Abstract
The development of osteoporosis with advancing age in man is a widespread if not a universal phenomenon. The average loss between youth and old age amounts to about 15% of the skeleton but involves a much larger proportion of trabecular than of cortical bone.
The principal clinical manifestation of osteoporosis is fracture, and three osteoporotic fracture syndromes can be defined: the lower forearm fracture, which predominantly affects women between the ages of 50 and 65; the fracture of the proximal femur, which affects both sexes over the age of 70; and the relatively rare vertebral crush fracture syndrome, which may present at any age but is most common in elderly women.
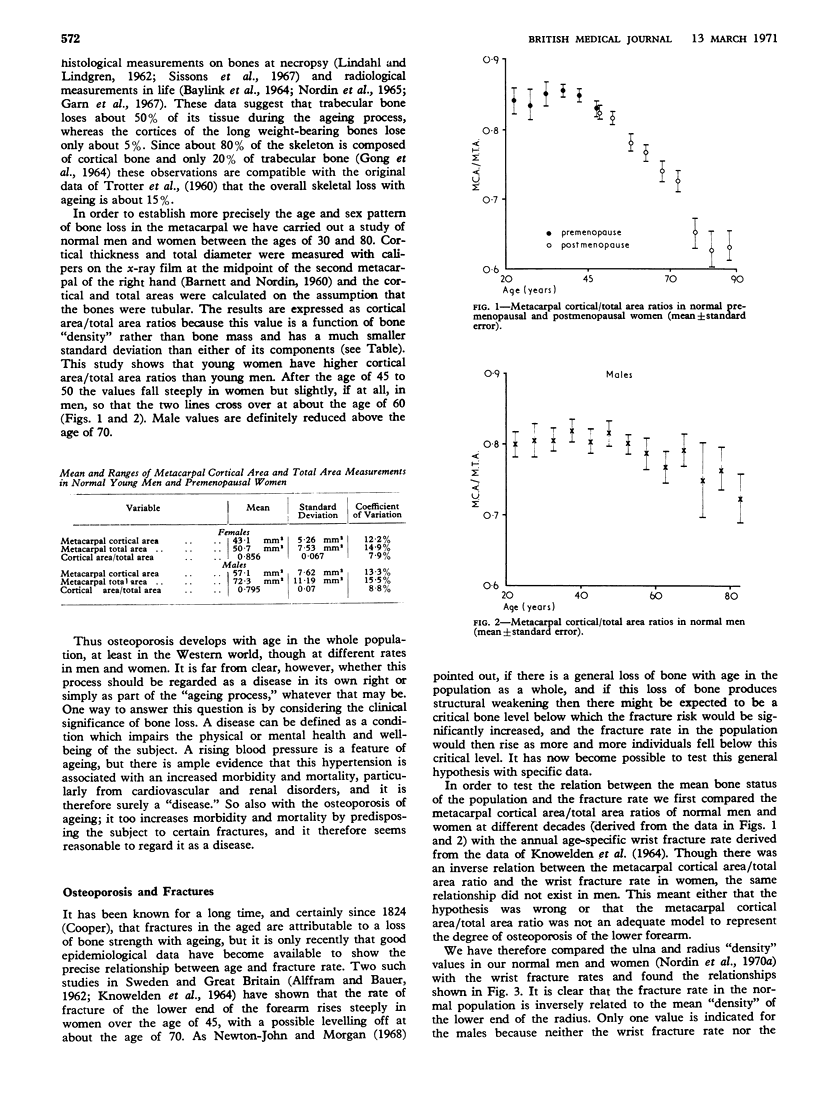
The lower forearm fracture rate is inversely related to the mean normal lower forearm x-ray “density” of the wrist, which falls by about 30% in the 15 years following the menopause. This process, which is associated with corresponding trabecular bone loss elsewhere in the skeleton, is associated with a corresponding rise in the fasting urinary calcium excretion. Some degree of negative calcium balance, and consequent bone resorption, probably occurs in everyone during the later part of the night because calcium absorption is completed within about three to five hours of a meal. In postmenopausal women, however, the sensitivity of the bone to parathyroid hormone appears to be increased, and their nocturnal negative calcium balance therefore comes to exceed the positive balance which can be achieved during the waking hours.
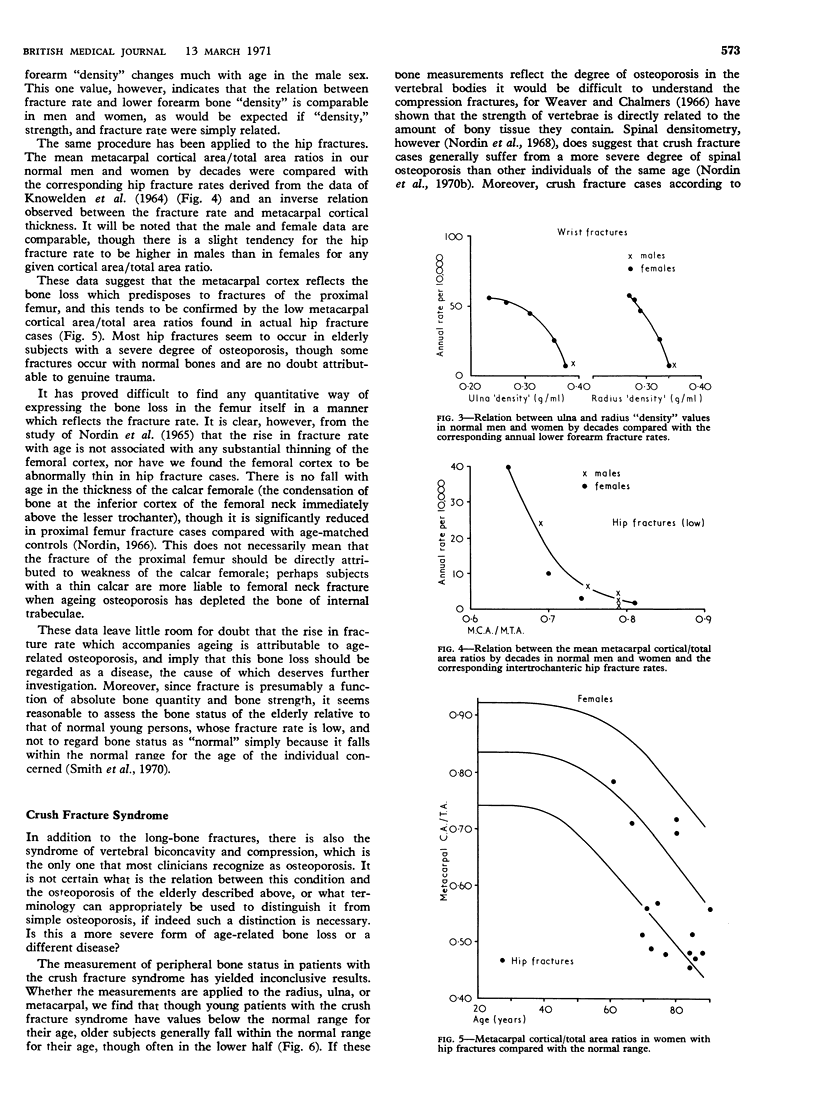
Femoral neck fractures in old people reflect the further progression of osteoporosis with advancing age since the fracture rate is inversely correlated with the mean thickness of the metacarpal cortex in the normal population. This progressive osteoporosis is associated with and could well result from a steady decline in calcium absorption which is at least partially attributable to vitamin-D deficiency and reversible on vitamin-D treatment.
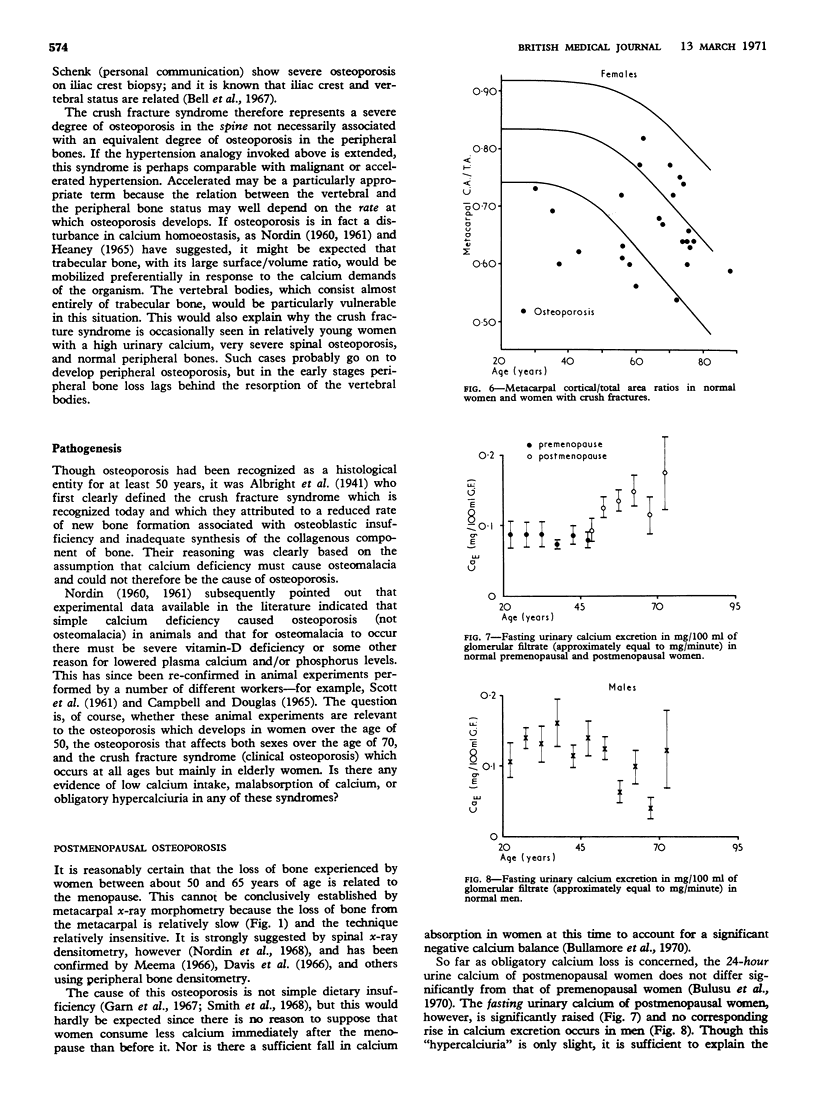
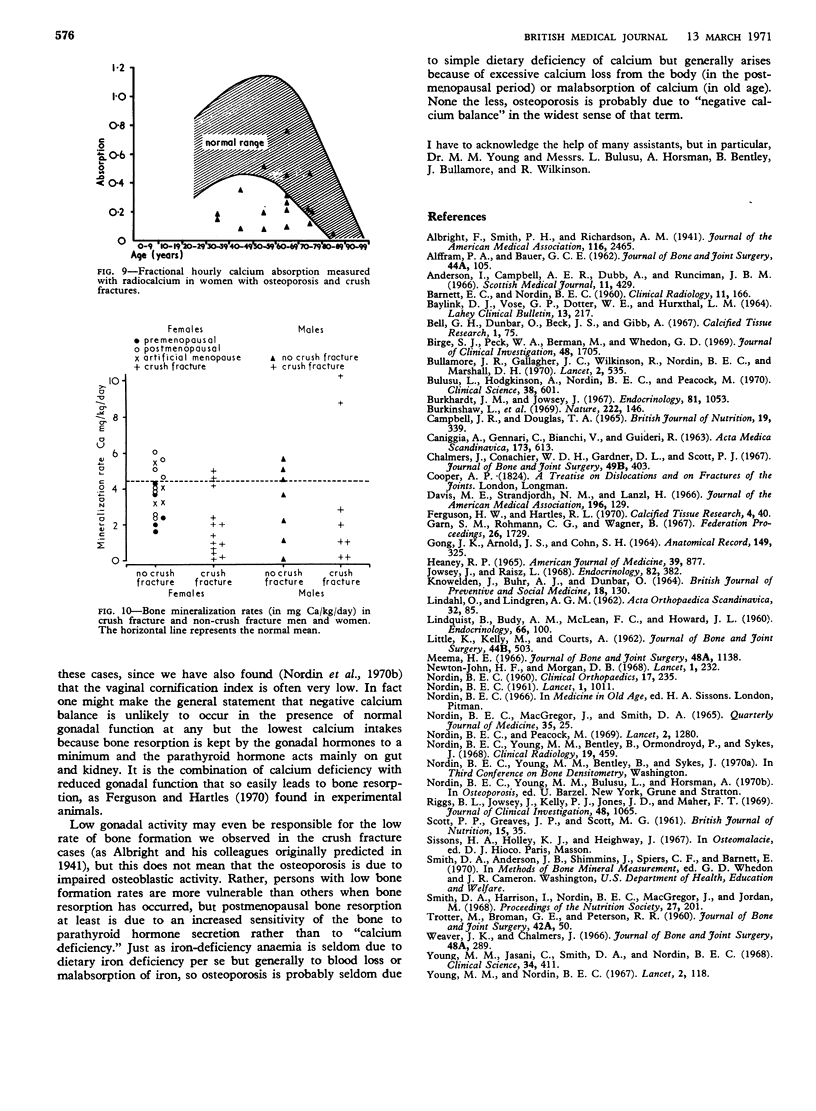
The vertebral crush fracture syndrome represents a severe degree of spinal osteoporosis which may be associated with relatively normal peripheral bones. It probably results from an accelerated negative calcium balance which mobilizes trabecular bone preferentially. Some of the factors which may contribute to this accelerated negative balance have been identified and include a reduced rate of bone turnover, impaired calcium absorption, and low oestrogen activity as judged by vaginal smears, but there may well be others as yet unidentified.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNETT E., NORDIN B. E. The radiological diagnosis of osteoporosis: a new approach. Clin Radiol. 1960 Jul;11:166–174. doi: 10.1016/s0009-9260(60)80012-8. [DOI] [PubMed] [Google Scholar]
- Bell G. H., Dunbar O., Beck J. S., Gibb A. Variations in strength of vertebrae with age and their relation to osteoporosis. Calcif Tissue Res. 1967;1(1):75–86. doi: 10.1007/BF02008077. [DOI] [PubMed] [Google Scholar]
- Birge S. J., Peck W. A., Berman M., Whedon G. D. Study of calcium absorption in man: a kinetic analysis and physiologic model. J Clin Invest. 1969 Sep;48(9):1705–1713. doi: 10.1172/JCI106136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullamore J. R., Wilkinson R., Gallagher J. C., Nordin B. E., Marshall D. H. Effect of age on calcium absorption. Lancet. 1970 Sep 12;2(7672):535–537. doi: 10.1016/s0140-6736(70)91344-9. [DOI] [PubMed] [Google Scholar]
- Bulusu L., Hodgkinson A., Nordin B. E., Peacock M. Urinary excretion of calcium and creatinine in relation to age and body weight in normal subjects and patients with renal calculus. Clin Sci. 1970 May;38(5):601–612. doi: 10.1042/cs0380601. [DOI] [PubMed] [Google Scholar]
- Burkhart J. M., Jowsey J. Parathyroid and thyroid hormones in the development of immobilization osteoporosis. Endocrinology. 1967 Nov;81(5):1053–1062. doi: 10.1210/endo-81-5-1053. [DOI] [PubMed] [Google Scholar]
- Burkinshaw L., Marshall D. H., Oxby C. B., Spiers F. W., Nordin B. E., Young M. M. Bone turnover model based on a continuously expanding exchangeable calcium pool. Nature. 1969 Apr 12;222(5189):146–148. doi: 10.1038/222146a0. [DOI] [PubMed] [Google Scholar]
- CANIGGIA A., GENNARI C., BIANCHI V., GUIDERI R. Intestinal absorption of 45Ca in senile osteoporosis. Acta Med Scand. 1963 May;173:613–617. doi: 10.1111/j.0954-6820.1963.tb17445.x. [DOI] [PubMed] [Google Scholar]
- Campbell J. R., Douglas T. A. The effect of low calcium intake and vitamin D supplements on bone structure in young growing dogs. Br J Nutr. 1965;19(3):339–351. doi: 10.1079/bjn19650032. [DOI] [PubMed] [Google Scholar]
- Chalmers J., Conacher W. D., Gardner D. L., Scott P. J. Osteomalacia--a common disease in elderly women. J Bone Joint Surg Br. 1967 Aug;49(3):403–423. [PubMed] [Google Scholar]
- Fonkalsrud E. W., Linde L. M., Longmire W. P., Jr Portal hypertension from idiopathic superior vena caval obstruction. JAMA. 1966 Apr 11;196(2):129–132. [PubMed] [Google Scholar]
- GONG J. K., ARNOLD J. S., COHN S. H. COMPOSITION OF TRABECULAR AND CORTICAL BONE. Anat Rec. 1964 Jul;149:325–331. doi: 10.1002/ar.1091490303. [DOI] [PubMed] [Google Scholar]
- Garn S. M., Rohmann C. G., Wagner B. Bone loss as a general phenomenon in man. Fed Proc. 1967 Nov-Dec;26(6):1729–1736. [PubMed] [Google Scholar]
- Heaney R. P. A unified concept of osteoporosis. Am J Med. 1965 Dec;39(6):877–880. doi: 10.1016/0002-9343(65)90109-9. [DOI] [PubMed] [Google Scholar]
- KNOWELDEN J., BUHR A. J., DUNBAR O. INCIDENCE OF FRACTURES IN PERSONS OVER 35 YEARS OF AGE. A REPORT TO THE M.R.C. WORKING PARTY ON FRACTURES IN THE ELDERLY. Br J Prev Soc Med. 1964 Jul;18:130–141. doi: 10.1136/jech.18.3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDAHL O., LINDGREN A. G. Grading of osteoporosis in autopsy specimens. A new method. Acta Orthop Scand. 1962;32:85–100. doi: 10.3109/17453676208989565. [DOI] [PubMed] [Google Scholar]
- LINDQUIST B., BUDY A. M., MCLEAN F. C., HOWARD J. L. Skeletal metabolism in estrogen-treated rats studied by means of Ca45. Endocrinology. 1960 Jan;66:100–111. doi: 10.1210/endo-66-1-100. [DOI] [PubMed] [Google Scholar]
- Meema H. E. Menopausal and aging changes in muscle mass and bone mineral content. A roentgenographic study. J Bone Joint Surg Am. 1966 Sep;48(6):1138–1144. [PubMed] [Google Scholar]
- NORDIN B. E. The pathogenesis of osteoporosis. Lancet. 1961 May 13;1(7185):1011–1015. doi: 10.1016/s0140-6736(61)91827-x. [DOI] [PubMed] [Google Scholar]
- Nordin B. E., MacGregor J., Smith D. A. The incidence of osteoporosis in normal women: its relation to age and the menopause. Q J Med. 1966 Jan;35(137):25–38. [PubMed] [Google Scholar]
- Nordin B. E., Peacock M. Role of kidney in regulation of plasma-calcium. Lancet. 1969 Dec 13;2(7633):1280–1283. doi: 10.1016/s0140-6736(69)90813-7. [DOI] [PubMed] [Google Scholar]
- Nordin B. E., Young M. M., Bentley B., Ormondroyd P., Sykes J. Lumbar spine densitometry methodology and results in relation to the menopause. Clin Radiol. 1968 Oct;19(4):459–464. doi: 10.1016/s0009-9260(68)80107-2. [DOI] [PubMed] [Google Scholar]
- Riggs B. L., Jowsey J., Kelly P. J., Jones J. D., Maher F. T. Effect of sex hormones on bone in primary osteoporosis. J Clin Invest. 1969 Jun;48(6):1065–1072. doi: 10.1172/JCI106062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT P. P., GREAVES J. P., SCOTT M. G. Nutrition of the cat. 4. Calcium and iodine deficiency on a meat diet. Br J Nutr. 1961;15:35–51. doi: 10.1079/bjn19610006. [DOI] [PubMed] [Google Scholar]
- Smith D. A., Harrison I. Mineral metabolism in relation to ageing. Proc Nutr Soc. 1968 Sep;27(2):201–210. doi: 10.1079/pns19680048. [DOI] [PubMed] [Google Scholar]
- TROTTER M., BROMAN G. E., PETERSON R. R. Densites of bones of white and Negro skeletons. J Bone Joint Surg Am. 1960 Jan;42-A:50–58. [PubMed] [Google Scholar]
- Weaver J. K., Chalmers J. Cancellous bone: its strength and changes with aging and an evaluation of some methods for measuring its mineral content. J Bone Joint Surg Am. 1966 Mar;48(2):289–298. [PubMed] [Google Scholar]



