Abstract
Shugoshin (SGO) is a family of proteins that protect centromeric cohesin complexes from release during mitotic prophase and from degradation during meiosis I. Two mammalian SGO paralogues—SGO1 and SGO2—have been identified, but their distribution and function during mammalian meiosis have not been reported. Here, we analysed the expression of SGO2 during male mouse meiosis and mitosis. During meiosis I, SGO2 accumulates at centromeres during diplotene, and colocalizes differentially with the cohesin subunits RAD21 and REC8 at metaphase I centromeres. However, SGO2 and RAD21 change their relative distributions during telophase I when sister-kinetochore association is lost. During meiosis II, SGO2 shows a striking tension-dependent redistribution within centromeres throughout chromosome congression during prometaphase II, as it does during mitosis. We propose a model by which the redistribution of SGO2 would unmask cohesive centromere proteins, which would be then released or cleaved by separase, to trigger chromatid segregation to opposite poles.
Keywords: meiosis, mitosis, shugoshin 2, sister-chromatid cohesion
Introduction
Sister-chromatid cohesion is maintained by a conserved complex, termed cohesin, which consists of two structural maintenance of chromosomes family proteins, Smc1 and Smc3, and two non-Smc subunits, Scc1/Rad21 and Scc3/SA (Nasmyth & Haering, 2005). During vertebrate mitosis, the bulk of cohesin associated with the chromosome arms is removed during prophase and prometaphase by phosphorylation of the SA2 subunit (Hauf et al, 2005). Then, at the onset of anaphase, centromeric cohesin is removed by separase cleavage of the SCC1/RAD21 subunit (Waizenegger et al, 2000). During meiosis, arm cohesion is lost during anaphase I to allow the segregation of recombined homologues, whereas centromere cohesion dissolves at the onset of anaphase II (Watanabe, 2004). Thus, centromeric cohesin complexes might be resistant to phosphorylation during mitotic prometaphase and to cleavage by separase during meiosis I.
Recent studies have characterized a conserved family of centromere-associated proteins called Shugoshin (Sgo; Kitajima et al, 2004). It has been proposed that Sgo1 in budding yeast (Katis et al, 2004; Marston et al, 2004), MEI-S332 in Drosophila (Clarke et al, 2005) and SGO1 in vertebrates (Salic et al, 2004; McGuinness et al, 2005) contribute to the maintenance of centromere cohesion until mitotic metaphase. Similarly, Sgo proteins protect centromeric cohesin complexes from degradation during meiosis I (Katis et al, 2004; Kitajima et al, 2004; Marston et al, 2004; Rabitsch et al, 2004).
In mammals, two SGO paralogues, SGO1 and SGO2, have been identified (Kitajima et al, 2004). Although the function of SGO1 has been studied in mitosis (Salic et al, 2004; McGuinness et al, 2005), the distribution and function of SGO1 and SGO2 during mammalian meiosis have not been reported. Here, we have characterized the dynamics of SGO2 during male mouse meiosis and mitosis.
Results
To analyse the distribution of SGO2 during meiosis in the male mouse, we generated two rabbit antibodies. By using western blotting, both antibodies detected the same principal immunoreactive band of approximately 130 kDa in nuclear protein extracts from mouse testis and 3T3 somatic cells (supplementary Fig 1 online). This size is consistent with the expected molecular mass of 128 kDa.
SGO2 at metaphase I centromeres
We carried out a double immunolabelling of SGO2 with an anti-centromere autoantibody (ACA) serum recognizing kinetochores. We also double immunolocalized SGO2 and the cohesin subunit RAD21 to study their possible colocalization, and to determine the spermatocyte staging, as RAD21 cohesin axes are coincident with the lateral elements of the synaptonemal complex. SGO2 was first detected at the centromeres during late diplotene (Fig 1A–C), underlying the kinetochore signals (inset in Fig 1B). SGO2 signals appeared at one end of each RAD21-labelled desynapsed lateral element (Fig 1D, inset). This SGO2 labelling at the centromeres persisted during diakinesis (Fig 1E–H). During metaphase I, SGO2 was located below the kinetochore signals (Fig 1I–K). A closer examination of the side view of metaphase I centromeres showed that the SGO2 signals had a T- or Y-like appearance below the associated sister kinetochores (Fig 2A–A″,B,B′). A top view of the centromeres showed two side-by-side associated SGO2 rings encircling sister kinetochores (Fig 2C,C′). Thus, SGO2 appeared as two rings and a longitudinal projection towards the inner centromere region that emerged from the contact zone between the rings. Interestingly, SGO2 and RAD21 colocalized at the inner centromere domain when metaphase I centromeres were viewed from the side (Figs 1L, 2F–F″,G,G′) and top (Fig 2H,H′). RAD21 was additionally observed as faint patches at the interchromatid domain of all bivalents (Figs 1L, 2F′,F″) and as large round agglomerates lying in the cytoplasm (Fig 1L).
Figure 1.
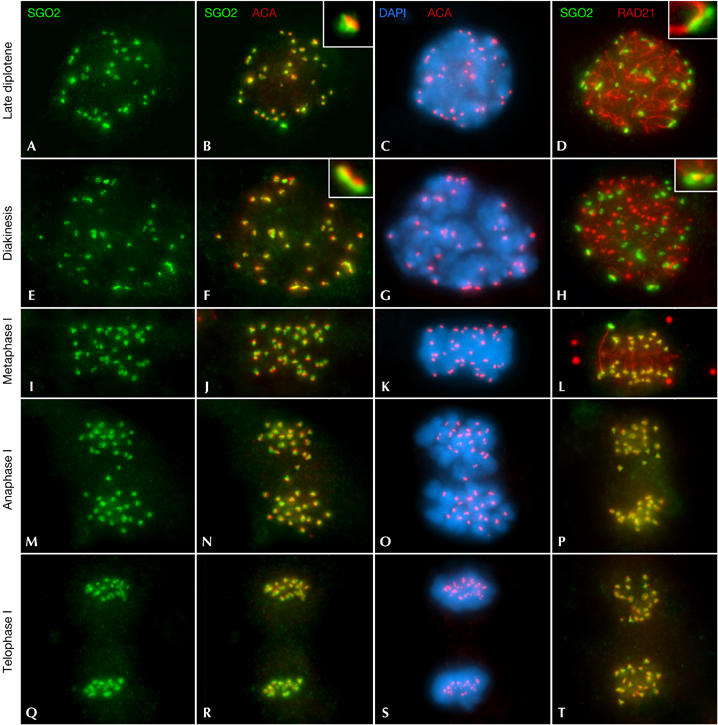
SGO2 distribution during meiosis I. Double immunolabellings of SGO2 (green) with kinetochores (ACA; red; columns 1–3); or RAD21 (red; column 4); and counterstaining with 4,6-diamidino-2-phenylindole (DAPI; blue; column 3). (A–D) Late diplotene; (E–H) diakinesis; (I–L) metaphase I; (M–P) anaphase I; and (Q–T) telophase I spermatocytes. All images are projections of different focal planes throughout the cell volume. ACA, anti-centromere autoantibody.
Figure 2.

SGO2 and RAD21, but not REC8, show a complex arrangement at the inner domain of metaphase I centromeres and change their relative distributions during telophase I. Double immunolabellings of SGO2 (green) with kinetochores (ACA; pseudocoloured in pink; A–E′), RAD21 (red; F–J′) or REC8 (blue; K–O′) in metaphase I (A–C′,F–H′,K–M′) and telophase I (D–E′,I–J′,N–O′) centromeres. (A–A″,F–F″,K–K″) Side views of autosomal metaphase I bivalents. (B,B′,G,G′,L,L′) Side views of metaphase I centromeres. (C,C′,H,H′,M,M′) Top views of metaphase I centromeres. (D,D′,I,I′,N,N′) Side views and (E,E′,J,J′,O,O′) top views of telophase I centromeres. ACA, anti-centromere autoantibody.
We next analysed the relative distribution of SGO2 and REC8 in metaphase I bivalents. REC8 was found at the interchromatid domain as a series of bright patches, whereas SGO2 was observed only at the centromeres (Fig 2K–K″). Inspection of the centromeres viewed from the side showed that REC8 was also present at their inner domains, but it colocalized only with the vertical region of the T-shaped SGO2 signals (Fig 2L,L′). In centromeres viewed from the top, REC8 colocalized only with the region of contact between the two SGO2 rings (Fig 2M,M′).
SGO2 at telophase I centromeres
During anaphase I and early telophase I, SGO2 was still detected at the centromeres (Fig 1M–O,Q–S). In centromeres viewed from the side, SGO2 persisted as T-shaped signals beneath the individualized sister kinetochores (Fig 2D,D′), whereas in top views, it was visible as two associated rings (Fig 2E,E′). Thus, SGO2 showed a ‘double cornet'-like three dimensional arrangement at the inner centromere domain from metaphase I up to early telophase I. RAD21 also showed this arrangement and colocalized with SGO2 during anaphase I (Fig 1P). However, SGO2 and RAD21 showed a different centromere distribution at early telophase I (Fig 1T). SGO2 presented a ‘double cornet'-like arrangement (Fig 2I,J), whereas RAD21 appeared as small elongated bars that partially colocalized with SGO2 signals (Fig 2I′,J′). Consequently, SGO2 and RAD21 change their relative distributions at the inner centromere domain during early telophase I, concomitant with the progressive separation between sister kinetochores.
With regard to the relative distributions of SGO2 and REC8 at early telophase I centromeres, we found that REC8 colocalized with the vertical region of the T-shaped SGO2 signals in side views (Fig 2N,N′) and top views (Fig 2O,O′). Interestingly, SGO2 and REC8 were no longer detected at the centromeres in late telophase I nuclei, whereas RAD21 still persisted between the separated sister kinetochores as elongated bars (data not shown).
SGO2 at metaphase II centromeres
We also analysed the expression of SGO2 during meiosis II. In early interkinesis nuclei, SGO2 was not detected but RAD21 still persisted at the chromocentres as elongated bars (Fig 3A–D). However, in late interkinesis nuclei, SGO2 was found at the chromocentres as large signals that did not colocalize with kinetochores (Fig 3E–G) or with RAD21 bars (Fig 3H, inset). In prophase II chromosomes, the SGO2 signals appeared at the inner centromere domain as bands between the separated sister kinetochores (Fig 3I–K, inset in J), whereas the RAD21 bars had been lost (Fig 3L). These SGO2 bands were occasionally observed in metaphase II, when most of the SGO2 signals were present as a pair of dots below each sister kinetochore (Fig 3M–O). By contrast, neither RAD21 (Fig 3P) nor REC8 (data not shown) was detected at metaphase II centromeres.
Figure 3.

SGO2 distribution during meiosis II. Double immunolabellings of SGO2 (green) with kinetochores (ACA; red; columns 1–3), or RAD21 (red; column 4), and counterstaining with 4,6-diamidino-2-phenylindole (DAPI; blue; column 3). (A–D) Early interkinesis; (E–H) late interkinesis; (I–L) prophase II; (M–P) metaphase II; (Q–T) anaphase II; and (U–X) telophase II spermatocytes. Arrowheads in (M,N) indicate an SGO2 band at one centromere. All images are projections of different focal planes throughout the cell volume. ACA, anti-centromere autoantibody.
To determine how SGO2 changed its centromere distribution, from a band during prophase II to a pair of dots in metaphase II, we analysed its appearance during chromosome congression. Our results showed three main SGO2 distributions at different prometaphase II centromeres. In early prometaphase II, all the chromosomes showed an SGO2 band that crossed the centromere and joined the separated sister kinetochores (Fig 4A,A′). Then, some centromeres showed that SGO2 was still present as a band at the inner centromere domain, but that concurrently a pair of round SGO2 signals became apparent below each sister kinetochore (Fig 4B,B′). Finally, the band at the inner centromere domain disappeared, and only a pair of SGO2 dots below each kinetochore was evident (Fig 4C,C′). This distribution was appreciated only when centromeres were viewed from the side. When metaphase II centromeres were viewed from the top, a single SGO2 ring was observed around each kinetochore (Fig 4D,D′).
Figure 4.
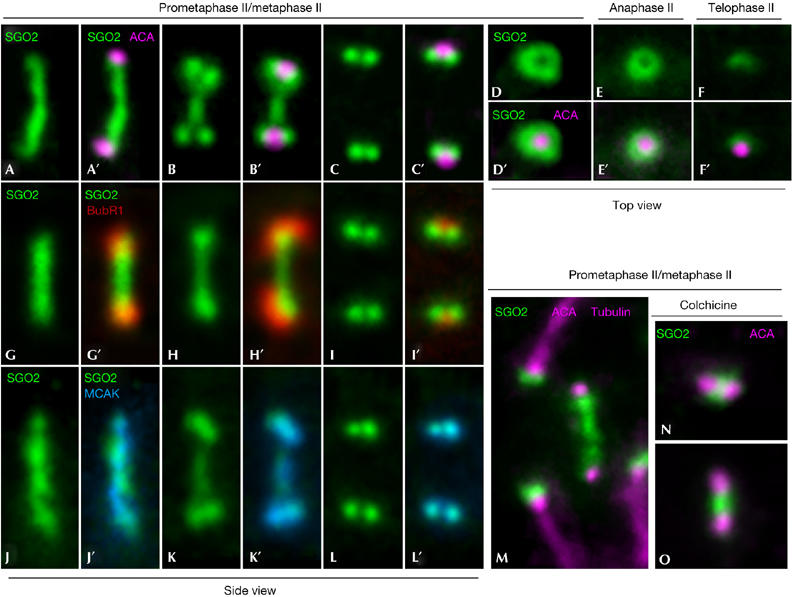
SGO2 redistributes within the centromeres throughout chromosome congression to the metaphase II plate depending on microtubule attachment to sister kinetochores. Double immunolabellings of SGO2 (green) with kinetochores (ACA; pseudocoloured in pink; A–F′), BubR1 (red; G–I′) or MCAK (blue; J–L′) in prometaphase II/metaphase II (A–D′,G–L′), anaphase II (E,E′) and telophase II (F,F′) enlarged centromeres. (A-C′,G-I′,J-L′) Side views of enlarged prometaphase II/metaphase II centromeres. (D,D′) Top views of metaphase II and (E,E′) anaphase II centromeres. (F,F′) Top view of a telophase II centromere. (M) Partial view of a prometaphase II spermatocyte labelled for SGO2 (green), kinetochores (ACA; pseudocoloured in pink) and microtubules (tubulin; pseudocoloured in pink). (N,O) Two selected centromeres from colchicine-treated prometaphase II spermatocytes labelled for SGO2 (green) and kinetochores (ACA; pseudocoloured in pink). ACA, anti-centromere autoantibody; MCAK, mitotic centromere-associated kinesin.
We next quantified the number of centromeres showing SGO2 bands between sister kinetochores in 100 prometaphase II/metaphase II spermatocytes (supplementary Table 1 online). These mouse spermatocytes possess 20 chromosomes. The results showed that SGO2 bands were present in 63% of prometaphase II/metaphase II spermatocytes. However, the number of SGO2 bands in these spermatocytes was low, and spermatocytes with more than ten SGO2 bands were never observed. In fact, 81% of these spermatocytes showed one to three centromeres with SGO2 bands, whereas in the remaining centromeres SGO2 was present as a pair of dots below the kinetochores. Thus, the redistribution of SGO2 at the inner centromere domain of congressing prometaphase II chromosomes is very fast.
We also studied the expression of SGO2 in somatic mouse 3T3 cells. SGO2 appeared at the centromeres just before prophase and disappeared during early anaphase (supplementary Figs 2,3 online). In 3T3 cells, SGO2 also redistributed at the inner centromere domain during chromosome congression. Thus, in presumptive unattached centromeres, SGO2 was visible as a band connecting sister kinetochores. By contrast, SGO2 was detected as pairs of dots beneath the kinetochores in aligned chromosomes, appearing as rings surrounding the kinetochores when centromeres were viewed from the top (supplementary Fig 3 online).
Our results indicate that tension across prometaphase II and mitotic prometaphase centromeres promotes the redistribution of SGO2. To corroborate this hypothesis in meiosis II, we double immunolabelled SGO2 and the spindle checkpoint protein BubR1, a kinase that localizes to unattached kinetochores and is mostly released from them once microtubules have stably attached (Taylor et al, 2001). We found that when SGO2 was present as a band at prometaphase II centromeres, sister kinetochores were intensely labelled by BubR1 (Fig 4G–H′). By contrast, when SGO2 appeared as pairs of dots, kinetochores were faintly labelled with BubR1 (Fig 4I,I′).
Recently, it has been proposed that the microtubule-depolymerizing kinesin mitotic centromere-associated kinesin (MCAK), located at the inner centromere domain of mitotic chromosomes, is involved in the correction of improper kinetochore–microtubule interactions. MCAK redistributes at HeLa mitotic and mouse prometaphase II centromeres depending on tension across centromeres (Andrews et al, 2004; Parra et al, 2006); therefore, we double immunolabelled SGO2 and MCAK to test whether their redistribution was comparable. Our results showed that both proteins redistributed in a similar manner during chromosome congression at prometaphase II (Fig 4J–L′). Next we double immunolabelled SGO2 and tubulin to test whether the redistribution of SGO2 was dependent on microtubule attachment at the kinetochores. We observed that when SGO2 appeared as a band at prometaphase II/metaphase II centromeres, their sister kinetochores were unattached, whereas SGO2 was visible as pairs of dots below the kinetochores when bundles of microtubules from opposite poles interacted with sister kinetochores (Fig 4M). Moreover, after treatment with colchicine, an microtubule-depolymerizing drug, SGO2 was detected as a patch between sister kinetochores, which in turn were closer than in aligned chromosomes. Altogether, our results indicate that the SGO2 redistribution at prometaphase II is dependent on tension across centromeres.
During anaphase II, SGO2 was still visible at the centromeres (Fig 3Q–S) but RAD21 was absent (Fig 3T). SGO2 signals were visualized as two spots below the kinetochores in centromeres viewed from the side (Fig 3Q–S) and as a ring surrounding them when viewed from the top (Fig 4E,E′). Finally, in telophase II spermatocytes, the SGO2 signals became diffuse and the rings disappeared (Figs 3U–X, 4F,F′).
Discussion
Our results show for the first time that mouse SGO2 localizes at the inner centromere domain during both meiotic and mitotic divisions, in the same way as its orthologue Sgo2 in fission yeast (Kitajima et al, 2004; Rabitsch et al, 2004). SGO2 and RAD21 colocalize and show a ‘double cornet' arrangement at the inner centromere domain below the closely associated sister kinetochores during metaphase I and anaphase I. By contrast, REC8 colocalizes only with the vertical region of the T-shaped SGO2 signals during these stages (supplementary Fig 4 online). These results show that there are two different cohesin complexes with either RAD21 or REC8 at the inner domain of metaphase I and anaphase I centromeres, and that these complexes coexist only at the vertical region of the T-shaped SGO2 signals. Thus, SGO2, as has been proposed for Sgo1 in Drosophila and yeast meiosis (Kitajima et al, 2004; Marston et al, 2004; Rabitsch et al, 2004; Clarke et al, 2005), could protect centromeric cohesin complexes with either RAD21 or REC8 against degradation by separase at the onset of anaphase I.
We have found that SGO2 and RAD21 change their relative distributions at the inner centromere domain during early telophase I, concomitant with the progressive separation between sister kinetochores (supplementary Fig 4 online). Sister kinetochores are intimately associated during metaphase I to operate as a single functional kinetochore per homologue and then permit the accurate biorientation of bivalents. However, sister kinetochores located at metaphase II centromeres appear back-to-back to attach microtubules emanating from opposite poles. Consequently, the centromere changes its structure between both meiotic metaphases to allow the separation of sister kinetochores while maintaining sister centromere cohesion up to the onset of anaphase II. We have previously shown that the redistribution of RAD21 at the inner centromere domain, from T-shaped structures to small elongated bars, is coincident with the ongoing separation between sister kinetochores during telophase I. As RAD21 was at the right place and redistributed at the right time, we proposed that this cohesin subunit maintained sister-kinetochore association until early telophase I (Parra et al, 2004). Our results show that SGO2, unlike RAD21, does not redistribute at the inner domain of early telophase I centromeres. This result supports a putative complex between SGO2 and RAD21 that dissociates during early telophase I, and that although both proteins colocalize temporally at the inner centromere domain, they might have different but probably coordinated functions. The disappearance of SGO2 from centromeres during late telophase I/early interkinesis suggests that SGO2 is released to allow a further separation between sister kinetochores through chromatin remodelling at the centromeres. Thus, RAD21 and SGO2 could be involved in maintaining sister-kinetochore association, from metaphase I up to telophase I, but they would be sequentially released. This proposal is supported by recent data obtained in fission yeast indicating that Sgo2 promotes sister-kinetochore association during meiosis I (Rabitsch et al, 2004; Vaur et al, 2005). We do not eliminate the possibility that REC8 could also collaborate in maintaining sister-kinetochore association during meiosis I in the mouse. In fact, REC8 maintains its centromere distribution from metaphase I up to late telophase I. Indeed, it has been proposed that, in fission yeast, Rec8 is required for sister-kinetochore association during meiosis I (Yokobayashi et al, 2003). During the telophase I/early interkinesis transition, SGO2 is lost from the centromeres. However, SGO2 reappears at chromocentres in late interkinesis nuclei (supplementary Fig 4 online). This result suggests that SGO2 might be degraded in late telophase I and resynthesized during early interkinesis. Alternatively, SGO2 might be released from late telophase I centromeres and loaded again at their inner domains during late interkinesis.
Our data indicate that SGO2 redistributes from a band at the inner centromere domain to a ring below each sister kinetochore during chromosome congression to the metaphase II plate (supplementary Fig 5 online) and also during mitosis. We have recently reported a similar redistribution for the microtubule-depolymerizing kinesin MCAK and the kinase Aurora B during meiosis II in the mouse (Parra et al, 2006). Moreover, SGO1 also redistributes at the centromeres during chromosome congression in HeLa cells (McGuinness et al, 2005). Our results show that the redistribution of SGO2, and MCAK with which it colocalizes, is very fast. It has been reported that MCAK redistributes at mitotic centromeres depending on tension across centromeres (Andrews et al, 2004). The results obtained after double immunolabelling of SGO2 with either BubR1 or tubulin indicate that the SGO2 redistribution at prometaphase II centromeres is dependent on the attachment of microtubules with opposite polarity to sister kinetochores. Our observations on the distribution of SGO2 in colchicine-treated spermatocytes also support this idea. Thus, we suggest that tension across prometaphase II and mitotic prometaphase centromeres, generated by pulling forces exerted by bundles of kinetochore microtubules from opposite poles during chromosome congression, promotes the redistribution of SGO2.
We have not detected either RAD21 or REC8 at metaphase II centromeres. Similarly, the cohesin subunits STAG3 (Prieto et al, 2001) and SMC3 (R.G. & J.A.S., unpublished data) are not detected at metaphase II centromeres. These results indicate that in mammalian meiosis, unlike in yeast meiosis (Watanabe, 2004; Nasmyth & Haering, 2005), known cohesin complexes are not necessary to maintain centromere cohesion until the onset of anaphase II. However, we cannot eliminate the possibility that cohesin complexes are present at metaphase II centromeres but in small quantities, or that the cohesin subunits are somehow modified so that antibodies are unable to reveal them. Accordingly, we propose a working model which suggests that SGO2 protects putative cohesive proteins at the inner centromere domain during prometaphase II and mitotic prometaphase (supplementary Fig 5 online). The occurrence of tension across centromeres would promote the redistribution of SGO2 so that those cohesive proteins would become unmasked to be then released or cleaved by separase, thus triggering chromatid segregation to opposite poles.
As depletion of SGO1 by RNA interference causes destabilization of kinetochore–microtubule interactions, the involvement of SGO1 has been suggested as a component of the tension-sensing machinery (Salic et al, 2004). This function has also been shown for its orthologue Sgo1 during budding yeast mitosis (Indjeian et al, 2005). Therefore, and considering our results, SGO2 might also be a component of the tension-sensing machinery during meiosis II and mitosis in the mouse.
Methods
Immunofluorescence on spermatocytes. Mouse testes were removed and detunicated, and seminiferous tubules were fixed and squashed as described previously (Parra et al, 2004). Immunofluorescence image stacks were collected on an Olympus BX61 microscope equipped with epifluorescence optics and an Olympus DP70 digital camera controlled by analySIS software (Soft Imaging System, Münster, Germany).
Primary and secondary antibodies. Rabbit polyclonal antisera K1058 and K1059 were raised against a synthetic peptide corresponding to the last 20 amino acids (KRQCVPLNLTEPSLRSKMRR) of the carboxy-terminal amino-acid sequence of mouse SGO2 protein (NCBI accession number NM 199007). Recently, an SGO2-like protein coding sequence (XM_143672.5) was reported in the mouse database, predicted by automated computational analysis from an annotated mouse genomic sequence by using gene prediction method GNOMON. Although we cannot eliminate a cross-reaction of mSGO2 antibodies with this predicted SGO2-like protein without further experiments, the relevant differences in the amino-acid sequence between the SGO2 peptide used as antigen to generate mSGO2 antibodies and the corresponding region of the SGO2-like protein support the antibody specificity. Doubtlessly, the study of the expression, localization and function of this new predicted mouse SGO2-like protein during mitotic and meiotic cell cycles will be of interest to future research.
The following primary antibodies were also used: K1059 mSGO2, K854 RAD21, mREC8 (kindly provided by J. Lee), kinetochores (15-235; Antibodies Incorporated, Davis, CA, USA), MCAK (kindly provided by L. Wordeman), hBubR1 (kindly provided by S. Taylor) and α-tubulin (T-5168; Sigma, Tres Cantos, Spain). We used the corresponding secondary antibodies against rabbit, human, mouse and sheep proteins conjugated with either fluorescein isothiocyanate or Texas Red (Jackson ImmunoResearch Labs, West Grove, PA, USA).
Materials, western blotting and immunofluorescence on somatic cells are presented in the supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We are grateful to R. Fernández for technical assistance and L. Gómez for animal work. We also acknowledge J. Lee, L. Wordeman and S. Taylor for the supply of antibodies. This work was supported by grants BFU2005-01266/BCM and BFU2005-05668-C03-01/BCM from Ministerio de Educación y Ciencia (MEC), and grants 1001160016 and 11/BCB/013 from Universidad Autónoma de Madrid (UAM) and Comunidad de Madrid. The Department of Immunology and Oncology was founded and is supported by the Spanish Council for Scientific Research (CSIC) and by Pfizer.
References
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR (2004) Aurora-B regulates MCAK at the mitotic centromere. Dev Cell 6: 253–268 [DOI] [PubMed] [Google Scholar]
- Clarke AS, Tang TT, Ooi DL, Orr-Weaver TL (2005) POLO kinase regulates the Drosophila centromere cohesion protein MEI-S332. Dev Cell 8: 53–64 [DOI] [PubMed] [Google Scholar]
- Hauf S, Roitinger E, Koch B, Dittrich CM, Mechtler K, Peters JM (2005) Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol 3: 419–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indjeian VB, Stern BM, Murray AW (2005) The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science 307: 130–133 [DOI] [PubMed] [Google Scholar]
- Katis VL, Galova M, Rabitsch KP, Gregan J, Nasmyth K (2004) Maintenance of cohesin at centromeres after meiosis I in budding yeast requires a kinetochore-associated protein related to MEI-S332. Curr Biol 14: 560–572 [DOI] [PubMed] [Google Scholar]
- Kitajima TS, Kawashima SA, Watanabe Y (2004) The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 427: 510–517 [DOI] [PubMed] [Google Scholar]
- Marston AL, Tham WH, Shah H, Amon A (2004) A genome-wide screen identifies genes required for centromeric cohesion. Science 303: 1367–1370 [DOI] [PubMed] [Google Scholar]
- McGuinness BE, Hirota T, Kudo NR, Peters JM, Nasmyth K (2005) Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol 3: 433–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH (2005) The structure and function of SMC and kleisin complexes. Annu Rev Biochem 74: 595–648 [DOI] [PubMed] [Google Scholar]
- Parra MT, Viera A, Gómez R, Page J, Benavente R, Santos JL, Rufas JS, Suja JA (2004) Involvement of the cohesin Rad21 and SCP3 in monopolar attachment of sister kinetochores during mouse meiosis I. J Cell Sci 117: 1221–1234 [DOI] [PubMed] [Google Scholar]
- Parra MT, Gómez R, Viera A, Page J, Calvente A, Wordeman L, Rufas JS, Suja JA (2006) A perikinetochoric ring defined by the microtubule-depolymerising kinesin MCAK as a new centromere domain in meiosis. PLoS Genet 2: 798–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto I, Suja JA, Pezzi N, Kremer L, Martínez-A C, Rufas JS, Barbero JL (2001) Mammalian STAG3 is a cohesin specific to sister chromatid arms during meiosis I. Nat Cell Biol 3: 761–766 [DOI] [PubMed] [Google Scholar]
- Rabitsch KP, Gregan J, Schleiffer A, Javerzat JP, Eisenhaber F, Nasmyth K (2004) Two fission yeast homologs of Drosophila Mei-S332 are required for chromosome segregation during meiosis I and II. Curr Biol 14: 287–301 [DOI] [PubMed] [Google Scholar]
- Salic A, Waters JC, Mitchison TJ (2004) Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118: 567–578 [DOI] [PubMed] [Google Scholar]
- Taylor SS, Hussein D, Wang Y, Elderkin S, Morrow CJ (2001) Kinetochore localisation and phosphorylation of the mitotic checkpoint components Bub1 and BubR1 are differentially regulated by spindle events in human cells. J Cell Sci 114: 4385–4395 [DOI] [PubMed] [Google Scholar]
- Vaur S et al. (2005) Control of shugoshin function during fission-yeast meiosis. Curr Biol 15: 2263–2270 [DOI] [PubMed] [Google Scholar]
- Waizenegger IC, Hauf S, Meinke A, Peters JM (2000) Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103: 399–410 [DOI] [PubMed] [Google Scholar]
- Watanabe Y (2004) Modifying sister chromatid cohesion for meiosis. J Cell Sci 117: 4017–4023 [DOI] [PubMed] [Google Scholar]
- Yokobayashi S, Yamamoto M, Watanabe Y (2003) Cohesins determine the attachment manner of kinetochores to spindle microtubules at meiosis I in fission yeast. Mol Cell Biol 23: 3965–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


