Abstract
Retinoic acid (RA) signalling ensures that vertebrate mesoderm segmentation is bilaterally synchronized, and corrects transient interferences from asymmetric left–right (L–R) signals involved in organ lateralization. Snail genes participate in both these processes and, although they are expressed symmetrically in the presomitic mesoderm (PSM), Snail1 transcripts are asymmetrically distributed in the L–R lateral mesoderm. We show that the alteration of the symmetric Snail expression in the PSM induces asynchronous somite formation. Furthermore, in the absence of RA signalling, normal asymmetric Snail1 expression in the lateral mesoderm is extended to the PSM, desynchronizing somitogenesis. Thus, Snail1 is the first cue corrected by RA in the PSM to ensure synchronized bilateral segmentation.
Keywords: Snail, retinoic acid, somitogenesis, left–right, pleiotropy
Introduction
The bilateral symmetry in the body plan of vertebrate embryos is apparent in the somites, which are aligned in rows on either side of the neural tube. The periodic segmentation of the presomitic mesoderm (PSM) generates each pair of epithelial somites. The periodicity of this segmentation by reflected by regular pulses in the expression of components of the Notch and Wnt signalling pathways (Pourquié, 2003). These cycles of expression are symmetric in the left and right PSM, although how they are bilaterally synchronized remains unclear. In the absence of retinoic acid (RA), transient asymmetry is observed in vertebrate somite formation (Kawakami et al, 2005; Vermot et al, 2005; Vermot & Pourquié, 2005). Thus, during a short temporal window—the ‘interference period'—symmetric somitogenesis is protected from left–right (L–R) asymmetric patterning cues by RA. However, the cues that RA buffers have not yet been identified.
Members of the Snail superfamily of transcription factors are expressed in distinct mesodermal territories, where they fulfil different roles (Nieto et al, 1994; Sefton et al, 1998; Carver et al, 2001). Owing to the high degree of modularity and reshuffling shown between Snail family members during vertebrate evolution (Locascio et al, 2002; Sefton et al, 1998), murine Snail and chicken Slug are the members expressed in the PSM (renamed Snail1 and Snail2, respectively; Barrallo-Gimeno & Nieto, 2005). They seem to be functionally equivalent (del Barrio & Nieto, 2002) and the participation of one or the other in a particular process is determined by tissue-specific enhancers in each species (Locascio et al, 2002). In addition to their symmetrical expression in mesoderm territories, the right-hand lateral plate mesoderm (LPM) transiently expresses higher levels of Snail1 than the left-hand side in both chick and mouse embryos (Sefton et al, 1998), reflecting its role in generating L–R asymmetry (Isaac et al, 1997).
Here, we show that the temporal window of L–R asymmetric expression of Snail1 in the LPM coincides with the ‘interference period'. Snail genes are expressed cyclically in the PSM, in which they integrate the Notch, Wnt and FGF signalling pathways and control somite epithelialization (Dale et al, 2006). We show here that their equivalent L–R levels in the PSM are necessary to maintain synchronic somitogenesis. Our data also show that RA blocks the asymmetric expression of Snail1 in the PSM, preventing desynchronization and helping to discriminate between the territories in which Snail fulfils different roles.
Results And Discussion
Asymmetric Snail1 levels at the ‘interference period'
Snail genes encode pleiotropic proteins that fulfil different functions during embryonic development and are simultaneously expressed in different mesodermal territories (Sefton et al, 1998). The expression of these genes in the PSM is cyclical, almost synchronous with genes of the Notch pathway and out of phase with Axin2—a cycling gene from the Wnt pathway (Dale et al, 2006). In addition, Snail1 is transiently expressed asymmetrically in the LPM of chick and mouse embryos, where it influences organ lateralization (Isaac et al, 1997; Sefton et al, 1998). The bilateral synchrony of somitogenesis is protected from the influence of organ lateralization during a short developmental window, known as the ‘interference period'. We show that the transient L–R asymmetric expression of Snail1 in the LPM occurrs at the 4–11 somite stage (HH8−HH10+) in chicken and mouse embryos (Fig 1A–D; data not shown), coinciding with the period in which RA offers protection from asymmetric signals.
Figure 1.
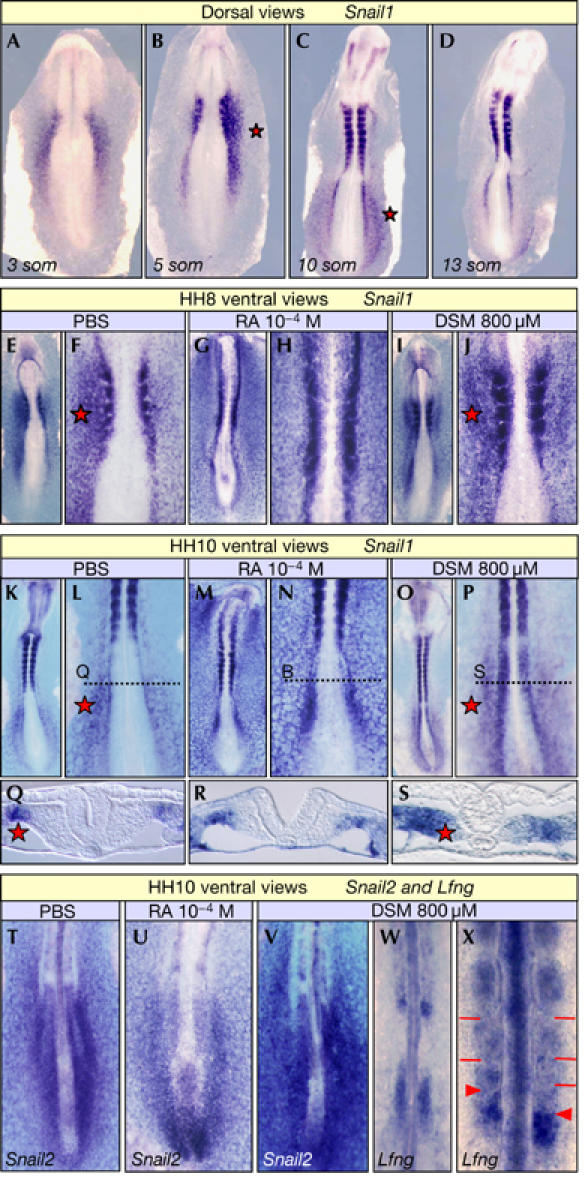
Retinoic acid signalling prevents asymmetric Snail1 expression in the anterior presomitic mesoderm. (A–D) Transient asymmetric left–right (L–R) Snail1 expression in the lateral plate mesoderm (LPM) of 4- to 11-somite (som) chicken embryos (Isaac et al, 1997). (E–X) Embryos incubated with PBS, retinoic acid (RA) or disulphiram (DSM) and analysed at the 4-somite (HH8; E–J) or 10-somite stage (HH10; K–X). (Q), (R) and (S) are sections through the LPM at the levels indicated in (L), (N) and (P), respectively. Embryos were hybridized for Snail1 (A–S), Snail2 (T–V) and Lfng (W,X). Exposure to RA abolishes asymmetric L–R Snail1 expression in the LPM (G,H,M,N) without affecting Snail2 expression in the presomitic mesoderm (PSM; T,U). Asymmetric L–R Snail1 expression invades the anterior PSM in HH10 embryos treated with DSM (O,P,S), which delayed segmentation on the side of highest Snail1 expression (X). Asymmetric L–R expression in the LPM is not affected in these embryos (P), where both Snail2 and Lfng continue cycling in the PSM (V–X). Red stars indicate asymmetric L–R expression, red bars the somite boundaries and red arrowheads the newly formed somite boundaries.
In both species, the territories expressing Snail1 are complementary to those with RA activity (Hochgreb et al, 2003; Vermot et al, 2005). Indeed, Snail1 is expressed asymmetrically in regions devoid of RA activity, the LPM, where it is required for organ lateralization. Conversely, Snail gene expression is bilaterally symmetric in the anterior PSM where RA signalling is active. The inverse correlation between the sites of Snail1 expression and RA signalling suggests that RA might regulate Snail1 expression.
RA prevents asymmetric Snail1 expression in the PSM
To determine whether RA signalling regulates asymmetric Snail1 expression, chicken embryos were exposed to RA or disulphiram (DSM), an Raldh2 inhibitor, at stages when organ lateralization cues emanate from the node (Raya & Izpisua Belmonte, 2004). When analysed just before the interference period (the 4-somite stage; HH8), the asymmetric L–R expression of Snail1 was lost in nearly 70% of the embryos treated with RA (18 out of 26; Fig 1E–H), indicating that RA signalling regulates Snail1 asymmetric expression. However, this asymmetry in Snail1 transcription was maintained in the presence of DSM (Fig 1I,J) and, as previously described, no alterations in bilateral synchronization were observed at this stage (Vermot & Pourquié, 2005).
Interestingly, when the embryos were analysed at the period of maximum interference (HH10), RA continued to abolish the asymmetric L–R Snail1 expression in the LPM (four out of seven; Fig 1K–N,Q,R) without affecting the PSM (Fig 1U). By contrast, downregulation of RA signalling by DSM provoked the appearance of asymmetric L–R Snail1 expression in the anterior PSM (three out of seven; Fig 1O,P,S) without affecting its expression in the LPM. Thus, RA administration exerted a strong influence in the LPM, a tissue devoid of endogenous RA signalling (Fig 1M,N,R), and DSM had a clear impact on the anterior PSM, a site of endogenous RA activity (Fig 1O,P,S). In DSM-treated embryos, both Snail2 and Lfng, a cycling gene from the Notch pathway (McGrew et al, 1998), continued to cycle although their expression was asymmetric (Fig 1V–X). Indeed, diminished RA activity led to asymmetric somitogenesis as described previously (Fig 1X; Vermot & Pourquié, 2005). Thus, our data show that RA signalling regulates Snail1 expression, and that when signalling is blocked Snail1 is expressed asymmetrically in the PSM and asynchronous somitogenesis occurs.
Unilateral Snail1 overexpression delays somitogenesis
We then investigated whether asymmetric Snail expression in the L–R PSM was sufficient to induce asynchronous somitogenesis. We misexpressed Snail1 in one side of the chicken PSM through in ovo electroporation and, as when RA signalling was abolished in chick embryos (Vermot & Pourquié, 2005), increased expression of Snail1 in one side desynchronized somite formation (n=15; Fig 2A–H). Although Snail2 continued cycling (data not shown), somitogenesis was delayed in the side with increased Snail1 expression in two-thirds of the embryos, as assessed by morphology and Uncx4.1 expression (10 out of 15; Fig 2E–H), whereas somite formation progressed synchronously in embryos electroporated with control constructs (n=8; Fig 2A–D). These data indicate that bilateral asymmetric expression of Snail1 is sufficient to induce desynchronization. Thus, it should be avoided in the chick PSM to maintain bilateral synchrony.
Figure 2.
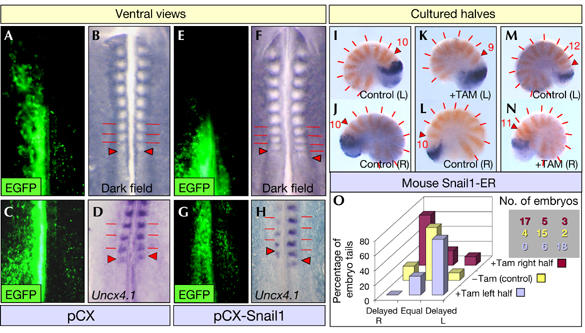
Unilateral Snail1 overexpression delays somite formation in chick and mouse embryos. Expression of EGFP (A,C,E,G), the morphology (B,F) and Uncx4.1 expression (D,H) in chicken embryos electroporated with the EGFP expression vector (pCX) (A–D) or pCX-Snail1 (E–H). Caudal halves of 10.5 dpc Snail1-ER transgenic mouse embryos cultured for 15 h in the presence (K,N) or absence (I,J,L,M) of 4′-OH-tamoxifen and analysed for Lfng (blue), and Uncx4.1 (orange) expression. Red bars indicate somite boundaries and red arrowheads the newly formed somite boundaries. (O) Diagram quantifying the percentage of embryos that showed synchronous or asynchronous somitogenesis after the different conditions in culture. Delayed R, embryos with delayed somitogenesis on the right side; Equal, embryos showing synchronic bilateral somitogenesis; Delayed L, embryos with delayed somitogenesis on the left. The number of embryos represented is also shown in a table that maintains the same colour code and relative position. dpc, days postconception.
In the mouse, Snail1 is also asymmetrically expressed in the LPM during the ‘interference period' (Sefton et al, 1998), but unlike in the chick, it is the family member that cycles synchronously in the L–R PSM (Dale et al, 2006). Thus, in the mouse, the expression of the Snail genes in the LPM and PSM is associated with Snail1. The asynchronous somitogenesis observed in the absence of RA signalling in the mouse (Vermot et al, 2005) suggests that the requirement for bilateral symmetrical Snail1 expression is evolutionarily conserved. However, because this remains to be shown, we generated a transgenic mouse carrying a tamoxifen-inducible form of Snail1 (hereafter Snail1 transgenic; see Methods and supplementary figure online). We cultured bisected caudal regions of Snail1 transgenic embryos 10.5 days postconception (dpc; n=70; Fig 2I–N). When the two halves of these embryos were cultured in medium alone (n=21), we observed asymmetric Lfng expression in less than one-third of the embryos (6 out of 21; Fig 2I,J,O), and there were no discrepancies in somite number in any of the embroys. By contrast, when one-half was cultured in the presence of tamoxifen and the other half was maintained in a control medium (n=49), somitogenesis was delayed in the half that were tamoxifen-treated (35 out of 49, 71%; Fig 2O); there was one somite less compared with the control half (Fig 2K–N). Out of the 49 embryo tails, in which Snail1 was specifically activated in one-half, 25 were right halves (shown in brown in Fig 2O) and 24 were left halves (shown in light purple in Fig 2O). Snail1 overexpression in either the left or right side produced similar results (Fig 2O). These data indicate that this effect was not lateralized and confirm that, as in the chick, increasing the levels of Snail1 expression in one side of the PSM causes a delay in somite formation.
Symmetric somitogenesis requires equal L–R Snail levels
As discussed earlier, and in contrast to the mouse, a Snail family member is expressed in the chick PSM. As they are thought to be functionally equivalent when expressed in similar territories, we checked whether increasing the levels of Snail2 expression in one side of the PSM would induce the same effects as Snail1. When control vectors were electroporated in one side of the embryonic PSM (n=10; Fig 3A,B), or when similar levels of Snail2 were misexpressed in both sides of the PSM (n=18; not shown), there was no clear effect on synchronization. By contrast, unequal misexpression of Snail2 in the left or right somitic mesoderm disrupted somite alignment (17 out of 34), with 66% of these embryos developing fewer somites in the side with higher levels of Snail2 (11 out of 17; Fig 3F,G). We analysed these asymmetries by defining the expression of Lfng and Hairy2, another cycling gene from the Notch pathway (Jouve et al, 2000), in embryos overexpressing Snail2 or a dominant-negative Snail2 construct lacking the zinc-fingers (ΔZf-Snail2; Aybar et al, 2003). Expression of the cycling genes was always bilaterally symmetrical in electroporated control embryos (ten out of ten for Lfng and seven out of seven for Hairy2; Fig 3C,D). By contrast, this symmetry was disrupted by unequal L–R expression in the PSM of either Snail2 (72%, 18 out of 25 for Lfng and 64%, 7 out of 11 for Hairy2; Fig 3H,I) or its dominant-negative form (61%, 14 out of 23 for Lfng and 47%, 8 out of 17 for Hairy2; Fig 3M,N). Like Snail1 overexpression in the mouse, delayed expression occurred in the side exhibiting higher Snail2 expression (83%, 15 out of 18 for Lfng and 86%, six out of seven for Hairy2). The delay in segmentation was also similar to that obtained after increasing Snail1 expression (Fig 2A–H), again reflecting that the two Snail proteins are functionally equivalent when expressed in similar territories (del Barrio & Nieto, 2002; Bolos et al, 2003). Interestingly, somitogenesis was more advanced in the side of the PSM with higher level of ΔZf-Snail2 (81%, 18 out of 22), and an extra somite developed in one-third of these embryos (Fig 3K–N). Thus, the phenotype induced by expressing a Snail2 dominant-negative form was complementary to that found after Snail2 overexpression.
Figure 3.
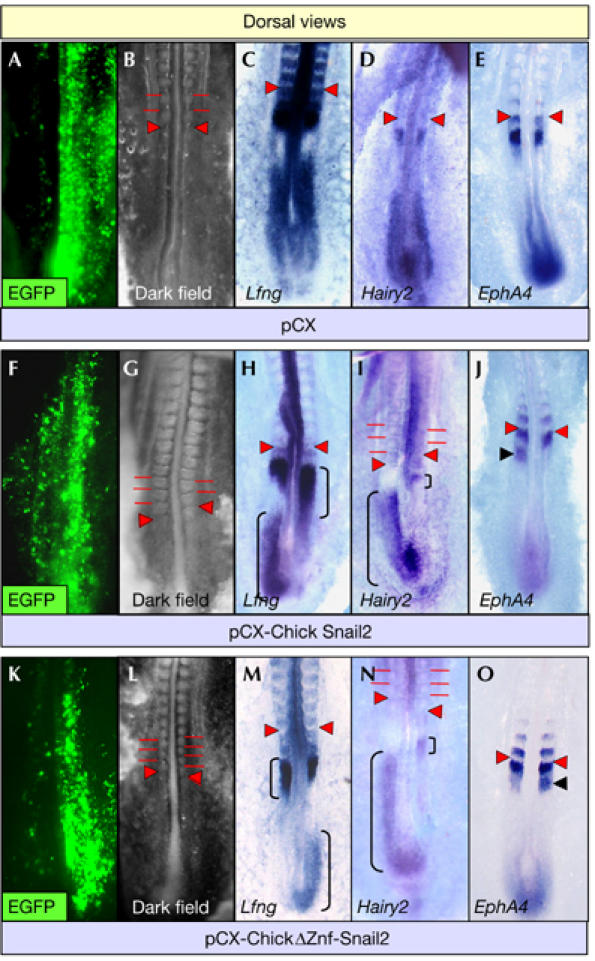
Delayed or accelerated somite formation provoked by Snail2 overexpression or dominant–negative Snail2 expression in the chick. Chicken embryos electroporated with pCX-dEGFP plus pCX (A–E), pCX-Snail2 (F–J) or a dominant-negative form of Snail2 (pCX-ΔZf-Snail2; K–O) showing dEGFP expression (A,F,K), their morphology (B,G,L) and the expression of Lfng (C,H,M), Hairy2 (D,I,N) and EphA4 (E,J,O). High levels of ectopic expression were observed in the right presomitic mesoderm (PSM) of the embryos. Brackets indicate the progress of PSM expression. The asymmetric phase of the cycling genes represented in this figure corresponds to embryos in which the differences were more apparent. Red bars indicate somite boundaries, red arrowheads the newly formed boundaries and black arrowheads indicate an extra band of EphA4 expression.
The EphA4 receptor is a marker of somite epithelialization and boundary formation (Barrios et al, 2003), and its symmetric expression (10 out of 11; Fig 3E) was also disrupted by these constructs. One stripe was lost in the side with higher Snail2 expression (7 out of 13; Fig 3J), whereas an extra stripe appeared in the side with higher levels of ΔZf-Snail2 (three out of nine; Fig 3O). Our data from studies on chick and mouse embryos confirm that Snail genes regulate somite boundary formation and that increased Snail activity delays epithelialization. This is in agreement with recent data showing that the downregulation of Snail genes in the anterior PSM determines the time of epithelialization (Dale et al, 2006), and the role of Snail in maintaining the mesenchymal phenotype of undifferentiated cells (reviewed by Nieto, 2002).
Significantly, we observed the same phenotypes in embryos electroporated at different developmental times, up to the 30-somite stage (HH16). Thus, regardless of the role of the cyclical expression of Snail genes in the posterior mesoderm and in epithelialization, equivalent L–R levels of Snail expression in the PSM are necessary to maintain synchronous bilateral segmentation during somitogenesis in both chick and mouse embryos. This reflects the requirement that the L–R asymmetric Snail1 expression is excluded from the anterior PSM and also explains the temporal coincidence of the ‘interference period' with the asymmetric L–R Snail1 expression in the embryo. Before this period, the inhibition of RA signalling does not have any effect on somite synchronization or Snail1 expression. At the period of maximal interference, when RA signalling is inhibited, asymmetric Snail1 expression develops in the anterior PSM and induces desynchronization. In conclusion, endogenous RA activity in the anterior PSM acts as a barrier that prevents the entry of the asymmetric Snail1 expression directed by the L–R patterning signals in the region where somite boundaries form, thereby ensuring bilateral symmetry during somitogenesis (Fig 4).
Figure 4.
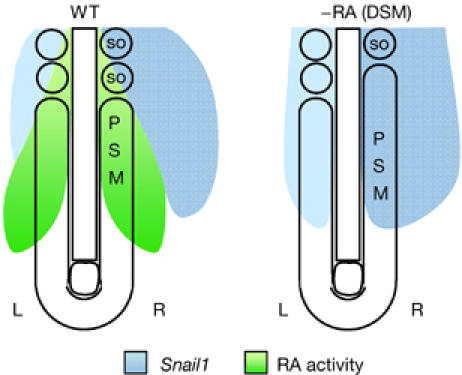
Inhibition of retinoic acid signalling provokes asymmetric Snail1 expression in the presomitic mesoderm inducing desynchronization in somite formation. Diagram depicting RA signalling activity and Snail1 expression in the wild-type embryo and after inhibiting RA signalling. Endogenous RA prevents the invasion of the left–right (L–R) asymmetric Snail1 expression in the PSM and ensures synchronic somitogenesis. DSM, disulphiram; PSM, presomitic mesoderm; RA, retinoic acid; WT, wild type.
The Snail gene family is a good example of pleiotropic genes that might cause interferences when the different developmental processes in which they are involved occur simultaneously in adjacent or overlapping regions. Under these circumstances, correction mechanisms, such as bilaterally symmetric somitogenesis and asymmetric organ lateralization, are required when the processes conflict.
Methods
Embryo dissection and in situ hybridization. Chicken and mouse embryo staging, and the method for caudal bilateral dissections and explant culture are described by Morales et al (2002). Whole-mount in situ hybridization was carried out as described by Sefton et al (1998) by using the chick Snail1, Snail2, Lfng and Lfng intronic, Hairy2, EphA4 and Uncx4.1, and the mouse Snail1, Lfng and Uncx4.1 riboprobes (Irving et al, 1996; Sefton et al, 1998; Jouve et al, 2000; Morales et al, 2002; Uncx4.1, BBSRC chicken clone ChEST47F8). In some cases, 40 μm vibratome sections were used from gelatin-embedded embryos.
Plasmids. The EGFP expression vector (pCX-dEGFP) contains a destabilized EGFP construct (d1EGFP, Clontech, Mountain View, CA, USA) with a half-life of approximately 1 h in the pCAAGS expression vector (Niwa et al, 1991). The full-length chicken Snail1 and Snail2 coding sequences (Sefton et al, 1998) and a truncated Snail2 construct (aa 1–134) were cloned into the pCAAGS expression vector (pCX-Snail1/2 and pCX-ΔZf-Snail2, respectively). These pCX plasmids were electroporated at concentrations of 3 μg/μl with 1 μg/μl of pCX-EGFP. The empty expression vector and pCX-dEGFP were electroporated into control embryos. The fusion protein between Snail1 and a modified human oestrogen receptor (pCMVSnail1-ERT2) was generated by cloning the mouse Snail1 coding region into the pCre-ERT2 expression vector (a generous gift from Dr Pierre Chambon of Feil et al, 1997) before transferring it into pcDNA3.
In ovo electroporation and chicken embryo culture. Stage HH5 embryos were electroporated as described previously (Dubrulle et al, 2001). A train of electric pulses (six pulses, 30 V, 50 ms) was applied by using a square wave electroporator (Intracel TSS20). Embryos were left for 20–40 h (mostly 30 h) and assayed for dEGFP expression. Embryos with a normal overall morphology and good levels of EGFP expression in the PSM were processed for in situ hybridization. Chicken embryos were explanted at stage HH4 and cultured as described by Chapman et al (2001). Where appropriate, the embryos were exposed to 100 μl of RA (100 μM) and DSM (800 μM) in PBS and the treated embryos were processed for in situ hybridization.
Transgenic mice, PSM culture and tamoxifen induction. A transgenic mouse for pCMV-Snail1-ERT2 was generated (Hogan et al, 1994) in which the constitutively expressed protein is only functional when translocated into the nucleus upon tamoxifen administration (see supplementary figure online). Bisected 10.5 dpc caudal halves (PSM plus three somites) were cultured for 15 h as described by Morales et al (2002), with 50 μg/ml of gentamycin (Gibco, Carlsbad, CA, USA) in the culture medium. In culture, 6–7 new somites were formed and, where appropriate, the cultured halves were exposed to 600 nM 4-OH-tamoxifen (Sigma, St Louis, MO, USA; Feil et al, 1997).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank M. Torres and J. Galceran for comments on the manuscript; B. Lázaro, J. Chuliá and C. López for technical assistance; K. Dale for assistance with electroporation; and M. Okabe, J. Miyazaki, O. Pourquié, H. Peters and P. Chambon for reagents. This work was funded by grant BFU2005-00762 to A.V.M., and grants BFU2004-02665, BFU2005-05772 and NAN2004-09230-C04-04 to M.A.N. C.A.F. was supported by BFU2004-02665 and NAN2004-09230-C04-04, A.V.M. by Advancell S.L. and the Ramon y Cajal Programs. A.V.M. and H.A. were also supported by the I3P Programme (European Social Fund/MEC), H.A. and O.H.O. by Spanish Ministry fellowships and V.G. by the Institute of International Cooperation.
References
- Aybar MJ, Nieto MA, Mayor R (2003) Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development 130: 483–494 [DOI] [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Nieto MA (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132: 3151–3161 [DOI] [PubMed] [Google Scholar]
- Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW (2003) Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr Biol 13: 1571–1582 [DOI] [PubMed] [Google Scholar]
- Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A (2003) The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci 116: 499–511 [DOI] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, Gridley T (2001) The mouse snail gene encodes a key regulator of the epithelial–mesenchymal transition. Mol Cell Biol 21: 8184–8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A (2001) Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dyn 220: 284–289 [DOI] [PubMed] [Google Scholar]
- Dale JK, Malapert P, Chal J, Vilhais-Neto G, Maroto M, Johnson T, Jayasinghe S, Trainor P, Herrmann B, Pourquié O (2006) Oscillations of the snail genes in the presomitic mesoderm coordinate segmental patterning and morphogenesis in vertebrate somitogenesis. Dev Cell 10: 355–366 [DOI] [PubMed] [Google Scholar]
- del Barrio MG, Nieto MA (2002) Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development 129: 1583–1593 [DOI] [PubMed] [Google Scholar]
- Dubrulle J, McGrew MJ, Pourquié O (2001) FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell 106: 219–232 [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P (1997) Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun 237: 752–757 [DOI] [PubMed] [Google Scholar]
- Hochgreb T, Linhares VL, Menezes DC, Sampaio AC, Yan CY, Cardoso WV, Rosenthal N, Xavier-Neto J (2003) A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development 130: 5363–5374 [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Constantini F, Lacy E (1994) Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Irving C, Nieto MA, DasGupta R, Charnay P, Wilkinson DG (1996) Progressive spatial restriction of Sek-1 and Krox-20 gene expression during hindbrain segmentation. Dev Biol 173: 26–38 [DOI] [PubMed] [Google Scholar]
- Isaac A, Sargent MG, Cooke J (1997) Control of vertebrate left–right asymmetry by a snail-related zinc finger gene. Science 275: 1301–1304 [DOI] [PubMed] [Google Scholar]
- Jouve C, Palmeirim I, Henrique D, Beckers J, Gossler A, Ish-Horowicz D, Pourquié O (2000) Notch signalling is required for cyclic expression of the hairy-like gene HES1 in the presomitic mesoderm. Development 127: 1421–1429 [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Raya A, Raya RM, Rodriguez-Esteban C, Belmonte JC (2005) Retinoic acid signalling links left-right asymmetric patterning and bilaterally symmetric somitogenesis in the zebrafish embryo. Nature 435: 165–171 [DOI] [PubMed] [Google Scholar]
- Locascio A, Manzanares M, Blanco MJ, Nieto MA (2002) Modularity and reshuffling of Snail and Slug expression during vertebrate evolution. Proc Natl Acad Sci USA 99: 16841–16846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew MJ, Dale JK, Fraboulet S, Pourquié O (1998) The lunatic fringe gene is a target of the molecular clock linked to somite segmentation in avian embryos. Curr Biol 8: 979–982 [DOI] [PubMed] [Google Scholar]
- Morales AV, Yasuda Y, Ish-Horowicz D (2002) Periodic Lunatic fringe expression is controlled during segmentation by a cyclic transcriptional enhancer responsive to notch signaling. Dev Cell 3: 63–74 [DOI] [PubMed] [Google Scholar]
- Nieto MA (2002) The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 3: 155–166 [DOI] [PubMed] [Google Scholar]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J (1994) Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264: 835–839 [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108: 193–199 [DOI] [PubMed] [Google Scholar]
- Pourquié O (2003) The segmentation clock: converting embryonic time into spatial pattern. Science 301: 328–330 [DOI] [PubMed] [Google Scholar]
- Raya A, Izpisua Belmonte JC (2004) Unveiling the establishment of left–right asymmetry in the chick embryo. Mech Dev 121: 1043–1054 [DOI] [PubMed] [Google Scholar]
- Sefton M, Sanchez S, Nieto MA (1998) Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development 125: 3111–3121 [DOI] [PubMed] [Google Scholar]
- Vermot J, Pourquié O (2005) Retinoic acid coordinates somitogenesis and left–right patterning in vertebrate embryos. Nature 435: 215–220 [DOI] [PubMed] [Google Scholar]
- Vermot J, Gallego Llamas J, Fraulob V, Niederreither K, Chambon P, Dolle P (2005) Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science 308: 563–566 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


