Abstract
This study evaluated the utility of human blood micronucleated reticulocyte (MNCD71+) frequency measurement as a cytogenetic damage biomarker. The analytical methodology was flow cytometry in conjunction with a previously described three color fluorescence labeling technique that includes anti-CD71 to focus analyses on the most immature fraction of reticulocytes [Dertinger et al., Environ. Molec. Mutagen., 44:427–435 (2004)]. Blood specimens from fifty self-reported healthy adult volunteers were studied. In addition to MNCD71+ measurements, blood plasma folate and B12 levels were assessed, since these variables tend to influence other indices of cytogenetic damage. Time-course data are also provided for ten cancer patients undergoing treatment. For these subjects, frequency of MNCD71+ was measured immediately before therapy, and daily during the first week of chemotherapy and/or fractionated radiotherapy. For the group of healthy volunteers, the variables of age, and folate and B12 levels demonstrated no significant effect on MNCD71+ frequency. In addition, no difference was observed between pre-treatment MNCD71+ values for cancer patients compared with healthy volunteers. Regarding chemotherapy and/or partial body radiotherapy, elevated frequencies were observed upon initiation of treatment for 9 of the 10 patients studied. Maximal effects were observed three to five days following initiation of therapy. The largest increases in frequency of MNCD71+ (up to 25.9-fold) were observed in those patients exposed to anti-neoplastic drugs, presumably due to the systemic red marrow exposure provided by these agents. Taken together, these data support the hypothesis that the MNCD71+ endpoint represents a valuable biomarker of cytogenetic damage that does not require cell culture or microscopy-based scoring.
Keywords: Micronuclei, cytogenetic damage, DNA damage, flow cytometry, CD71 antigen
1. INTRODUCTION
In vivo cytogenetic damage assays have proven useful for myriad human health applications, including assessments of product safety, occupational and environmental exposures, dietary and life-style choices, and the effect of genetic polymorphisms on chromosomal integrity (1–6). Furthermore, the current gold standard techniques for estimating radiation dose in the absence of physical dosimetry are based on chromosomal damage endpoints, especially dicentric and micronucleus formation in peripheral blood lymphocytes (7–10). Whatever the application, it is well recognized that conventional assays are too cumbersome to efficiently assess large populations (11–12). Thus, there is a need for new methods capable of quantifying chromosomal damage that are significantly more amenable to application on a mass scale.
Whereas chromosomal effects measured in peripheral blood lymphocytes have traditionally involved cell culture in the presence of mitogens, erythrocyte-based micronucleus assays do not share this requirement. Rather, harvested cells are immediately ready for analysis following minimal processing. As the assay was originally described, target cells (i.e., reticulocytes, or Retics) were obtained from the bone marrow compartment of rodents (13–14). MacGregor and colleagues (15) demonstrated that micronucleated erythrocytes formed in the bone marrow persist in the peripheral circulation of mice, paving the way for mouse blood-based assays. Since the spleen of most other species eliminate micronucleated erythrocytes from circulation, the use of rat, canine, non-human primate, and human blood was considered counterintuitive. Indeed, although splenic filtration does dilute the effect observed in blood relative to the bone marrow compartment, much data have been reported establishing that circulating Retics may represent a suitable target population for studying genotoxicant-induced micronuclei, even for species with efficient splenic activity (16–29). It has been reported that assay sensitivity can be realized for blood-based analyses by restricting interrogation to the most immature fraction of Retics (16, 19, 21–22, 25), and also by increasing the number of Retics evaluated (30). Both of these modifications to the traditional assay are readily made using various flow cytometry-based techniques for measuring the frequency of micronucleated reticulocytes.
The current study was designed to address two deficiencies that exist in the human blood micronucleated reticulocyte literature: sparse information regarding the degree of inter-individual variation, and an incomplete understanding of the sensitivity and kinetics of the endpoint when exposure to known clastogenic agents occurs. To address these remaining questions, a flow cytometry-based assay developed by this laboratory was applied to blood specimens from 50 self-reported healthy volunteers in order to score: (i) young Retics (i.e., CD71-positive erythrocytes, or ReticCD71+), (ii) micronucleus-containing young reticulocytes (MNCD71+), and (iii) micronucleus-containing mature erythrocytes (MNCD71−). As folate and B12 levels have been shown to affect other endpoints of cytogenetic damage (31–32), the influence of these factors on MNCD71+ incidence was evaluated for the health volunteer population. Finally, flow cytometric analyses were performed for blood specimens obtained from cancer patients before and during chemotherapy and/or partial body radiotherapy.
2. MATERIALS AND METHODS
2.1. Reagents
Prototype Human MicroFlow®PLUS Kits (Litron Laboratories, Rochester, NY) contained anti-human-CD71-FITC, anti-human-CD61-PE, diluent solution, fixative, buffer solution, RNase stock solution, anti-rat-CD71-FITC, propidium iodide solution, and fixed malaria-infected rodent blood (“malaria biostandard”).
2.2. Blood Specimen Acquisition, Processing, and Storage
2.2.1. Healthy Subjects
This study was approved by the University of Rochester Institutional Review Board and the Western Institutional Review Board (WIRB, Olympia, WA); informed consent was acquired from each of the 50 healthy volunteers recruited from the University of Rochester Medical Center. This group comprised twenty-five males and twenty-five females, ranging in age from 21 to 63 years (mean ± std dev = 41 ± 11.4 yrs). Approximately 6 mL of venous blood was collected in green-capped vacutainers (sodium heparin). Approximately 5 mL of blood suspension was pelleted by centrifugation and the plasma was frozen for subsequent measurement of B12 and folate levels , while approximately 1 mL whole blood cell suspension was transferred to tubes containing 5 mL diluent solution. Aliquots (1 mL) of diluted blood were then forcefully injected into 15 mL polypropylene tubes containing 11 mL ultracold fixative solution (−80°C). The tubes were vortexed for several seconds and returned to a −80°C freezer. Fixed samples were stored at −80°C for at least one day before being transported to Litron Laboratories on dry ice. At Litron, samples were stored at −85°C until flow cytometric analysis.
2.2.2. Cancer Patients
This study was approved by the University of Rochester Institutional Review Board and the WIRB; informed consent was acquired from each of the cancer patients recruited from the James P. Wilmot Cancer Center, University of Rochester. As with specimens from healthy volunteers, these samples were collected into heparin vacutainers, diluted, and fixed into ultracold fixative. Blood was obtained just prior to initiation of therapy and again at approximately 24 hour intervals during the first week of treatment. For two patients, blood specimens were collected during the first week of induction chemotherapy without radiation (i.e., cisplatin plus docetaxel) and again four weeks later when concomitant chemotherapy and radiotherapy were initiated. Only those patients able to provide four or more specimens are presented here (n = 10). Subject characteristics are presented inTable 1. Fixed samples were stored at −80°C for at least one day before being transported to Litron Laboratories on dry ice. At Litron, samples were stored at −85°C until flow cytometric analysis. (Cancer patient blood pspecimens were not processed for plasma B12 or folate measurements.)
Table I.
Cancer Patient Information.
| ID | Sex | Age | Diagnosis | Treatment | Radiation Dose (Gy/day) |
|---|---|---|---|---|---|
| CP10/12 | M | 75 | NSCL Cancer IIIB | 1st Rx = cisplatin (75 mg/m2) plus docetaxel (75 mg/m2) | N/A |
| 2nd Rx = RT Mon. – Fri. (chest) plus Mon. & Thurs. docetaxel (12 mg/m) | 1.8 | ||||
| CP11/14 | M | 56 | NSCL Cancer IIIB | 1st Rx = cisplatin (75 mg/m2) plus docetaxel (75 mg/m2) | N/A |
| 2nd Rx = RT Mon. – Fri. (chest) plus Mon. & Thurs. docetaxel (12 mg/m) | 1.8 | ||||
| CP15 | M | 75 | Mesothelioma | RT Mon. – Fri. (chest) plus Mon. & Wed. & Fri. paclitaxel (20 mg/m2) | 1.8 |
| CP17 | M | 64 | NSCL Cancer, UC | RT Mon. – Fri. (chest) | 2.5 |
| CP21 | M | 71 | Multiple Myeloma | RT Mon. – Fri. (right and left femur, partial pelvis) | 3.0 |
| CP22 | F | 46 | NSCL Cancer IIIA | RT Mon. – Fri. (chest) plus Mon. & Wed. & Fri. paclitaxel (20 mg/m2) | 1.8 |
| CP23 | F | 52 | NSCL Cancer IIIA | RT Mon. – Fri. (chest) plus Mon. cisplatin (25 mg/m2) | 1.8 |
| CP24 | M | 52 | NSCL Cancer IIIB | RT Mon. – Fri. (chest) plus Tues. docetaxel (20 mg/m2) | 1.8 |
| CP25 | F | 61 | SCL Cancer | Tues. carboplatin (AUC 5) plus Tues. – Thurs. etoposide (100 mg/m2) | N/A |
| CP26 | M | 76 | SCL Cancer | Mon. carboplatin (AUC 5) plus Mon. – Wed. etoposide (75 mg/m2) | N/A |
Abbreviations: RT = radiotherapy (megavoltage external-beam photon radiation delivered with linear accelerators); UC = Undifferentiated Carcinoma; NSCL = non-small cell lung; SCL = small cell lung; AUC = area under the curve.
2.3. Measurement of Plasma Folate and B12 Levels
Plasma was allowed to thaw at room temperature and 200 μl of each specimen was added to 1 mL freshly prepared Borate-KCN buffer solution with dithiothreitol and ligand-labeled folate, according to instructions included with the Immulite® B12 and Folic Acid kits (Diagnostic Products Corp. Los Angeles, California). After a heat denaturation step, levels of B12 and folate were measured using the chemiluminescence-based Immulite Instrument (Diagnostic Products Corp.).
2.4. Micronuclei Fluorescence Labeling Technique
Fixed human blood specimens (2 mL) were added to tubes containing 12 mL ice-cold buffer solution and cells were collected by centrifugation. Washed cells were concentrated with vigorous decanting, and entire cell pellets were added to tubes containing 100 μl of an antibody/RNase solution (anti-human-CD71-FITC, anti-human-CD61-PE, RNase A), which was prepared according to MicroFlow kit specifications. Following successive 30 min incubations at 4°C and room temperature, cells were washed with 5 mL buffer containing 1% v/v fetal bovine serum. Finally, cells were resuspended in 1 to 1.5 mL working propidium iodide solution. Stained samples were stored at 4°C or on ice until analysis (same day).
2.5. Flow Cytometry Data Acquisition
At the beginning of each day of flow cytometric analysis, instrumentation and acquisition/analysis software parameters were calibrated based on the fluorescence of a biological standard—blood from Plasmodium berghei infected rats. A 20 μl aliquot of this fixed and washed cell suspension was incubated with 80 μl of antibody/RNase solution, according to manufacturer specifications. These samples guided photomultiplier tube voltage and electronic compensation settings to optimally resolve parasitized Retics (MNCD71+ mimicking cells), and also guided the position of the quadrant delineating erythrocytes with and without MN (33–34).
Data acquisition and analysis were performed using a FACSCalibur flow cytometer providing 488 nm excitation, running CellQuest software (v3.3) (instruments and software from BD-Biosciences, San Jose, CA). Anti-CD71-FITC, anti-CD61-PE, and propidium iodide fluorescence signals were detected in the FL1, FL2, and FL3 channels, respectively (log scale). Human blood specimens were labeled and resuspended with propidium iodide solution at high densities. Each specimen was analyzed two times, the first at a reduced cell density (50 to 100 μL high density specimen added to 400 μl ice cold propidium iodide solution). A second flow cytometric analysis was performed on the undiluted, high density sample using an FL1 threshold. Set sufficiently high, this had the effect of eliminating the majority of events (i.e., mature, CD71-negative erythrocytes) from consideration, facilitating rapid evaluation of immature Retics for the presence of micronuclei (35). Figure 1 andTable 2 provide more detail regarding these low and high density analyses.
Figure 1.
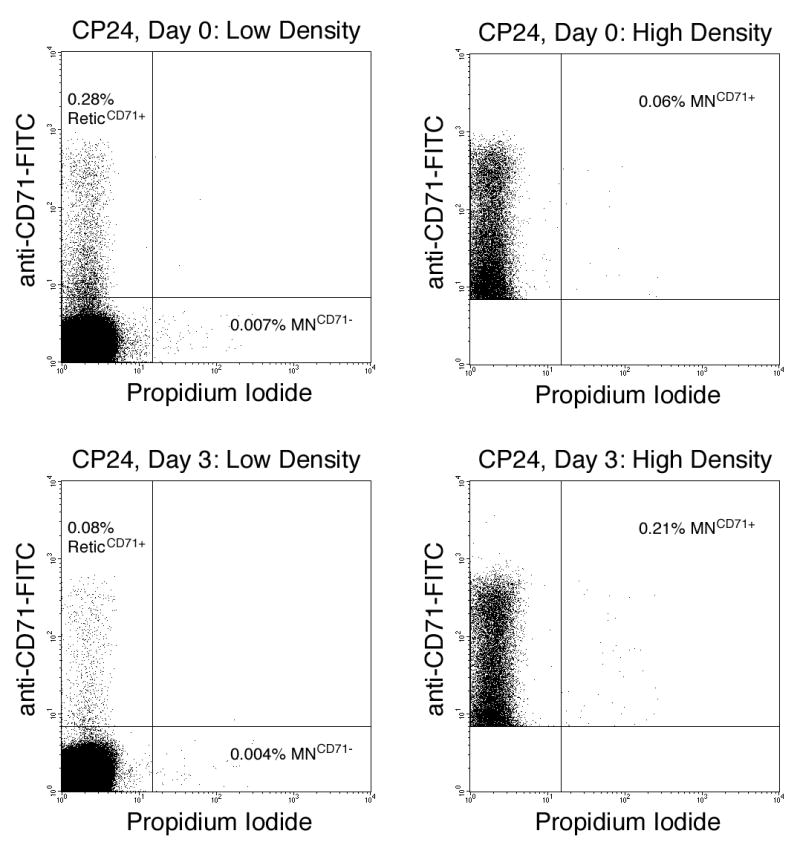
Bivariate graphs illustrate the fluorescent resolution of human erythrocyte subpopulations (nucleated cells and platelets have been excluded from these plots based on their light scatter and fluorescence staining characteristics). Upper left and upper right plots: low and high density analyses of Cancer Patient CP24. Lower left and lower right plots: low and high density analyses of Cancer Patient CP24, three days following initiation of treatment. The low density analyses occurred with a forward scatter threshold, facilitating enumeration of young reticulocytes (ReticCD71+), and also CD71-negative micronucleated erythrocytes (MNCD71−). The high density analyses occurred with FL1-thresholding, permitting rapid measurement of CD71-positive micronucleated reticulocytes (MNCD71+).
Table II.
Flow Cytometric Measurements.
| Analysis | Relative Cell Density | Threshold | Stop Mode | Statistic Measured | Endpoint |
|---|---|---|---|---|---|
| 1 | Low | FSC | 106 RBCs | %Retic
CD71+
= CD71+ reticulocytes among total RBCs
%MN CD71− = micronucleated CD71- RBCs among total CD71- RBCs |
Erythropoiesis function
Erythrophagocytic activity, primarily splenic |
| 2 | High | FL1 | 20,000 ReticCD71+ | %MN CD71+ = micronucleated CD71+ reticulocytes among total CD71+ reticulocytes | Genotoxicity (recent) |
Abbreviations: FSC = forward light scatter; RBCs = red blood cells; FL1 = fluorescence channel 1 (corresponds to anti-CD71-FITC fluorescence).
2.6. Statistical Analyses
The precision of flow cytometic data acquired by the method employed herein, as well as other analytical performance characteristics of the technique, have been described in detail previously (36–37). Statistical analyses were performed with JMP Software (v5, SAS Institute, Cary, NC). For healthy volunteers, the range, mean, and standard deviation for percent ReticCD71+, MNCD71−, and MNCD71+, and levels of folate and B12 in plasma were calculated. The variables age, and plasma folate and B12 levels were evaluated for possible effects on frequency of MNCD71+ with the JMP program’s regression analyses platform. The associated ANOVA tables partitioned the total variation into components, and compared the linear-fit equation with a simple mean response model. A p value < 0.05 was used to indicate a significant regression effect. Additionally, all cancer patients’ pre-treatment MNCD71+ frequencies were compared with healthy volunteers’ MNCD71+ values using a two-tailed, unpaired t-test (significance indicated by p < 0.05).
3. RESULTS AND DISCUSSION
3.1. Healthy Subjects
Specimens from 50 self-reported healthy adult volunteers were analyzed for ReticCD71+, MNCD71−, and MNCD71+ frequencies. Fluorescence profiles for these erythrocyte subpopulations are shown in representative bivariate plots (Figure 1), and frequency data are presented inTable 3. Whereas classically defined Retics (i.e., RNA-positive erythrocytes) are typically found in circulation of healthy adults on the order of 1% to 2%, the mean ReticCD71+ value was 0.10%. Previous studies with anti-human-CD71 (25–26) have resulted in similar findings, suggesting that it is approximately the youngest 10% to 20% of Retics that label with this immunoglobulin reagent. Presumably, the measurement of micronucleus frequencies in a young age cohort such as this is desirable to help minimize the impact that splenic filtration function has on genotoxicant-induced micronucleus frequencies.
Table III.
Healthy Adult Subjects, n = 50
| Parameter | Min. | Max. | Avg. | Std. Dev. | P value* |
|---|---|---|---|---|---|
| %ReticCD71+ | 0.02 | 0.55 | 0.10 | 0.093 | |
| %MNCD71+ | 0.04 | 0.28 | 0.12 | 0.062 | |
| %MNCD71− | 0.001 | 0.006 | 0.002 | 0.001 | |
| Age (years) | 21 | 63 | 41 | 11.4 | 0.3721 |
| B12 (pg/ml) | 99 | 990 | 392 | 205 | 0.4059 |
| Folate (ng/ml) | 6.4 | 57.1 | 22.6 | 11.0 | 0.6735 |
Linear regression analyses evaluating the factors Age, B12, and Folate on MNCD71+ frequency; these P values (> 0.05) indicate that these factors do not affect %MNCD71+.
The average frequency of MNCD71+ in the plasma of healthy subjects was similar (although somewhat lower) to previously reported values observed in the bone marrow or in the peripheral blood circulation of splenectomized human subjects (0.12% compared with approximately 0.2% to 0.3%, respectively) (3, 38–43). This is likely related to splenic filtration function, which may not be fully negated by restricting analyses to ReticCD71+ (44). Even so, when compared with MNCD71− values (mean = 0.002%), the average MNCD71+ frequency of 0.12% provides evidence that the analytical system described herein does effectively minimize the impact that spleen function has on peripheral blood micronucleus frequency.
Similar to other reports (3, 19, 41), we observed a considerable range of MNCD71+ frequencies in presumably healthy volunteers (0.04% – 0.28%). Linear regression analyses demonstrated that the variation in these baseline readings could not be correlated with age, or levels of B12 and folate. This is in contrast to other studies reporting significant effects for each of these factors on the incidence of micronuclei (3, 31–32). These discrepancies may be related to differences in target cells (erythroblasts versus lymphocytes) or some other factor(s), such as the relative homogeneity of these subjects, the great majority of which exhibited normal/healthy B12 and folate levels (all recruited from a University hospital setting). The design of biomonitoring studies will benefit from further characterizations of inter-individual variation.
3.2. Cancer Patients
As indicated byTable 1, the ten cancer patients provided a cross-section of treatment modalities for evaluating MNCD71+ responses. As expected, these therapies tended to reduce the frequency of ReticCD71+ within one to two days post-treatment (Figure 2, yy-axis). The proportion of red marrow space that was subjected to treatment was likely an important determinant for the range of responses observed. For instance, those patients that received systemic chemotherapy showed the greatest reduction in frequency of ReticCD71+. In fact, in the case of subjects CP10/12 and CP11/14, cisplatin plus docetaxel treatment caused peripheral blood frequency of ReticCD71+ to drop to a level that precluded accurate determination of MNCD71+ frequency beyond two or three days post-treatment.
Figure 2.
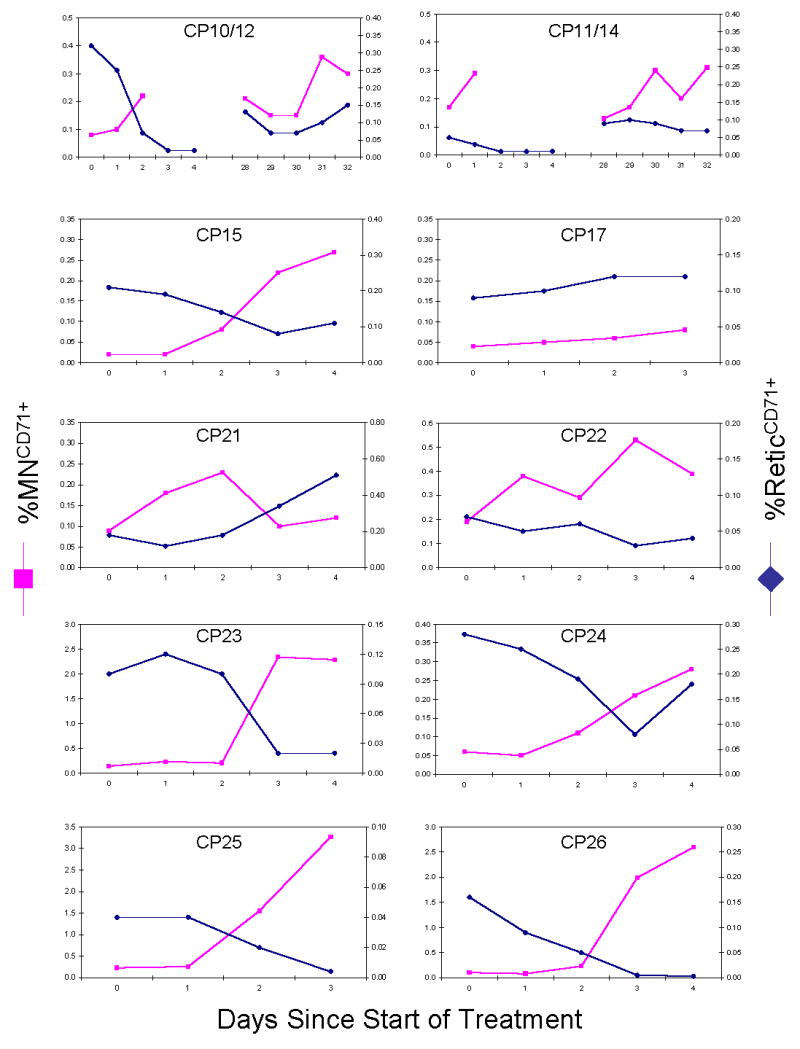
Percent CD71-positive micronucleated reticulocytes (MNCD71+) and percent CD71-positive reticulocytes (ReticCD71+) are graphed for each cancer therapy patient. Treatment details are shown inTable 1. While the frequency of ReticCD71+ was generally found to decline over the first week of treatment, higher incidences of MNCD71+ were observed.
Regarding cancer patients’ MNCD71+ frequencies, 9 of 10 patients demonstrated elevated levels over the course of therapy (see Figure 2, y-axis). As with ReticCD71+, MNCD71+ frequencies are expected to be influenced by the proportion of red marrow space exposed. Thus, in the case of radiotherapy, it is likely that micronucleus induction is muted to the extent that non-exposed sites of erythropoiesis supplied the peripheral blood compartment with MNCD71+ at a baseline frequency. This likely explains the nil effect that radiotherapy had on CP17’s MNCD71+ frequency. This subject exhibited no reduction to %ReticCD71+, suggesting the presence of little or no active red marrow space in the treatment field. Conversely, the higher micronucleus responses observed for patients undergoing chemotherapy can likely be attributed to the large amount of red marrow exposure achieved (e.g., 25.9-fold for CP26). Subject CP21 was an exceptional case in that the frequency of MNCD71+ was observed to increase over the week of therapy, even as the frequency of ReticCD71+ values increased markedly. It is tempting to speculate that this patient’s diagnosis of multiple myeloma, a condition that impacts the red marrow space, may have contributed to this unusual response to treatment.
Despite differences in health status and mean ages, cancer patients did not exhibit a statistically significant difference in pre-treatment MNCD71+ frequency relative to the group of 50 self-reported healthy volunteers.
3.3. Conclusions
Data presented herein support the hypothesis that the frequency of MNCD71+ in human peripheral blood circulation can be used to index recent chromosomal damage induced by agents and dose levels/intensities used in the cancer clinic. This is made possible by a high throughput analytical system capable of restricting analyses to the most immature fraction of Retics. The rarity of ReticsCD71+, coupled with the low rate of micronucleus formation, makes high analysis rates an essential characteristic. These data, coupled with recent reports (27–29), suggest that ReticsCD71+ may represent a viable alternative to lymphocyte-based analyses. Retics offer several advantages, including a low blood volume requirement, no need for cell culture, and compatibility with an automated scoring methodology. Of course, when exposures occur acutely, the short-lived nature of genotoxicant-induced MNCD71+ relative to more persistent expression of chromosome damage in lymphocytes may favor analysis of one cell type over the other, depending on the experimental question being asked. Since MNCD71+ induction was evident in clinical specimens originating from the cancer clinic, it will be important to assess the utility of the endpoint for studying the genotoxic consequences of occupational, environmental, nutritional, and/or genetic factors. Use of this method for biomonitoring applications such as these is less certain, but reports by Grawé et al. (28) and Stopper et al. (29) are encouraging.
Acknowledgments
This work was supported in part by grants from the National Institute of Health/National Institute of Environmental Health Sciences (S.D.D., No. R44ES010752) and by the Center for Medical Countermeasures against Radiation Program, a grant from the National Institute of Health/National Institute of Allergy and Infectious Disease (Y.C., No. U19AI067733-010004). The contents are the sole responsibility of the authors, and do not necessarily represent the official views of NIEHS or NIAID. The authors would like to thank the volunteers who generously donated blood specimens; we also would like to extend our appreciation to those Litron and URMC staff who critiqued this manuscript and supplied many valuable suggestions that enhanced its readability, especially Drew Tometsko and Amy Huser. Disclosure statement: Litron Laboratories holds patents pertaining to the flow cytometric analysis of micronucleated erythrocytes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anwar WA, Salama SI, Serafy MM, Hermida S, Hafez AS. Chromosomal aberrations and micronucleus frequency in nurses occupationally exposed to cytotoxic drugs. Mutagenesis. 1994;9:315–7. doi: 10.1093/mutage/9.4.315. [DOI] [PubMed] [Google Scholar]
- 2.Ilyinskikh NN, Eremich AV, Ivanchuk II, Ilyinskikh EN. Micronucleus test of erythrocytes and lymphocytes in the blood of the people living in the radiation pollution zone as a result of the accident at the Siberian chemical plant on April 6, 1993. Mutat Res. 1996;36:173–8. doi: 10.1016/s0165-1161(96)90252-6. [DOI] [PubMed] [Google Scholar]
- 3.MacGregor JT, Wehr CM, Hiatt RA, Peters B, Tucker JD, Langlois RG, et al. “Spontaneous” genetic damage in man: evaluation of interindividual variability, relationship among markers of damage, and influence of nutritional status. Mutat Res. 1997;377:125–35. doi: 10.1016/s0027-5107(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 4.Maffei F, Angelini S, Forti GC, Lodi V, Violante FS, Mattioli S, et al. Micronuclei frequencies in hospital workers occupationally exposed to low levels of ionizing radiation: influence of smoking status and other factors. Mutagenesis. 2002;17:405–9. doi: 10.1093/mutage/17.5.405. [DOI] [PubMed] [Google Scholar]
- 5.Fenech M. Biomarkers of genetic damage for cancer epidemiology. Toxicology. 2002;181–182:411–416. doi: 10.1016/s0300-483x(02)00480-8. [DOI] [PubMed] [Google Scholar]
- 6.Bonassi S, Ugolini D, Kirsh-Volders M, Strömberg U, Vermeulen R, Tucker JD. Human population studies with cytogenetic biomarkers: review of the literature and future prospectives. Environ Molec Mutagen. 2005;45:258–70. doi: 10.1002/em.20115. [DOI] [PubMed] [Google Scholar]
- 7.Bender MA, Gooch PC. Somatic chromosome aberrations induced by human whole-body irradiation: The “Recuplex” criticality accident. Radiat Res. 1966;29:568–82. [PubMed] [Google Scholar]
- 8.International Atomic Energy Agency. Technical Report 260. Vienna: IAEA; 1986. Dosimetry: chromosome aberration analysis for dose assessment. [Google Scholar]
- 9.Fenech M, Perepetskaya G, Mikhalevich L. A more comprehensive application of the micronucleus technique for biomonitoring of genetic damage rates in human populations--experiences from the Chernobyl catastrophe. Environ Mol Mutagen. 1997;30:112–8. [PubMed] [Google Scholar]
- 10.Amundson SA, Bittner M, Meltzer P, Trent J, Fornace A., Jr Biological indicators for the identification of ionizing radiation exposure in humans. Expert Rev Mol Diagn. 2001;1:89–97. doi: 10.1586/14737159.1.2.211. [DOI] [PubMed] [Google Scholar]
- 11.Thierens H, Vral A, De Ridder L, Touil N, Kirsch-Volders M, Lambert T, et al. Inter-laboratory comparison of cytogenetic endpoints for the biomonitoring of radiological workers. Int J Radiat Biol. 1999;75:23–34. doi: 10.1080/095530099140771. [DOI] [PubMed] [Google Scholar]
- 12.Mettler FA, Jr, Voelz GL. Major radiation exposure--what to expect and how to respond. N Engl J Med. 2002;346(20):1554–61. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 13.Heddle J. A rapid in vivo test for chromosome damage. Mutat Res. 1973;18:187–90. doi: 10.1016/0027-5107(73)90035-3. [DOI] [PubMed] [Google Scholar]
- 14.Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 15.MacGregor J, Wehr C, Gould G. Clastogen-induced micronuclei in peripheral blood erythrocytes: the basis of an improved micronucleus test. Environ Mutagen. 1980;2:509–14. doi: 10.1002/em.2860020408. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi M, Kodama Y, Awogi T, Suzuki T, Asita AO, Sufuni T. The micronucleus assay using peripheral blood reticulocytes from mitomycin C- and cyclophosphamide-treated rats. Mutat Res. 1992;278:209–13. doi: 10.1016/0165-1218(92)90236-s. [DOI] [PubMed] [Google Scholar]
- 17.Asanami S, Shimono K, Sawamoto O, Kurisu K, Uejima M. The suitability of rat peripheral blood in subchronic studies for the micronucleus assay. Mutat Res. 1995;347:73–78. doi: 10.1016/0165-7992(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 18.Wakata A, Miyamae Y, Sato S, Suzuki T, Morita T, Asano N, et al. Evaluation of the rat micronucleus test with bone marrow and peripheral blood: summary of the 9th collaborative study by CSGMT/JEMS. MMS. Collaborative Study Group for the Micronucleus Test. Environmental Mutagen Society of Japan. Mammalian Mutagenicity Study Group. Environ Molec Mutagen. 1998;32:84–100. [PubMed] [Google Scholar]
- 19.Abramsson-Zetterberg L, Zetterberg G, Bergqvist M, Grawé J. Human cytogenetic biomonitoring using flow-cytometric analysis of micronuclei in transferrin-positive immature peripheral blood erythrocytes. Environ Molec Mutagen. 2000;36:22–31. doi: 10.1002/1098-2280(2000)36:1<22::aid-em4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Abramsson-Zetterberg L, Grawé J, Zetterberg G. The micronucleus test in rat erythrocytes from bone marrow, spleen and peripheral blood: the response to low doses of ionizing radiation, cyclophosphamide and vincristine determined by flow cytometry. Mutat Res. 1999;423:113–124. doi: 10.1016/s0027-5107(98)00233-4. [DOI] [PubMed] [Google Scholar]
- 21.Torous DK, Dertinger SD, Hall NE, Tometsko CR. Enumeration of micronucleated reticulocytes in rat peripheral blood: a flow cytometric study. Mutat Res. 2000;465:91–9. doi: 10.1016/s1383-5718(99)00216-8. [DOI] [PubMed] [Google Scholar]
- 22.Torous DK, Hall NE, Murante FG, Gleason SE, Tometsko CR, Dertinger SD. Comparative scoring of micronucleated reticulocytes in rat peripheral blood by flow cytometry and microscopy. Tox Sci. 2003;74:309–14. doi: 10.1093/toxsci/kfg143. [DOI] [PubMed] [Google Scholar]
- 23.Hamada S, Sutou S, Morita T, Wakata A, Asanami S, Hosoya S, et al. Evaluation of the rodent micronucleus assay by a 28-day treatment protocol: Summary of the 13th collaborative study by the collaborative study group for the micronucleus test (CSGMT)/Environmental Mutagen Society of Japan (JEMS)—Mammalian Mutagenicity Study Group (MMS) Environ Molec Mutagen. 2001;37:93–110. doi: 10.1002/em.1017. [DOI] [PubMed] [Google Scholar]
- 24.Hynes GM, Torous DK, Tometsko CR, Burlinson B, Gatehouse DG. The single laser flow cytometric micronucleus test: a time course study using colchicines and urethane in rat and mouse peripheral blood and acetaldehyde in rat peripheral blood. Mutagenesis. 2002;17:15–23. doi: 10.1093/mutage/17.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Dertinger SD, Torous DK, Hall NE, Murante FG, Gleason SE, Miller RK, et al. Enumeration of micronucleated CD71-positive human reticulocytes with a single-laser flow cytometer. Mutat Res. 2002;515:3–14. doi: 10.1016/s1383-5718(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 26.Dertinger SD, Chen Y, Miller RK, Brewer KJ, Smudzin T, Torous DK, et al. Micronucleated CD71-positive reticulocytes: A blood-based endpoint of cytogenetic damage in humans. Mutat Res. 2003;542:77–85. doi: 10.1016/j.mrgentox.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Offer T, Ho E, Traber MG, Bruno RS, Kuypers FA, Ames BN. A simple assay for frequency of chromosome breaks and loss (micronuclei) by flow cytometry of human reticulocytes. FASEB J. 2005;19:485–487. doi: 10.1096/fj.04-2729fje. [DOI] [PubMed] [Google Scholar]
- 28.Grawé J, Biko J, Lorenz R, Reiners C, Stopper H, Vershenya S, et al. Evaluation of the reticulocyte micronucleus assay in patients treated with radioiodine for thyroid cancer. Mutat Res. 2005;583:12–25. doi: 10.1016/j.mrgentox.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Stopper H, Hempel K, Reiners C, Vershenya S, Lorenz R, Vukicevic V, et al. Pilot study for comparison of reticulocyte-micronuclei with lymphocyte-micronuclei in human biomonitoring. Toxicol Letters. 2005;156:351–360. doi: 10.1016/j.toxlet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Schlegel R, MacGregor JT. The persistence of micronucleated erythrocytes in the peripheral circulation of normal and splenectomized Fisher 344 rats: Implications for cytogenetic screening. Mutat Res. 1984;127:169–74. doi: 10.1016/0027-5107(84)90018-6. [DOI] [PubMed] [Google Scholar]
- 31.Fenech M. Important variables that influence base-line micronucleus frequency in cytokinesis-blocked lymphocytes a biomarker for DNA damage in human populations. Mutat Res. 1998;404:155–165. doi: 10.1016/s0027-5107(98)00109-2. [DOI] [PubMed] [Google Scholar]
- 32.Bonassi S, Fenech M, Lando C, Lin Y-P, Ceppi M, Chang WP, et al. The Human MicroNucleus project. International data base comparison for results with the cytokinesis-block micronucleus assay in human lymphocytes. I. Effect of laboratory protocol, scoring criteria, and host factors on the baseline frequency of micronuclei. Environ Molec Mutagen. 2001;37:31–45. [PubMed] [Google Scholar]
- 33.Tometsko AM, Torous DK, Dertinger SD. Analysis of micronucleated cells by flow cytometry. 1. Achieving high resolution with a malaria model. Mutat Res. 1993;292:129–135. doi: 10.1016/0165-1161(93)90140-u. [DOI] [PubMed] [Google Scholar]
- 34.Dertinger SD, Torous DK, Hall NE, Tometsko CR, Gasiewicz TA. Malaria-infected erythrocytes serve as biological standards to ensure reliable and consistent scoring of micronucleated erythrocytes by flow cytometry. Mutat Res. 2000;464:195–200. doi: 10.1016/s1383-5718(99)00183-7. [DOI] [PubMed] [Google Scholar]
- 35.Dertinger SD, Camphausen K, MacGregor JT, Bishop ME, Torous DK, Avlasevich S, et al. Three-Color Labeling Method for Flow Cytometric Measurement of Cytogenetic Damage in Rodent and Human Blood. Environ Molec Mutagen. 2004;44:427–35. doi: 10.1002/em.20075. [DOI] [PubMed] [Google Scholar]
- 36.Torous D, Asano N, Tometsko C, Sugunan S, Dertinger S, Morita T, Hayashi M. Performance of flow cytometric analysis for the micronucleus assay—a reconstruction model using serial dilutions of malaria-infected cells with normal mouse peripheral blood. Mutagenesis. 2006;21:11–13. doi: 10.1093/mutage/gei053. [DOI] [PubMed] [Google Scholar]
- 37.Dertinger SD, Bishop ME, McNamee JP, Hayashi M, Suzuki T, Asano N, Nakajima M, Saito J, Moore M, Torous DK, MacGregor JT. Flow cytometric analysis of micronuclei in peripheral blood reticulocytes: I. Intra- and inter-laboratory comparison with microscopic scoring. Tox Sci. 2006 doi: 10.1093/toxsci/kfl075. in press. [DOI] [PubMed] [Google Scholar]
- 38.Goetz P, Sram RJ, Dohnalova J. Relationship between experimental results in mammals and man: cytogenetic analysis of bone marrow injury induced by a single dose of cyclophosphamide. Mutat Res. 1975;31:247–54. doi: 10.1016/0165-1161(75)90007-2. [DOI] [PubMed] [Google Scholar]
- 39.Krogh Jensen M, Nyfors A. Cytogenetic effect of methotrexate on human cells in vivo. Mutat Res. 1979;64:339–43. doi: 10.1016/0165-1161(79)90126-2. [DOI] [PubMed] [Google Scholar]
- 40.Abe T, Isemura T, Kikuchi Y. Micronuclei in human bone marrow cells: evaluation of the micronucleus test using human leukemia patients treated with antileukemic agents. Mutat Res. 1984;130:113–20. doi: 10.1016/0165-1161(84)90111-0. [DOI] [PubMed] [Google Scholar]
- 41.Smith DF, MacGregor JT, Hiatt RA, Hooper NK, Wehr CM, Peters B, et al. Micronucleated erythrocytes as an index of cytogenetic damage in humans: demographic and dietary factors associated with micronucleated erythrocytes in splenectomized subjects. Cancer Res. 1990;50:5049–5054. [PubMed] [Google Scholar]
- 42.Tucker JD, Vanderlaan M, Kwan TC, Moore DH, II, Felton JS. Effects of diet and folate on levels of micronucleated polychromatic erythrocytes. Mutat Res. 1993;301 :19–26. doi: 10.1016/0165-7992(93)90051-v. [DOI] [PubMed] [Google Scholar]
- 43.Zuniga G, Torres-Bugarin O, Ramirez-Munoz MP, Delgado-Lamas JL, De Loza-Saldana R, Cantu JM. Micronucleated erythrocytes in splenectomized patients with and without chemotherapy. Mutat Res. 1996;361:107–12. doi: 10.1016/s0165-1161(96)90244-7. [DOI] [PubMed] [Google Scholar]
- 44.MacGregor JT, Bishop ME, McNamee JP, Hayashi M, Asano N, Wakata A, Nakajima M, Aidoo A, Moore MM, Dertinger SD. Flow cytometric analysis of micronuclei in peripheral blood reticulocytes: II. An efficient method of monitoring chromosomal damage in the rat. Tox Sci. 2006 doi: 10.1093/toxsci/kfl076. in press. [DOI] [PubMed] [Google Scholar]


