Abstract
DNA double-strand breaks formed during the assembly of antigen receptors or after exposure to ionizing radiation are repaired by proteins important for nonhomologous end joining that include Ku86, Ku70, DNA-PKCS, Xrcc4, and DNA ligase IV. Here we show that ku86-mutant mice, compared with control littermates, prematurely exhibited age-specific changes characteristic of senescence that include osteopenia, atrophic skin, hepatocellular degeneration, hepatocellular inclusions, hepatic hyperplastic foci, and age-specific mortality. Cancer and likely sepsis (indicated by reactive immune responses) partly contributed to age-specific mortality for both cohorts, and both conditions occurred earlier in ku86−/− mice. These data indicate that Ku86-dependent chromosomal metabolism is important for determining the onset of age-specific changes characteristic of senescence in mice.
Some segmental progeroid syndromes suggest that chromosomal metabolism plays an important role during senescence (1). Werner’s syndrome (WS), an inherited autosomal recessive disease caused by a mutation in WRN, is of particular interest because of its similarity to ordinary senescence (2). WRN is homologous to the RecQ family of DNA helicases (3) and catalyzes DNA unwinding (4) and 3′–5′ exonuclease activity (5). WS patients prematurely exhibit signs of senescence, including atrophic skin, graying and hair loss, osteoporosis, malignant neoplasms, and shortened lifespan. Cells derived from WS patients prematurely undergo replicative senescence (6), which describes the limited lifespan of cells grown in tissue culture (7). Even though there is some discordance between WS and ordinary senescence, the WS phenotype suggests chromosomal metabolism is a part of a genetic process of senescence.
Chromosomal metabolism appears to be associated with senescence in mice. Chromosomal aberrations progressively increase in bone marrow cells as some strains of senescence accelerated mice age (8) and in liver cells as C57BL/6J mice age (9). Interestingly, cells deleted for some chromosomal metabolism proteins exhibit premature replicative senescence. For example, murine cells deleted for a segment of the murine WRN homologue exhibit hypersensitivity to topoisomerase inhibitors and premature replicative senescence (10). Additionally, murine cells deficient for the recombinational repair proteins, Atm (11) and Brca2 (12–14), and the nonhomologous end-joining (NHEJ) proteins, Ku70 (15), Ku86 (16), Xrcc4 (17), and DNA ligase IV (18), undergo premature replicative senescence. Yet an early onset of senescence has not been reported for mice harboring mutations in any of these genes.
Here, we investigate the role of Ku86 during senescence in the whole mouse. Ku86 is important for the repair of DNA double-strand breaks (DSB) by NHEJ in association with Ku70 (19, 20). The Ku86–Ku70 heterodimer (Ku) binds to DNA ends, nicks, gaps, and hairpins. In vitro, Ku forms a complex called DNA-dependent protein kinase by associating with a 450-kDa catalytic subunit (DNA-PKCS). These subunits, together with Xrcc4 and DNA ligase IV, are important for repairing DNA DSB formed during the assembly of antigen receptors and after exposure to ionizing radiation (15–18, 21–23).
Further analysis of ku86-mutant mice shows early onset of age-specific changes. Compared with control littermates, ku86-mutant mice prematurely exhibit osteopenia, epiphyses closure, atrophic skin and hair follicles, hepatocellular degeneration, and age-specific mortality. The age-specific diseases: cancer and likely sepsis (suggested by reactive immune responses), are partly responsible for age-specific mortality in both cohorts. Both diseases occur earlier in ku86-mutant mice. These data suggest that Ku86 influences the process of senescence.
MATERIALS AND METHODS
Ku86 Genotypic Analysis by PCR.
The wild-type allele was detected with the sense primer (5′-GAGAGTCTACGACAACTGTGC-3′) and the antisense primer (5′-AGAGGGACTGCAGCCATATTA-3′) located in sequences deleted in the mutant Ku86 allele described as xrcc5M1 (21). The mutant allele was detected with a sense primer (5′-GGTTGCCAGTCATGCTACGGT-3′), which anneals to intronic sequences upstream of the positive selection cassette, and an antisense primer (5′-CCAAAGGCCTACCCGCTTCCATT-3′), which anneals to the PGK promoter in the positive selection cassette. PCR reactions were preincubated at 94°C for 5 min and then 30 cycles of amplification at 94°C for 30 sec, 59°C for 1 min (with 1-min ramp), and 72°C for 30 sec in a Perkin–Elmer DNA Thermal Cycler 480. Wild-type (0.3-kb) and mutant (0.4-kb) fragments were separated on a 1.2% ethidium bromide stained gel by electrophoresis. PCR products were sequenced to prove they were not artifacts, and results were confirmed by Southern analysis (21).
Histological Analysis.
Tissues were fixed in 10% buffered formalin phosphate, paraffin embedded, cut into 4 μM sections, and stained with haematoxylin and eosin by standard procedures.
Immunohistochemistry for Liver Sections.
Immunohistochemistry was performed by using 3-μm paraffin sections that were deparaffinized, hydrated, blocked in 3% methanol/hydrogen peroxide, then incubated with the primary antibody against glutamine synthetase (GS) (gift from James W. Campbell, Rice University); fumarylacetoacetatehydrolase (FAH) (gift from Robert Tanguay, University of Laval, Quebec); and mouse α fetoprotein (MAFP) (ICN catalogue no. 645611), Ki-67 [Immunotech (Westbrook, ME) Mib-1 clone]. All primary antibodies are rabbit polyclonal. Secondary anti-rabbit antibody was applied as SuperSensitive MultiLink Anti-rabbit (BioGenex/ABN no. HK326-UR) followed by Super Sensitive Label (BioGenex/ABN no. HK330–9K) for FAH and MAFP, and with secondary anti-rabbit and strepavidin-alkaline phosphatase for GS and Ki-67, followed by 3-amino-9-ethylcarbozole (BioGenex/ABN no. HK129–5K) for colorimetric detection, then hydrated, counterstained with hematoxylin, and covered with coverslip.
Serum Alanine Aminotransferase Concentration (ALT).
Blood was taken from anesthetized mice by intracardiac puncture. Serum was isolated by centrifugation and analyzed by using a standard assay of ALT activity (Vitrosslides, Johnson and Johnson, New Brunswick, NJ).
RESULTS
Ku86 was deleted in mice by mutating the gene Xrcc5 (21). This mutation was previously designated xrcc5M1; however, for clarity, it is called ku86−/− or Ku86+/− in the homozygous or heterozygous condition, respectively. A cohort of 47 control mice (wild type and Ku86+/−) and 89 ku86−/− mice, in a C57BL/6 × 129Sv crossbred background, were observed for age-specific changes associated with advanced age.
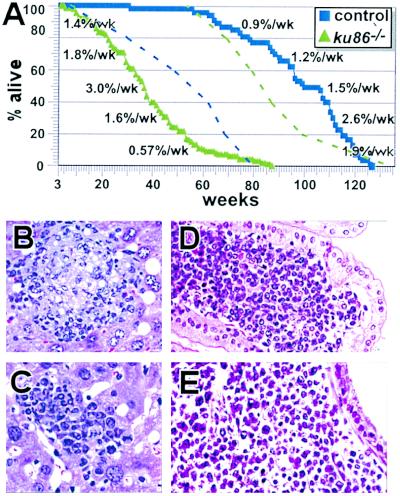
Shortened Lifespan for ku86−/− Mice. The relationship between chronological age and mortality is an important indicator of senescence (24); therefore, a survival curve was established starting with 3-wk-old mice. The lifespan of ku86−/− mice is shorter than that of control mice, primarily because of an early onset of age-specific mortality, which is about 8 and 56 wk, respectively (Fig. 1A). About 50% of ku86−/− and control mice die by 36 and 102 wk, respectively. The average lifespan is 38 +/− 14 wk for ku86−/− mice and 97 +/− 17 wk for control mice. Within the cohorts observed, the most long-lived ku86−/− and control mice died at 87 and 127 wk, respectively. By comparing survival curves, there is a difference in the rate of age-specific mortality (P < 0.005). The first 60% of the ku86−/− population died at a slightly faster rate than controls, whereas the remaining 40% died at a progressively slower rate (Fig. 1A).
Figure 1.
Lifespan and potential causes of age-specific death. (A) Survival curve (100%× number of mice alive after each week/total number of mice at beginning of study). The survival curve begins after weaning (3 wk) because ku86−/− pups are less fit to compete for resources than control littermates, and about 50% die before weaning unless control pups are removed soon after birth (16). Control mice, blue squares; ku86−/− mice, green triangles. A dashed blue line representing the control survival curve is superimposed onto the ku86−/− curve, and a dashed green line representing the ku86−/− survival curve is superimposed onto the control curve, to illustrate their differences. The percent of population that dies each week is displayed to the left of the ku86−/− survival curve and the right of the control survival curve for each interval of 20% starting at the onset of age-specific mortality. Number of mice observed: control, 47; ku86−/−, 89. (B and C) Section of liver with reactive immune response from (B) 89-wk-old control mouse and (C) 61-wk-old ku86−/− mouse. Note infiltration of mononuclear cells for B and neutrophils for C. (D and E) Section of malignant lymphoma from (D) 71-wk-old control mouse (infiltrating intestine) and (E) 37-wk-old ku86−/− mouse (subjacent to bronchial epithelium). (B–E, ×205.)
Mortality is not necessarily caused by senescence; however, age-specific illnesses like sepsis likely result from a progressive deteriorative process associated with advanced age (25). Age-specific acute and chronic immune reactions were observed in a variety of organs that include the liver (Fig. 1 B and C), kidney, spleen, urogenital tract, oropharynx, skin, and submandibular glands for both cohorts. These reactive immune responses were observed at a younger age in ku86−/− mice than control mice (observation of reactive immune responses for the liver are described in Table 1). Sepsis is implicated through the activation of reactive immune responses, especially in the liver and kidney, and could progress into a lethal condition.
Table 1.
Summary for ku86−/− (mt) and control (con) mice
| Genotype | Age, wk | Osteopenia | Epiphysis closure | Skin and follicular atrophy | ALT,* units/liter | Inclusions† | Reactive immune response‡ | Nodular hyperplasia§ |
|---|---|---|---|---|---|---|---|---|
| mt | 1–15 | 0/3 | 0/6 | 0/4 | 57 ± 1 | 0/7 | 0/7 | 0/7 |
| con | 0/5 | 0/7 | 0/7 | 56 ± 17 | 0/12 | 0/12 | 0/12 | |
| mt | 22 | nd | 2/2 | nd | nd | nd | nd | nd |
| con | nd | 0/2 | nd | nd | nd | nd | nd | |
| mt | 31–50 | 3/3 | 1/1 | 3/3 | 81 ± 4 | 2/4 | 1/5 | 2/4 |
| con | 0/4 | 0/1 | 0/4 | 40 | 0/4 | 0/4 | 0/4 | |
| mt | 51–70 | nd | nd | nd | 93 ± 30 | 3/8 | 2/7 | 5/8 |
| con | nd | nd | nd | 38 ± 6 | 0/9 | 0/9 | 1/9 | |
| mt | >70 | nd | nd | nd | nd | 1/1 | 0/1 | 1/1 |
| con | 4/5 | 4/4 | 8/8 | 126 ± 109 | 6/23 | 19/23 | 9/23 |
The number of mice affected (left) compared to the total number observed (right). nd, not done.
Number observed: for ku86−/− at 1–15 wk, 3; 31–50 wk, 3; 51–70 wk, 6 and for control at 1–15 wk, 6; 31–50 wk, 1; 50–70 wk, 6; >70 wk, 11.
Cytoplasmic hepatocellular inclusions observed for all ku86−/− mice and four control mice, nuclear hepatocellular inclusions observed for two control mice.
Includes microabscesses (acute response with neutrophils) and granulamatous inflamation (chronic response with mononuclear cells) observed in the liver.
Nodules were grossly observed in seven livers from control mice and in two livers from ku86−/− mice, the remainder could only be observed by histology (micronodular).
It is possible that exogenous opportunistic infection caused the reactive immune responses in ku86−/− mice because they are immune deficient because of defective V(D)J recombination (21). However, this is not likely for the following reasons. First, fatal systemic opportunistic infection derived from normal flora increase with advanced age (25). Second, ku86−/− mice were housed in a specific pathogen-free environment (tests negative for all known pathogens). These ku86−/− mice lived much longer than ku86−/− mice housed in a conventional colony contaminated with mouse hepatitis virus (MHV) and Taielers encephalomyelitis (50% die by 17 wk, 57 ku86−/− mice observed). MHV likely caused morbidity in at least two ku86−/− mice housed in the conventional colony, because they suffered from necrosis and inflammation of the liver (not shown). Third, reactive immune responses were commonly observed in the immune-competent control mice housed in the same cages. Therefore, the etiology of reactive immune responses in ku86−/− mice is likely to be similar to that for control mice. Even though the reactive immune responses are similar for both cohorts, it is possible that some differences exist, such as predisposition to certain pathological agents. A more detailed analysis of the pathogens in these animals is warranted in future studies.
Cancer incidence progressively increases with age in both mice (26) and humans (27) and causes morbidity in both cohorts. Compared with control mice, ku86−/− mice exhibited an early onset of cancer. Both cohorts exhibited malignant lymphoma (Fig. 1 D and E): eight of 47 control mice (75–119 wk) and two of 89 ku86−/− mice (37 and 54 wk). In addition to lymphoma, the following cancers were found in one of the 47 control mice: harderian gland adenocarcinoma (86 wk), squamous-cell carcinoma (90 wk), angiosarcoma (94 wk), adenocarcinoma of the lung (100 wk), mammary adenocarcinoma (102 wk), germ-cell neoplasm (110 wk), and hemangiopericytoma (119 wk). The mice with adenocarcinoma and germ-cell neoplasm also had lymphoma (included in the eight mice). These forms of cancer were not observed in the ku86−/− cohort. Thus, 13 of 47 control mice had cancer (27.6%), whereas two of 89 ku86−/− mice had cancer (2.2%).
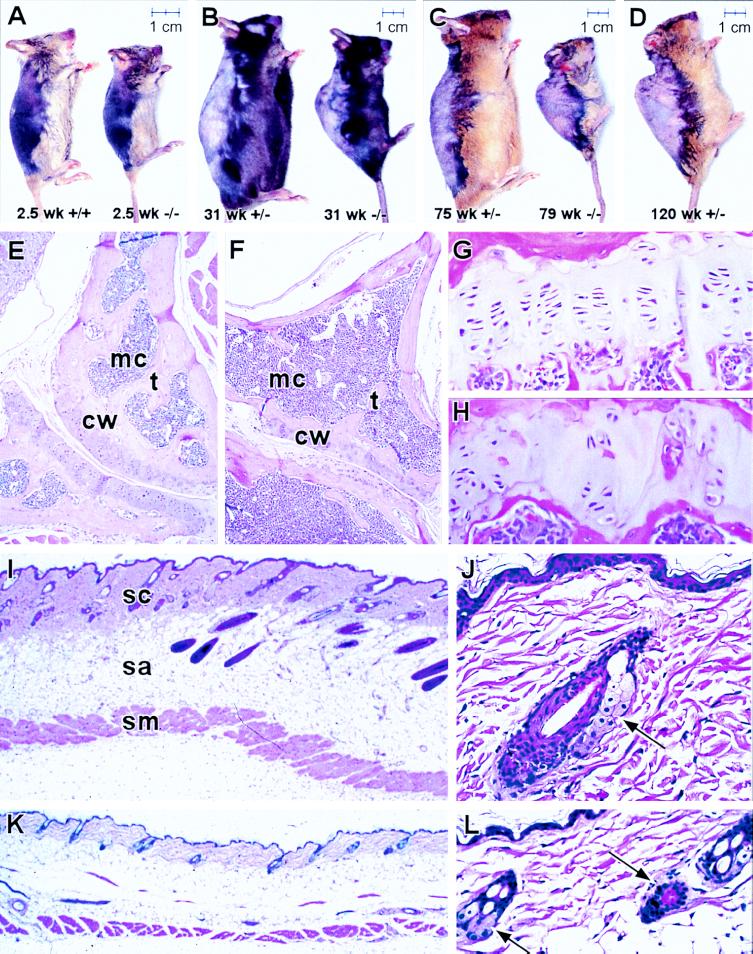
Early Onset of Age-Specific Changes Observed in ku86−/− Mice. Control and ku86−/− mice were observed for outward changes associated with age. Both cohorts exhibited kyphosis (abnormally increased convexity in the curvature of the thoracic spine from a lateral view) and decreased skin thickness as they aged with an earlier onset for ku86−/− mice (Fig. 2A–D). These outward changes occurred, by varying degrees, for the most long-lived 30% of the population of both cohorts.
Figure 2.
Age-specific changes in control and ku86−/− mice. (A–D) Outward signs of senescence. The dorsal region was shaved to enhance visualization of kyphosis. (A) Control (+/+ and +/−) and ku86−/− (−/−) mice at 2.5 wk. No kyphosis observed. (B) Control and ku86−/− mice at 31 wk. Kyphosis observed in only ku86−/− mouse. (C) Control and ku86−/− mice at 75 and 79 wk, respectively. Kyphosis observed in only ku86−/− mouse. (D) Control mouse at 120 wk. Kyphosis observed. (E and F) Section of vertebral articular processes from (E) 45-wk-old control and (F) 49-wk-old ku86−/− mouse. For ku86−/− bone, compared with control, the cortical wall (cw) and trabeculae (t) is thinner, the number of trabeculae are reduced and the medullary cavity (mc) is expanded. (G and H) Section of epiphysis from 22-wk-old (G) control and (H) ku86−/− mouse. For ku86−/− epiphysis, compared with control, the number of chondrocytes is reduced and the columnar organization of chondrocytes is lost. (I–L) Section of skin (dorsal region over cranial to mid-thorax) from (I and J) 45-wk-old control and (K and L) 49-wk-old ku86−/− mouse. For ku86−/− skin, compared with control, all subcutaneous elements, including superficial collagen (sc), subcutaneous adipose (sa), and skeletal muscle (sm) are reduced and hair follicles and sebaceous glands (arrow in J and L) atrophied. (Bar = A–D 1 cm.) (E and F, ×65; G and H, ×200; I and K, ×35; J and L, ×175.)
Bone, epiphyses, skin, and hair follicles were observed for age-specific histological changes as both cohorts aged (Table 1). Bone was observed for osteopenia (28), epiphyses for closure, and skin and follicles for atrophy (29). No difference was observed for bone, epiphyses, skin, or hair follicles between 1- to 15-wk-old control and ku86−/− mice. However, ku86−/− mice, but not control mice, exhibited osteopenia by 37 wk (Fig. 2 E and F), epiphyseal closure by 22 wk (Fig. 2 G and H) and skin and follicular atrophy by 37 wk (Fig. 2 I–L). Control mice, greater than 70 wk of age, exhibited osteopenia, epiphyses closure, and skin and follicular atrophy (Table 1). Osteopenia and skin and follicular atrophy are commonly associated with advanced age in both mice and humans (28–30). Even though epiphyses closure is not considered to be characteristic of senescence, it is an age-specific change that results in cellular decline of function in reproductively-mature animals; therefore, the genetic process of epiphyses closure may be similar to, or overlap with, that of senescence.
The liver was examined for signs of senescence because genomic rearrangements accumulate in the liver as mice age (9). ALT was performed to assess hepatocellular necrosis as an indicator of liver damage. ALT concentration was not elevated for control and ku86−/− mice between 1–15 wk (Table 1). However, ku86−/− mice, but not control mice, exhibited mildly elevated ALT concentrations by 33 wk of age. Control mice greater than 70 wk of age exhibited mildly elevated ALT concentrations (Table 1).
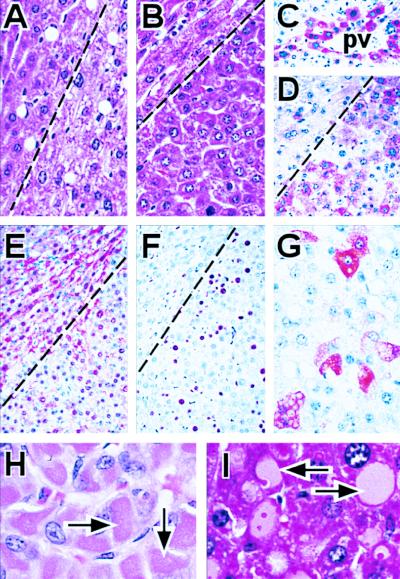
Livers were examined grossly and by histology for age-specific changes. Nodules appeared in the livers of both control and ku86−/− mice. These lesions are best observed microscopically, represent a response to unspecified hepatocellular injury, and represent proliferative activity that is potentially preneoplastic; however, there is no evidence that these lesions impact mortality. Hyperplastic foci were observed at a younger age for ku86−/− mice than control mice (Table 1). Histology shows the nodules compress normal liver for control (Fig. 3A) and ku86−/− mice (Fig. 3B). Special stains performed on a section at the border of normal liver and liver nodule from a ku86−/− mouse showed dysregulation, proliferation, and dedifferentiation of hepatocytes. Abnormalities were detected only in hepatocytes within the nodule. Improper expression of GS and FAH demonstrates hepatocellular dysregulation. GS is normally expressed in hepatocytes that are two deep from the portal veins (Fig. 3C); however, GS was expressed in nodular hepatocytes not bordering the portal veins (Fig. 3D). FAH is normally expressed in a diffuse pattern; however, FAH was expressed in a mosaic pattern for nodular hepatocytes (Fig. 3E). Proliferation of nodular hepatocytes was shown by a Ki-67 stain (stains cells in late G1, S, G2, and M; Fig. 3F). Additionally, dedifferentiation of nodular hepatocytes was shown by expression of MAFP, which is normally expressed in fetal, but not adult liver (Fig. 3G). Thus, these changes demonstrate this liver nodule is adenomatous.
Figure 3.
Age-specific changes in liver. (A and B) Liver nodules from (A) 110-wk-old control mouse and (B) 61-wk-old ku86−/− mouse. Normal part of liver (Upper Left) is compressed by nodule (Lower Right), separated by dashed line. (C) Expression of GS in normal part of liver. GS present in cells only two deep from portal vein (pv). (D–G) Dysregulation, proliferation, and dedifferentiation of cells in a liver nodule from a 61-wk-old ku86−/− mouse. (D) GS stain. Diffuse hepatocellular staining in liver nodule, but not normal liver. (E) FAH stain. Loss of FAH diffuse positivity resulting in mosaic expression pattern in liver nodule but not normal liver. (F) Anti-Ki-67 stain. Cellular proliferation in nodular hepatocytes, but not normal liver. (G) MAFP stain. MAFP is not normally expressed in adult hepatocytes. (H and I) Hepatocellular cytoplasmic inclusions (arrows) from: (H) 111-wk-old control mouse and (I) 65-wk-old ku86−/− mouse. (A–F ×115; G, ×210; H and I, ×420.)
In addition to hyperplastic foci, histology revealed another age-specific change in the liver. Both control (Fig. 3H) and ku86−/− (Fig. 3I) mice exhibit hepatocellular inclusions as they age with an earlier onset for ku86−/− mice (Table1). Cytoplasmic and nuclear inclusions in hepatocytes are characteristic of age-specific changes in mice (31).
DISCUSSION
Do ku86−/− mice truly exhibit an early onset of senescence? To answer this question, it is important to understand the distinction between aging and senescence. Aging encompasses all time-related changes that may have positive, neutral, or deteriorative effects. Senescence, as defined by Edward Masoro, describes only the “deteriorative changes [that occur] with time during postmaturational life that underlie an increasing vulnerability to challenges, thereby decreasing the ability of an organism to survive” (24). By this definition, ku86−/− mice exhibit an early onset of senescence.
Proposed theories for a genetic basis of evolutionary aging generally agree that senescence is not subject to natural selection (24). Data presented here do not dispute these theories; however, they suggest that a genetic process influenced by Ku86 determines the onset of senescence that would be subject to natural selection. It is interesting to note that for ku86−/− mice, the onset of age-specific mortality begins shortly after sexual maturity, possibly accounting for their reduced fecundity (P.H., unpublished results). Therefore, Ku86 increases fitness (ability to survive and reproduce) and would be subject to selective pressure.
Ku86, Chromosomal Metabolism, and Senescence. Data presented here support models that propose senescence is influenced by chromosomal metabolism. These models include accumulation of genetic damage induced by reactive oxygen species (32) and maintenance of telomeres (33) and/or ribosomal DNA (34, 35).
Analyses of senescence in the budding yeast, Saccharomyces cerevisiae offer intriguing possibilities for the relationship of DNA DSB repair and the onset of senescence. Yeast cells, deficient for either NHEJ or recombinational repair, have shortened lifespans. Sir proteins (Sir2, Sir3, Sir4), important for NHEJ (36), delay the onset of senescence by translocating from telomeres to the nucleolus (37) to reduce the accumulation of extrachromosomal ribosomal DNA (rDNA) circles (ERCs) (38). yKu may participate with the Sir complex by virtue of the Sir4–yKu70 association (36). Recent publications describe the yKu and Sir proteins translocating from telomeres to an induced DSB in DNA as a part of a Mec1, Rad9 response (39, 40). Thus, onset of senescence may be delayed by a cell-cycle response to DSB in rDNA. By comparison, a deficiency of recombinational repair prematurely translocates Sir3 from telomeres, however without accumulation of ERCs. Therefore, a general response to DSB and not just DSB in rDNA may regulate onset of senescence.
It will be interesting to determine whether a similar model applies to mammals. Perhaps the wide variance in lifespan within a species is partly caused by subtle differences or requirements within the population to repair DSB by NHEJ and recombinational repair. If this were true, then ku86−/− mice would be particularly sensitive to subtle differences or requirements in recombinational repair because of absence of NHEJ, which could contribute to their altered lifespan curve.
Ku86 and Cancer Incidence. Cancer is a cause of mortality for both ku86−/− and control cohorts. Even though cancer was observed earlier in the ku86−/− cohort, cancer incidence was reduced by 13-fold. This reduction may be simply a consequence of insufficient time for cancer to develop in ku86−/− mice because of their shortened lifespan. Alternatively, it is possible that deletion of Ku86 reduces cancer incidence in accord with the theory that senescence is antioncogenic (41), perhaps because of increased cell-cycle responses to inefficient repair of DSB. Several observations support the latter view. First, ku86−/− mice exhibit an early onset of a variety of age-specific conditions (including cancer) that are not usually observed in control mice until their age exceeds that of the longer-lived ku86−/− mice. Second, the mortality rate significantly declines for the most long lived 40% of the ku86−/− cohort compared with control, indicating diminished rate or occurrence of some lethal condition. Third, ku86−/− mice have a sufficiently long lifespan for a large fraction to develop cancer, exemplified by mice deleted for p53 (42), Atm (43), Brca2 (12, 44), Ku70 (15, 45), and some mismatch repair proteins (46).
Regardless whether deletion of Ku86 reduces cancer incidence, its deletion does not significantly increase cancer incidence. This observation is surprising given that deletion of DNA repair proteins frequently increase the risk of cancer (47). Most surprising is that mutation of Ku86 affects cancer risk differently than mutation of Ku70 (high incidence of CD4+ CD8+ T cell lymphoma) and suggests that one or both of these proteins work independently of the other for certain functions (15, 45). Independent function is possible because Ku86 is present at low levels in ku70−/− mice (15) and vice versa (16). T cell lymphoma may be caused by leaky T cell development that occurs exclusively in ku70−/− mice (15, 45). Alternatively, the difference in cancer incidence may be because of different genetic backgrounds; however, this seems unlikely because the genetic backgrounds are very similar (129Sv × C57BL/6).
Acknowledgments
We thank Drs. Mariana Yaneva and Greg Donoho for helpful discussions; Drs. Molly A. Bogue, David B. Roth, Mariana Yaneva, Greg Donoho, Brian Zambrowicz for critical review of the manuscript; Mrs. Shirley Jackson and Mr. Darrin Shiver for technical assistance; and Drs. James W. Campbell and Robert Tanguay for the generous gifts of anti-GS and anti-FAH, respectively. Lexicon Genetics Inc. and the National Cancer Institute (1RO1CA76317-01, to P.H.) supported this work.
ABBREVIATIONS
- WS
Werner’s syndrome
- DSB
double-strand break
- NHEJ
nonhomologous end joining
- Ku
the Ku86–Ku70 heterodimer
- GS
glutamine synthetase
- FAH
fumarylacetoacetatehydrolase
- MAFP
mouse α fetoprotein
- ALT
serum alanine aminotransferase concentration
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Martin G M. Birth Defects Orig Artic Ser. 1978;14:5–39. [PubMed] [Google Scholar]
- 2.Epstein C J, Martin G M, Schultz A L, Motulsky A G. Medicine. 1966;45:177–221. doi: 10.1097/00005792-196605000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Yu C-E, Oshima J, Fu Y-H, Wijsman E M, Hisama F, Alisch R, Matthews S, Nakura J, Miki T, Ousais S, et al. Science. 1996;272:258–262. doi: 10.1126/science.272.5259.258. [DOI] [PubMed] [Google Scholar]
- 4.Gray M D, Shen J-C, Kamath-Loeb A S, Blank A, Sopher B L, Martin G M, Oshima J, Loeb L A. Nat Genet. 1997;17:100–103. doi: 10.1038/ng0997-100. [DOI] [PubMed] [Google Scholar]
- 5.Huang S, Li B, Gray M D, Oshima J, Mian I S, Campisi J. Nat Genet. 1998;20:114–116. doi: 10.1038/2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin, G. M., Sprague, C. A. & Epstein, C. J. (1970) Lab. Invest.23, No. 1, 86–92. [PubMed]
- 7.Hayflick L. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 8.Nisitani S, Hosokawa M, Sasaki M S, Yasuoka K, Naiki H, Matsushita T, Takeda T. Mutat Res. 1990;1990:221–228. doi: 10.1016/0921-8734(90)90003-a. [DOI] [PubMed] [Google Scholar]
- 9.Dolle M E T, Giese H, Hopkins C L, Martus H-J, Hausdorff J M, Vijg J. Nat Genet. 1997;17:431–434. doi: 10.1038/ng1297-431. [DOI] [PubMed] [Google Scholar]
- 10.Lebel M, Leder P. Proc Natl Acad Sci USA. 1998;95:13097–13102. doi: 10.1073/pnas.95.22.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawley R S, Friend S H. Genes Dev. 1996;10:2383–2388. doi: 10.1101/gad.10.19.2383. [DOI] [PubMed] [Google Scholar]
- 12.Connor F, Bertwistle D, Mee P J, Ross G M, Swift S, Grigorieva E, Tybulewicz L J, Ashworth A. Nat Genet. 1997;17:423–430. doi: 10.1038/ng1297-423. [DOI] [PubMed] [Google Scholar]
- 13.Patel K J, Yu V P C C, Lee H, Corcoran A, Thistlethwaite F C, Evans M J, Colledge W H, Friedman L S, Ponder B A J, Venkitaraman A R. Mol Cell. 1998;1:347–357. doi: 10.1016/s1097-2765(00)80035-0. [DOI] [PubMed] [Google Scholar]
- 14.Morimatsu M, Donoho G, Hasty P. Cancer Res. 1998;58:3441–3447. [PubMed] [Google Scholar]
- 15.Gu Y, Seidl K J, Rathbun G A, Zhu C, Manis J P, van der Stoep N, Davidson L, Cheng H-L, Sekiguchi J M, Frank K, et al. Immunity. 1997;7:653–665. doi: 10.1016/s1074-7613(00)80386-6. [DOI] [PubMed] [Google Scholar]
- 16.Nussenzweig A, Chen C, Soares C d C, Sanchez M, Sokol K, Nussenzweig M C, Li G C. Nature (London) 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Sun Y, Frank K M, Dikkes P, Y, F, Seidl K J, Sekiguchi J M, Rathbun G A, Swat W, Wang J, Bronson R T, et al. Cell. 1998;95:891–902. doi: 10.1016/s0092-8674(00)81714-6. [DOI] [PubMed] [Google Scholar]
- 18.Frank K M, Sekiguchi J M, Seidl K J, Sat W, Rathbun G A, Cheng H-L, Davidson L, Kangaloo L, Alt F W. Nature (London) 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- 19.Lieber M R. Am J Pathol. 1998;153:1323–1332. doi: 10.1016/s0002-9440(10)65716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith G C M, Jackson S P. Genes Dev. 1999;13:916–934. doi: 10.1101/gad.13.8.916. [DOI] [PubMed] [Google Scholar]
- 21.Zhu B, Bogue M A, Lim D-S, Hasty P, Roth D B. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 22.Gu Y, Jin S, Gao Y, Weaver D T, Alt F W. Proc Natl Acad Sci USA. 1997;94:8076–8081. doi: 10.1073/pnas.94.15.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouyang H, Nussenzweig A, Kurimasa A, da Costa Soares V, Li X, Cordon-Cardo C, Li W-h, Cheong N, Nussenzweig M, Iliakis G, et al. J Exp Med. 1997;186:921–929. doi: 10.1084/jem.186.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masoro E J. In: Handbook of Physiology. Masoro E J, editor. Vol. 11. New York: Am. Phys. Soc. by Oxford Univ. Press; 1995. pp. 3–21. [Google Scholar]
- 25.Hyde S R, Stith R D, McCallum R E. Infect Immun. 1990;58:619–624. doi: 10.1128/iai.58.3.619-624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith, G. S., Walford, R. L. & Mickey, M. R. J. Natl. Cancer Inst.50, 1195–1213. [DOI] [PubMed]
- 27.Ershler W B, Longo D L. J Natl Cancer Inst. 1997;89:1489–1497. doi: 10.1093/jnci/89.20.1489. [DOI] [PubMed] [Google Scholar]
- 28.Weiss A, Arbell I, Steinhagen-Thiessen E, Silbermann M. Bone (NY) 1991;12:165–172. doi: 10.1016/8756-3282(91)90039-l. [DOI] [PubMed] [Google Scholar]
- 29.Fenske N A, Lober C W. J Am Acad Dermatol. 1986;15:571–585. doi: 10.1016/s0190-9622(86)70208-9. [DOI] [PubMed] [Google Scholar]
- 30.Osman A-H A, Bassiouni H, Koutri R, Nijs J, Geusens P, Dequeker J. Bone (NY) 1994;15:437–442. doi: 10.1016/8756-3282(94)90822-2. [DOI] [PubMed] [Google Scholar]
- 31.Percy D H, Barthold S W. Pathology of Laboratory Rodents and Rabbits. Ames: Iowa State Univ. Press; 1993. [Google Scholar]
- 32.Harman D. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 33.Lindsey, J., McGill, N. I., Lindsey, L. A., Green, D. K. & Cooke, H. J. (1991) Mutat. Res.4548. [DOI] [PubMed]
- 34.Strehler B L. Exp Geront. 1986;21:283–319. doi: 10.1016/0531-5565(86)90038-0. [DOI] [PubMed] [Google Scholar]
- 35.Sinclair D A, Mills K, Guarente L. Science. 1997;277:1313–1316. doi: 10.1126/science.277.5330.1313. [DOI] [PubMed] [Google Scholar]
- 36.Tsukamoto Y, Kato J-i, Ikeda H. Nature (London) 1997;388:900–902. doi: 10.1038/42288. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy B K, Gotta M, Sinclair D A, Mills K, McNabb D S, Murthy M, Pak S M, Laroche T, Gasser S M, Guarante L. Cell. 1997;89:381–391. doi: 10.1016/s0092-8674(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 38.Johnson F B, Sinclair D A, Guarente L. Cell. 1999;96:291–302. doi: 10.1016/s0092-8674(00)80567-x. [DOI] [PubMed] [Google Scholar]
- 39.Martin S G, Laroche T, Suka N, Grunstein M, Gasser S M. Cell. 1999;97:621–633. doi: 10.1016/s0092-8674(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 40.Mills K D, Sinclair D A, Guarente L. Cell. 1999;97:609–620. doi: 10.1016/s0092-8674(00)80772-2. [DOI] [PubMed] [Google Scholar]
- 41.Campisi J. J Am Geriatr Soc. 1997;45:482–488. doi: 10.1111/j.1532-5415.1997.tb05175.x. [DOI] [PubMed] [Google Scholar]
- 42.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A J, Butel J S, Bradley A. Nature (London) 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 43.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley J N, Ried T, Tagle D, et al. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 44.Friedman L S, Thistlethwaite F C, Patel K J, Yu C P C C, Lee H, Venkitaraman A R, Abel K J, Carlton M B L, Hunter S M, Colledge W H, et al. Cancer Res. 1998;58:1338–1343. [PubMed] [Google Scholar]
- 45.Li G C, Ouyang H, Li X, Nagasawa H, Little J B, Chen D J, Ling C C, Fuks Z, Cordon-Cardo C. Mol Cell. 1998;2:1–8. doi: 10.1016/s1097-2765(00)80108-2. [DOI] [PubMed] [Google Scholar]
- 46.Prolla T A, Baker S M, Harris A C, Tsao J-L, Yao X, Bronner C E, Zheng B, Gordon M, Reneker J, Arnheim N, et al. Nat Genet. 1998;18:276–279. doi: 10.1038/ng0398-276. [DOI] [PubMed] [Google Scholar]
- 47.Lengauer C, Kinzler K W, Vogelstein B. Nature (London) 1998;396:643. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]