Abstract
Three experiments were conducted using a lick-suppression preparation with rats to determine whether temporal and physical context shifts modulate the effectiveness of 2 sequentially trained blocking stimuli. Experiment 1 ascertained that it is possible to obtain blocking by conditioning rats to react to a target cue using 2 different blocking cues, each trained with a single-phase blocking paradigm. Experiment 2 showed that the more recently trained blocking stimulus was more effective (i.e., showed a recency effect) when testing was conducted immediately after training, but a long retention interval attenuated blocking by the most recently trained blocking stimulus and increased blocking by the initially trained blocking stimulus (i.e., a recency-to-primacy shift). This shift from recency to primacy affected in Experiment 2 by varying the retention interval was replicated in Experiment 3 by changing the physical context between training and testing. Taken together, the results indicate that the effectiveness of sequentially trained competing stimuli follows the same recency-to-primacy shift rule that is seen in traditional interference phenomena.
Keywords: blocking, Pavlovian conditioning, retrieval
Pairing a conditioned stimulus (CS) with an unconditioned stimulus (US) ordinarily results in conditioned responding to the CS. However, if the pairings occur in the presence of another cue, responding to the target CS is attenuated. Miller and Matzel’s (1988) comparator hypothesis offers one account of this sort of cue competition. The comparator hypothesis states that responding to a CS during testing is determined by three associations. The first is the traditional association between the target CS and the US. The second association is between the target CS and any other salient stimulus (excluding the US) that was present during training (called a comparator stimulus). The third association is between this comparator stimulus and the US. The model posits that when the target CS is presented at test, it directly activates a representation of the US based on the strength of the CS–US association. The presentation of the target CS also activates a representation of its comparator stimulus, which in turn activates a representation of the US. The strength of this indirectly activated US representation is dependent on the product of the strengths of the associations between the target CS and the comparator stimulus and between the comparator stimulus and the US. Cue competition occurs because the indirectly activated representation down modulates responding to the target CS, which is otherwise dictated by the strength of the directly activated US representation. A central assumption of the comparator hypothesis is that cues do not compete during the acquisition of the associative strength but at the time of testing. Thus, during a test of the target CS after training that leads to cue competition, there is a response deficit due to a failure to express an acquired association, not a deficit in associative acquisition during training. This account contrasts with acquisition-focused associative theories of cue competition, such as that of Rescorla and Wagner (1972), that posit that cue competition occurs during acquisition.
One prediction of the comparator hypothesis is that the post-training exposure of a comparator cue will result in a recovery from cue competition because there is a decrease in the associative strength between the comparator stimulus and the US. This prediction is supported by a number of studies that show a recovery from cue competition produced by extinguishing the comparator stimulus after training (e.g., recovery from blocking in Blaisdell, Gunther, & Miller, 1999; recovery from overshadowing in Kaufman & Bolles, 1981; attenuation of conditioned inhibition in Lysle & Fowler, 1985). In addition, such posttraining extinction could not only extinguish the association between the comparator stimulus and the US but would also extinguish any within-compound association between the comparator stimulus and the target stimulus (see Arcediano, Escobar, & Miller, 2005, for evidence of bidirectional extinction).
Recovery from cue competition after posttraining extinction of a comparator (competing) stimulus can also be explained by some contemporary acquisition-focused associative models (e.g., Dickinson & Burke, 1996; Van Hamme & Wasserman, 1994). For example, Dickinson and Burke’s revision of Wagner’s (1981) SOP model, SOP–Revised (SOP-R), assumes that stimuli are represented by multiple nodes in memory that exist in one of three states at any moment in time: an inactive state (I), a highly active state (A1), and a low activity state (A2). Presentation of an unexpected stimulus causes a transition from I to A1 for some proportion of the nodes representing that stimulus, which then decay back through A2 to I. When a node is activated by an associate of the stimulus, the node transitions directly from I to A2. The temporal overlap of nodes in various states affects changes in the associative connections between these nodes. When nodes of a given CS and US are concurrently in A1, there is an increment in the strength of an excitatory connection between them, whereas when nodes of the cue are in A1 while those of the US are in A2, an inhibitory connection from the CS to the US is strengthened. An added assumption unique to Dickinson and Burke’s revision of SOP is that if nodes of both a CS and a US are in A2, the excitatory association between the two stimuli will be incremented. Furthermore, if nodes of the CS are in A2 and those of the US are in A1, the inhibitory association between the CS and US will be incremented. Thus, according to this assumption of SOP-R, when a competing stimulus is presented alone after single-phase blocking treatment, the competing CS enters into the A1 state, while the US and target CS are activated in A2. Thus, incrementing the association between the US and the target CS results in retrospective revaluation (i.e., a recovery of the response to the target stimulus).
In contrast to the many published studies that show evidence of retrospective revaluation, a number of other experiments have failed to observe retrospective revaluation (e.g., Holland, 1999). One reason for this disparity suggested by Holland is that the mechanisms of overshadowing and blocking are affected by the conditioning preparation used (appetitive or aversive). However, this account does not completely address the inconsistencies in the literature. Many other parameters must play a role in contributing to the differences in the stimulus selection mechanisms. For example, Shevill and Hall (2004), also using a conditioned suppression procedure, investigated retrospective revaluation. In their Experiment 1, they expected to observe a recovery of responding to a target stimulus after extinction of an overshadowing stimulus. However, they found the opposite results, that is, a small mediated-extinction effect. Conversely, when they repeated the procedure in their Experiment 2 using different parameters (serial A–X compound during training), they did observe recovery from overshadowing. In summary, the exact conditions and parameters that yield retrospective revaluation have not yet been identified. Considering that both effects have been repeatedly demonstrated, this presents an interesting question for future research. The present research is not intended to resolve this issue. Instead, we focus on instances of recovery from blocking instead of mediated extinction.
Most of the research investigating cue competition has involved competition between a single competing stimulus and a single target CS. For example, studies of overshadowing (e.g., Pavlov, 1927), blocking (e.g., Kamin, 1969), Pavlovian conditioned inhibition (e.g., Pavlov, 1927), and overexpectation (e.g., Kremer, 1978) all involve situations in which responding to a single target CS is reduced because it is paired during training with one other CS that has a strong association with the US. Although less well-known, some recent studies have investigated the influence of two competing stimuli that are both trained with a single target cue. In most of these studies, the two competing stimuli were trained simultaneously; consequently, there was a direct association between them (e.g., Blaisdell, Bristol, Gunther, & Miller, 1998; Stout, Chang, & Miller, 2003; Urcelay & Miller, 2006; Urushihara, Stout, & Miller, 2004). However, to our knowledge, only one published study (Grahame, Barnet, & Miller, 1992) has examined the interaction between two competing stimuli that were trained sequentially with a single target CS such that there was no direct association between the two competing stimuli. Competing stimuli presumably include all CSs present during training of the target CS. Thus, when a CS is presented alone (i.e., with no other simultaneously presented punctate stimuli) during training, the context is the only potentially effective competing stimulus. In Grahame et al., a target CS was trained sequentially in two distinct contexts that could have served as two competing stimuli for the target CS. However, a test of the target stimulus revealed that the two contexts did not act as competing stimuli. To test the effectiveness of the different contexts, Grahame et al. extinguished the first-trained context, the second-trained context, or neither context before testing the target CS in a neutral context. According to the comparator hypothesis (and select other models, such as SOP-R), posttraining extinction of a competing context should reduce its effectiveness. When Grahame et al. extinguished the contexts after training, only the extinction of the most recently experienced training context was effective in enhancing responding to the target CS. That is, animals for which training of the CS occurred first in one context and then in a second context showed enhanced fear (i.e., recovery from cue competition) only as a result of extinguishing the second context, not the first one.
Grahame et al. (1992) seemed to observe a recency effect, that is, the superior expression of a more recently acquired memory relative to a memory acquired earlier. Furthermore, the recency effect seemed to produce retroactive interference, reducing the influence of the first-experienced competing stimulus. This interpretation of the results of Grahame et al. suggests that the interference between competing stimuli of a common stimulus resembles interference effects observed in the serial learning literature. A vast amount of research has been conducted to examine retroactive and proactive interference effects in learning and memory. One effect that characterizes interference phenomena in a number of different fields is the recency-to-primacy shift, which consists of a shift from better retrieval of memory for a recent training event (i.e., recency) to the equal or better retrieval of an initial training event (i.e., primacy) as a result of a delay in testing. Many studies have been performed in which an improvement in the memory of humans for initial (i.e., first learned) events was observed with an increasing delay between learning and test (e.g., Knoedler, Hell-wing, & Neath, 1999; Neath, 1993; Postman, Stark, & Fraser, 1968). The shift from recency to primacy has been reported not only in humans using serial lists of words but also with other species in analogous tasks (e.g., Urushihara, Wheeler, & Miller, 2004; Wright, Santiago, Sands, Kendrick, & Cook, 1985). The recency-to-primacy shift has also been observed using a variety of other tasks. For example, recovery over time of the first learned response to a single cue has been observed in traditional Pavlovian conditioning. Bouton and Peck (1992) examined the effects of interposing a retention interval on performance by rats in a counterconditioning paradigm. First they paired a stimulus with a shock, and then they paired the same cue with food. After training, if they tested after 1 day, the rats showed food-appropriate behavior; however, after 28 days, the tone elicited more shock-appropriate behavior. Thus, with a delay, there was a shift toward the earlier learned behavior. More recently, similar recency-to-primacy shifts have been reported after both extinction and latent inhibition procedures (e.g., Wheeler, Stout, & Miller, 2005).
Recently, Urushihara, Wheeler, and Miller (2004) found that the outcome-alone exposure effects, which are considered by most contemporary learning theories to be a form of competition between the context and the target cue, also obey the recency-to-primacy principle. With a sensory preconditioning preparation, they found that outcome-alone exposure after training (i.e., outcome postexposure) had a stronger deleterious effect relative to outcome-alone exposure prior to training (i.e., outcome preexposure), that is, a recency effect. They also found that when testing was considerably delayed, outcome-alone preexposure had a stronger deleterious effect than did outcome postexposure (i.e., a recency-to-primacy shift presumably caused by an increase in retention interval). Their results can be taken as evidence that the effect of cue competition is also strongly influenced by differential retrieval of competing cue–outcome associations caused by various factors such as shifts in the temporal context or the presentation of a priming cue. These results are problematic for both the acquisition-focused theories and the comparator hypothesis and suggest the importance of investigating the mechanism of differential retrieval of acquired associations with a common element.
The previously mentioned studies all examined the effect of retention interval on sequentially learned information. However, there is also a literature demonstrating that changes in physical context between training and testing can affect recency-to-primacy shifts. For example, Bouton and Ricker (1994) trained and extinguished conditioned responding in rats in one context and then tested them in the same context or a different context. They found a recovery of conditioned responding when testing occurred outside of the training and extinction context (AAB renewal), that is, a recency-to-primacy shift based on a change in physical context. Bouton (1993) proposed that renewal is similar to spontaneous recovery from extinction, which occurs when a long period of time passes after extinction treatment (Pavlov, 1927). He suggested that an instant in time can be regarded as an element of the context, thereby creating a spatiotemporal context. Presumably any appreciable change in the spatiotemporal context that follows sequential learning will result in a recency-to-primacy shift. In the present research, we examined this assertion with respect to both temporal and physical contexts for sequentially trained competing stimuli.
On the basis of Grahame et al.’s (1992) and Urushihara, Wheeler, and Miller’s (2004) data and Bouton’s (1993) theory, we further investigated the effect of differential retrieval of competing associations in a cue competition situation. Unlike Grahame et al., the present study focused on blocking by two discrete stimuli rather than two contexts. Our fundamental question was whether a recency-to-primacy shift could be observed with respect to the effectiveness of sequentially trained competing stimuli. Thus, we compared the effectiveness of two competing stimuli on responding to a target cue and investigated how the relative effectiveness of each cue changed as a function of increasing the retention interval or changing the physical context between training and testing. Because studies conducted in our laboratory have shown that overshadowing (which is the simplest cue competition treatment) wanes quickly as a function of the number of training trials when many training trials are presented (Stout, Arcediano, Escobar, & Miller, 2003), we used an alternative cue competition paradigm less vulnerable to trial number, single-phase blocking. In a single-phase blocking procedure, trials in which the blocking stimulus (A) is paired alone with the US are interspersed with reinforced pairings of the blocking and target CSs (AX–US; Wagner, 1969). Thus, in the present research, we used two sequential single-phase blocking procedures to determine whether a recency-to-primacy shift would occur with respect to the effectiveness of the competing stimuli. Extrapolating from Grahame et al. and Urushihara, Wheeler, and Miller, we anticipated that there would be a shift in the effective competing stimulus from the most recent one to the earliest after a shift in either the temporal or the physical context, as is observed in interference between simple excitors.
Experiment 1
Experiment 1 was preliminary to Experiments 2 and 3. It was designed to determine if two successively trained competing stimuli would successfully block responding to a target CS in a single-phase blocking paradigm. That is, in Phase 1, we trained the target cue (X) with one competing stimulus (A) in a single-phase blocking design, and then, in Phase 2, we trained the same target cue with a different competing stimulus (B) in a single-phase blocking design (see Table 1). Assuming blocking was observed in Experiment 1, we planned to use this preparation in Experiments 2 and 3 to examine the potential shift in the effective competing stimulus from CS B (most recent relative to testing) to CS A (earliest) as a function of test context.
Table 1.
Design of Experiment 1
| Group | Phase 1,Blocking A, 3 days | Phase 2,Blocking B, 3 days | Test |
|---|---|---|---|
| Blocking | 12A+/3AX+ | 12B+/3BX+ | X |
| Control | 12C+/3AX+ | 12D+/3BX+ | X |
Note. A, B, C, and D = competing stimuli; X = target cue; + = footshock. Numbers next to the pairings indicate total number of trials in that phase. Slashes indicate interspersed trials.
Method
Subjects
The subjects were 12 male (305–350 g) and 12 female (215–240 g) Sprague–Dawley, experimentally naive, young adult rats bred in our colony. The rats were randomly assigned to one of two groups (blocking and control; for each group, n = 12), counterbalanced for sex. Subjects were individually housed and maintained on a 16:8-hr light–dark cycle. Experimental sessions occurred roughly midway through the light portion of the cycle. Subjects had free access to food in the home cages. Prior to initiation of the experiment, water availability was progressively reduced to 30 min per day, provided approximately 2 hr after any scheduled treatment.
Apparatus
Because Experiment 3 required two distinct contexts, six identical copies of each of two different types of experimental chambers were used for training in all experiments. Chamber Rectangular (R) was a clear, Plexiglas, rectilinear chamber, measuring 23.0 × 8.5 × 12.5 cm (length × width × height). The floor was constructed of 0.48-cm diameter stainless steel rods, spaced 1.5 cm apart, center to center. The rods were connected by NE-2H neon bulbs that allowed a constant-current footshock to be delivered by means of a high-voltage alternating current (AC) circuit in series with a 1.0-MΩ resistor. Each copy of Chamber R was housed in a separate light- and sound-attenuating environmental isolation chest, which was dimly illuminated by a 2-W (nominal at 120 VAC) incandescent bulb driven at 60 VAC. This house light was mounted on the ceiling of the environmental chest approximately 26 cm from the center of the experimental chamber.
Chamber V-shaped (V) was a 22.1-cm long box in the shape of a vertical truncated V (25.3 cm height, 21.3 cm wide at the top, 5.1 cm wide at the bottom). The floor and long sides were constructed of stainless steel sheets, the short sides were constructed of black Plexiglas, and the ceiling was constructed of clear Plexiglas. The floor consisted of two parallel metal plates, each 2.0 cm wide, with a 1.1-cm gap between them, which permitted the delivery of constant-current footshock. Each V-shaped chamber was housed in its own environmental isolation chest, which was dimly illuminated by a 7.5-W (nominal at 120 VAC) incandescent house light driven at 60 VAC mounted on an inside wall of the environmental chest approximately 30 cm from the center of the experimental chamber. The light entering the animal chamber was primarily that reflected from the roof of the environmental chest, which was white sound-attenuating material. The light intensities in the two types of chambers (R and V) were approximately equal because of the differences in opaqueness of the walls.
Each chamber (R and V) was also equipped with three 45-Ω speakers widely separated on the inside walls of the environmental chest. Each speaker could deliver a different auditory stimulus. One speaker mounted on the right sidewall was used to deliver a complex tone stimulus (800 and 1,000 Hz) 8 dB above background. A second speaker mounted on the back sidewall of the experimental chamber was used to deliver a click stimulus (6/s) 8 dB above background, which served as Stimulus X. A third speaker mounted on the left sidewall of the chamber was used to deliver a white noise stimulus 8 dB above background. Additionally, a SonAlert (Mallory SonAlert Products, Inc., Indianapolis, IN) mounted on each environmental chest was able to deliver a high-frequency (approximately 1,900 Hz) tone 8 dB above the background sound level. Ventilation fans in each enclosure provided constant 74-dB background noise. Each chamber could also provide a flashing-light stimulus (0.17 s on, 0.17 s off). In Chamber R, the flashing light was provided by a 25-W bulb (nominal at 120 VAC) driven at 60 VAC, whereas the flashing light in Chamber V was provided by a 100-W bulb (nominal at 120 VAC) driven at 60 VAC. The bulbs were located on the back wall of each environmental chest. Because of differences in the opaqueness of the experimental walls, these two stimuli produced approximately equal illumination of the R and the V chambers. All CS durations were 5 s except during testing. The white noise and SonAlert served as Stimuli A and C, counterbalanced. The tone and flashing light served as Stimuli B and D, counterbalanced. The unconditioned stimulus was a 0.7-mA, 0.5-s footshock.
Each chamber (R and V) could be equipped with a water-filled lick tube that extended 1 cm from the rear of a cylindrical niche that was 4.5 cm in diameter, left–right centered in one short wall with its axis perpendicular to the wall and positioned with its center 4.25 cm above the floor of the chamber. Each niche had a horizontal infrared photobeam horizontally traversing it parallel to the wall on which the niche was mounted, 1 cm in front of the lick tube. To drink from the tube, subjects had to insert their heads into the niche, thereby breaking the infrared photobeam. Thus, we could record when subjects had their heads in the niche to access the water tube. Ordinarily, they broke the beam only when they were drinking. Disruption of ongoing drinking by a test stimulus served as our dependent variable. Chambers R and V were counterbalanced within groups and each subject was trained and tested exclusively in one chamber.
Procedure
Acclimation
On Day 1, each subject was exposed to the experimental context for 60 min with the lick tube present. No nominal stimuli were presented. At the end of this session, the lick tube was removed.
Blocking by A (Phase 1)
On each of Days 2–4, subjects in the blocking group were exposed to one reinforced presentation of Stimulus A simultaneously paired with Stimulus X (AX–US) and four reinforced presentations of Stimulus A alone (A–US). The subjects in the control group received similar treatment, except that Stimulus C instead of Stimulus A was reinforced alone (C–US and AX–US). On Day 2, the reinforced presentation of the compound AX occurred 41 min into the 60-min session and the 4 reinforced presentations of stimulus A or C alone occurred 8, 19, 30, and 50 min into the 60-min session. On Day 3, the reinforced presentation of the compound AX occurred 30 min into the 60-min session and the 4 reinforced presentations of Stimulus A or C alone occurred 8, 19, 41, and 50 min into the 60-min session. On Day 4, the reinforced presentation of the compound AX occurred 19 min into the 60-min session and the 4 reinforced presentations of Stimulus A or C alone occurred 8, 30, 41, and 50 min into the 60-min session. In all cases, the CS or CSs were immediately followed by a US presentation.
Blocking by B (Phase 2)
On each of Days 5–7, subjects in the blocking group were exposed to one reinforced presentation of Stimulus B simultaneously paired with Stimulus X (BX–US) and four reinforced presentations of Stimulus B alone (B–US). The subjects in the control group received similar treatment, except that Stimulus D instead of Stimulus B was reinforced alone (D–US and BX–US). The reinforced presentation of the compound BX on Days 5, 6, and 7 occurred at the same times as the compound AX occurred on Days, 2, 3, and 4, respectively, into the 60-min session. Similarly, the four reinforced presentations of Stimulus B or D alone on Days 5, 6, and 7 occurred at the same times as the Stimulus A or C occurred on Days 2, 3, and 4, respectively, into the 60-min session. In all cases, the CS or CSs were immediately followed by a US presentation.
Reacclimation
On Days 8 and 9, to stabilize baseline drinking rates that might have been perturbed by fear conditioning, all subjects received a daily 60-min session with no nominal stimuli being presented.
Test
On Day 10, all subjects were tested for suppression to CS X in an 11-min test session. During this session, there was a 10-min continuous presentation of X alone starting on completion of the first 5 cumulative seconds of licking. Thus, all rats were drinking at the moment of CS X onset. Time to complete the first 5 cumulative seconds of drinking provided a pre-CS score. The time to complete another 5 cumulative seconds, this time in the presence of the CS, provided a CS score. As is the custom of our laboratory, animals with pre-CS scores greater than 60 s were eliminated from the study because of their exhibiting excessive fear of the context. On the basis of this criterion, 1 subject from each of the two groups was excluded from the analyses. All scores were capped at 10 min and subjected to a log (base 10) transformation to improve the normality of the within-group distributions and allow the use of parametric statistics.
Results and Discussion
Figure 1 shows that the blocking group suppressed less during the presentation of CS than did the control group. A t test performed on the transformed pre-CS scores showed no effect, t(20) = 0.81, p = 43; therefore, any differences in responding to the CS were not appreciably influenced by differences in fear of the context. A t test analysis of the transformed latencies in the presence of the CS revealed an effect of blocking, t(20) = 5.98, p < .01. Thus, we observed a cue competition effect with two different competing stimuli sequentially presented in two phases of single-phase blocking. However, an alternative interpretation of these results is possible. In this experiment, the blocking group received training with two stimuli in addition to X (A–US and AX–US followed by B–US and BX–US), while the control group received four additional stimuli during training (C–US and AX–US followed by D–US and BX–US). Thus, the stronger responding to X in the control group compared with the blocking group may have been a result of greater generalization from the training stimuli instead of the effect of blocking. Although such an explanation of the results of Experiment 1 is possible, it does not explain the results of Experiments 2 and 3.
Figure 1.
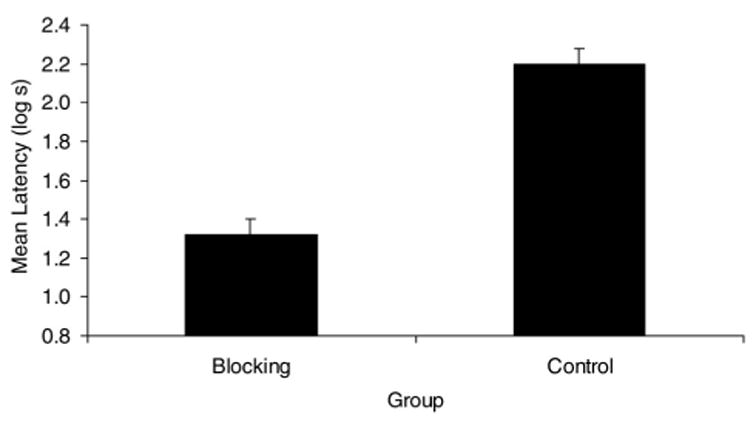
Mean times to complete 5 cumulative seconds of drinking on presentation of the target conditioned stimulus (X) in Experiment 1. See Table 1 for treatments of the two groups. Error bars denote standard errors of the means.
Experiment 2
The purpose of Experiments 2 and 3 was to examine the effects of posttraining extinction of the blocking CSs A or B as a function of the retention interval (Experiment 2) and changes in the physical context (Experiment 3) between blocking treatment and extinction treatment. In the framework of the comparator hypothesis, we assessed relative value as a comparator stimulus of A and B by observing the effects on responding to X as a consequence of posttraining extinction of these cues. In Experiment 2, we assessed the effect of a temporal context switch on the identity of the effective competing stimulus. In this experiment, we used the single-phase blocking paradigm that was used in Experiment 1. Table 2 summarizes the design of Experiment 2. In Phase 1, the blocking occurred with competing stimulus A and in Phase 2, blocking occurred with a second, different competing stimulus, B. After training, half of the groups received a short retention interval of 1 day, and the other half received a long interval of 22 days. In both the short condition and the long condition, one group experienced extinction of the first competing stimulus (Ext A), another group experienced extinction of the second competing stimulus (Ext B), and a control group did not experience extinction of either stimulus (No Ext). Both extinction and testing occurred after the retention interval. If posttraining extinction of one blocking stimulus but not the other had substantial effect on responding to the target cue, we could conclude that that blocking stimulus was responsible for the weak responding observed without the extinction treatment. On the basis of the findings of Blaisdell et al. (1999) and Grahame et al. (1992), we anticipated that after a short retention interval, only the subjects that received extinction to the second competing stimulus (Ext B) would show a recovery from blocking. Therefore, a strong response to the blocked CS was expected in Group Short–Ext B relative to Group Short–No Ext and Group Short–Ext A. Moreover, if the effectiveness of a competing stimulus is subject to a recency-to-primacy shift as a consequence of a long retention interval as suggested by Urushihara, Wheeler, and Miller (2004), in the long condition, only the group that received extinction to the first competing stimulus A should show a recovery from blocking. Such a result would indicate that the effective competing stimulus reflects a primacy effect after a long retention interval.
Table 2.
Design of Experiment 2
| Group | Phase 1, Blocking A, 3 days | Phase 2, Blocking B, 3 days | Phase 3, retention interval | Phase 4, extinction | Test |
|---|---|---|---|---|---|
| Short–Ext A | 12A+/3AX+ | 12B+/3BX+ | 1 day | 400 A− | X |
| Short–Ext B | 12A+/3AX+ | 12B+/3BX+ | 1 day | 400 B− | X |
| Short–No Ext | 12A+/3AX+ | 12B+/3BX+ | 1 day | Context | X |
| Long–Ext A | 12A+/3AX+ | 12B+/3BX+ | 22 days | 400 A− | X |
| Long–Ext B | 12A+/3AX+ | 12B+/3BX+ | 22 days | 400 B− | X |
| Long–No Ext | 12A+/3AX+ | 12B+/3BX+ | 22 days | Context | X |
Note. A and B = competing stimuli; X = target cue; + = footshock; Short = 1-day retention interval; Long = 22-day retention interval; Ext = extinction. Numbers next to the stimuli indicate the total number of trials or days in that phase. Slashes indicate interspersed trials.
Method
Subjects and Apparatus
The subjects were 36 male (213–334 g) and 36 female (160–233 g) Sprague–Dawley, experimentally naive, young adult water-restricted rats (n = 12 per group) bred in our colony. Apparatus and Phases 1 and 2 of treatment were identical to those used in Experiment 1. The white noise and tone served as Stimuli A and B, counterbalanced. The click train served as Stimulus X.
Procedure
Acclimation and blocking by A (Phase 1) and B (Phase 2)
On Days 1–7, acclimation, Phase 1 (single-phase blocking by A), and Phase 2 (single-phase blocking by B) were conducted in the same manner as in Experiment 1. There were no blocking control groups in Experiment 2; that is, all subjects received the blocking treatments.
Retention interval (Phase 3)
Over Days 8–28, subjects in the long condition stayed in their home cages and received no experimental treatment except for 30 s of handling three times per week.
Extinction (Phase 4)
On each of Days 8 and 9, subjects in Group Short–Ext A were exposed to 200 nonreinforced presentations of Stimulus A, 1 every 36 s in the 120-min session. Subjects in Group Short–Ext B were exposed daily to 200 nonreinforced presentations of Stimulus B, on an average interval of 1 every 36 s in the 120-min session. Group Short–No Ext was exposed daily to context alone for 120 min. The total number of extinction trials was based on the results of Blaisdell et al. (1999), which demonstrated recovery from single-phase blocking with a total of 200 nonreinforced presentations of the competing stimulus. We gave twice that number of extinction trials to assure robust recovery. At the end of the extinction treatment, the lick tubes were restored to the apparatus. On Days 29 and 30, subjects in Groups Long–Ext A and Long–Ext B received extinction treatment with A and B, respectively, that was identical to that previously received by Groups Short–Ext A and Short–Ext B.
Reacclimation
On Days 10 and 11, the subjects in the short condition were reacclimated to stabilize baseline drinking rates as in Experiment 1. Subjects in the long condition received identical treatment on Days 31 and 32.
Test
On Day 12, subjects in the short conditions were tested for suppression to CS X as in Experiment 1. On Day 33, subjects in the long condition were similarly tested. Subjects that took longer than 60 s to complete 5 cumulative seconds of drinking before the onset of the CS were eliminated from the study; on the basis of this criterion, 1 subject from Group Short–Ext B and 1 from Group Long–Ext B were excluded.
Results and Discussion
In Figure 2, one can observe that Groups Short–Ext B and Long–Ext A showed a stronger suppression to the target stimulus X than did Groups Long–No Ext, Short–No Ext, Short–Ext A, and Long–Ext B. That is, with a short retention interval, recovery from blocking was observed only when the most recently trained blocking cue was extinguished (a recency effect). In contrast, with a long retention interval, recovery from blocking occurred only when the first-trained blocking cue was extinguished (a primacy effect). A 2 (interval: short vs. long) × 3 (extinction: A, B, or none) analysis of variance (ANOVA) performed on pre-CS scores showed no main effects or interactions, ps > .17. A 2 (interval) × 3 (extinction) ANOVA on the CS scores revealed no main effect of interval, p = .63, and no main effect for extinction, p= .19. However, the ANOVA revealed an interaction between extinction and interval, F(2, 64) = 21.57, p< .01, MSE = 1.86. Planned comparisons detected a difference among the three groups in the short condition. The group extinguished to CS B (Short–Ext B) suppressed more than both the group extinguished to CS A (Short–Ext A), F(1, 64) = 17.34, p< .01, and the no-extinction control group (Short–No Ext), F(1, 64) = 7.25, p<.01. Further planned comparisons among the three groups in the long condition, which experienced a retention interval of 22 days, revealed that the group extinguished to CS A (Long–Ext A) suppressed more than both the group extinguished to CS B (Long–Ext B), F(1, 64) = 23.61, p< .01, and the control group (Long–No Ext), F(1, 64) = 16.31, p<.01.
Figure 2.

Mean times to complete 5 cumulative seconds of drinking on presentation of the target conditioned stimulus (X) in Experiment 2. See Table 2 for treatments of the six groups. Ext A = extinguished to Stimulus A; Ext B = extinguished to Stimulus B; No Ext = did not experience extinction of either stimulus. Error bars denote standard errors of the means.
These results suggest that, although the two blocking stimuli were identically trained except for their order, they differed in their potential to block the target CS. As anticipated, after the short retention interval, extinction of the more recently trained blocking stimulus (B) produced a recovery from blocking, resulting in strong suppression to the target CS X as compared with the no-extinction control group and the group that received extinction to A. In fact, there was no appreciable recovery from blocking when the first blocking stimulus (A) was extinguished. This demonstrates that recency is a determinant of the effective competing stimulus. By contrast, after a 22-day retention interval, we observed the opposite results. That is, extinction of A resulted in recovery from blocking of the target stimulus, producing strong suppression of X, as compared with both the group that received extinction to the second-trained blocking stimulus (B) and the no-extinction control group. This suggests that there was a primacy effect with respect to the effective competing stimulus after a 22-day delay. In summary, the present results demonstrate that a shift in the temporal context yields a shift in the effective competing stimulus from the most recently trained one to the first trained one. Also, as mentioned previously, the results of this experiment contraindicate the interpretation of weaker responding to X as a result of blocking treatment being the consequence of less stimulus generalization in the blocking group than in the control group. If responding to X was augmented by generalized excitation from the other excitatory stimuli (i.e., A and B in the blocking groups and A, B, C, and D in the control groups of Experiment 1), then extinction of A and B should have reduced responding to X. Instead, responding to X was, if anything, inversely related to the excitatory status of A or B. One could still argue that the blocking effect observed in Experiment 1 was augmented by generalization from C and D in the control group. However, this possibility does not significantly affect the interpretation of our results because it is clear in Experiments 2 and 3 that A and B do act as competing stimuli for X.
Experiment 3
Experiment 2 demonstrated that after a 22-day retention interval, there was a shift from recency to primacy in the identity of the effective competing stimulus. The time delay that was introduced by the retention interval can be viewed as a shift in the temporal context. Bouton (1993) suggested that as time passes after training, the context provided by internal and sometimes external cues constituting the background also changes. Thus, the passage of time presumably is accompanied by a gradual change in context. Consequently, a long interval can be viewed as a temporal context change in the same manner as a physical context change is viewed, and both treatments can produce a recency-to-primacy shift with respect to simple excitation (Bouton, 1993). To extend our finding of a recency-to-primacy shift in cue competition over a long retention interval, in Experiment 3, we evaluated the effect of a physical context shift by varying the context of extinction relative to that of testing (see Table 3). That is, we tested whether a change in physical context between training and testing would produce a shift in the effectiveness of two competing stimuli after sequential training. As in Experiment 1, we sequentially trained two competing stimuli using single-phase blocking and tested with a short retention interval; however, the physical context was manipulated. When the subjects were trained, extinguished on A or B, and then tested on X all in the same context (Context 1), we expected to observe recovery from blocking only when we extinguished the second-trained competing stimulus. Such a result with a short retention interval would partially replicate Experiment 2 and indicate that without a context shift, the most effective competing stimulus is B. We also anticipated that, if we trained the subjects in Context 1 and then extinguished and tested them in a different context (Context 2), we would observe a shift to primacy, such that the first-trained competing stimulus (A) would be more effective, thereby producing a recovery from blocking in the group that was extinguished on the first blocking stimulus. Additionally, to make Experiment 3 parallel to Experiment 2, we had the physical context shift in Experiment 3 occur just prior to extinction treatment.
Table 3.
Design of Experiment 3
| Group | Phase 1, 3 Days | Phase 2, 3 Days | Phase 3, extinction | Test |
|---|---|---|---|---|
| Same–Ext A | (12A+/3AX+ )1 | (12B+/3BX+ )1 | (400 A− )1 | X1 |
| Same–Ext B | (12A+/3AX+ )1 | (12B+/3BX+ )1 | (400 B− )1 | X1 |
| Same–No Ext | (12A+/3AX+ )1 | (12B+/3BX+ )1 | (Context)1 | X1 |
| Different–Ext A | (12A+/3AX+ )1 | (12B+/3BX+ )1 | (400 A− )2 | X2 |
| Different–Ext B | (12A+/3AX+ )1 | (12B+/3BX+ )1 | (400 B− )2 | X2 |
| Different–No Ext | (12A+/3AX+ )1 | (12B+/3BX+ )1 | (Context)2 | X2 |
Note. A and B = competing stimuli; X = target cue; + = footshock; Same = trained and tested in the same context; Different = trained in Context 1 and tested in Context 2; Ext = extinction. Numbers next to the stimuli indicate the total number of trials in that phase. Subscripts indicate different contexts. Slashes indicate interspersed trials.
Method
Subjects and Apparatus
The subjects were 36 male (264–378 g) and 36 female (190–254 g) Sprague–Dawley, experimentally naive, young adult water-restricted rats (n = 12 per group) bred in our colony. The apparatus, stimuli, and Phases 1 and 2 of treatment were identical to those used in Experiment 2. The subjects that were trained and tested in different contexts were either trained in an R chamber and extinguished and then tested in a V chamber or vice versa.
Procedure
Acclimation
On Days 1 and 2, all subjects were exposed to both contexts. Within each group, for half the subjects, Context 1 was an R chamber and for the other half it was a V chamber. On Day 1, all subjects were exposed to Context 1 (the training context) for 30 min and then Context 2 for 30 min, both with lick tubes present. On Day 2, subjects were first exposed to Context 2 for 30 min and then Context 1 for 30 min. For each subject, these sessions were separated by 180 min. No nominal stimuli were presented. At the end of these sessions, the lick tubes were removed.
Blocking by A (Phase 1) and B (Phase 2)
On Days 3–8, Phases 1 and 2 were conducted in the same manner as they were in Experiment 2. Training occurred in Context 1 for all subjects.
Extinction (Phase 3)
On Days 9–12, subjects in Groups Same–Ext A and Different–Ext A were exposed daily to 100 nonreinforced presentations of Stimulus A, one every 36 s in the daily 60-min sessions. Subjects in Groups Same–Ext B and Different–Ext B were exposed daily to 100 nonreinforced presentations of Stimulus B, one every 36 s in the daily 60-min sessions. Groups Same–Ext A and Same–Ext B received these extinction trials in Context 1, whereas Groups Different–Ext A and Different–Ext B received them in Context 2. Groups Same–No Ext and Different–No Ext were merely exposed to Contexts 1 and 2, respectively, for 60 min. At the end of this session, the lick tubes were restored to the apparatus.
Acclimation
On Days 13 and 14, each subject was exposed to its test context to stabilize its baseline drinking rate after training. Times to complete the first 5 cumulative seconds of drinking were recorded. On Day 13, each subject was exposed to Context 1 for 30 min with lick tubes present and then Context 2 for 30 min. On Day 14, each subject was first exposed to Context 2 for 30 min and then Context 1 for 30 min. No nominal stimuli were presented.
Test
On Day 15, subjects were tested for suppression to Stimulus X in an 11-min test session. Subjects in the same condition were tested in Context 1, and subjects in the different condition were tested in Context 2 (testing for all groups occurred in the same context as Phase 3 treatment and reshaping). Other than this, testing was conducted as in the prior experiments. As in the previous experiments, animals taking more than 60 s to complete their initial 5 cumulative seconds of drinking were eliminated from the study. On the basis of this criterion, 1 subject from Group Same–Ext B was excluded from the analyses.
Results and Discussion
The results of Experiment 3 indicated a recency-to-primacy shift in cue competition as a consequence of a change in physical context from training to extinction and testing (see Figure 3). A 2 (context: same vs. different) × 3 (extinction: A, B, or none) ANOVA of the pre-CS scores to complete 5 cumulative seconds of drinking did not show any main effects or an interaction, all ps > .24. A 2 (context) × 3 (extinction) ANOVA on CS scores revealed a marginally significant main effect of context, p= .05, and a robust main effect for extinction, p< .01. More important, the ANOVA detected an interaction between extinction and context, F(2, 65) = 41.37, p< .01, MSE = 2.2. Planned comparisons revealed differences among the three groups in the condition in which subjects were trained and tested in the same context. Group Same–Ext B exhibited greater suppression than did both Group Same–Ext A, F(1, 65) = 44.19, p< .01, and Group Same–No Ext, F(1, 65) = 25.85, p< .01. Additional planned comparisons detected differences among the three groups in the condition in which subjects were trained in Context 1 but tested in Context 2. The group that was extinguished to CS A showed greater suppression than both the group extinguished to CS B, F(1, 65) = 36.82, p< .01, and the No Ext control group, F(1, 65) = 40.39, p< .01.
Figure 3.
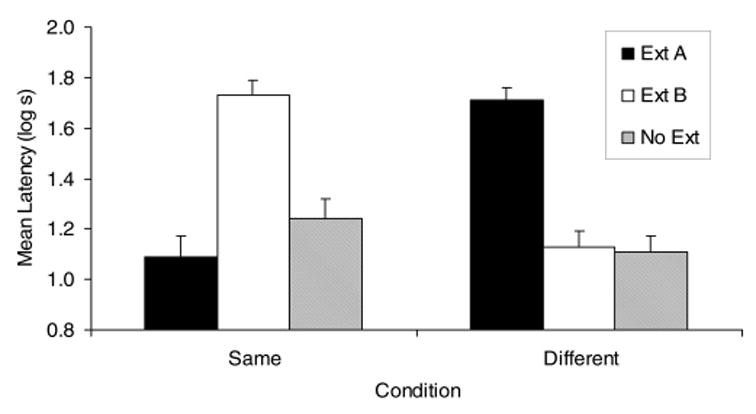
Mean times to complete 5 cumulative seconds of drinking on presentation of the target conditioned stimulus (X) in Experiment 3. See Table 3 for treatments of the six groups. Ext A = extinguished to Stimulus A; Ext B = extinguished to Stimulus B; No Ext = did not experience extinction of either stimulus; Same = trained and tested in the same context; Different = trained and tested in different contexts. Error bars denote standard errors of the means.
In summary, the subjects in the condition in which they were trained and tested in the same context showed strong responding to Target Stimulus X (recovery from blocking) only when the second-trained (most recent) competing stimulus was extinguished. This indicates that the most recent competing stimulus was the most effective one. However, when the subjects were trained in one context and extinguished and tested in a different context, the first-trained competing stimulus was the more effective competing stimulus. With this context shift, only the subjects in the group that was extinguished to the first blocking stimulus showed recovery from blocking, suggesting that primacy is a critical determinant of the effectiveness of a competing stimulus after a switch in physical context. Consistent with our expectations, we observed that after a change in physical context, there was an attenuation of the effectiveness of the most recently trained competing stimulus and an enhancement of the effectiveness of the first-trained competing stimulus.
General Discussion
Previous studies have shown that in situations involving sequential training, a recency-to-primacy shift in simple conditioned responding occurs after a shift of either the temporal or the physical context between training and testing. In the present series of experiments, we investigated the recency-to-primacy shift with regard to cue competition. In Experiment 1, we demonstrated that cue competition effects could be obtained in a single-phase blocking paradigm, given training of the target stimulus with two different sequentially trained competing stimuli. In Experiments 2 and 3, we evaluated the relative effectiveness of these two sequentially trained competing stimuli. In Experiment 2, we examined the effect of interposing long or short retention intervals between training and testing and observed that when subjects were tested shortly after training, extinction of the most recently experienced competing stimulus was effective in reducing blocking, but extinction of the first-trained competing stimulus was not effective. This phenomenon of recency determining cue competition after sequential training of the target cue with two different competing stimuli has been observed previously (Grahame et al., 1992). We also observed in Experiment 2 that after a long retention interval, extinction of the first-trained competing stimulus was effective in reducing blocking, whereas extinction of the most recently trained competing cue was not effective. This result suggests that the effectiveness of the competing stimuli was determined by primacy after a long retention interval. In Experiment 3, we observed the same sort of recency-to-primacy shift after a switch of the physical context between training and testing.
One model that speaks to part of the present results is Dickinson and Burke’s (1996) revision of SOP (Wagner, 1981). SOP-R was specifically designed to explain retrospective revaluation effects such as the recovery from blocking observed in the present series of experiments. According to SOP-R, on AX–US trials, the mental representations of Stimulus A, Stimulus X, and the US are activated into the A1 state. All the nodes that are simultaneously active in A1 come to be linked by binary excitatory associations. This means that the organisms learn an A–US association, an X–US association, and also an A–X within-compound association (along with US–A, US–X, and X–A associations). Thus, in the case of extinction of A after AX–US compound training, when Stimulus A is presented in the absence of X and the US, the representation of A is activated into A1. Moreover, although Stimulus X and the US are not actually present, A is able to activate nodes of both stimuli into A2 on the basis of the A–US association and the A–X within-compound association. The simultaneous activation of X and the US in A2 caused by exposure to A alone presumably strengthens the excitatory association between X and the US. This should result in an increase in conditioned responding to X that was in fact observed at test after A was extinguished in Experiments 2 and 3.
SOP-R in its original form, however, remains unable to completely account for the results of the present experiments. After AX–US and BX–US training, either presentation of A alone or presentation of B alone should result in an increase in the conditioned response to X. Although extinction of A or B might not yield the same amount of retrospective revaluation (e.g., the within-compound association should be strongest between the elements of the most recently trained compound, and this should make extinction of B especially apt to support retrospective revaluation, i.e., a recency effect), this consequence should not change because of other factors such as retention interval or the physical context of testing. In other words, no recency-to-primacy shift is expected to affect the relative influences of the two competing stimuli.
Although SOP-R cannot account for the effect of a recency-to-primacy shift, it can be adapted to allow for a more complete interpretation of the present results. Assuming that a CS (such as X) has been sequentially associated with two other CSs (e.g., as a result of AX–US trials during Phase 1 and BX–US trials during Phase 2), the potential of the A–X and B–X within-compound associations to mediate retrospective revaluation could change with time or physical context. Initially, the most recently trained competing stimulus would be assumed to be more effective than the first-trained one. But after extensive time has passed or a physical context change, this situation might be expected to be altered, with the first-trained competing stimulus becoming the more effective in producing retrospective revaluation. In this case, the resulting primacy effect would be a consequence of proactive interference with associations to B by associations to A. In the framework of SOP-R, this would mean that immediately after sequential training with the compounds AX and BX, Stimulus B should be more effective than Stimulus A in activating the representation of X into A2. But with the passage of time or with a switch to a context other than that of training, the relative potentials of A and B to activate X into A2 are reversed, making Stimulus A more effective than Stimulus B in activating X into A2. We can only speculate how this process might take place. However, a plausible assumption is that the first-learned association involving a specific element (in this case, X) is the strongest one but is initially subject to retroactive interference by the most recently acquired association. It appears that when extinction and testing occur directly after training and lack any change in physical context, the subjects revalue the associative status of X primarily on the basis of the most recent information (i.e., the B–X association). This recency effect suggests that the B–X association retroactively interferes with the A–X association. Presumably, recency effects result from the similarity of the spatiotemporal context of recent training to the spatiotemporal context of testing, thereby allowing the latter context to serve as an occasion setter (e.g., Holland, 1992; Miller & Oberling, 1998), which favors retrievability of the B–X association at the expense of retrievability of the A–X association (e.g., Bjork, 2001). However, once the similarity between training and testing is weakened by a long retention interval or a switch in physical context, potential retrospective revaluation depends more on the first-trained competing stimulus, thereby allowing the A–X association to proactively interfere with expression of the B–X association.
An alternative perspective on the present data is provided by the comparator hypothesis (Miller & Matzel, 1988). Without any additional assumptions, the comparator hypothesis anticipates that posttraining extinction of a competing stimulus after blocking treatment will produce recovery from blocking. But, like SOP-R, it does not speak to why extinction of A or B should differentially impact responding to X or why this differential effect changes with retention interval or test context. However, one could modify it by applying known principles of interference theory to memories of training episodes instead of to dyadic associations as is more commonly done. The results of the present experiments demonstrate that the rules of the comparator hypothesis are subject to variations, such as the effects of recency and primacy. For the comparator hypothesis to account for the present results, one might assume that there is interference between the memories of different episodes of training. It can be inferred from the present observations that when two comparator stimuli are sequentially trained with a target stimulus, the effective comparator stimulus is dependent on the temporal and physical contexts of testing. If neither the temporal nor the physical context is shifted between training and testing, the memories of the most recent training with X (Phase 2 in Experiments 2 and 3) seem to retroactively interfere with the retrieval or expression of memories from the first phase of training. That is why, in Experiments 2 and 3, without a temporal or physical context shift, posttraining extinction of the first-trained competing stimulus (A) had no appreciable effect on conditioned responding to CS X. That is, only the most recent memories of X (in which X was paired with B) were expressed when X was tested. In contrast, after a physical or temporal context shift, memories of the most recent phase of training involving X were not expressed when X was tested, as evidenced by posttraining extinction of B not recovering responding to X. Instead, only extinction of A recovered responding to X. This suggests that the interference between memories based on the different phases of training was effectively reversed from retroactive to proactive with a physical or temporal context shift. It is important to note that most of the published research concerning the comparator hypothesis in relation to interference has dealt with interference between associations (e.g., Miller & Escobar, 2002). However, in this case, it must be assumed that interference is not occurring between associations but between the memory complexes created during each phase of training. Although this concept of interference is not widely used in the associative learning literature, it is prevalent in other areas of study, such as serial list learning (e.g., Neath, 1993).
Notably, there is a distinction between the two accounts offered above. According to our modification of the comparator hypothesis, any interference effects must occur between different training episodes that involve X. Interference does not occur between specific associations. If interference did occur between the A–X and B–X associations, extinction of one of these associations would presumably eliminate the interference when X is subsequently tested. In contrast, our extension of SOP-R is compatible with a mechanism involving interference between specific associations. For example, when A is extinguished and there is a short retention interval as in Experiment 2, the B–X association may interfere with the expression of the A–X association, which would prevent revaluation of X. This distinction does allow the two proposed accounts to make at least one differential prediction. According to our version of SOP-R, any recency-to-primacy shift (i.e., B to A) should not affect responding to X if a temporal or physical context shift occurs after extinction of A or B. However, the proposed modification of the comparator hypothesis suggests that any recency-to-primacy shift will be effective regardless of whether the context shift occurs before or after extinction of the competing stimuli. These predictions could be tested experimentally, but such an experiment would involve changing the physical or temporal context after the extinction of A or B, which could result in spontaneous recovery or renewal with respect to A or B (i.e., poor expression of Phase 3 extinction).
The effects of recency-to-primacy shifts have been broadly discussed. Recency-to-primacy shifts are ordinarily manifest as increasing retrieval for the first items on a list as a direct function of delay in time. Bjork (2001) proposed that this shift might be adaptive with respect to the skills and knowledge one needs to access in real-world situations. The immediate future is best predicted by the immediate past, which makes the recency effect useful. But with changes in time or place, the most recent relevant past event is not as apt to foreshadow the immediate future. Consequently, it is functional for recency effects to wane. Functionally, recency phenomena should give way to equal weighting of all prior events, not necessarily a primacy effect. To account for this, we must assume a privileged status of first-learned events.
Empirically, here and in many other situations, we see evidence suggesting that the first learned memory is the strongest (all other things being equal), and thus any changes in either physical or temporal context will favor a shift away from transitory recency effects to the original information learned. Such recency-to-primacy shifts are evident among various procedures such as free recall from a list of words; probe (memory-search) procedures; competing habits; paired associate tasks; and, in the clinical treatment of fears, the determination of which behaviors are elicited by fear-inducing stimuli. These examples all suggest that the retrievability of a specific association from a sequentially acquired set of associations is subject to a recency-to-primacy shift. The present research indicates that in the same way, the retrievability of an association to a competing stimulus is subject to the same shift.
Footnotes
Olga Lipatova, Daniel S. Wheeler, Miguel A. Vadillo, and Ralph R. Miller, Department of Psychology, State University of New York at Binghamton.
National Institute of Mental Health Grant 33881 provided support for this research. Miguel A. Vadillo was supported by Fellowship BF 101.31 from the Department of Education, Universities and Research of the Basque Government. We thank Jeffrey C. Amundson, Gonzalo Urcelay, and Kouji Urushihara for their comments on an earlier version of this article. We also thank James Esposito for assistance with the collection of data.
References
- Arcediano F, Escobar M, Miller RR. Bidirectional associations in humans and rats. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:301–318. doi: 10.1037/0097-7403.31.3.301. [DOI] [PubMed] [Google Scholar]
- Bjork RA. Recency and recovery on human memory. In: Roediger HL III, Nairne JS, Neath I, Suprenant AM, editors. The nature of remembering: Essays in honor of Robert G. Crowder. Washington DC: American Psychological Association; 2001. pp. 211–232. [Google Scholar]
- Blaisdell AP, Bristol AS, Gunther LM, Miller RR. Overshadowing and latent inhibition counteract each other: A comparator hypothesis analysis. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:335–351. doi: 10.1037//0097-7403.24.3.335. [DOI] [PubMed] [Google Scholar]
- Blaisdell AP, Gunther LM, Miller RR. Recovery from blocking achieved by extinguishing the blocking CS. Animal Learning & Behavior. 1999;27:63–76. [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;144:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Peck CA. Spontaneous recovery in cross-motivational transfer (counterconditioning) Animal Learning & Behavior. 1992;20:313–321. [Google Scholar]
- Bouton ME, Ricker ST. Renewal of extinguished responding in a second context. Animal Learning & Behavior. 1994;22:317–324. [Google Scholar]
- Dickinson A, Burke J. Within-compound associations mediate the retrospective revaluation of causality judgements. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology. 1996;49B:60–80. doi: 10.1080/713932614. [DOI] [PubMed] [Google Scholar]
- Grahame JN, Barnet CR, Miller RR. Pavlovian conditioning in multiple contexts: Competition between contexts for comparator status. Animal Learning & Behavior. 1992;20:329–338. [Google Scholar]
- Holland PC. Occasion setting in Pavlovian conditioning. In: Medin DL, editor. The psychology of learning and motivation. Vol. 28. San Diego, CA: Academic Press; 1992. pp. 69–125. [Google Scholar]
- Holland PC. Overshadowing and blocking as acquisition deficits: No recovery after extinction of overshadowing or blocking cues. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology. 1999;52B:307–333. doi: 10.1080/027249999393022. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. Predictability, surprise, attention, and conditioning. In: Campbell BA, Church RM, editors. Punishment and aversive behavior. New York: Appleton-Century-Crofts; 1969. pp. 279–296. [Google Scholar]
- Kaufman MA, Bolles RC. A nonassociative aspect of overshadowing. Bulletin of the Psychonomic Society. 1981;18:318–320. [Google Scholar]
- Knoedler AJ, Hellwig KA, Neath I. The shift from recency to primacy with increasing delay. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:474–487. [Google Scholar]
- Kremer EF. The Rescorla–Wagner model: Losses in associative strength in compound conditioned stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1978;4:22–36. doi: 10.1037//0097-7403.4.1.22. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Fowler H. Inhibition as a “slave” process: Deactivation of conditioned inhibition through extinction of conditioned excitation. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:71–94. doi: 10.1037//0097-7403.11.1.71. [DOI] [PubMed] [Google Scholar]
- Miller RR, Escobar M. Associative interference between cues and between outcomes presented together and presented apart: An integration. Behavioural Processes. 2002;57:163–185. doi: 10.1016/s0376-6357(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. San Diego, CA: Academic Press; 1988. pp. 51–92. [Google Scholar]
- Miller RR, Oberling P. Analogies between occasion setting and Pavlovian conditioning. In: Schmajuk NA, Holland PC, editors. Occasion setting: Associative learning and cognition in animals. Washington, DC: American Psychological Association; 1998. pp. 3–35. [Google Scholar]
- Neath I. Distinctiveness and serial position effects in recognition. Memory & Cognition. 1993;21:689–698. doi: 10.3758/bf03197199. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes. Anrep GV, translator. New York: Dover; 1927. Original work published 1927. [Google Scholar]
- Postman L, Stark K, Fraser J. Temporal changes in interference. Journal of Verbal Learning and Verbal Behavior. 1968;7:672–694. [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonrein-forcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Shevill I, Hall G. Retrospective revaluation effects in the conditioned suppression procedure. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology. 2004;57B:331–347. doi: 10.1080/02724990344000178. [DOI] [PubMed] [Google Scholar]
- Stout SC, Arcediano F, Escobar M, Miller RR. Overshadowing as a function of trial number: Dynamics of first- and second-order comparator stimuli. Learning & Behavior. 2003;31:85–97. doi: 10.3758/bf03195972. [DOI] [PubMed] [Google Scholar]
- Stout SC, Chang R, Miller RR. Trial spacing is a determinant of cue interaction. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:23–38. [PubMed] [Google Scholar]
- Urcelay GP, Miller RR. Counteraction between overshadowing and degraded contingency treatments: Support for the extended comparator hypothesis. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:21–32. doi: 10.1037/0097-7403.32.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara K, Stout SC, Miller RR. The basic laws of conditioning differ for elemental cues and cues trained in compound. Psychological Science. 2004;15:268–271. doi: 10.1111/j.0956-7976.2004.00664.x. [DOI] [PubMed] [Google Scholar]
- Urushihara K, Wheeler DS, Miller RR. Outcome pre- and postexposure effects: Retention interval interacts with primacy and recency. Journal of Experimental Psychology: Animal Behavior Processes. 2004;30:283–298. doi: 10.1037/0097-7403.30.4.283. [DOI] [PubMed] [Google Scholar]
- Van Hamme LJ, Wasserman EA. Cue competition in causality judgments: The role of nonpresentation of compound stimulus elements. Learning and Motivation. 1994;25:127–151. [Google Scholar]
- Wagner AR. Stimulus validity and stimulus selection in associative learning. In: Mackintosh NJ, Honig WK, editors. Fundamental issues in associative learning. Halifax, Nova Scotia, Can-ada: Dalhousie University Press; 1969. pp. 90–122. [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Wheeler DS, Stout SC, Miller RR. Interaction of retention interval with CS-preexposure and extinction treatments: Symmetry with respect to primacy. Learning & Behavior. 2005;32:335–347. doi: 10.3758/bf03196032. [DOI] [PubMed] [Google Scholar]
- Wright AA, Santiago HC, Sands SF, Kendrick DF, Cook RG. Memory processing of serial lists by pigeons, monkeys, and people. Science. 1985 July 19;229:287–289. doi: 10.1126/science.9304205. [DOI] [PubMed] [Google Scholar]


