Abstract
Rab5, a small guanosine triphosphatase, is known to regulate the tethering and docking reaction leading to SNARE (soluble NSF attachment protein receptors)-mediated fusion between endosomes. However, it is uncertain how the signal of the activated Rab5 protein is transduced by its downstream effectors during endosome fusion. Here, we show that the Sec1/Munc18 gene vps-45 is essential for not only viability and development but also receptor-mediated and fluid-phase endocytosis pathways in Caenorhabditis elegans. We found that VPS-45 interacts with a Rab5 effector, Rabenosyn-5 (RABS-5), and the mutants of both vps-45 and rabs-5 show similar endocytic phenotypes. In the macrophage-like cells of vps-45 and rabs-5 mutants, aberrantly small endosomes were accumulated, and the endosome fusion stimulated by the mutant RAB-5 (Q78L) is suppressed by these mutations. Our results indicate that VPS-45 is a key molecule that functions downstream from RAB-5, cooperating with RABS-5, to regulate the dynamics of the endocytic system in multicellular organisms.
Keywords: vps-45, rabenosyn-5, Sec1/Munc-18, endocytosis, Caenorhabditis elegans
Introduction
Endocytosis is an important cellular process in all eukaryotic cells. In endocytic pathways, endosomes undergo continuous fusion and fission for sorting internalized materials (Zerial & McBride, 2001). Membrane fusion is controlled by three main protein families: Rab [GTPase] (guanosine triphosphatase), SNARE (soluble NSF attachment protein receptors) and SM (Sec1/Munc18; Jahn et al, 2003). It is widely accepted that Rab GTPase proteins regulate specific tethering and docking reactions (initial recognition between two membranes) and SNARE proteins execute fusion reactions as the core of the fusion machinery (Jahn et al, 2003). However, the functions of SM proteins have not yet been clarified.
An SM family is a group of hydrophilic proteins of 60–80 kDa, which consist of three domains (Misura et al, 2000). The loss of activity of each SM member leads to severe impairment of specific transport pathways in various species (Toonen & Verhage, 2003). For example, loss of Sly1 blocks the ER-to-Golgi transport, loss of Vps45 and Vps33 blocks the Golgi-to-vacuole transport and loss of Munc18-1 blocks the neurotransmitter release in neuronal cells (Toonen & Verhage, 2003). Intensive studies have shown that SM proteins interact with some syntaxin proteins, which are classified as Qa-SNAREs (Fasshauer et al, 1998; Toonen & Verhage, 2003). However, it is unknown whether SM proteins function primarily by their interaction with the cognate syntaxins, as the mechanisms underlying the interactions are divergent (Dulubova et al, 2003; Gallwitz & Jahn, 2003).
Previous studies using yeast have demonstrated that the SM genes interact genetically with the Rab/Ypt GTPase genes in various transport pathways (Dascher et al, 1991; Tall et al, 1999; Toonen & Verhage, 2003). Although no physical interactions between these families have been found so far, several SM proteins bind directly to Rab effectors (Tall et al, 1999; Coppola et al, 2002). A recent report has suggested that the human SM protein (VPS45)—whose orthologous protein in yeast (Vps45) is required for a transport pathway from the Golgi to vacuoles (biosynthetic pathway)—binds to the Rab5 effector Rabenosyn-5 (RABS-5; Nielsen et al, 2000). It has been recognized that Vps45 is not required for endocytic transport, as deletion of Vps45 does not affect trafficking of Ste3 receptors and FM4–64 from the plasma membrane to vacuoles in yeast cells (Bryant et al, 1998). Conversely, the fact that Rab5 functions in endocytic transport in mammalian cells (Zerial & McBride, 2001) implies that Vps45 in multicellular organisms might have a further role in the endocytic pathway. However, there was no functional evidence that SM proteins have a role in the endocytic pathways of multicellular organisms. Also, in vivo functions of the Vps45 orthologues have not yet been investigated in a model system of the metazoan. In this study, we analysed the Caenorhabditis elegans orthologues of Vps45 and its interacting proteins by using a reverse genetics approach.
Results and Discussion
vps-45 mutation causes severe endocytosis defects
The C. elegans genome has a single gene (vps-45) that is highly homologous to yeast Vps45 and human VPS45 (Bock et al, 2001). We found that a translational fusion gene (vps-45∷EGFP) is expressed in all main tissues from the early embryonic stage to adulthood (supplementary Fig 2 online), indicating that the vps-45 protein is ubiquitously expressed during development. To gain an insight into the function of VPS-45, we isolated a deletion allele vps-45(tm246) in this genomic locus (supplementary Fig 1 online). tm246 has a deletion extending from the promoter to the fourth exon and is probably a null allele. Homozygous progenies segregating from heterozygous mothers grow to fertile adulthood. However, their self-progenies show ts (temperature sensitive) lethality, as they reach adulthood at 15°C but are arrested in the early larval stage at 25°C (supplementary Fig 2 online). A shift to a higher temperature at any stage results in developmental arrest. The arrested larvae show intestinal phenotypes such as an enlarged lumen and accumulation of large refractile granules, which are thought to be lysosome-related organelles in the intestine (supplementary Fig 2 online). Electron microscopy showed the presence of multilamellar bodies (MLBs) in the mutant intestine, which are typical structures observed in the cells that accumulate that cholesterol in lysosomes (Lajoie et al, 2005; supplementary Fig 2 online). In addition, the arrested larvae are often encased in the old cuticle (supplementary Fig 2 online). The genetic properties and the moulting-defective phenotype in the vps-45 mutant resemble those of wild-type (WT) worms grown on cholesterol-depleted medium. As cholesterol is endocytosed with yolk proteins into oocytes (Matyash et al, 2001), we tested whether receptor-mediated endocytosis of yolk proteins is defective in vps-45 mutants.
Oocyte uptake of yolk proteins was visualized by the vitellogenin∷EGFP (YP170/EGFP; EGFP for enhanced green fluorescent protein) reporter (Grant & Hirsh, 1999). We found that the vps-45 mutant exhibits the receptor-mediated endocytosis-defective (Rme) phenotype—that is, YP170/EGFP is not endocytosed by oocytes at 25°C (Fig 1A). This phenotype was rescued by expression of vps-45 (+) complementary DNA (Fig 1A). To investigate whether defective endocytosis is also observed in the other tissues of vps-45 mutant worms, we checked the fluid-phase endocytosis by scavenger cells called coelomocytes. We used Pmyo-3∷ssGFP transgenic worms in which GFP expressed in body wall muscles is secreted into the pseudocoelom (Fares & Greenwald, 2001). In the WT background, pseudocoelomic GFP was efficiently endocytosed by coelomocytes and bright fluorescent vesicles were seen in these cells (Fig 1B). Secreted GFP accumulated in the pseudocoelom at 25°C in the vps-45 mutant (Fig 1B), which is consistent with its phenotype showing defects in coelomocyte uptake (Cup). The Cup phenotype was rescued when vps-45 (+) cDNA was expressed in the vps-45 mutant coelomocytes, indicating a cell-autonomous function of VPS-45 protein (data not shown).
Figure 1.
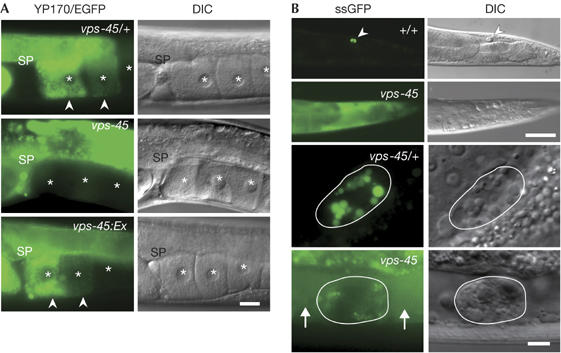
Endocytosis defects in the vps-45 mutant. (A) Receptor-mediated endocytosis-defective (Rme) phenotype. Adult hermaphrodites expressing the YP170/EGFP are shown. Arrowheads indicate oocytes endocytosing the fusion protein. Asterisks show the nucleus of oocytes. SP, spermatheca. Scale bar, 20 μm. (B) Coelomocyte uptake-defective (Cup) phenotype. Adult hermaphrodites carrying Pmyo-3∷ssGFP secrete GFP from their muscles into the body cavity. The upper panels show worms at a low magnification and the lower panels show individual coelomocytes at a high magnification. Arrowheads indicate coelomocytes endocytosing GFP. Arrows indicate pseudocoelomic GFP. Individual coelomocytes are outlined in white. Scale bars, 50 μm (upper) and 5 μm (lower). DIC, differential interference contrast; GFP, green fluorescent protein (EGFP, enhanced GFP).
To investigate further which step is defective when endocytosed materials are transported through different endosomal compartments in a vps-45 mutant, we carried out a pulse–chase analysis by using Texas-Red-conjugated BSA (TR–BSA; Zhang et al, 2001). TR–BSA was injected into the pseudocoeloms of WT and vps-45 worms expressing an endosomal marker RME-8/GFP (Zhang et al, 2001). TR–BSA was initially present in RME-8/GFP–labelled endosomes in both WT and vps-45 coelomocytes (Fig 2). In the WT, TR–BSA subsequently accumulated into discrete foci, which might represent endocytic intermediates, and finally reached RME-8-negative compartments by 30 min (Zhang et al, 2001; Fig 2A). The RME-8-negative compartments are probably lysosomes, because TR–BSA reached lysosomal-associated membrane protein orthologue (LMP-1)/GFP-labelled compartments at 45 min after injection (supplementary Fig 3 online). By contrast, TR–BSA remained in RME-8-positive endosomes in vps-45 mutant coelomocytes at 30 min after TR–BSA injection, and thus delivery of endocytic cargoes from endosomes to lysosomes is retarded (13 out of 21 coelomocytes; Fig 2B). In some cases (8 out of 21 coelomocytes), we could see little or no uptake of TR–BSA (data not shown; supplementary Fig 3 online). Thus, Rme and Cup phenotypes of the vps-45 mutant and the impairments in uptake of TR–BSA in the vps-45 mutant coelomocytes indicate that VPS-45 might affect a common molecular step in both receptor-mediated endocytosis and fluid-phase endocytosis.
Figure 2.
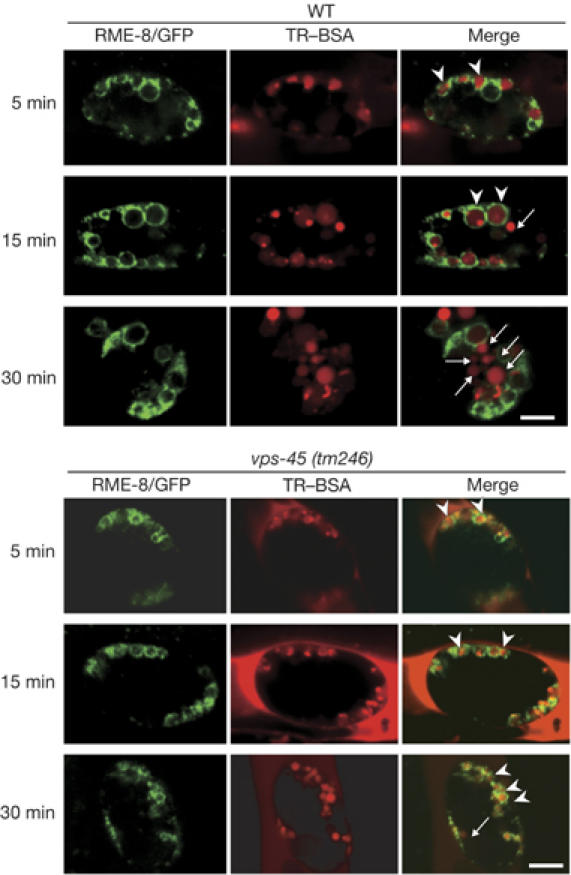
Impairments of Texas-Red-conjugated BSA uptake by vps-45 mutant coelomocytes. The confocal micrographs show a typical pattern of TR-BSA fluorescence (red) endocytosed by coelomocytes. After microinjection of TR-BSA into the pseudocoelom, animals expressing RME-8/GFP for an endosomal marker were incubated for the indicated time (left). The upper panels show WT and the lower panels show the vps-45 mutant. Arrowheads indicate concentrations of TR-BSA in compartments labelled with RME-8/GFP. Arrows indicate RME-8/GFP-negative vesicles, probably lysosomes (see supplementary Fig 3 online). Scale bar: 5 μm in all images. Rme, receptor-mediated endocytosis defective; TR–BSA, Texas-Red-conjugated BSA.
rabs-5 causes the same endocytic phenotypes as vps-45
To explain the mechanism of the endocytic function of vps-45, we first analysed the cognate syntaxin genes for vps-45. Vps45 interacts with several syntaxins: Pep12 and Tlg2 in yeast (Burd et al, 1997; Nichols et al, 1998), and Pep12-like syntaxin 13, Tlg2-like syntaxin 16 and syntaxin 4 in mammals (Nielsen et al, 2000; Yamaguchi et al, 2002). We found that C. elegans VPS-45 interacts specifically with SYN-13 (orthologue of syntaxin 13) and SYN-16 (orthologue of syntaxin 16) among all nine syntaxins on the C. elegans genome (Bock et al, 2001; supplementary Fig 4 online). The syn-13(tm2037), syn-16(tm1560) and syn-13(tm2037);syn-16(tm1560) deletion mutants showed no obvious endocytic defects, including Cup and Rme (data not shown; supplementary Fig 5 online).
We next investigated whether vps-45 functions with C. elegans Rabenosyn-5 (rabs-5) in vivo. We found that VPS-45 interacts physically with RABS-5, as reported previously in mammals (Nielsen et al, 2000; Fig 3A). Neither RAB-5 nor early endosomal antigen 1, which is another Rab5 effector with the FYVE domain, interacted with VPS-45 (Fig 3A), suggesting the specificity of interaction between VPS-45 and RABS-5. The rabs-5 mutant worms (tm2036 and ok1513) were indistinguishable from the vps-45 mutant worms in both the ts lethality (data not shown) and the ts endocytic defects (Fig 3B). In addition, defects in rabs-5; vps-45 double mutants were not more severe than the single mutants (data not shown). These results indicate that the VPS-45 and RABS-5 proteins might form a complex and function in the same endocytic pathway.
Figure 3.
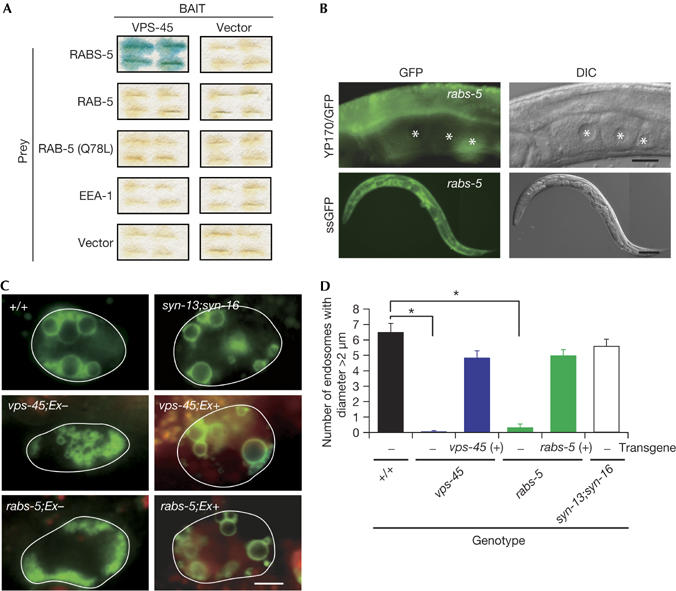
C. elegans Rabenosyn-5 mutant shows a phenocopy of the vps-45 mutant in various endocytic defects. (A) Yeast two-hybrid assay. (B) Receptor-mediated endocytosis-defective (Rme) and coelomocyte uptake (Cup) phenotypes in Rabenosyn-5 (rabs-5) mutant. (C) Individual coelomocytes from animals expressing RME-8/GFP. (D) Quantitative analysis of (C). Error bars indicate s.e.m. *P<0.001. Scale bars, 20 μm (B, upper), 50 μm (B, lower) and 5 μm (C). DIC, differential interference contrast; GFP, green fluorescent protein.
VPS-45 is required for endosomal dynamics
Previous work using in vitro fusion assay has suggested that Rabenosyn-5 might regulate homotypic fusion between endosomes in mammalian cells (Nielsen et al, 2000). To test whether VPS-45 and RABS-5 are involved in regulating endosomal dynamics, we investigated the size of endosomes using the RME-8/GFP endosomal marker in the vps-45 and rabs-5 mutant coelomocytes. We constructed transgenic mosaic strains, which have vps-45 (+) or rabs-5 (+) cDNA on the extrachromosomal (Ex) array in the respective mutant backgrounds. Both vps-45 and rabs-5 mutant coelomocytes, in which Ex arrays are lost (vps-45;Ex−, rabs-5;Ex−), contained aberrantly small RME-8/GFP vesicles accumulated in the peripheral regions (Fig 3C,D). These endosomal phenotypes were restored in mutant coelomocytes with Ex arrays (vps-45;Ex+, rabs-5;Ex+; Fig 3C,D). We found that endosomes labelled with another endosomal marker containing 2 × FYVE domain (Dang et al, 2004) are also small in the vps-45 mutant coelomocytes (data not shown). However, there was no obvious reduction in the size of LMP-1/GFP-labelled lysosomes (supplementary Fig 3 online), suggesting that the defects of endosomes are specific in the endocytic compartments of vps-45 mutants. The size of endosomes in syn-13, syn-16 and the syn-13;syn-16 mutant coelomocytes was in the normal range (Fig 3C,D; data not shown).
Taken together, the above data suggest that VPS-45 might function in Rab5-dependent fusion between endosomes, probably with RABS-5. To ascertain this, we expressed a GTP-locked, dominant active form of RAB-5 (Q78L), which promotes homotypic endosome fusion in C. elegans (Patton et al, 2005). RAB-5 (Q78L) expression induced enlarged endosomes filled with GFP in WT coelomocytes (Fig 4). However, we rarely detected these enlarged endosomes in both vps-45 and rabs-5 mutant coelomocytes (Fig 4). These results indicate that VPS-45 and RABS-5 proteins are required for Rab5-mediated endosome fusion. By contrast, the endosomal enlargement was not suppressed by the syn-13, syn-16 and syn-13;syn-16 mutations (Fig 4; supplementary Fig 6 online).
Figure 4.
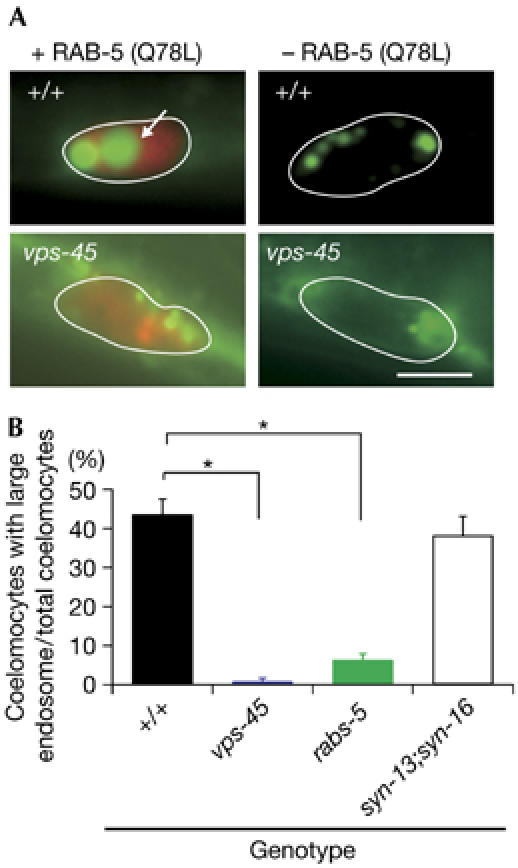
vps-45 and rabs-5 mutations suppress RAB-5 (Q78L)-induced endosome fusion. (A) Individual coelomocytes from wild-type (WT) or vps-45, carrying the Pmyo-3∷ssGFP array and RAB-5 (Q78L) array (+RAB-5Q78L), or carrying only the Pmyo-3∷ssGFP array (−RAB-5 (Q78L)). RAB-5 (Q78L)-induced large endosomes accumulating GFP were detectable in WT (arrow) but not in the vps-45 mutant background. Scale bar, 5 μm. (B) Quantitative analysis of (A). In coelomocytes with red fluorescence, percentages of the coelomocyte with large endosomes per total coelomocyte were calculated. Error bars indicate s.e.m. *P<0.001. GFP, green fluorescent protein; RAB-5, Rab5 homologue; RABS-5, Rabenosyn-5; SYN-13 and SYN-16, Syntaxin; WT, wild type.
Our results provide strong evidence that VPS-45 has a crucial role in the endocytic system of C. elegans, cooperating with RAB-5 effector RABS-5. Specific interactions of VPS-45 with its cognate syntaxins (supplementary Fig 4 online) suggest that these SNAREs might also act in endocytic trafficking. However, unlike severe endocytic phenotypes of both vps-45 and rabs-5 mutants, endocytic events in syn-13;syn-16 double mutants were not affected. To examine whether the other syntaxins are necessary for RAB-5-dependent endocytic transport, we carried out a large endosome assay for all C. elegans syntaxins. We did not detect any depletions in syntaxins that resulted in defects in this assay (supplementary Fig 6 online). The lack of syntaxins might be compensated by the redundant functions of other syntaxins and/or non-syntaxin SNAREs in mutant cells. syn-13 and syn-16 are not essential in endocytic pathways, indicating that the binding of VPS-45 to them might not necessarily be required for the proper function of VPS-45. Consistent with our results, the mutant forms of SM proteins, which abolish the high-affinity interactions with their cognate syntaxins, are fully functional in yeast (Peng & Gallwitz, 2004; Carpp et al, 2006). They also indicated that SM proteins bind directly not only to cognate syntaxins but also to several SNAREs including nonsyntaxin t-SNAREs and v-SNAREs (Peng & Gallwitz, 2004; Carpp et al, 2006). One possibility is that the primary role of VPS-45 might be mediated by binding to RABS-5 and/or SNAREs other than syntaxins in endocytic pathways. By contrast, studies using mammalian cells have suggested that the interaction between SM and syntaxin is biologically important and required for transport in the early secretory pathway (Yamaguchi et al, 2002; Dulubova et al, 2003; Williams et al, 2004). Further studies with the C. elegans system are needed for understanding the essential function of VPS-45, uncovering the physiological significance of interactions with its multiple partners.
Speculations
We found that the murine orthologue (VPS45) is functionally interchangeable with vps-45, but the yeast orthologue is not (supplementary Fig 7 online). vps-45/Vps45 might have evolved to gain the endocytic function in multicellular organisms, as their transport pathways are required to be more complex than monocellular organisms. An additional uncoordinated movement (Unc) phenotype of the vps-45 mutant (data not shown) might imply the presence of vesicle recycling defects in neuronal synapses. Future investigations into the roles of VPS-45 in integrating upstream RAB-5-dependent regulation and execution of membrane fusion by SNARE will allow us to understand not only the regulatory mechanism of endosome fusion but also human diseases associated with the defects of endocytic transport in various tissues.
Methods
C. elegans strains and culture conditions. C. elegans strains were cultured using standard techniques. All strains were derived from the WT Bristol strain N2. Strains carrying vps-45(tm246), rabs-5(ok1513) and rabs-5(tm2036) were grown at the permissive temperature 15°C. Various phenotypes of these strains were examined when worms were shifted to the restrictive temperature 25°C. For Rme phenotypes of vps-45 and rabs-5 mutants, homozygous animals (m+z−) derived from heterozygous mothers were examined.
Genetics. Genetic crosses were carried out by standard genetic protocols. The deletion alleles were identified by PCR amplification with primers spanning the deleted region.
Pulse–chase analysis in coelomocytes. Texas-Red-conjugated BSA (TR–BSA, Sigma) (1 mg/ml) was injected into the pseudocoelom of adult worms, incubated at 25°C for the indicated time period and assayed as described previously (Zhang et al, 2001). vps-45(tm246);bIs34 and vps-45(tm246);pwIs50 were shifted to the restrictive temperature (25°C) for 48 h before this assay.
Analysis of the RME-8/GFP-positive endosomes. bIs34, vps-45(tm246);bIs34;tmEx1315, rabs-5(ok1513);bIs34;tmEx1387 and syn-13(tm2037);syn-16(tm1560);bIs34 were used for analysing the size of endosomes in coelomocytes. tmEx1315 has an extrachromosomal array that rescues the tm246 mutation, and the tmEx1387 has an extrachromosomal array that rescues the ok1513 mutation. Confocal images of the coelomocytes expressing RME-8/GFP from adult hermaphrodites were taken by z-sectioning at 1 μm intervals. The average number of RME-8/GFP-positive endosomes with a diameter of 2 μm or more was counted by analysing a series of z-sectioning images with IP-lab software. All strains were grown at 25°C. In the vps-45(tm246) and rabs-5 (ok1513) mutations, two types of coelomocyte were analysed: the coelomocytes that carry the array (DsRed positive, depicted as vps-45;Ex+ or rabs-5;Ex+ in Fig 3) and the coelomocytes that have lost the array (DsRed negative, depicted as vps-45;Ex− or rabs-5;Ex− in Fig 3). At least 20 coelomocytes were analysed for each strain.
Statistics. Statistical analysis was carried out using analysis of variance followed by Tukey–Kramer test to localize the significant difference. P<0.001 was considered significant. All statistics were run with R (R project http://www.r-project.org/).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Fig 1
Supplementary Fig 2
Supplementary Fig 3
Supplementary Fig 4
Supplementary Fig 5
Supplementary Fig 6
Supplementary Fig 7
Supplementary Information
Acknowledgments
Sadly, Kazushi Fujimoto died on 28 November 2003. We thank Dr DH Hall for helpful advice on electron microscopy. rabs-5 (ok1513) was kindly provided by the Caenorhabditis elegans Gene Knockout Consortium (Oklahoma Medical Research Foundation, Oklahoma City). bIs34[rme-8∷GFP] was kindly provided by Dr B Grant (Zhang et al, 2001). arIs37[Pmyo-3∷ssGFP] (Fares & Greenwald, 2001) and pwIs50 [lmp-1∷GFP] (Treusch et al, 2004) were obtained from the Caenorhabditis Genetics Center. This study was supported in part by grants from Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Japan Science and Technology Agency (JST) to S.M. and K.G.-A.
References
- Bock JB, Matern HT, Peden AA, Scheller RH (2001) A genomic perspective on membrane compartment organization. Nature 409: 839–841 [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Piper RC, Gerrard SR, Stevens TH (1998) Traffic into the prevacuolar/endosomal compartment of Saccharomyces cerevisiae: a VPS45-dependent intracellular route and a VPS45-independent, endocytic route. Eur J Cell Biol 76: 43–52 [DOI] [PubMed] [Google Scholar]
- Burd CG, Peterson M, Cowles CR, Emr SD (1997) A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol Biol Cell 8: 1089–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpp LN, Ciufo LF, Shanks SG, Boyd A, Bryant NJ (2006) The Sec1p/Munc18 protein Vps45p binds its cognate SNARE proteins via two distinct modes. J Cell Biol 173: 927–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola T, Frantz C, Perret-Menoud V, Gattesco S, Hirling H, Regazzi R (2002) Pancreatic β-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis. Mol Biol Cell 13: 1906–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang H, Li Z, Skolnik EY, Fares H (2004) Disease-related myotubularins function in endocytic traffic in Caenorhabditis elegans. Mol Biol Cell 15: 189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C, Ossig R, Gallwitz D, Schmitt HD (1991) Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol 11: 872–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Yamaguchi T, Arac D, Li H, Huryeva I, Min SW, Rizo J, Sudhof TC (2003) Convergence and divergence in the mechanism of SNARE binding by Sec1/Munc18-like proteins. Proc Natl Acad Sci USA 100: 32–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares H, Greenwald I (2001) Genetic analysis of endocytosis in Caenorhabditis elegans: coelomocyte uptake defective mutants. Genetics 159: 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D, Sutton RB, Brunger AT, Jahn R (1998) Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA 95: 15781–15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D, Jahn R (2003) The riddle of the Sec1/Munc-18 proteins – new twists added to their interactions with SNAREs. Trends Biochem Sci 28: 113–116 [DOI] [PubMed] [Google Scholar]
- Grant B, Hirsh D (1999) Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell 10: 4311–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Lang T, Sudhof TC (2003) Membrane fusion. Cell 112: 519–533 [DOI] [PubMed] [Google Scholar]
- Lajoie P, Guay G, Dennis JW, Nabi IR (2005) The lipid composition of autophagic vacuoles regulates expression of multilamellar bodies. J Cell Sci 118: 1991–2003 [DOI] [PubMed] [Google Scholar]
- Matyash V, Geier C, Henske A, Mukherjee S, Hirsh D, Thiele C, Grant B, Maxfield FR, Kurzchalia TV (2001) Distribution and transport of cholesterol in Caenorhabditis elegans. Mol Biol Cell 12: 1725–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misura KM, Scheller RH, Weis WI (2000) Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature 404: 355–362 [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Holthuis JC, Pelham HR (1998) The Sec1p homologue Vps45p binds to the syntaxin Tlg2p. Eur J Cell Biol 77: 263–268 [DOI] [PubMed] [Google Scholar]
- Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M (2000) Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J Cell Biol 151: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton A, Knuth S, Schaheen B, Dang H, Greenwald I, Fares H (2005) Endocytosis function of a ligand-gated ion channel homolog in Caenorhabditis elegans. Curr Biol 15: 1045–1050 [DOI] [PubMed] [Google Scholar]
- Peng R, Gallwitz D (2004) SNARE interactions of an SM protein: Sed5p/Sly1p binding is dispensable for transport. EMBO J 23: 3939–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall GG, Hama H, DeWald DB, Horazdovsky BF (1999) The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting. Mol Biol Cell 10: 1873–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toonen RF, Verhage M (2003) Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol 13: 177–186 [DOI] [PubMed] [Google Scholar]
- Treusch S, Knuth S, Slaugenhaupt SA, Goldin E, Grant BD, Fares H (2004) Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proc Natl Acad Sci USA 101: 4483–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AL, Ehm S, Jacobson NC, Xu D, Hay JC (2004) rsly1 binding to syntaxin 5 is required for endoplasmic reticulum-to-Golgi transport but does not promote SNARE motif accessibility. Mol Biol Cell 15: 162–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Dulubova I, Min SW, Chen X, Rizo J, Sudhof TC (2002) Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev Cell 2: 295–305 [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Grant B, Hirsh D (2001) RME-8, a conserved J-domain protein, is required for endocytosis in Caenorhabditis elegans. Mol Biol Cell 12: 2011–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig 1
Supplementary Fig 2
Supplementary Fig 3
Supplementary Fig 4
Supplementary Fig 5
Supplementary Fig 6
Supplementary Fig 7
Supplementary Information


