Abstract
Nitric oxide (NO) and reactive oxygen species (ROS) act independently as well as cooperatively to induce neuronal death in acute neurological disorders. Inhibition of neuronal nitric oxide synthase (nNOS) and inhibition of lipid peroxidation induced by ROS have both been proposed as neuroprotective strategies in stroke and trauma. Recently, in our laboratory, the combination of the two strategies was found to be synergistic in reducing neuronal damage. Here, we report that BN 80933 [(S)-N-{4-[4-[(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-yl)carbonyl]-1-piperazinyl]phenyl}-2-thiophenecarboximidamide], a compound that combines potent antioxidant and selective nNOS inhibitory properties in vitro, affords remarkable neuronal protection in vivo. Intravenous administration of BN 80933 significantly reduced brain damage induced by head trauma in mice, global ischemia in gerbils, and transient focal ischemia in rats. Treatment with BN 80933 (0.3–10 mg/kg) significantly reduced infarct volume (>60% protection) and enhanced behavioral recovery in rats subjected to transient (2-h) middle cerebral artery occlusion and 48-h or 7-day reperfusion. Furthermore, treatment with BN 80933 commencing up to 8 h after the onset of ischemia resulted in a significant improvement of neurological outcome. All these results indicate that BN 80933 represents a class of potentially useful therapeutic agents for the treatment of stroke or trauma and possibly neurodegenerative disorders that involve both NO and ROS.
Considerable research effort has been devoted to the development of neuroprotective agents to save neurons from the biochemical and metabolic consequences of ischemic brain injury. The different neuroprotective strategies are based on the identification of the effectors and the sequence of events that lead to neuronal death. Each step of the ischemic cascade is a potential target for therapeutic intervention. Among the initial events are the widespread neuronal depolarization and massive release of glutamate, which activates N-methyl-d-aspartate (NMDA) receptors, leading to calcium influx. Numerous secondary processes occur thereafter to amplify ischemic neuronal damage, including activation of proteases, phospholipases, nitric oxide synthases (NOSs), and protein kinases and the generation of reactive oxygen species (ROS). Approaches concentrating on blocking presynaptic glutamate release, glutamate receptors, or voltage-sensitive channels have so far failed to demonstrate clinical efficacy (1–4). Because the mechanisms of neurotoxicity are multifactorial, involving numerous interdependent and sequential processes, the monomodal nature of such therapies may partly explain their lack of clinical efficacy. In this context, new therapeutic strategies focused on multiple downstream events may provide efficacy and a wider therapeutic window for effective intervention.
We have recently tested the association of NOS inhibitors and antioxidants in transient focal ischemia in rats and found a synergistic reduction of infarct size when we used a combined treatment of NG-nitro-l-arginine (l-NA), a nonselective NOS inhibitor, and the anti-oxidant/superoxide anion scavenger di(tert-butyl)hydroxybenzoic acid (5). Both NO and ROS are potent effectors of the ischemic cascade and act independently as well as cooperatively to induce cellular damage (6–12). Stimulation of excitatory amino acid (e.g., NMDA) receptors results in activation of neuronal NOS (nNOS) (13), and their persistent stimulation may lead to overproduction of NO, with deleterious consequences. ROS arising from infiltrating activated leukocytes, neurons, and astroglia during ischemia–reperfusion injury (14–16) promote lipid peroxidation. Our results suggested that controlling both ROS and NO is more efficient than the inhibition of only one of these (5). The combined treatment protected rat brain from the ischemic damage even when delayed up to 4 h after the occlusion, indicating the possibility of a time-window for therapeutic intervention in stroke patients. On the basis of these observations, we have developed a therapeutic concept of combining, in the same molecule, activities inhibiting nNOS and lipid peroxidation. Here, we describe the in vitro and in vivo neuroprotective properties of BN 80933 (Fig. 1), a representative of this class of compounds.
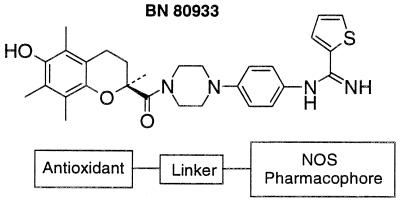
Figure 1.
Chemical structure of BN 80933. The antioxidant moiety (Trolox) is linked to the NOS inhibitor pharmacophore, thiopheneamidine, by a piperidine residue.
MATERIALS AND METHODS
BN 80933.
The structure of BN 80933 [(S)-N-{4[4-[(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-yl)carbonyl]-1-piperazinyl]phenyl}-2-thiophenecarboximidamide] is shown in Fig. 1. Note that the antioxidant group (vitamin E or Trolox derivative) is coupled by a piperazine linker to a “NOS” pharmacophore (thiopheneamidine). The preparation and purification of the compound will be described elsewhere.
In Vitro Enzymatic Assay (Ki Determination).
Activity of NOS was determined by monitoring the conversion of l-[3H]arginine into l-[3H]citrulline (17). Endothelial NOS (eNOS) was a bovine endothelial recombinant enzyme purchased from Spibio (Cayman Chemical), nNOS was obtained from rat cerebellum homogenate, and inducible NOS (iNOS) was obtained from the murine macrophage cell line J774 after 18-h incubation in culture medium containing lipopolysaccharide (1 μg/ml) and interferon γ (25 units/ml).
In Vitro Functional Selectivity for nNOS in Rat.
Organ chamber experiments were used to examine the tissue permeability properties of BN 80933 and its functional selectivity on the neuronal versus the endothelial isoenzyme. Inhibition of eNOS and nNOS was, respectively, assayed as change in tone in the endothelium-dependent relaxation of the rat aorta in response to carbachol and in the nitrergic relaxation elicited by electrical field stimulation of the rat gastric fundus, as described previously (18).
In Vitro Antioxidant Properties.
For determination of the ability to inhibit iron-dependent lipid peroxidation, membrane brain homogenate (0.13 g/ml) from the cerebral cortex of male Sprague–Dawley rats (200–250 g; Charles River, St Aubin les Elboeuf, France) was exposed to 100 μM Fe2+ plus 400 μM ascorbic acid. The resulting lipid peroxidation was evaluated by the formation of malonaldehyde (19), the main decomposition product of peroxides derived from polyunsaturated fatty acids, using the chromogenic reagent N-methyl-2-phenylindole, which reacts with malonaldehyde to yield a stable chromophore with maximal absorbance at a wavelength of 586 nm.
Transient Focal Ischemia in Rat.
Transient ischemia was induced in isoflurane (1% in O2) -anaesthetized male Sprague–Dawley rats (300–330 g, Charles River, France) by 2-h occlusion of the left middle cerebral artery (MCAO), as previously described (5). After ligation of the pterygopalatine artery, a 1.8- to 2-cm length of a 4-0 monofilament nylon suture (ethilon; Ethicon, Brussels) coated with poly(l-lysine) (Sigma) was inserted from the internal carotid artery, effectively occluding the middle cerebral artery. After 2 h the filament was retracted to allow reperfusion of the ischemic region. Forty-eight hours after MCAO, the brains were sliced into 2-mm coronal sections and stained with 2,3,5-triphenyltetrazolium chloride for 20 min at room temperature. The infarct volumes were calculated by integration of the area of infarct and the distance between slices by using an image analysis system (Biocom, Paris). To determine the dose–response relationship, BN 80933 was administered as an i.v. bolus (0.1, 0.3, 1, or 10 mg/kg; 2 ml/kg) at 4 and 24 h after the onset of MCAO. For analysis of duration of the neuroprotective action, the size of the infarct was estimated 7 days after MCAO by using thionin and alizarin staining. For determination of the therapeutic time window, BN 80933 (10 mg/kg) was administered 1, 4, 6 or 8, and 24 h after MCAO.
Neurological Deficits.
Rats submitted to 2-h focal ischemia were tested for neurological deficit by using a 15-point neurological deficit scoring system (0 = no deficit), modified from ref. 20. This scale involves activity level (2-point scale), a rope platform test (3-point scale), motility (5-point scale), and pain reflex (1-point scale). A score of 15 was attributed to animals that died for reasons unrelated to subarachnoid hemorrhage secondary to suture-induced rupture of the internal carotid artery, as assessed by postmortem examination.
Global Ischemia in Mongolian Gerbils.
Transient global ischemia was induced in isoflurane (2% in O2) -anaesthetized male Mongolian gerbils (80–100 g; Tumblebrook Farm, West Brookfield, MA) by bilateral occlusion of the common carotid arteries (21). An atraumatic clasp was loosely placed around each carotid artery and tightened for 5 min. At the end of the ischemic period, blood flow was restored and the patency of the carotid arteries was checked by direct visualization. Sham-operated animals were subjected to simple exposure of the carotid arteries. BN 80933 (1 mg/kg, i.v.) was administered 5 min, 5 h, 24 h, and 48 h after reperfusion. Body temperature was maintained at 38°C for the first 5 h. Seven days after global ischemia, the gerbils were killed and the brains were sliced into coronal sections of 20 μm and stained with cresyl violet (Nissl stain). For each animal, measurements were made with an image analyzer (Imstar, Paris, France) of the area of preserved neurons from both hippocampi in three coronal sections corresponding to CA1 sector from the hippocampal fissure to the alveus.
Traumatic Brain Injury.
Male CD1 mice (22–26 g), lightly anaesthetized with isoflurane (about 3% in air), were subjected to head injury by means of a falling weight (50 g) (22). BN 80933 was injected i.v. (1, 3, or 10 mg/kg, 0.1 ml/kg) 5 min after trauma. One hour after injury, the neurological status of the mice was evaluated (22), according to a “grip test,” which measured the length of time they could remain on a horizontal bar 60 cm long suspended 40 cm above a table within a 30-sec time period.
Data Analysis.
Data are expressed as mean ± SEM or 95% confidence limits. Statistical comparisons were carried out with Fisher’s test (two-tailed), the Mann–Whitney test (two-tailed), Dunnett’s test (two-tailed), or Student’s t test (two-tailed) as appropriate.
RESULTS
Inhibition of NOS Enzyme Activities.
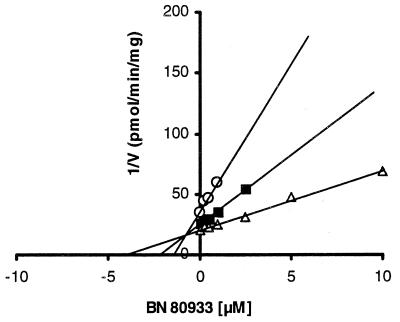
Both BN 80933 (Ki = 0.92 μM) and l-NA (Ki = 0.21 μM) were found to inhibit the activity of nNOS in a manner that was competitive with l-arginine (Table 1; Fig. 2). l-NA was 1/5 as potent an inhibitor of eNOS as of nNOS, whereas BN 80933 selectively inhibited nNOS, being 1/120 as potent on eNOS as on nNOS. In addition, unlike l-NA, BN 80933 exerted no significant effect on iNOS up to a concentration of 300 μM (Table 1).
Table 1.
In vitro inhibition of NOS activities by BN 80933
| Inhibitor |
Ki, μM
|
||
|---|---|---|---|
| nNOS | eNOS | iNOS | |
| BN 80933 | 0.92 | 111 | >300 |
| l-NA | 0.21 | 1.15 | 12.6 |
For NOS activity assay, the standard reaction mixture contained 100 mM Hepes (pH 7.4), 2.5 mM CaCl2, 10 μg/ml calmodulin, 2 mM dithiothreitol, 2 mM NADPH, 10 μM FAD, 10 μM FMN, and 10 μM tetrahydrobiopterin in a final volume of 200 μl, l-[3H]arginine (62.5 nM for nNOS or 100 nM for iNOS and eNOS, specific activity 40–50 Ci/mmol; Amersham), l-arginine as indicated, l-NA or BN 80933 at various concentrations, and 50 μl of the enzyme. Reaction mixtures for iNOS were similar, but CaCl2 and calmodulin were omitted. Reaction was initiated by addition of the enzyme. After a 15-min incubation at 37°C the reaction was stopped by 2 ml of 20 mM Hepes (pH 5.5)/2 mM EDTA. The reaction mixture was applied to a 1-ml column of Dowex AG50WX-8 (Na+ form). l-[3H]citrulline was quantified by liquid scintillation counting of the 2-ml flow-through. Ki values shown are averages of two or three experiments made in duplicate. Duplicate measurements agreed within ±5%.
Figure 2.
Dixon plot of inhibition of nNOS by BN 80933. ○, ■, and ▵ represent assay points at 10, 20, and 40 μM l-arginine, respectively.
Effects on Rat Fundus and Rat Aorta.
BN 80933 dose-dependently (IC50 = 18 ± 4.5 μM) inhibited the nitrergic relaxation elicited by electrical field stimulation of fundus strips. In contrast, BN 80933 had no significant effect on endothelium-dependent relaxation in response to carbachol in rat aorta, even at the highest concentration tested (300 μM), confirming the selectivity observed in enzyme assays. The functional selectivity of BN 80933 for the fundus was not observed with l-NA, which was actually slightly more potent on the aorta (IC50 = 3.7 μM) than on the gastric fundus (IC50 = 7.4 μM) (18).
Antioxidant Properties.
Butylated hydroxytoluene (BHT) was found to be about 8 times more potent than Trolox in inhibiting iron-dependent lipid peroxidation in a rat brain membrane preparation. This finding is in agreement with previous results (23). BN 80933 was 136 and 17 times more potent than the parent compound Trolox and BHT, respectively (Table 2).
Table 2.
The in vitro antioxidant actions of BN 80933, Trolox, and BHT
| Antioxidant | IC50, μM |
|---|---|
| BN 80933 | 0.29 (0.22–0.38) |
| Trolox | 39.4 (31–49) |
| BHT | 4.9 (2.2–11) |
Rat brain homogenate (0.5 ml) was incubated for 15 min at 37°C with test compound or vehicle (10 μl). Lipid peroxidation was initiated by the addition of 50 μl of 1 mM FeCl2, 1 mM EDTA, and 4 mM ascorbic acid. After 30-min incubation at 37°C, the reaction was stopped by adding 50 μl of 0.2% BHT. Lipid peroxidation was determined by the LPO method (Bioxytech, Gagny, France) using a chromogenic reagent, N-methyl-2-phenylindole, which reacts with malonaldehyde at 45°C to yield a stable chromophore with maximal absorbance at a wavelength of 586 nm. Results represent the mean of two or three determinations, with 95% confidence limits in parentheses.
Transient Focal Ischemia in Rats. A series of experiments was performed in rats with transient (2-h) MCAO to assess the protective properties of BN 80933. The compound was administered i.v. (2 ml/kg) at 4 and 24 h after ischemia at doses ranging from 0.1 to 10 mg/kg, the latter being the maximum tolerated dose.
BN 80933 significantly reduced (60–70% reduction) the infarct volume at doses from 0.3 to 10 mg/kg (Table 3). There were no statistical changes in physiological variables such as PaCO2 PaO2, pH, glucose, or body temperature, and only a slight and transient elevation of mean arterial blood pressure was observed at the highest dose, 10 mg/kg (data not shown).
Table 3.
Dose–response of BN 80933 in rat model of focal ischemia
| Treatment | Infarct volume, mm3
|
|||
|---|---|---|---|---|
| 0.1 mg/kg | 0.3 mg/kg | 1 mg/kg | 10 mg/kg | |
| Vehicle | 295.36 ± 31.99 (8) | 311.70 ± 34.05 (5) | 269.81 ± 32.37 (9) | 295.36 ± 31.99 (8) |
| BN 80933 | 299.56 ± 14.27 (8) | 93.90 ± 31.94** (7) | 86.77 ± 29.94** (7) | 92.23 ± 25.66*** (8) |
Dose–response study of BN 80933 treatment on the infarct volume and on the total neurologic score after 2 h of MCAO. Rats were subjected to i.v. administration of vehicle or the indicated dose of BN 80933 at 4 and 24 h after onset of ischemia. The infarct volume was assessed 48 h after MCAO; six coronal sections of the brain were stained with 2,3,5-triphenyltetrazolium chloride for 20 min at room temperature and the infarct size was determined by an indirect method for calculating infarct volume (40), using an image analysis system. Results are mean ± SEM with the number of measurements in parentheses. ∗∗, P < 0.01 and ∗∗∗, P < 0.001 for comparison between vehicle-treated group and BN 80933-treated group.
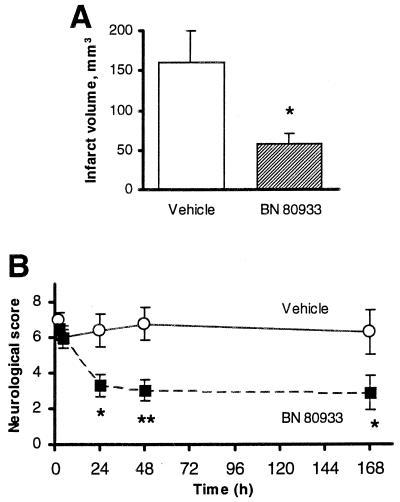
The neuroprotection afforded by BN80933 was long-lasting, since the histological assessment performed 7 days after ischemia showed that BN 80933 (0.3 mg/kg i.v.) administered 4 and 24 h after occlusion reduced the total infarct volume by 62% (Fig. 3A). Cortical (91.56 ± 25.87 mm3; n = 9 vs. 16.23 ± 9.40 mm3; n = 11, P < 0.01) and subcortical (62.45 ± 12.08 mm3; n = 9 vs. 40.19 ± 5.95 mm3; n = 11) infarct sizes were reduced by 82% and 35% respectively. To assess whether reduction of infarct size results in functional recovery, neurological function was measured. As shown in Fig. 3B, 4 h after MCAO (just before treatment), the two groups of animals exhibited comparable neurological deficits (neurological score = 6.0 ± 0.62, n = 13, for the vehicle-treated group and 6.0 ± 0.43, n = 12, for the BN 80933-treated group). The overall neurological score at 24 h (Fig. 3B) in the BN 80933-treated rats (3.29 ± 0.62) was significantly better than that of the vehicle-treated group (6.35 ± 0.92, P < 0.05). By the second and seventh day, the total neurological score remained significantly improved in the BN 80933-treated group (Fig. 3B).
Figure 3.
Effect of BN 80933 treatment on the infarct volume (A) and on the total neurological score (B) after 2 h of MCAO. Vehicle or 0.3 mg/kg BN 80933 was administered i.v. to rats at 4 and 24 h after onset of ischemia. The infarct volume (A) was assessed 7 days after MCAO; coronal sections of the brain were stained with thionin and alizarin and the infarct size was determined by image analysis. P < 0.05 for comparison between vehicle-treated rats (n = 9) and BN 80933-treated rats (n = 11) by a two-tailed Student’s t test. The neurologic score (B) was evaluated on a 15-point scale such that the higher the score, the greater was the neurological deficit. ∗, P ≤ 0.05; ∗∗, P ≤ 0.01 for comparison between vehicle-treated rats (n = 13) and BN 80933-treated rats (n = 12) by a two-tailed Mann–Whitney test.
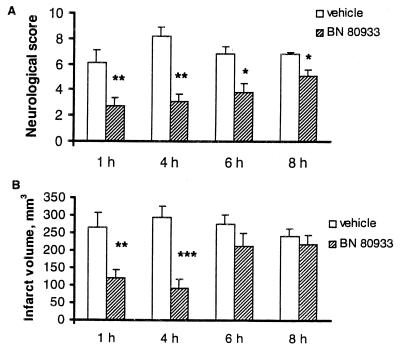
In a separate group of experiments the effect of BN 80933 was studied in a situation in which its first administration was delayed for 1 to 8 h. The neurological score immediately prior to BN 80933 administration was comparable between vehicle- and BN 80933-treated rats (vehicle = 8.5 ± 0.3; n = 8; BN 80933 = 8.7 ± 0.2; n = 9 for 1-h study; vehicle = 7.7 ± 0.7; n = 9; BN 80933 = 8.8 ± 0.4; n = 8 for 4-h study; vehicle = 6.8 ± 0.5; n = 8; BN 80933 = 6.1 ± 0.7; n = 10 for 6-h study; vehicle = 7.2 ± 0.3; n = 10; BN 80933 = 7.3 ± 0.5; n = 9 for 8-h study). At any of the time studies of BN 80933 (10 mg/kg) improved the neurological deficit significantly 48 h after ischemia compared with the vehicle group (Fig. 4A). This neurological deficit improvement was coupled with a significant reduction in infarct volume when treatment was delayed for 1 h (54% reduction) and 4 h (69% reduction) after MCAO (Fig. 4B). However, when treatment was started 6 h after MCAO a reduction of 22% in the size of the infarct was observed, but this was not significant compared with the vehicle control (P = 0.19). At 8 h after MCAO almost no effect was observed (9% reduction) on histological measurement. This result contrasts with the neurological deficit, which was protected significantly at all times after treatment with BN 80933, although an overall decline of efficacy with time was observed.
Figure 4.
Time window for BN 80933 treatment in rats subjected to 2 h of MCAO. Vehicle or 10 mg/kg BN 80933 was administered i.v. at 1, 4, 6, or 8 h and then 24 h after onset of ischemia. The neurologic score (A) was evaluated on a 15-point scale 48 h after MCAO. ∗, P < 0.05; ∗∗, P < 0.01 for comparison between vehicle-treated rats and BN 80933-treated rats, Mann–Whitney test (two-tailed). The infarct volume (B) was assessed 48 h after MCAO. ∗∗, P < 0.01 and ∗∗∗, P < 0.001 for comparison between vehicle-treated rats (n = 7, 8, 8, and 10 for 1, 4, 6, and 8 h, respectively) and BN 80933-treated rats (n = 9, 8, 10, and 9 for 1 , 4, 6, and 8 h, respectively) by a two-tailed Student’s t test.
Global Ischemia in Gerbils.
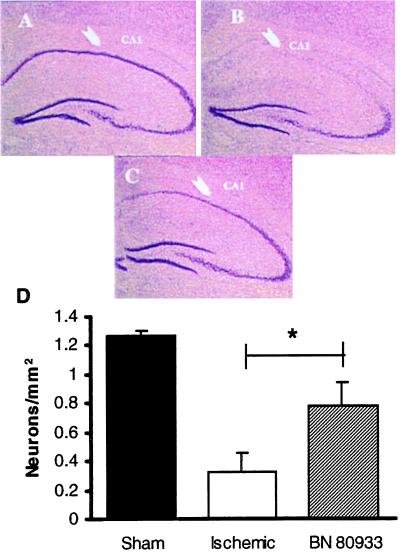
In ischemic gerbils, the most pronounced damage of all examined areas was in the CA1 region, which is known to be selectively vulnerable to ischemia. In the sham-operated gerbils, the mean area of intact neurons in the CA1 pyramidal cell layer was 1.27 ± 0.037 mm2 (n = 6), whereas 7 days after 5-min global ischemia most of the pyramidal neurons disappeared, and the area of surviving neurons was decreased to 25.9% (0.33 ± 0.127 mm2; n = 6). Administration of BN 80933 (1 mg/kg i.v.) 5 min, 5 h, 24 h, and 48 h after reperfusion increased significantly (P < 0.05) the area of surviving neurons (Fig. 5) so that the BN 80933-treated gerbils had 61.4% (0.78 ± 0.165 mm2) of neurons surviving in the CA1 pyramidal cell layer.
Figure 5.
(A–C) Low-power (×7.5) light micrographs of 20-μm coronal sections stained with cresyl violet, showing a normal CA1 subfield of the hippocampus in a sham-operated gerbil (A), in a gerbil subjected to 5-min bilateral occlusion of the common carotid arteries without (B) or with (C) treatment by BN 80933, 1 mg/kg i.v. 5 min, 5 h, 24 h, and 48 h after occlusion. (D) Effect of BN 80933 treatment on CA1 neuronal count after 5-min bilateral occlusion of the common carotid arteries. The extent of degeneration was assessed 7 days after occlusion in cresyl violet-stained coronal sections at the hippocampal level. The number of surviving neurons was counted. ∗, P < 0.05, when compared with vehicle-treated gerbils. Sham, sham-operated gerbils (n = 6); Ischemic, vehicle-treated gerbils (n = 6); BN 80933, BN 80933-treated gerbils (n = 7).
Head Trauma in Mice.
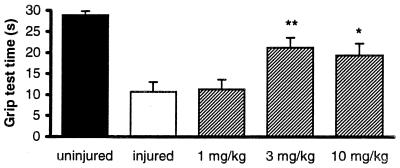
One hour after injury, the grip score reflected a severe impairment of sensory motor function in the injured mice (10.68 ± 2.32 sec; n = 22) as compared with uninjured mice (28.93 ± 0.83 sec; n = 16). Administration of 3 and 10 mg/kg i.v. BN 80933, 5 min after injury, significantly reduced the neurological deficit by 58% and 48%, respectively, in comparison with injured mice not treated with BN 80933 (Fig. 6).
Figure 6.
Male CD1 mice were subjected to head injury and within 3–5 min after injury were treated with either vehicle or BN 80933 (1–10 mg/kg, i.v., 0.1 ml/10 g). At 1 h after injury, the neurological recovery was tested by using a grip test. Experimental groups consisted of 16–22 mice. ∗, P < 0.05; ∗∗, P < 0.01 vs. injured mice.
DISCUSSION
We hereby report the neuroprotective actions of a dual inhibitor of lipid peroxidation and nNOS, BN 80933. This compound is the first representative of this class of neuroprotective agents. The potent neuroprotection afforded by BN 80933 in vivo suggests the importance of NO and ROS and their interaction in the cascade of events that leads to neuronal death. Both reactive oxygen (e.g., superoxide anion) and reactive nitrogen species (e.g., NO) are involved in glutamate neurotoxicity and inflammatory processes and contribute to neuronal death (9–11, 13, 24, 25). These species can act independently but also interact directly to form the highly reactive oxidant peroxynitrite (12).
Although the formation of NO is increased in brain after induction of ischemia, the administration of nonselective inhibitors of NOS has variously been reported to reduce, enhance, or not affect cerebral ischemic damage (26). To explain these discrepancies, it has been proposed that activation of nNOS by the massive increase of calcium after excitotoxic amino acid receptor stimulation leads to neuronal death, whereas NO generated from eNOS is protective, presumably by maintaining cerebral blood flow and reducing the adhesion of platelets and white cells to cerebral vessels (10, 11). The development of knockout mice deficient in eNOS and nNOS isoforms has confirmed these assumptions, clearly showing that excessive NO generated by nNOS contributes to cell damage, whereas eNOS has a neuroprotective role (27, 28).
Few selective nNOS inhibitors are available that have been tested in stroke models. Relatively selective nNOS inhibitors such as 7-nitroindazole or 1-(2-trifluoromethylphenyl)imidazole (TRIM) reduce ischemic brain damage in transient focal or global ischemia (29–31). The more selective (100-fold versus eNOS) and highly potent (6-fold more potent than l-NA) nNOS inhibitor ARL 17477 effectively reduces infarct size at 1 mg/kg (i.v.) but is ineffective at 3 and 10 mg/kg (29). This bell-shaped dose–response curve, revealing a narrow range of efficacy, is also observed with the nonselective inhibitor l-NA (5, 32, 33). The reason for this effect is unclear but may be lack of in vivo selectivity for nNOS resulting in a reduction of cerebral blood flow (29). Additionally, it may not be necessary to block nNOS synthesis fully to achieve optimal protection with NOS inhibitors, as shown by Carreau et al. (33). BN 80933 is a competitive inhibitor of nNOS (Ki = 0.92 μM), less potent than l-NA (Ki = 0.21 μM) but highly selective for nNOS (120-fold versus eNOS, more than 300-fold versus iNOS) in acellular enzymatic assays. Its ability to block nNOS and its selectivity are well validated in functional models of rat aorta (eNOS) and gastric fundus (nNOS). The lack of inhibitory effect of BN 80933 on the endothelium dependent-relaxation induced by carbachol is particularly relevant because it indicates that this compound does not affect the NO pathway in vascular tissue. This conclusion is in accordance with the fact that, in contrast to ARL 17477, BN 80933 (10 mg/kg) does not affect cerebral blood flow (data not shown).
Evidence accumulated over the last two decades supports the idea that ROS such as hydroxyl radical, superoxide anion, and hydrogen peroxide play a major role in the pathophysiology of acute neurological conditions (6, 34). Once formed, ROS cause oxidative damage such as lipid peroxidation, inactivation of transport proteins, and impairment of energy production by mitochondria. Because of the presence of the benzopyran moiety (Trolox-like) in the molecule, BN 80933 is a potent and dose-dependent inhibitor of lipid peroxidation, with an IC50 of 0.30 μM on rat brain membrane preparation. Similar potencies against lipid peroxidation and inhibition of nNOS were important criteria in designing a dual inhibitor such as BN 80933, so that both actions occur within the same concentration range. Interestingly, BN 80933 was more potent in inhibiting lipid peroxidation than either the parent compound Trolox or BHT. In addition, it is possible that BN 80933, by being targeted to the nNOS, is delivered at the site of injury near the point of free radical production and thus achieves an optimal antioxidant activity.
Intravenous injection of 0.3 mg/kg BN 80933 affords a significant (62% protection) and long-lasting reduction in the infarct volume in a MCAO model, as assessed 7 days after reperfusion. This observation is significant in view of the fact that the protective effect of some agents decreases or disappears with time. The histological indication of protection is associated with a highly significant improvement in the neurological score from 24 h to 7 days.
Most antiischemic drugs that have failed in clinical studies are not protective in MCAO when delayed treatment is introduced (2). However, when administered 4 h after the onset of ischemia, BN 80933 significantly reduced infarct size (up to 70%) and enhanced behavioral recovery from 0.3 mg/kg up to 10 mg/kg. To our knowledge, such a wide dose range of neuroprotection has not been previously described for nNOS inhibitors or antioxidants. Moreover, BN 80933 may be delivered as long as 8 h after initiation of the insult and still provide significant neurological improvement. Interestingly, our studies are in agreement with other authors who have shown that, when the MCAO model is used, infarct volume does not always correlate with neurological deficit (20, 35, 36). In our hands improvement was more readily detected by neurological outcome than by histological measurement; these observations emphasize the necessity of performing parallel histological and neurological studies.
The neuroprotective activity of BN 80933 was confirmed in global ischemia in gerbils and in the mouse head trauma model. In the model of global ischemia, BN 80933 offered histological improvement as seen by decreasing hippocampal damage, and in the contusion brain trauma model BN 80933 elicited behavioral improvement. Although the in vivo mechanism of action of BN 80933 has not been addressed in this paper, the pharmacological efficacy of BN 80933 suggests that the involvement of NO, ROS, or both is an early as well as a downstream event implicated in the neurotoxic cascade that occurs in several acute neurological diseases.
In summary, because in acute neurological disorders the mechanisms of neurotoxicity involve numerous processes, targeting a single event may be insufficient to provide effective neuroprotection. Acting at several sites in the neurotoxic cascade is likely to be more effective, as suggested by several studies in which different treatments have been associated (5, 37–39). Our results suggest that a better efficacy may be obtained with the use of a dual inhibitor. BN 80933, which combines antioxidant and specific nNOS inhibitory properties in the same molecule, may thus offer an alternative for the treatment of stroke and perhaps other neurological disorders in humans.
Acknowledgments
We gratefully acknowledge the excellent technical help of Ms. Marie Anne Barthelemy and Christian Carré and also Annie Higgs for her help in the preparation of the manuscript. We are grateful to Jérome Marsais for statistical data analysis.
ABBREVIATIONS
- BHT
butylated hydroxytoluene
- l-NA
NG-nitro-l-arginine
- ROS
reactive oxygen species
- NOS
nitric oxide synthase
- nNOS
neuronal NOS
- eNOS
endothelial NOS
- iNOS
inducible NOS
- MCAO
middle cerebral artery occlusion
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
A Commentary on this article begins on page 10557.
References
- 1.Dyker A G, Lees K R. Stroke. 1998;29:535–542. doi: 10.1161/01.str.29.2.535. [DOI] [PubMed] [Google Scholar]
- 2.Parsons C G, Danysz W, Quack G. Drug News Perspect. 1998;11:523–569. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- 3.Hickenbottom S L, Grotta J. Semin Neurol. 1998;18:485–492. doi: 10.1055/s-2008-1040901. [DOI] [PubMed] [Google Scholar]
- 4.del Zoppo G J. Neurology. 1998;51:S59–S61. doi: 10.1212/wnl.51.3_suppl_3.s59. [DOI] [PubMed] [Google Scholar]
- 5.Spinnewyn B, Cornet S, Auguet M, Chabrier P-E. J Cereb Blood Flow Metab. 1999;19:139–143. doi: 10.1097/00004647-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hall E D. Crit Care Clin. 1989;5:793–805. [PubMed] [Google Scholar]
- 7.Malinski T, Bailey F, Zhang Z G, Chopp M. J Cereb Blood Flow Metab. 1993;13:355–358. doi: 10.1038/jcbfm.1993.48. [DOI] [PubMed] [Google Scholar]
- 8.Kumura E, Yoshimine T, Kubo S, Tanaka S, Hayakawa T, Shiga T, Kosaka H. Neurosci Lett. 1995;200:137–140. doi: 10.1016/0304-3940(95)12099-p. [DOI] [PubMed] [Google Scholar]
- 9.Chan P H. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 10.Iadecola C. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- 11.Samdani A F, Dawson T M, Dawson V L. Stroke. 1997;28:1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- 12.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garthwaite J, Charles S L, Chess-Williams R. Nature (London) 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- 14.Copin J C, Ledig M, Tholey G. Neurochem Res. 1992;17:677–682. doi: 10.1007/BF00968004. [DOI] [PubMed] [Google Scholar]
- 15.Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. Nature (London) 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo Y, Kihara T, Ikeda M, Ninomiya M, Onodera H, Kogure K. J Cereb Blood Flow Metab. 1995;15:941–947. doi: 10.1038/jcbfm.1995.119. [DOI] [PubMed] [Google Scholar]
- 17.Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilmard C, Auguet M, Chabrier P-E. Nitric Oxide. 1998;2:147–154. doi: 10.1006/niox.1998.0170. [DOI] [PubMed] [Google Scholar]
- 19.Esterbauer H, Cheeseman K H. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 20.Lolic M M, Fiskum G, Rosenthal R E. Ann Emerg Med. 1997;29:758–765. doi: 10.1016/s0196-0644(97)70197-5. [DOI] [PubMed] [Google Scholar]
- 21.Domingo M T, Spinnewyn B, Chabrier P-E, Braquet P. Brain Res. 1994;640:268–276. doi: 10.1016/0006-8993(94)91882-1. [DOI] [PubMed] [Google Scholar]
- 22.Hall E D. J Neurosurg. 1985;62:882–887. doi: 10.3171/jns.1985.62.6.0882. [DOI] [PubMed] [Google Scholar]
- 23.Callaway J K, Beart P M, Jarrott B. J Pharmacol Toxicol Methods. 1998;39:155–162. doi: 10.1016/s1056-8719(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 24.Hall E D, Braughler J M. Free Radic Biol Med. 1989;6:303–313. doi: 10.1016/0891-5849(89)90057-9. [DOI] [PubMed] [Google Scholar]
- 25.Siesjo B K, Agardh C D, Bengtsson F. Cerebrovasc Brain Metab Rev. 1989;1:165–211. [PubMed] [Google Scholar]
- 26.Moncada S, Palmer R M, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 27.Huang Z, Huang P L, Panahian N, Dalkara T, Fishman M C, Moskowitz M A. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 28.Huang Z, Huang P L, Ma J, Meng W, Ayata C, Fishman M C, Moskowitz M A. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z G, Reif D, Macdonald J, Tang W X, Kamp D K, Gentile R J, Shakespeare W C, Murray R J, Chopp M. J Cereb Blood Flow Metab. 1996;16:599–604. doi: 10.1097/00004647-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 30.O’Neill M J, Hicks C, Ward M. Eur J Pharmacol. 1996;310:115–122. doi: 10.1016/0014-2999(96)00387-1. [DOI] [PubMed] [Google Scholar]
- 31.Escott K J, Beech J S, Haga K K, Williams S C, Meldrum B S, Bath P M. J Cereb Blood Flow Metab. 1998;18:281–287. doi: 10.1097/00004647-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Nagafuji T, Sugiyama M, Matsui T, Koide T. Eur J Pharmacol. 1993;248:325–328. doi: 10.1016/0926-6917(93)90007-d. [DOI] [PubMed] [Google Scholar]
- 33.Carreau A, Duval D, Poignet H, Scatton B, Vige X, Nowicki J P. Eur J Pharmacol. 1994;256:241–249. doi: 10.1016/0014-2999(94)90549-5. [DOI] [PubMed] [Google Scholar]
- 34.Kuroda S, Siesjo B K. Clin Neurosci. 1997;4:199–212. [PubMed] [Google Scholar]
- 35.Wahl F, Allix M, Plotkine M, Boulu R G. Stroke. 1992;23:267–272. doi: 10.1161/01.str.23.2.267. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi T, Suzuki M, Yamamoto M. Brain Res. 1995;669:107–114. doi: 10.1016/0006-8993(94)01268-m. [DOI] [PubMed] [Google Scholar]
- 37.Aronowski J, Strong R, Grotta J C. Stroke. 1996;27:1571–1576. doi: 10.1161/01.str.27.9.1571. [DOI] [PubMed] [Google Scholar]
- 38.Zhang R L, Zhang Z G, Chopp M. Neurology. 1999;52:273–279. doi: 10.1212/wnl.52.2.273. [DOI] [PubMed] [Google Scholar]
- 39.Ma J, Endres M, Moskowitz M A. Br J Pharmacol. 1998;124:756–762. doi: 10.1038/sj.bjp.0701871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Q, Chopp M, Zhang Z G, Helpern J A, Ordidge R J, Ewing J, Jiang P, Marchese B A. J Cereb Blood Flow Metab. 1994;14:732–741. doi: 10.1038/jcbfm.1994.94. [DOI] [PubMed] [Google Scholar]