of muscle degeneration in dystrophin-deficient muscle have been proposed and include defects in signaling and/or intracellular ion homeostasis (40, 46). Specifically, changes in calcium leak channel activity (58, 59) and in stretch-inactivated calcium channels (60) have been characterized. Recently, an ecto-ATPase activity was ascribed to α-sarcoglycan and implied that this ATPase activity may directly affect the entry of extracellular calcium into the muscle (61), consistent with a direct signaling role for sarcoglycan.
Because there are both mechanical and signaling roles emerging for the dystrophin–glycoprotein complex, this may constitute a coupled system not only to transmit mechanical forces from the contractile apparatus to the extracellular matrix, but also to provide intracellular signals that report the mechanical activity. Such a coupling is needed in skeletal muscle to explain the fact that maintained muscle mass and muscle hypertrophy relies on periodic stress being placed on the muscle. Clearly, activity per se, in the absence of load on a muscle, is not sufficient to either preserve normal mass or signal hypertrophy. Dystrophin and its associated complex of proteins, either through direct interaction with integrin complexes or through indirect coupling via the cortical cytoskeleton, is well placed to provide mechanical modulation of signaling. Hypothetically, this signaling could trigger processes involved in cytoskeletal remodeling and growth. Intriguingly, the muscles of the mdx mouse are not as severely affected as the muscles of the gsg−/− mouse. Thus, an aberrant dystrophin/sarcoglycan complex may provide a normal mechanical linkage to the extracellular matrix, but also may lead to aberrant signaling that is more damaging to mouse muscle than complete loss of the dystrophin–glycoprotein complex and both its mechanical and signaling functions.
We have shown that the absence of sarcoglycan is sufficient to cause muscle degeneration and is likely to play a causative role in both DMD and LGMD. Although the absence of dystrophin may result in a mechanical deficit in mdx and DMD muscle, it may not be the only deficit contributing to the dystrophic process. These results underscore the functional importance of sarcoglycan in muscle survival and help to clarify the functional organization of the DGC. Furthermore, these findings raise the possibility that the loss of sarcoglycan may contribute to muscle degeneration in both dystrophin- and sarcoglycan-associated muscular dystrophies. Gene therapy for the muscular dystrophies likely will need to restore sarcoglycan expression to fully correct the dystrophic process. This is of critical importance to therapeutic efforts designed at treating a diverse group of muscular dystrophies as well as to human gene therapy trials for LGMD and DMD.
Acknowledgments
We acknowledge the technical support of Kirsten S. Sigrist. E.M.M. is a Culpeper Medical Scholar. A.A.H. is supported by the Medical Scientist Training Program. This work was supported by grants from the Heart Research Foundation (E.M.M.), the Muscular Dystrophy Association (E.M.M. and H.L.S.), and the National Institutes of Health (E.M.M. and H.L.S.).
ABBREVIATIONS
- DGC
dystrophin–glycoprotein complex
- DMD/BMD
Duchenne/Becker muscular dystrophy
- LGMD
limb-girdle muscular dystrophy
- ECC
eccentric contraction
- EDL
extensor digitorum longus
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Campbell K P. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- 2.Bonnemann C G, McNally E M, Kunkel L M. Curr Opin Peds. 1996;8:569–582. [PubMed] [Google Scholar]
- 3.Straub V, Campbell K P. Curr Opin Neurol. 1997;10:168–175. doi: 10.1097/00019052-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Lim L E, Campbell K P. Curr Opin Neurol. 1998;11:443–452. doi: 10.1097/00019052-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Burghes A H, Logan C, Hu X, Belfall B, Worton R G, Ray P N. Nature (London) 1987;328:434–437. doi: 10.1038/328434a0. [DOI] [PubMed] [Google Scholar]
- 6.Monaco A P, Neve R L, Colletti F C, Bertelson C J, Kurnit D M, Kunkel L M. Nature (London) 1986;323:646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- 7.Koenig M, Hoffman E, Bertelson C, Monaco A, Feener C, Kunkel L. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 8.Campbell K P, Kahl S D. Nature (London) 1989;338:259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida M, Ozawa E. J Biochem (Tokyo) 1990;108:748–752. doi: 10.1093/oxfordjournals.jbchem.a123276. [DOI] [PubMed] [Google Scholar]
- 10.Ervasti J M, Campbell K P. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- 11.Ibraghimov-Beskrovnaya O, Ervasti J M, Leveille C J, Slaughter C A, Sernett S W, Campbell K P. Nature (London) 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 12.Ervasti J M, Campbell K P. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams M E, Butler M H, Dwyer T M, Peters M F, Murnane A A, Froehner S C. Neuron. 1993;11:531–540. doi: 10.1016/0896-6273(93)90157-m. [DOI] [PubMed] [Google Scholar]
- 14.Ahn A H, Yoshida M, Anderson M S, Feener C A, Selig S, Hagiwara Y, Ozawa E, Kunkel L M. Proc Natl Acad Sci USA. 1994;91:4446–4450. doi: 10.1073/pnas.91.10.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang B, Ibraghimov-Beskrovnaya O, Moomaw C R, Slaughter C A, Campbell K P. J Biol Chem. 1994;269:6040–6044. [PubMed] [Google Scholar]
- 16.Carr C, Fischbach G D, Cohen J B. J Cell Biol. 1989;109:1753–1764. doi: 10.1083/jcb.109.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner K R, Cohen J B, Huganir R L. Neuron. 1993;10:511–522. doi: 10.1016/0896-6273(93)90338-r. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida M, Yamamoto H, Noguchi S, Mizuno Y, Hagiwara Y, Ozawa E. FEBS Lett. 1995;367:311–314. doi: 10.1016/0014-5793(95)00574-s. [DOI] [PubMed] [Google Scholar]
- 19.Sadoulet-Puccio H M, Khurana T S, Cohen J B, Kunkel L M. Hum Mol Genet. 1996;5:489–496. doi: 10.1093/hmg/5.4.489. [DOI] [PubMed] [Google Scholar]
- 20.Peters M F, O’Brien K F, Sadoulet-Puccio H M, Kunkel L M, Adams M E, Froehner S C. J Biol Chem. 1997;272:31561–31569. doi: 10.1074/jbc.272.50.31561. [DOI] [PubMed] [Google Scholar]
- 21.Blake D J, Nawrotzki R, Loh N Y, Gorecki D C, Davies K E. Proc Natl Acad Sci USA. 1998;95:241–246. doi: 10.1073/pnas.95.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sicinski P, Geng Y, Ryder-Cook A S, Barnard E A, Darlison M G, Barnard P J. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 23.Roberds S L, Leturcq F, Allamand V, Piccolo F, Jeanpierre M, Anderson R D, Lim L E, Lee J C, Tome F M, Romero N B, et al. Cell. 1994;78:625–633. doi: 10.1016/0092-8674(94)90527-4. [DOI] [PubMed] [Google Scholar]
- 24.Noguchi S, McNally E M, Ben Othmane K, Hagiwara Y, Mizuno Y, Yoshida M, Yamamoto H, Bonnemann C G, Gussoni E, Denton P H, et al. Science. 1995;270:819–822. doi: 10.1126/science.270.5237.819. [DOI] [PubMed] [Google Scholar]
- 25.Bonnemann C G, Modi R, Noguchi S, Mizuno Y, Yoshida M, Gussoni E, McNally E M, Duggan D J, Angelini C, Hoffman E P. Nat Genet. 1995;11:266–273. doi: 10.1038/ng1195-266. [DOI] [PubMed] [Google Scholar]
- 26.Lim L E, Duclos F, Broux O, Bourg N, Sunada Y, Allamand V, Meyer J, Richard I, Moomaw C, Slaughter C, et al. Nat Genet. 1995;11:257–265. doi: 10.1038/ng1195-257. [DOI] [PubMed] [Google Scholar]
- 27.Nigro V, de Sa Moreira E, Piluso G, Vainzof M, Belsito A, Politano L, Puca A A, Passos-Bueno M R, Zatz M. Nat Genet. 1996;14:195–198. doi: 10.1038/ng1096-195. [DOI] [PubMed] [Google Scholar]
- 28.Ettinger A J, Feng G, Sanes J R. J Biol Chem. 1997;272:32534–32538. doi: 10.1074/jbc.272.51.32534. [DOI] [PubMed] [Google Scholar]
- 29.McNally E M, Ly C T, Kunkel L M. FEBS Lett. 1998;422:27–32. doi: 10.1016/s0014-5793(97)01593-7. [DOI] [PubMed] [Google Scholar]
- 30.McNally E M, Duggan D, Gorospe J R, Bonnemann C G, Fanin M, Pegoraro E, Lidov H G, Noguchi S, Ozawa E, Finkel R S, et al. Hum Mol Genet. 1996;5:1841–1847. doi: 10.1093/hmg/5.11.1841. [DOI] [PubMed] [Google Scholar]
- 31.Rybakova I N, Amann K J, Ervasti J M. J Cell Biol. 1996;135:661–672. doi: 10.1083/jcb.135.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rybakova I N, Ervasti J M. J Biol Chem. 1997;272:28771–28778. doi: 10.1074/jbc.272.45.28771. [DOI] [PubMed] [Google Scholar]
- 33.Amann K J, Renley B A, Ervasti J M. J Biol Chem. 1998;273:28419–28423. doi: 10.1074/jbc.273.43.28419. [DOI] [PubMed] [Google Scholar]
- 34.Sunada Y, Bernier S M, Kozak C A, Yamada Y, Campbell K P. J Biol Chem. 1994;269:13729–13732. [PubMed] [Google Scholar]
- 35.Pasternak C, Wong S, Elson E L. J Cell Biol. 1995;128:355–361. doi: 10.1083/jcb.128.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menke A, Jockusch H. Nature (London) 1991;349:69–71. doi: 10.1038/349069a0. [DOI] [PubMed] [Google Scholar]
- 37.Menke A, Jockusch H. J Cell Sci. 1995;108:727–733. doi: 10.1242/jcs.108.2.727. [DOI] [PubMed] [Google Scholar]
- 38.Carpenter S, Karpati G. Brain. 1979;102:147–161. doi: 10.1093/brain/102.1.147. [DOI] [PubMed] [Google Scholar]
- 39.Petrof B J. Mol Cell Biochem. 1998;179:111–123. doi: 10.1023/a:1006812004945. [DOI] [PubMed] [Google Scholar]
- 40.Duncan C J. Experientia. 1978;34:1531–1535. doi: 10.1007/BF02034655. [DOI] [PubMed] [Google Scholar]
- 41.Bodensteiner J B, Engel A G. Neurology. 1978;28:439–446. doi: 10.1212/wnl.28.5.439. [DOI] [PubMed] [Google Scholar]
- 42.Glesby M J, Rosenmann E, Nylen E G, Wrogemann K. Muscle Nerve. 1988;11:852–856. doi: 10.1002/mus.880110809. [DOI] [PubMed] [Google Scholar]
- 43.Weller B, Karpati G, Carpenter S. J Neurol Sci. 1990;100:9–13. doi: 10.1016/0022-510x(90)90005-8. [DOI] [PubMed] [Google Scholar]
- 44.Moens P, Baatsen P H, Marechal G. J Muscle Res Cell Motil. 1993;14:446–451. doi: 10.1007/BF00121296. [DOI] [PubMed] [Google Scholar]
- 45.Petrof B J, Shrager J B, Stedman H H, Kelly A M, Sweeney H L. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hack A A, Ly C T, Jiang F, Clendenin C J, Sigrist K S, Wollmann R L, McNally E M. J Cell Biol. 1998;142:1279–1287. doi: 10.1083/jcb.142.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooks S V, Faulkner J A. J Physiol (London) 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stead C V. In: Intracellular Staining in Neurobiology. Kater S, Nicholson C, editors. Berlin: Springer; 1973. pp. 41–59. [Google Scholar]
- 49.Bradley W G, Fulthorpe J J. Neurology. 1978;28:670–677. doi: 10.1212/wnl.28.7.670. [DOI] [PubMed] [Google Scholar]
- 50.Hutter O F. J Inher Metab Dis. 1992;15:565–577. doi: 10.1007/BF01799615. [DOI] [PubMed] [Google Scholar]
- 51.Carlson C G. Neurobiol Dis. 1998;5:3–15. doi: 10.1006/nbdi.1998.0188. [DOI] [PubMed] [Google Scholar]
- 52.McCully K, Giger U, Argov Z, Valentine B, Cooper B, Chance B, Bank W. Muscle Nerve. 1991;14:1091–1098. doi: 10.1002/mus.880141109. [DOI] [PubMed] [Google Scholar]
- 53.Brussee V, Tardif F, Tremblay J P. Neuromuscul Disord. 1997;7:487–492. doi: 10.1016/s0960-8966(97)00115-6. [DOI] [PubMed] [Google Scholar]
- 54.Vilquin J T, Brussee V, Asselin I, Kinoshita I, Gingras M, Tremblay J P. Muscle Nerve. 1998;21:567–576. doi: 10.1002/(sici)1097-4598(199805)21:5<567::aid-mus2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 55.Duclos F, Straub V, Moore S A, Venzke D P, Hrstka R F, Crosbie R H, Durbeej M, Lebakken C S, Ettinger A J, van der Meulen J, et al. J Cell Biol. 1998;142:1461–1471. doi: 10.1083/jcb.142.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vainzof M, Passos-Bueno M R, Canovas M, Moreira E S, Pavanello R C, Marie S K, Anderson L V, Bonnemann C G, McNally E M, Nigro V, et al. Hum Mol Genet. 1996;5:1963–1969. doi: 10.1093/hmg/5.12.1963. [DOI] [PubMed] [Google Scholar]
- 57.Straub V, Rafael J A, Chamberlain J S, Campbell K P. J Cell Biol. 1997;139:375–385. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fong P Y, Turner P R, Denetclaw W F, Steinhardt R A. Science. 1990;250:673–676. doi: 10.1126/science.2173137. [DOI] [PubMed] [Google Scholar]
- 59.Turner P R, Fong P Y, Denetclaw W F, Steinhardt R A. J Cell Biol. 1991;115:1701–1712. doi: 10.1083/jcb.115.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franco A J, Lansman J B. Nature (London) 1990;344:670–673. doi: 10.1038/344670a0. [DOI] [PubMed] [Google Scholar]
- 61.Betto R, Senter L, Ceoldo S, Tarricone E, Biral D, Salviati G. J Biol Chem. 1999;274:7907–7912. doi: 10.1074/jbc.274.12.7907. [DOI] [PubMed] [Google Scholar]