Abstract
The fundamental principle of cardiac behaviour is described by the Frank-Starling law relating force of contraction during systole with end-diastolic volume. While both work and respiration rates increase linearly with imposed load, the basis of mechano-energetic coupling in heart muscle has remained a long-standing enigma. Here, we highlight advances made in understanding of complex cellular and molecular mechanisms that orchestrate coupling of mitochondrial oxidative phosphorylation with ATP utilization for muscle contraction. Cardiac system bioenergetics critically depends on an interrelated metabolic infrastructure regulating mitochondrial respiration and energy fluxes throughout cellular compartments. The data reviewed indicate the significance of two interrelated systems regulating mitochondrial respiration and energy fluxes in cells: (1) the creatine kinase, adenylate kinase and glycolytic pathways that communicate flux changes generated by cellular ATPases within structurally organized enzymatic modules and networks; and (2) a secondary system based on mitochondrial participation in cellular calcium cycle, which adjusts substrate oxidation and energy-transducing processes to meet increasing cellular energy demands. By conveying energetic signals to metabolic sensors, coupled phosphotransfer reactions provide a high-fidelity regulation of the excitation–contraction cycle. Such integration of energetics with calcium signalling systems provides the basis for ‘metabolic pacing’, synchronizing the cellular electrical and mechanical activities with energy supply processes.
Living cells are open systems exchanging energy and metabolic substrates and products with the environment (Westerhoff & Van Dam, 1987; Nicholls & Ferguson, 2002). This allows cells to perform all the work needed for their biological functions, and to keep entropy low to maintain a high and specific structural organization (Schroedinger, 2000). Evolution has selected ATP along with ADP and Pi as the main system for free energy conversion in cells – a high ratio of [ATP]/[ADP]×[Pi] makes available free energy both for cellular work and anabolic reactions (Garlid, 2001; Nicholls & Ferguson, 2002). This high ratio is maintained by mitochondrial oxidative phosphorylation and by glycolysis. How the free energy transduction and energy supply/demand balance is maintained and which signals and mechanisms ensure the precise and timely coupling of ATP-consuming and ATP-producing processes is a major question in cellular energetics (Bose et al. 2003; Dzeja & Terzic, 2003; Hochachka, 2003; Schlattner & Wallimann, 2004; Saks et al. 2005). Significant progress has been made in understanding molecular mechanisms of muscle contraction, cell motility, ion transport and the ATP synthesis machinery in mitochondria (Mitchell, 1979; Gordon et al. 2001; Nicholls & Ferguson, 2002; Berridge et al. 2003; Bianchi et al. 2004). However, the integration of different energetic units within the cell and the nature of signalling pathways that regulate their activities and provide linkage with ATP-utilizing and ATP-sensing components are a growing field of investigation. Here, we analyse paradoxes of cellular energetics and controversies existing at present in this field, and highlight recent advances in cardiac energetics. We particularly underscore the emerging understanding of energy transfer and metabolic signalling networks that secure mechano-energetic coupling, and underlie heart physiology and the relationship between respiration rate and cardiac work in the classical Frank-Starling law.
Cardiac energetics: the Frank-Starling law and the ‘stability paradox’
Under normoxia, cardiac energy metabolism relies on aerobic oxidation of fatty acids and carbohydrate substrates in mitochondria with the majority of ATP consumed by contractile machinery and ion pumps (Opie, 1998; Stanley et al. 2005; Taegtmeyer et al. 2005). Therefore, mechanisms of intracellular feedback communication and regulation of mitochondrial respiration must be deciphered in order to understand the balanced coupling of ATP-producing and ATP-consuming processes in vivo. The phenomenological aspects of this regulation were initially recognized by the Starling laboratory in the period between 1914 and 1926, before ATP was discovered and long before the discovery of oxidative phosphorylation (Evans & Matsuoka, 1915; Starling & Visscher, 1926). The heart maintains normal blood circulation under a wide range of workloads, a function governed by the Frank-Starling law (Opie, 1998; Fuchs & Smith, 2001) originally described by Otto Frank (Frank, 1885) and Ernest Starling (Patterson et al. 1914; Starling & Visscher, 1926). This law states that cardiac performance increases with an increase in end-diastolic ventricular volume. In this way, the heart responds to increases in venous filling pressure (Patterson et al. 1914; Katz, 2002). However, this law has wider implications as it addresses the principles of cardiac mechano-energetic coupling (Opie, 1998). In their original experiments, Starling and co-workers used heart–lung preparations and measured the rate of oxygen consumption, which was taken as a measure of ‘the total energy set free in the heart during its activity’, an assumption recalling the XVIIIth century definition of biological oxidation by Lavoisier and Laplace (Evans & Matsuoka, 1915; Starling & Visscher, 1926; Vignais, 2005). As both work and respiration rates increased linearly with increase of left ventricular end-diastolic volume, the conclusion was made that ‘any increase in the work demanded of the heart is met by a corresponding increase in the oxygen consumption and in the amount of chemical changes taking place’ (Starling & Visscher, 1926). This is the metabolic underpinning of the Frank-Starling law. Within the century that followed Starling's work, detailed characteristics of the contraction cycle, energy consumption and heat production were determined as summarized elsewhere (Gibbs, 1978; Suga, 1990). One of the main parameters studied has been V̇o2, the rate of oxygen consumption, described as a linear function of the pressure–volume area (PVA):
where, D is unloaded V̇o2, PVA consists of the mechanical work within the contraction cycle and so-called ‘potential energy’ term, and A is a constant (which inversely describes the efficiency of the heart) (Suga, 1990). Another principal characteristic of heart energetics is that the linear increase in V̇o2 with workload is observed in the absence of measurable changes in the ATP and phosphocreatine (PCr) cellular content (Neely et al. 1972). This remarkable metabolic stability, also called ‘metabolic homeostasis’ (Balaban, 2002), justifies the use of V̇o2 as a parameter of energy consumption. Using the Frank-Starling mechanism and increasing the rate of left ventricular filling, Williamson and colleagues have shown that the rate of respiration in isolated working hearts can be changed by more than an order of magnitude, about 15–20 times, from an unloaded V̇o2 of around 8–12 μmol min−1 (g dry wt)−1 to a maximal value of 170 μmol min−1 (g dry wt)−1 (see Fig. 1; Williamson et al. 1976). This occurs under conditions of remarkable metabolic stability (Williamson et al. 1976). In the face of large changes in muscle work and respiration, this observed metabolic stability or homeostasis underlying Frank-Starling law is referred to as the ‘stability paradox’ (Hochachka, 2003).
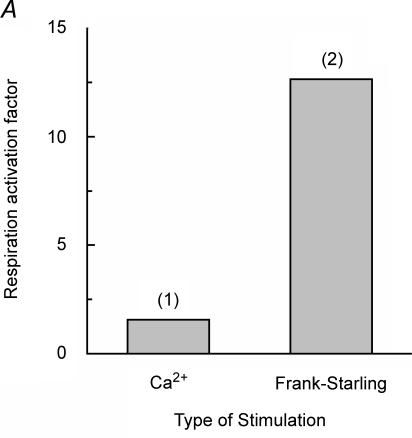
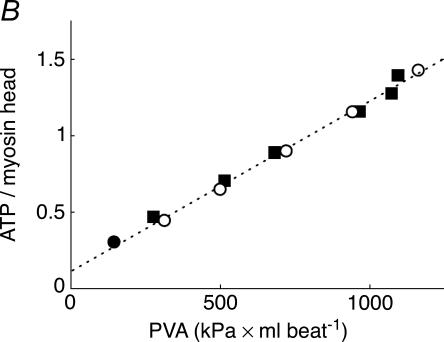
Figure 1. Conflict between the calcium hypothesis of regulation of mitochondrial oxidative phosphorylation in vivo and the high degree of activation of cardiac respiration by the Frank-Starling mechanism.
A, the regulatory effects on the mitochondrial respiration rate of calcium in isolated mitochondria in vitro (1) (Territo et al. 2001), and that of changing left ventricle filling in isolated perfused rat hearts (2) (Williamson et al. 1976). The respiratory activation factor means the ratio of the maximal respiration rate to that in the absence of calcium (1) or in the arrested, non-contracting hearts (2). At zero calcium, the respiration rate of isolated mitochondria is already about 60% of Vmax observed at calcium concentrations higher than 600 nm (Territo et al. 2001). The difference between the degrees of activation of respiration by calcium and by Frank-Starling mechanism is of an order of magnitude. B, simulations demonstrating the influence of a mechanical loading protocol on the ATP consumption rate of muscle fibres in the left ventricle. ATP consumption by the left ventricle is shown as a function of pressure–volume area (PVA). The linear relationship (dashed line) between ATP consumption and PVA is the same for ejecting (•) and isovolumetric (□) contractions. (Adapted from Vendelin et al. 2002.)
The mitochondrial calcium cycle and respiration regulation paradox
Currently, central to the explanation of cellular mechanisms regulating mitochondrial respiration is the assumption of parallel activation of contraction and respiration by calcium (McCormack et al. 1990; Jouaville et al. 1999; Territo et al. 2001; Balaban, 2002; Bianchi et al. 2004; Brookes et al. 2004; Gunter et al. 2004).
Calcium liberated from local intracellular stores by calcium-induced calcium release mechanisms during excitation–contraction coupling activates the contraction cycle by binding to troponin C in the troponin–tropomyosin complex of thin filaments (Gordon et al. 2001). The mitochondrial calcium cycle, elucidation of which was started with pioneering works of Lehninger, Hunter and Carafoli (for a review see Carafoli, 2003), includes a calcium entry-and-export system that: (a) regulates calcium concentration in the mitochondrial matrix, where calcium is an important activator of the Krebs cycle dehydrogenases, thus increasing the capacity of oxidative phosphorylation (Denton et al. 1978; McCormack et al. 1990; Hansford & Zorov, 1998; Balaban, 2002); (b) allows active regulation of compartmentalized cytoplasmic calcium fluctuations and related signalling (Berridge et al. 2003; Mackenzie et al. 2004); and (c) protects mitochondria from calcium overload (Hansford & Zorov, 1998; Bernardi, 1999; Rizzuto et al. 2000; Gunter et al. 2004; Jacobson & Duchen, 2004).
It is assumed that calcium signals match the demand for ATP in the cell to its production in mitochondria, and thus to the control of respiration (Jouaville et al. 1999; Territo et al. 2001; Balaban, 2002; Brini, 2003; Bianchi et al. 2004; Gunter et al. 2004; Brookes et al. 2004).
Although the ‘calcium-only’ hypothesis of respiration regulation has become popular, quantitative estimates of calcium effects in mitochondria are in conflict with the magnitude of changes in the respiratory rate in vivo (Fig. 1). Both experimental studies of calcium effects on the mitochondrial respiration in vitro (Territo et al. 2001) and mathematical modelling of mitochondrial metabolism (Cortassa et al. 2003) have shown that changes in calcium concentration can at maximum double respiration. This degree of activation of mitochondrial respiration is far too small to explain the energy flux changes of more than an order of magnitude that are observed in muscle cells in vivo (Fig. 1A). In cells with small fluctuations of energy fluxes, direct regulation of mitochondrial activity by calcium may be sufficient (Jouaville et al. 1999), but for excitable cells with high and rapidly fluctuating energy fluxes, such as the heart, skeletal muscle, brain and other cells, this is not the case. The decreased NADH/NAD ratio at higher workloads induced by increased ventricular filling, as observed by Williamson and colleagues (Williamson et al. 1976), is consistent with the metabolic feedback signalling regulating respiration in vivo. In the case of calcium-induced activation, an opposite effect is expected (Balaban, 2002): in this ‘push’ mechanism, the NADH/NAD ratio should be increased first, followed by an increase of respiration due to increased availability of this substrate to the respiratory chain.
Mitochondria are not only the main sites of cellular ATP generation, but also have a regulatory role in apoptosis and cell necrosis, thus making decisions on cell life and death (Bernardi, 1999; Rizzuto et al. 2000; Jacobson & Duchen, 2004; Brookes et al. 2004). Further increase of the mitochondrial calcium content above the optimal level would be extremely dangerous for the cell, since it results in inhibition of ATP synthesis (Holmuhamedov et al. 2001) and opening of the mitochondrial permeability transition pore (PTP) that would finally lead to cell death (Bernardi, 1999; Rizzuto et al. 2000; Brookes et al. 2004). This damaging effect of calcium is amplified by production of reactive oxygen species (ROS) within the respiratory chain (Brookes et al. 2004).
Most paradoxically, the ‘calcium-only’ hypothesis of respiration regulation contradicts conclusions made in cardiac physiology in the studies of the cellular basis of the Frank-Starling law. It was discovered by Hibberd and Jewell and then confirmed by many others that the cellular mechanism behind the Frank-Starling law is the force–length relationship, a length-dependent activation of myofilaments due to increased sensitivity of the thin filaments to calcium at greater sarcomere length (Hibberd & Jewell, 1982; Allen & Kurihara, 1982; Landesberg, 1996; Landesberg & Sideman, 1999; Gordon et al. 2001; Fuchs & Smith, 2001; Robinson et al. 2002; Katz, 2002). Intracellular calcium probes have revealed that practically no changes in the intracellular calcium transients accompany the stretching of sarcomeres sufficient to activate myofilaments (Kentish & Wrzosek, 1998; Shimizu et al. 2002). Only a slow increase of calcium transients after a length change was observed in the experiments of Allen & Kurihara (1982). However, it has clearly been shown in Suga's laboratory that the Frank-Starling mechanism does not affect intracellular calcium recirculation between sarcoplasm and sarcoplasmic reticulum (Mizuno et al. 2001). The number of strongly bound crossbridges and the force generated after calcium binding depend upon the length of sarcomeres (Gordon et al. 2001; Fuchs & Smith, 2001; Katz, 2002; Robinson et al. 2002). The mechanism of this length-dependent activation is complex and still under active study. It includes changes in myofilament lattice spacing with possible involvement of titin (Fukuda et al. 2001), resulting in a decrease of the distance between actin and myosin filaments and increased probability of crossbridge formation with an increase of sarcomere length (Millman, 1998; Fukuda et al. 2001), positive co-operativity of crossbridge binding to actin (Robinson et al. 2002) and an increase of the affinity of the troponin complex for calcium, induced by strong binding of crossbridges (Landesberg, 1996; Landesberg & Sideman, 1999; Gordon et al. 2001; Robinson et al. 2002). Due to these mechanisms, sarcomere stretch at submaximal calcium concentrations in cytoplasm results in an increase in the number of active crossbridges, and thus increased release of the products of ATP hydrolysis, first Pi during the power stroke, and then ADP (Landesberg & Sideman, 1999; Gordon et al. 2000, 2001). By this mechanism of sarcomere length-dependent activation of contraction, the steady-state rates of ATP consumption can change by an order of magnitude without any changes in the calcium cycle (Fig. 1B).
The question of how the rate of ATP production is increased by a factor of 15–20 to match the rates of ATP consumption in cardiomyocytes, while cardiac work is increased by a mechanism of length-dependent activation without the need of alteration of calcium signals, remains unresolved. If there are no changes in cytoplasmic calcium concentrations and/or in high-energy phosphate levels, which intracellular signal could prime mitochondria to increase the respiratory rate in a ‘very energy-rich’ intracellular environment (Colombini, 2004)?
The Km (ADP) paradox and regulation of mitochondrial respiration
In many studies, cytoplasmic ADP concentration is calculated by assuming that creatine kinase (CK) is in full equilibrium in muscle cells in vivo (Meyer et al. 1984; Ingwall, 2002). The popularity of this approach lies in its simplicity to calculate global cellular ADP concentrations, found to be in the range of 50 to 100 μm, and then to deduce the value for the phosphorylation potential – the amount of free energy available in the ATP + ADP + Pi system (Meyer et al. 1984; Gard et al. 1985; Unitt et al. 1992; Ingwall, 2002). However, since the rate of oxidative phosphorylation is regulated by the ADP availability to the adenine nucleotide translocator (ANT) and since the affinity of ANT for ADP is very high with an apparent Km(ADP) between 5 and 20 μm even in the presence of ATP at physiological concentration (3–5 mm) (Vignais, 1976; Jacobus et al. 1982), respiration should always proceed, even at rest, with a rate ≥ 80% of Vmax (between 120 and 130 μmol of O2 min−1 (g dry wt)−1. This would leave no margin for respiration regulation during workload changes, in conflict with experimental observations. Metabolic stability (or homeostasis) at vastly different workloads and respiration rates only confirms this conclusion: cytoplasmic ADP concentrations calculated from the supposed CK equilibrium are completely dissociated from the rates of respiration (From et al. 1986). Mathematical modelling has shown that the assumption of a CK equilibrium imposes an unnecessary limitation, masking the real mechanism of metabolic regulation of respiration (Saks & Aliev, 1996; see below). [There may still be some justification in calculating a virtual cytosolic phosphorylation potential from these ADP values, if this parameter is taken to show the possible average level or reserve of free energy potentially available in the ATP–ADP–Pi system in the cell cytoplasm, where compartmentalized creatine kinase may approach the equilibrium state in cytoplasmic space in the diastolic phase of contraction (Aliev & Saks, 1997). However, for quantitative analysis of kinetic and energetic aspects of regulation of cellular respiration, this assumption is not appropriate. Instead, local ADP concentrations and phosphorylation potentials in different compartments calculated from mathematical models should be taken into consideration (Kinding et al. 2005a).]
The respiration rate in vivo seems to be controlled by processes and/or structures that can respond linearly to the ADP and Pi liberation within the contraction cycle. Such a regulatory mechanism also should be extremely efficient in removing cytosolic ADP. MgADP, as a close structural analogue of MgATP, is an efficient competitive inhibitor of ATPases, e.g. with a Ki close to 200 μm for filament sliding (Yamashita et al. 1994). In addition, MgADP induces the actomyosin–ADP complex which cooperatively promotes strong-binding crossbridges such as the rigor complexes and increases myofilament Ca2+ sensitivity, thus masking the length-dependent activation (Fukuda et al. 2000). The reversible ion pumps of the sarcoplasmic reticulum and sarcolemma are very sensitive to inhibition by MgADP (de Meis & Inesi, 1982). Therefore, accumulation of MgADP in the vicinity of any ATPase slows down the contraction cycle (Yamashita et al. 1994; Sata et al. 1996) and impairs the Frank-Starling mechanism (Fukuda et al. 1998, 2000; Robinson et al. 2002). In conclusion, the cell needs to be protected from an excess in cytosolic free calcium, as well as from ADP, both potential regulators of mitochondrial respiration. The apparent affinity of oxidative phosphorylation for cytoplasmic ADP may be modified also by regulating the permeability of the voltage-dependent anion channel (VDAC) in the outer mitochondrial membrane (Saks et al. 1994; Lemeshko & Lemeshko, 2000; Colombini, 2004). In any case, local availability of ADP to ANT is a potential key regulator of respiration.
As will be outlined in the following, intracellular energy and metabolic signalling phosphotransfer networks within a defined cellular structural organization have the potential to both: (1) protect cells from the excess of cytosolic free calcium and ADP; and (2) regulate respiratory ATP production in close correspondence to ATP consumption.
Creatine kinase isoenzymes constitute a local and global energy transfer network
The major cellular energy transferring networks relies on isoenzymes of CK and the rapidly diffusable phosphocreatine (PCr) as a ‘high-energy’ intermediate (Fig. 2). CK catalyses the reversible transfer of ‘high-energy’ phosphoryl groups from PCr to ADP to generate ATP, or alternatively, from ATP to creatine (Cr) to generate PCr. CK acts as a very sensitive ADP sensor and the enzyme is able to remove ADP in situ by rephosphorylating it to ATP, tapping the large cellular PCr pool. Creatine kinase occurs in the form of multiple isoenzymes, with a cytosolic and a mitochondrial isoenzyme coexpressed in most tissues (Wallimann et al. 1992; Schlattner et al. 1998; Schlattner & Wallimann, 2004). These isoenzymes form an intricate high-energy phosphotransfer system not only for ‘temporal buffering’, but also for ‘energy transport’ or ‘spatial energy-buffering’ functions (Fig. 2a–d) (Bessman & Carpenter, 1985; Wallimann et al. 1992; Saks et al. 1994; Schlattner et al. 1998; Wyss & Kaddurah-Daouk, 2000; Saks et al. 2004; Schlattner & Wallimann, 2004), due to their subcellular localization at distinct sites and interaction with a number of cellular proteins, enzymes, biological membranes or organelles.
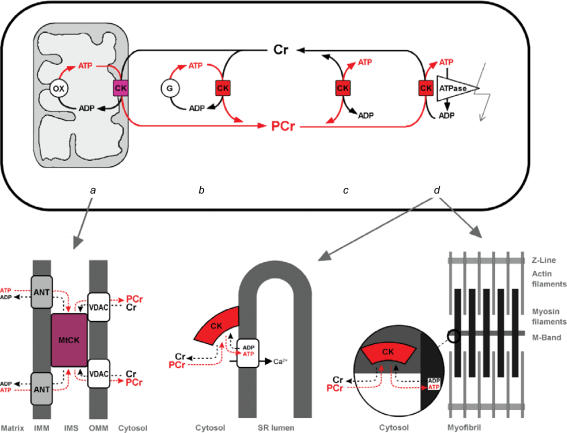
Figure 2. The creatine kinase–phosphocreatine energy transfer system and selected CK microdomains.
Overview (top): isoenzymes of creatine kinase (CK) are found in different compartments like mitochondria (a) and cytosol (b–d), in soluble form (c) or associated to a different degree with ATP delivering (a and b) or -consuming processes (d). A large cytosolic phosphocreatine (PCr) pool of up to 30 mm is built up by CK using creatine (Cr) and ATP preferentially from oxidative phosphorylation (OX), e.g. in heart (a) or from glycolysis (G), e.g. in fast-twitch glycolytic muscle (b). PCr is then used to buffer global (c) and local (d) ATP/ADP ratios. In cells that are polarized and/or have very high or localized ATP consumption, these CK isoenzymes, together with easily diffusible PCr, also maintain an energy shuttle between ATP-providing or -consuming processes (a and d) (Wallimann et al. 1992). Metabolite channelling occurs where CK is associated with ATP-providing or -consuming transporters, pumps or enzymes (a, b and d) (Schlattner & Wallimann, 2004). Substrate and product fluxes between MtCK and the associated proteins are depicted as arrows. MtCK metabolite channelling in mitochondrial microcompartments (lower left): octameric MtCK is found in two locations: (i) in the mitochondrial intermembrane space (IMS), where it associates in the so-called contact sites with the adenine nucleotide translocator (ANT) as well as the voltage-dependent anion channel (VDAC, or porin), and (ii) in the cristae where it associates with ANT only (not shown). In contact sites, octameric MtCK binds simultaneously to inner (IM) and outer mitochondrial membranes (OM), due to the identical top and bottom faces of the octamer. The binding partner in the IM is the 2-fold negatively charged cardiolipin, which allows a functional interaction with adenine nucleotide translocator (ANT) that is situated in cardiolipin membrane patches (Schlattner et al. 2004). In the OM, MtCK interacts with other acidic phospholipids and, in a calcium-dependent manner, directly with the regulated pore-forming VDAC (Schlattner et al. 2001). In contact sites, the metabolite channelling allows for a constant supply of substrates and removal of products at the active sites of MtCK. By preventing the dissipation of mitochondrially generated ATP into the cytosol, its energy content is directly transferred by MtCK to Cr, to give PCr, a metabolically inert compound. PCr then leaves via VDAC into the cytosol, where it is available to CKs at the various locations for in situ regeneration of ATP. Vice versa, intramitochondrial regeneration of ADP stimulates oxidative phosphorylation (OX) (Schlattner et al. 1998). Microcompartments of cytosolic MM-CK with the SR Ca2+-ATPase pump (lower middle): a fraction of total cytosolic muscle MM-CK is bound to the SR where it maintains a high local ATP/ADP ratio and is functionally coupled to the Ca2+-ATPase pump (Rossi et al. 1990). ATP provided to the pump is preferentially delivered from PCr by bound CK and the apparent Km for ATP for the calcium pump is lowered by this functional interaction. The energetically demanding Ca2+ pump operates close to thermodynamic equilibrium and thus, for efficient sequestration of Ca2+, depends on a high local ATP/ADP ratio. Microcompartments of MM-CK in the sarcomeric M-band of the myofibrillar contractile apparatus (lower right): a further fraction of cytosolic muscle MM-CK is bound to the sarcomeric M-band of myofibrils, sufficient in activity to fully regenerate the ATP hydrolysed by the acto-myosin ATPase at maximal contraction velocity. MM-CK is specifically anchored in an isoenzyme-specific manner at the M-band via two ‘lysine charge clamps’ that facilitate the binding of the enzyme to two structural proteins, M-protein, as well as myomesin, at this sarcomeric location (Hornemann et al. 2000).
Mitochondrial CKs (MtCK) form octamers that are present (1) between the outer and inner mitochondrial membrane and (2) in the cristae space (Wallimann et al. 1992; Schlattner et al. 1998; Schlattner & Wallimann, 2005). The kinase catalyses the direct transphosphorylation of intramitochondrially produced ATP and cytosolic creatine into ADP and PCr. ADP enters the matrix space to stimulate oxidative phosphorylation, giving rise to mitochondrial recycling of a specific pool of ATP and ADP, while PCr is the primary ‘high-energy’ phosphoryl compound that leaves mitochondria to enter the cytosol (Jacobus & Lehninger, 1973). The molecular basis for such directed metabolite flux is channelling between the large MtCK octamer (Schlattner et al. 1998; Schlattner & Wallimann, 2005) and two transmembrane proteins, adenine nucleotide translocator (ANT), and mitochondrial porin or voltage-gated anion channel (VDAC) (Fig. 2). The MtCK-linked metabolite channelling is based on: (1) co-localization, (2) direct interactions, and (3) diffusion barriers (Vendelin et al. 2004b; Saks et al. 2005). MtCK tightly binds to cardiolipin, an acidic phospholipid that is specific for the mitochondrial inner membrane. Since ANT is situated in a cardiolipin patch, this leads to co-localization and metabolite channelling between both proteins in the cristae and intermembrane space (Schlattner et al. 2004). MtCK in the intermembrane space further interacts with outer membrane phospholipids and VDAC (Schlattner et al. 2001), thus virtually cross-linking the inner and outer membrane and contributing to the mitochondrial contact sites (Fig. 2, lower left). The limited permeability of VDAC and thus of the entire outer membrane creates a dynamic micro-compartmentation of metabolites in the intermembrane space (Gellerich et al. 1987) that contributes to MtCK-linked channelling and separate mitochondrial ATP and ADP pools (Andrienko et al. 2003).
In muscle, a significant fraction of the non-mitochondrial enzyme (MM-CK) is structurally and functionally associated or co-localized with: (1) different ion pumps in the plasma membrane, (2) the sarcomeric M-band of the myofibrils, and (3) the calcium pump of the sarcoplasmic reticulum (Rossi et al. 1990; Wallimann et al. 1992; Hornemann et al. 2000). Tight functional coupling of CK to ATPases, e.g. acto-myosin ATPase and ion pumps, such as the K+/Na+-ATPase or the Ca2+-ATPase, has the advantage: (1) that product inhibition of the ATPase by ADP and H+ is avoided, since the latter two are both substrates of the CK reaction (PCr2−+ MgADP−+ H+↔ Cr + MgATP2−), and (2) that the high free energy of ATP hydrolysis (ΔGATP) at sites of ATP hydrolysis is preserved by keeping very high ATP/ADP ratios locally due to coupling of CK with those ATPases in situ and thus preventing energy dissipation caused by ATP transport and mixing with the bulk surroundings (Wallimann et al. 1992; Wyss & Kaddurah-Daouk, 2000; Dzeja & Terzic, 2003). There is increasing evidence for a restriction of ATP (and ADP) diffusion in the cell, particularly pronounced in the subsarcolemmal area (Abraham et al. 2002; Karpen & Rich, 2004; Selivanov et al. 2004; Vendelin et al. 2004a). These restrictions are bypassed by the CK–PCr system due to the attachment of the MM-CK isoenzyme to the sarcolemma. It was shown directly that the CK system maintains a close functional coupling between mitochondria and ion channels in sarcolemma (Sasaki et al. 2001; Abraham et al. 2002).
Thus, the CK–PCr circuit represents an efficient regulator of energy flux and uses metabolite channelling as a fine-tuning device of local ATP levels. The significance of such a regulated channelling circuit operating at the high total PCr and Cr pools lies in its high sensitivity towards ADP that it prevents, especially in excitable cells, the accumulation of ADP, and consequently AMP through the adenylate kinase reaction (Dzeja et al. 1998; Dzeja & Terzic, 2005), unless severe stress, such as hypoxia or ischaemia is imposed. In the latter case, AMP-activated protein kinase (AMPK) and other AMP-sensitive components would be activated by free AMP initiating signalling cascades that would turn on compensatory mechanisms for increasing energy supply and reducing energy consumption (Pucar et al. 2004; Kahn et al. 2005).
Integrated phosphotransfer and signalling networks in regulation of cellular energy homeostasis
Besides the CK–PCr system described above, additional and complementary phosphotransfer networks comprising adenylate kinase isoforms and glycolytic enzymes as well as the energy sensor AMP-activated protein kinase (AMPK), work hand in hand with CK (Dzeja & Terzic, 2003; Neumann et al. 2003; Kahn et al. 2005). One of the major outcomes resulting from CK and AK1 knockout studies was the discovery that glycolytic enzymes have the ability to provide a network capacity for transferring and distributing high-energy phosphoryls (Dzeja et al. 1998, 2004; de Groof et al. 2001; Pucar et al. 2002; Janssen et al. 2003a). This new ‘spatial’ look at glycolytic metabolism together with the robust phosphotransfer capacity, especially by the GAPDH–PGK (glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase) couple (From et al. 1986; Kingsley-Hickman et al. 1987), renders glycolysis an equal partner in the cellular energy distribution network along with CK and AK phosphotransfer pathways (Dzeja & Terzic, 2003; Dzeja et al. 2004). The function of the newly discovered adaptor protein DRAL/FHL-2, which is involved in anchoring CK, adenylate kinase and glycolytic enzymes to sites of high-energy consumption (Lange et al. 2002), further highlights the significance of maintaining the structural integrity of intracellular phosphotransfer networks for matching cellular energy needs.
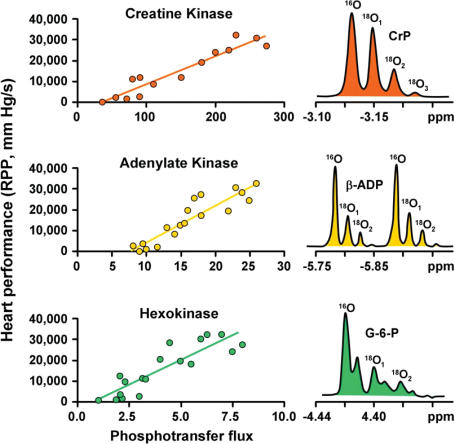
Further insights and key support for the current understanding of metabolic signalling networks in their full complexity have come with the development of new methodologies (Dzeja et al. 1998). High-energy phosphoryl fluxes through CK, adenylate kinase and glycolytic phosphotransfer, captured with 18O-assisted 31P NMR, tightly correlate with the performance of the myocardium under various conditions of stress load (Pucar et al. 2004) (Fig. 3), implicating phosphotransfer reactions as indispensable routes that direct flow of high-energy phosphoryls between cellular ATPases and the ATP production machinery in mitochondria. Another unique feature of the 18O methodology is that it allows direct monitoring of intracellular energetic communication by following the [18O]Pi/[18O]γ-ATP labelling ratio, a novel parameter of energetic signalling between cellular compartments (Pucar et al. 2001). Changes in this parameter indicate that communication between cellular ATPases and ATPsynthases is reduced by myocardial ischaemia, heart failure and transgenic phosphotransfer enzyme deficiencies (Pucar et al. 2001; Janssen et al. 2003a,b; Dzeja et al. 2004). This new methodology allows quantitative evaluation of cellular ATP turnover and the distribution of energy fluxes between different phosphotransfer pathways in vivo. In the experiments with Langendorff-perfused hearts shown in Fig. 3, the creatine kinase flux increased linearly with cardiac performance (evaluated as the rate–pressure product), and reached the value close to 300 nmol min−1 (mg protein)−1 with the heart performing at about 30 000 mmHg min−1, corresponding to a rate of oxygen consumption of about 40 μmol O2 min−1 (g dry weight)−1 (Dos Santos et al. 2000). Taking into account the CK flux in the equilibrium state (in arrested heart) (Fig. 3), protein content of tissue of about 150 mg (g wet weight)−1, and the wet weight/dry weight ratio of 5, these data show the PCr/O2 ratio close to 5. This supports the notion that the CK pathway carries the major part of energy flux out of mitochondria to the ATPases under normal physiological conditions in heart cells as described above (Fig. 2), in agreement with mathematical modelling of the compartmentalized energy transfer (Aliev & Saks, 1997). About 10–15% of cellular high-energy phosphoryls can be carried by the combined AK–glycolytic systems, whose contribution increases with muscle contraction and in failing hearts (Dzeja et al. 1998, 1999; Pucar et al. 2001). Such flux distribution between CK, AK and glycolytic systems is based on the assumption of parallel phosphotransfer pathways. However, in the cellular environment, these pathways are closely co-localized and interconnected allowing high-energy phosphoryls to flow from one system to another (Kupriyanov et al. 1984; Bessman & Carpenter, 1985; Wallimann et al. 1992; Janssen et al. 2000; Dzeja & Terzic, 2003; Dzeja et al. 2004). Indeed, labelling studies indicate that about 1/3 of phosphoryls in PCr come from the adenine nucleotide β-phosphoryls through the AK catalysis (Bessman & Carpenter, 1985). Also, there is an intimate interaction and complementation between AK, CK and glycolytic phosphotransfers at myofibrillar and mitochondrial sites (Dzeja et al. 1998; de Groof et al. 2001; Neumann et al. 2003; Dzeja & Terzic, 2005). The role and contribution of individual phosphotransfer enzymes also depend on the species, tissue, developmental stage or physiological state, underscoring the plasticity of the cellular energetic system in governing metabolic homeostasis (Dzeja et al. 2002; Dzeja & Terzic, 2005). The linear relationship between the creatine kinase flux and the workload (and thus the respiration rate) is also consistent with its measurements by the 31P-NMR saturation transfer method (Kupriyanov et al. 1984; Bittl & Ingwall, 1985). The latter method is, however, rather ‘global’ and the results of its use may sometimes be misinterpreted (Wallimann, 1996). Further complicating global flux analysis of the system, the directions of CK reaction fluxes are opposite at subcellular sites of energy utilization, where bound CK regenerates ATP, and at mitochondria, where CK produces PCr. Also, up to 35% of total CK flux, most probably corresponding to compartmentally bound CK, is ‘NMR-invisible’ (van Deursen et al. 1994; Wallimann, 1996). However, by a new method of NMR CK flux analysis (Joubert et al. 2001), it was possible to demonstrate multicompartment CK fluxes that are consistent with the PCr–CK shuttle (Joubert et al. 2004).
Figure 3. Phosphotransfer networks mediate coupling between heart functional activity and ATP generation.
Processing of high-energy phosphoryls through creatine kinase (CK), adenylate kinase (AK) and glycolytic (hexokinase) systems correlates linearly with heart functional activity (left panels). Phosphotransfer flux measurements obtained from 18O-induced shift in 31P NMR spectra of phosphocreatine (CrP), β-ADP and glucose-6-phosphate (G-6-P) (right panels) (Pucar et al. 2001). Appearance of 18O1, 18O2 and 18O3 peaks in corresponding phosphoryl-containing metabolites indicates phosphotransfer flux through the CK, AK and hexokinase steps in the glycolytic system (Dzeja et al. 2004). Control hearts and contractile function modulated by ischaemia–reperfusion are included in calculations (Pucar et al. 2001). RPP, rate pressure product; phosphotransfer flux is expressed as nmol min−1 (mg protein)−1 of corresponding metabolite.
The 18O-assisted 31P NMR and mass spectrometry have less complications compared with the 31P NMR saturation or inversion spin transfer methods (Dzeja et al. 1998, 2004; Pucar et al. 2001), allowing quantitative studies of in vivo distribution of high-energy phosphoryl fluxes through different metabolic pathways (Janssen et al. 2000). The high values of the CK fluxes and their linear relationship with workload measured by this method are consistent with a central role of the CK system in energo-mechanical coupling underlying the heart's Frank-Starling law.
Phosphotransfer systems in metabolic feedback regulation
Due to a high degree of structural organization and diffusional restrictions, intracellular energetic signalling employs distinct mechanisms of communication, ranging from substrate channelling, reaction-diffusion and ligand conduction, depending on the specific compartment, molecular organization and topological arrangement of enzymatic networks (Bessman & Carpenter, 1985; Saks et al. 1994, 2004; Dzeja & Terzic, 2003, 2005; Schlattner & Wallimann, 2004). While the spatial heterogeneity and directionality of enzyme-catalysed processes are not possible in well mixed conditions in vitro, this becomes a vital entity in highly organized living matter (Mitchell, 1979). In such organized enzymatic networks a wide range of metabolic flux changes can be induced and propagated at relatively stable levels of intermediates (Dzeja et al. 1998; Vance et al. 2002). The studies, initiated by the discovery of organized enzyme clusters, so-called metabolons by Srere some 30 years ago (for review see Srere, 2000), have significantly contributed to the development of a new, holistic approach to cell metabolism and regulation, for which the term ‘molecular system biology’ has been coined (Kitano, 2002; Noble, 2002). Cluster organization and the high rate of unidirectional phosphoryl exchange in phosphotransfer systems promotes ligand conduction and signal communication at cellular distances providing enhanced thermodynamic efficiency (Mitchell, 1979; Saks et al. 1994, 2004; Dzeja et al. 1998). Indeed, the surrounding of mitochondria is properly ordered in muscle cells in vivo to avoid the potential chaos of macromolecular crowding, and mitochondria are arranged in a very regular pattern (Vendelin et al. 2005), forming intracellular energetic units (ICEUs), functional complexes with adjacent MgATPases of sarcoplasmic reticulum and myofibrils (Saks et al. 2001; Seppet et al. 2001; Vendelin et al. 2004a). ICEUs are analogous to calcium release units (CRUs), structurally organized sites of Ca2+ microdomains (Ca2+ sparks) which form a discrete, stochastic system of intracellular calcium signalling in cardiac cells (Wang et al. 2004). The structural organization of ICEUs results in local confinement of adenine nucleotides and Cr–PCr couple in discrete dynamic energetic circuits between actomyosin ATPases and mitochondrial ATPsynthases (Dzeja et al. 1998; Saks et al. 2004). Similar discrete microdomains in cardiomyocytes have been shown for cAMP in the range of approximately 1 μm and with high local concentrations (Zaccolo & Pozzan, 2002). Assuming that all units function in a synchronized manner, it would be sufficient to describe quantitatively the regulation of mitochondrial function within one such ICEU unit (Aliev & Saks, 1997; Vendelin et al. 2000). Indeed, it has been shown by Aon et al. (2003) that depolarization by a two-photon laser of a small number of mitochondria in a very limited area of the cell did not change the polarization of mitochondria in other parts of the cell. However, the mitochondrial polarization state did begin to oscillate in a synchronized manner after a substantial delay, triggered by local release of reactive oxygen species which in turn generated cyclic changes in the action potential (Aon et al. 2003). This is consistent with multiple studies showing that in cardiac and many other type of cells, mitochondria are morphologically and functionally heterogenous (Zorov et al. 2000, 2004; Collins et al. 2002; Kuznetsov et al. 2004), resulting in depolarization of separate mitochondria (‘black hole’ phenomenon) in pathological states due to reactive oxygen species (ROS)-induced ROS release (Zorov et al. 2000) or mitochondrial permeability transition induced by calcium overload (Kuznetsov et al. 2004; Zorov et al. 2004). It is still an open question and an exciting task to discover which genes and proteins are responsible for the very regular arrangement of mitochondria in heart cells. The proteins controlling the fission and fusion of mitochondria are expressed in muscle cells, but these processes are strongly restricted by a highly organized cell structure and cytoskeletal arrangement (Yaffe, 1999; Rube & van der Bliek, 2004). Further studies should address how the regular arrangement of mitochondria into ICEUs in muscle cells is established from chaotic or stochastic mitochondrial dynamics during muscle development.
There are already several interesting mathematical models of mitochondrial function and cardiac energy metabolism, reviewed recently by Jafri et al. (2001). A quantitative analysis of respiration regulation in the ICEUs under the physiological conditions of the Frank-Starling law can be achieved with a mathematical model of compartmentalized energy transfer sustained by the coupled CK system (available at: http://cens.ioc.ee/~markov/etransfer/current_model.pdf) (Fig. 4A). This model shows that ADP released from crossbridges is rapidly rephosphorylated by myofibrillar CK, producing small but opposite changes in Cr and PCr concentrations (Vendelin et al. 2000). This results in cyclic changes in the concentration of ADP in the core of myofibrils in ICEUs (Fig. 4B) in a microcompartment containing myofibrillar-bound MMCK, where ADP is first produced by actomyosin–MgATPase during the contraction cycle of crossbridges, and then rephosphorylated by CK due to a non-equilibrium state of the CK reaction (Vendelin et al. 2000). Thus, cyclic changes in the local ADP production are immediately displacing the myofibrillar MM-CK reaction in the direction of local ATP regeneration (Fig. 4C). The amplitude of displacement of CK from equilibrium (as well as cyclic changes of ADP) is proportionally increased with workload (Fig. 4B and C) (Vendelin et al. 2000). Without CK, the changes of local ADP concentrations in these microcompartments will be much more dramatic (Aliev & Saks, 1997; Saks et al. 2005). If myoplasmic CK is structurally organized and bound to the cytoskeleton, these cyclic changes may be channelled to mitochondria by a mechanism of so-called ‘vectorial ligand conduction’– spatially directed group translocation down gradients of group potential (Mitchell, 1979; Saks et al. 1994, 2005; Dzeja & Terzic, 2005). The stimulatory effect of these CK ligands (Cr and/or ADP) on mitochondrial respiration is amplified by the functional coupling between MtCK and ANT (Schlattner et al. 1998; Saks et al. 2004, 2005; Schlattner & Wallimann, 2004). Because of this coupling, MtCK always catalyses the steady-state unidirectional PCr and ADP production from mitochondrial ATP and cytoplasmic creatine (Aliev & Saks, 1997; Vendelin et al. 2004b) (Fig. 4D). Within the whole contraction cycle these coupled reactions are in the steady state (Fig. 4D), in which the rates of ADP and ATP cycling and thus the respiration in mitochondria coupled to the PCr production (see Fig. 2) are increased with elevation of the workload (Fig. 4D). Accompanying changes in Cr, PCr and total ATP are within the experimental errors of their detection, thus giving an overall impression of metabolic stability (Vendelin et al. 2000; Wallimann, 1996). In this regard, CK, adenylate kinase and other phosphotransfer isoenzymes in different intracellular compartments are ‘pushed’ or ‘pulled’ from the equilibrium in opposite directions, depending on the activity of an associated process which drives steady-state high-energy phosphoryl flux (Saks et al. 1994; Dzeja et al. 1998).
Figure 4. Mathematical modelling of the regulation of mitochondrial respiration in cardiac cells in vivo under physiological conditions controlled by the Frank-Starling mechanism.
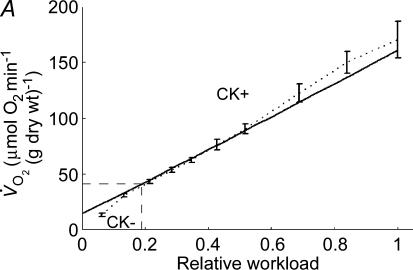
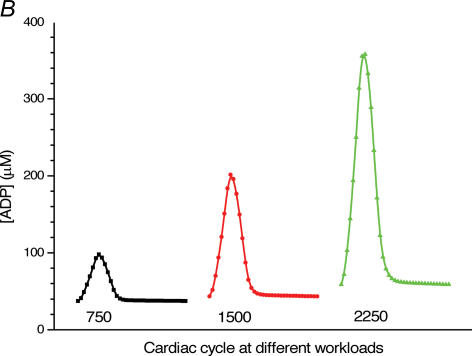
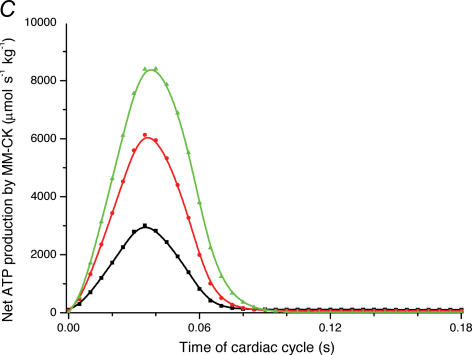
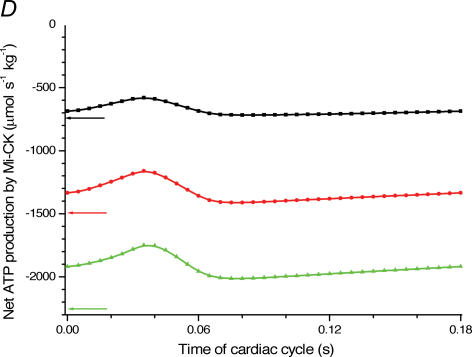
A, computed (continuous line) and experimental (points with standard deviations, from Williamson et al. (1976) oxygen consumption rates of working cardiac muscle. Relative workload is the fraction of maximal workload applied, in computations this is the rate of ATP hydrolysis by actomyosin–MgATPase. CK+ shows the calculations for active creatine kinase system, CK- shows the calculations for the cells with inactivated creatine kinases (results shown within the dashed square). B, ADP profiles in the myoplasmic space in the core of myofibrils of ICEUs over cardiac cycle at different workloads (in the presence of CK). The model includes the diffusion restriction of ADP through mitochondrial outer membrane (Vendelin et al. 2000). Profiles correspond to the workloads equivalent to 750 (black), 1500 (red) and 2250 (green) μmol ATP s−1 kg−1. Reproduced from Vendelin et al. (2000) with permission. C, workload dependence of the dynamics of net ATP production by MM-CK in myofibrils. The reaction rates for workloads of 750 (black), 1500 (red) and 2250 (green) μmol ATP s−1 kg−1 are shown. Net rate of the reaction means displacement from the equilibrium position of the creatine kinase reaction (observed in diastolic phase) as the result of local ADP production in the contraction cycle (Fig. 4B). Courtesy of M. Aliev. D, workload dependence of the dynamics of net PCr production by mito-CK. Workload values are indicated by the arrows of respective colour. Complete model of Dos Santos et al. (2000), used in simulations in Saks et al. (2005). Mitochondrial membrane potential ΔΨ=−160 mV, Vmax of ATP-synthase = 4269.3 μmol ATP s−1 kg−1. PCr export by mitochondria is 90.9, 89.1 and 85.8% of total mitochondrial energy export for workloads of 750, 1500 and 2250 μmol ATP s−1 kg−1, respectively. Due to the functional coupling between ANT and MtCK (Fig. 2), the reaction runs always out of equilibrium in the direction of PCr synthesis (Vendelin et al. 2004b), the steady-state values of the rates of this coupled reactions are increased by increasing the workload (cyclic changes of MgATP and MMCK reactions in myofibrils) and metabolic signalling of these changes to mitochondria by the creatine kinase–phosphocreatine energy transfer system (see Fig. 2 and the text). Courtesy of M. Aliev.
The complex organization of this regulatory network has, paradoxically, a very simple purpose: to respect the conservation equation of ATP synthesis: ADP3−+ Pi2−+ H+↔ ATP4− (Garlid, 2001). Under conditions of metabolic stability, an increase in the rate of ATP synthesis and of the coupled respiration (oxidative phosphorylation) is possible only if the supply of ADP and Pi to mitochondria is increased, making both metabolites good candidates for metabolic feedback signals controlling respiration. ADP is supplied by the metabolic signalling networks described above, to overcome the possible restriction of its diffusion and to avoid the inhibition of MgATPases. Additionally, the CK reaction, if running in the direction of ATP regeneration, compensates for pH changes due to MgATPase hydrolysis (Wallimann et al. 1992). Application of the principles of Metabolic Control Analysis, a universal methodological approach to study regulation of multienzyme systems (Westerhoff & VanDam, 1987; Bose et al. 2003), to the mathematical model showed that in parallel to the cyclic changes in ADP, at least at low workloads, Pi flux to mitochondria plays an important regulatory role (Saks et al. 2000). This conclusion was confirmed experimentally (Bose et al. 2003). Thus, the feedback metabolic signal has a complex nature, several of its components such as Pi, ADP and Cr may act in parallel, and their relative contribution changes with workload (Saks et al. 2000; Dzeja & Terzic, 2003). In any case, as a response to sarcomere stretch and crossbridge cycling, this complex signal leads to increases in the respiration rate, coupled mitochondrial PCr production, and energy flux via the CK–PCr system, while also maintaining metabolic stability (homeostasis) at such an elevated workload.
‘Metabolic pacing’: synchonization of electrical and mechanical activities with energy supply
It is equally important for the normal functioning of cardiac cells that the isoenzymes of adenylate kinase and CK regulate the activity of diverse metabolic sensors, such as the ATP-sensitive sarcolemmal K+ (KATP) channel, which opening reduces the duration of the action potential and thus the entry of calcium into the cell (Noma & Shibasaki, 1985; Weiss & Venkatesh, 1993; Dzeja et al. 1998; Carrasco et al. 2001; Abraham et al. 2002; Neumann et al. 2003; Hodgson et al. 2003; Pucar et al. 2004). In the heart, the KATP channel metabolic sensor function is manifested in a feedback response to cellular energetic signals adjusting membrane potential-dependent functions to harmonize myocardial functional activity (Weiss & Venkatesh, 1993; Sasaki et al. 2001; Alekseev et al. 2005). Genetic deficiency, pharmacological inhibition or altered metabolic sensing of KATP channels are associated with disturbed intracellular Ca2+ handling, excitation–contraction coupling and moladaptive stress response (Rajashree et al. 2002; Hodgson et al. 2003; Kane et al. 2004). In humans, mutations in KATP channel sub-units producing, defective metabolic sensing, increase susceptibility to dilated cardiomyopathy (Bienengraeber et al. 2004).
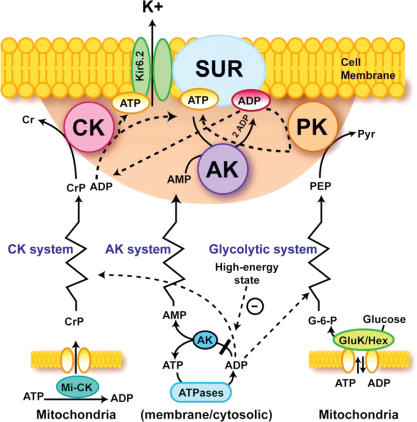
Basic principles of phosphotransfer-mediated metabolic signalling to metabolic sensors at the sarcolemma are presented in Fig. 5. The premembrane area, where diffusion of molecules is restricted due to an unstirred layer of structured water and molecular crowding (Abraham et al. 2002; Selivanov et al. 2004; Saks et al. 2005), represents the ‘sensing zone’ of a metabolic sensor, where intimate changes in nucleotide ratios are sensed and transduced into appropriate cellular responses (Alekseev et al. 2005; Dzeja & Terzic, 1998; Selivanov et al. 2004). Since cellular and even submembrane ATP concentrations are higher than required for half-maximal channel inhibition (between 10 and 100 μm) (Noma & Shibasaki, 1985; Abraham et al. 2002; Selivanov et al. 2004), production of ADP by AK or membrane ATPases and ADP scavenging by CK and PK reactions are critical for channel activity. It may be assumed that dynamic interaction between creatine kinase (CK), adenylate kinase (AK) and glycolytic enzymes (represented as pyruvate kinase, PK) phosphotransfer relays determines the behaviour of metabolic sensors such as the KATP channel – and subsequent cellular responses, such as excitability, hormone secretion, intracellular calcium homeostasis and vascular tone (Carrasco et al. 2001;
Figure 5. A paradigm of phosphotransfer-mediated energetic signalling: coupling cellular metabolic and electrical activities.
Dynamic interaction between creatine kinase (CK), adenylate kinase (AK) and glycolytic (represented by pyruvate kinase, PK) phosphotransfer relays determines a prototypic metabolic sensor – KATP channel behaviour and subsequent cellular responses, such as excitability, hormone secretion, intracellular calcium homeostasis and vascular tone. The shadowed area represents a metabolic sensor ‘sensing zone’, where intimate local changes in nucleotide ratios are sensed and transduced into an appropriate cellular response. Phosphotransfer circuits connect the ‘sensing zone’ with cellular processes. Dashed lines indicate pathways signalling the high-energy state, while continuous lines represent low-energy state signal transmission. Kir6.2, potassium channel subunit; SUR, sulphonylurea receptor; GluK/Hex, glucokinase and hexokinase. Used with permission from Am. J. Physiol 2005.
Sasaki et al. 2001; Abraham et al. 2002; Dzeja & Terzic, 2003; Alekseev et al. 2005; Dhar-Chowdhury et al. 2005; Kane et al. 2005). These phosphotransfer relays communicate metabolic signals originating in mitochondria or at cellular ATPases to metabolic sensors conveying information about ‘high’ or ‘low’ cellular energy, oxygen supply or hormonal states (Dzeja & Terzic, 1998; Tarasov et al. 2004). CK and AK deficiencies or their altered activity ratio compromise metabolic signalling to KATP channels (Carrasco et al. 2001; Abraham et al. 2002). Decreases in CK activity, such as in CK knockouts or failing hearts, can be partially compensated by the increase in glycolytic phosphotransfer to alleviate cardiomyocyte electrical instability (Dzeja et al. 1999; Abraham et al. 2002; Hodgson et al. 2003). Thus, phosphotransfer-governed nucleotide exchange and metabolic sensing is essential in processing cellular information and sustaining energy, ionic and hormonal homeostasis and in generating synchronized electrical and metabolic pacing ensuring cellular energetic homeostasis (Fig. 6). As such, during the cardiac cycle, an electrical impulse-induced action potential and membrane depolarization causes Ca2+ influx through L-type Ca2+ channels in the sarcolemma and T-tubules triggering Ca2+ release from intracellular stores in the sarcoplasmic reticulum (SR) through ryanodine receptor-channels (RyR) and cardiomyocyte contraction (Bers, 2002; Foell et al. 2004). ATP consumed during myofibrillar contraction and Ca2+ pumping is rapidly replenished by CK, AK and glycolytic phosphotransfer relays maintaining optimal local ATP/ADP ratios and free energy of ATP hydrolysis (Wallimann et al. 1992; Saks et al. 1994; Dzeja & Terzic, 2003; Ingwall, 2004). Simultaneously these systems generate and translate feedback signals to stimulate ATP production in mitochondria and to convey information to energy demand sensors, such as KATP channels (and also AMPK) (Dzeja & Terzic, 1998; Neumann et al. 2003; Dzeja et al. 2004; Schlattner & Wallimann, 2004; Saks et al. 2004). The amount of Ca2+ entering a cell is controlled by the action potential duration regulated by KATP channels located in sarcolemma and T-tubules (Sasaki et al. 2001; Kane et al. 2004; Alekseev et al. 2005). Conversely, the activity of this metabolic sensor is regulated by phosphotransfer reaction and Ca2+ feedbacks (Weiss & Venkatesh, 1993; Dzeja & Terzic, 1998; Wilson et al. 2000; Selivanov et al. 2004). Ca2+ entering the cell or released from intracellular stores activates substrate transport (recruitment of glucose transporters), dehydrogenase (DH) activity (such as pyruvate dehydrogenase, an entry point into the mitochondrial Krebs cycle), and glycogenolysis to meet incoming energy needs (Hansford & Zorov, 1998; Balaban, 2002; Cortassa et al. 2003). Simultaneous recruitment of different steps in the cellular energetic system and ligand conduction type handling of substrates in phosphotransfer systems increase metabolic flux without apparent changes in substrate concentrations (Mitchell, 1979; Saks et al. 1994; Dzeja et al. 1998).
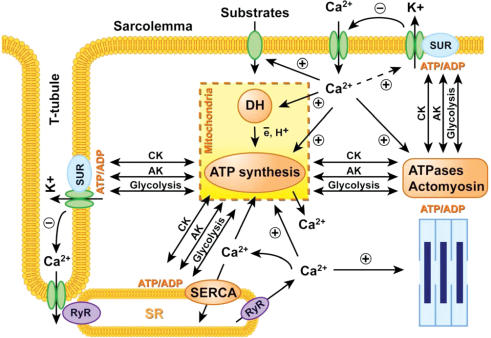
Figure 6. Cardiac excitation–contraction–energy coupling: synchronization electrical and metabolic pacing.
Electrical pacing induced action potential and membrane depolarization causes Ca2+ influx through L-type Ca2+ channels in sarcolemma and T-tubules triggering Ca2+ release from intracellular stores in sarcoplasmic reticulum (SR) through ryanodine receptor-channels (RyR) and cardiomyocyte contraction. Interplay between CK, AK and glycolytic phosphotransfer relays, energetic modules (mitochondria and ATPases), metabolic sensors (KATP channel) and Ca2+ transients, generate metabolic pacing signals in synchrony with the electrical and functional activity to ensure cellular energetic homeostasis. After contraction intracellular Ca2+ is sequestrated by SR Ca2+-ATPase (SERCA), by mitochondria, and removed from the cell by the Na+–Ca2+ exchanger and a Ca2+ pump in sarcolemma (see details in the text).
Thus, phosphotransfer-mediated metabolic feedback and Ca2+ signalling are important components in regulated signal transmission and coordinated dehydrogenase activation producing an optimal mitochondrial response to increased cellular energy demands.
Metabolic channelling is needed for a wide range of workloads and protects the cell from deleterious effects of calcium overload
The physiological mechanism of respiration regulation described above has the important advantage of ensuring effective control of free energy conversion across the whole physiological range of workloads, without requiring a severe increase in cytoplasmic calcium concentration. It thus avoids any danger of mitochondrial calcium overload that would open the mitochondrial permeability transition pore and thus lead to cell death. Without such a mechanism of metabolic signalling and protection (Dolder et al. 2003), the fate of mitochondria in heart and oxidative skeletal muscle cells would be left under the monopolized control of calcium. Under these metabolic circumstances an Olympic athlete would probably be dead before finishing the competition, because of mitochondrial calcium overload and PTP opening, accelerated by ROS production by mitochondria (Brookes et al. 2004). Indeed, any attempt to increase the respiration rate more than 2-fold by calcium would necessarily imply a heavy overload of mitochondria by this potentially dangerous cation. Very fortunately, the regulation of mitochondrial function by the efficient protective mechanism of metabolic feedback channelling was chosen by nature long ago: the mitochondrial CK octamer appeared in very early stages of biological evolution (Ellington, 2001).
The importance of CK function is corroborated by the phenotype of transgenic CK knock-out mice. Mice that do not express either muscle-type cytosolic or sarcomeric MtCK, or both, exhibit, despite some remarkable compensations, like-enhanced muscle glycogen content and a massive increase in mitochondrial volume fraction (Steeghs et al. 1998), marked difficulties with peak force development and burst activity (van Deursen et al. 1993), as well as disturbances both in frequency and power output of voluntary movements (Momken et al. 2005). Studies with CK double knock-out mice have shown that the CK system is critically necessary to maintain optimal heart function at increased workloads (Spindler et al. 2004). The strongest phenotype of CK double knock-out mice is characterized by significant perturbations in intracellular calcium handling and muscle relaxation, emphasizing the physiological importance of the CK system for the energetics of intracellular calcium homeostasis in general and for the delivery of ATP to the energetically demanding Ca2+-ATPase, in particular (Steeghs et al. 1998). Hearts with deficient cytosolic and mitochondrial CK isoforms exhibit impaired calcium homeostasis and increased susceptibility to ischaemia-reperfusion injury (Spindler et al. 2004). The M-CK gene knockout also shifts cellular energetics and signal delivery to KATP channels towards the glycolytic system, thus generating a phenotype with increased electrical vulnerability to disturbances in glucose metabolism (Abraham et al. 2002; Dzeja et al. 2004). Interestingly, fast-twitch CK-knockout skeletal muscles fatigue more slowly during repeated tetanic stimulation, probably due to an increase in mitochondrial volume and a decrease of crossbridge cycling as the result of actomyosin–ADP complex formation, which cooperatively promotes strong-binding crossbridges (Dahlstedt et al. 2000; Fukuda et al. 2000).
Adenylate kinase, whose phosphotransfer flux along with AMP concentration markedly increases under stress, contributes to the response of KATP channels under metabolic challenge (Carrasco et al. 2001). Deletion of the adenylate kinase AK1 gene compromises nucleotide exchange at the metabolic sensing KATP channel site and impedes communication between mitochondria-generated signals and KATP channels, rendering cellular metabolic sensing defective (Carrasco et al. 2001). Hearts deficient in the cytosolic adenylate kinase (AK1) isoform have aberrant AMP and adenosine metabolic signalling, compromised ability to maintain nucleotide pools and increased vulnerability to injury (Pucar et al. 2002), suggesting that normal adenylate kinase function contributes to stress resistance in the heart. Double genetic disruption of the cytosolic CK (M-CK)- and AK1-catalysed phosphotransfer pathways compromises intracellular metabolic communication and energetic efficiency reducing the cellular capability to maintain total ATP turnover under functional load (Janssen et al. 2003b) Thus, new methodologies and transgenic gene manipulations provide the opportunity to decipher regulatory mechanisms that underlie cardiac bioenergetic homeostasis, and have revealed their significance for normal muscle function.
Due to the central importance of this efficient and protective metabolic feedback signalling system, its alteration in pathological states of the cell contributes significantly to the development of cardiac failure, either due to a decrease of total creatine content (Nascimben et al. 1996; ten Hove et al. 2005a), alteration of CK expression (Nahrendorf et al. 2005), or changes of the coupling of CK in mitochondria and with MgATPases (Zoll et al. 2005; for review see Ventura-Clapier et al. 2004; Schlattner et al. 2005). Ingwall and colleagues have shown that the product of CK activity and total creatine content, CK ×[Cr], decreases by a factor of 3–6 in cardiomyopathy and in failing human hearts (Nascimben et al. 1996). This is consistent with earlier data showing that replacement of creatine by its much less productive analogue guanidino propionic acid, given in diet, decreases by more than half the maximal work performance capacity and oxygen consumption of isolated working perfused heart (Kapelko et al. 1988). Also, significant wash-out of creatine during long periods of perfusion of frog hearts has been shown to lead to a hypodynamic state with a 2- to 3-fold decrease of contractile force, which can be reversed by uptake of creatine (Saks et al. 1976; Ventura-Clapier & Vassort, 1980). Very recently, it was shown in Neubauer's laboratory that hearts with undetectable levels of creatine and phosphocreatine due to knock-out of a key enzyme of creatine biosynthesis, lost the ability to respond to inotropic stimulation even at low workload, and showed markedly impaired recovery of heart function during ischaemia/reperfusion (ten Hove et al. 2005b). Momken et al. (2005) found recently that the double M-CK and Mt-CK knock-out mice showed only 10% of work capacity in voluntary exercise in running wheels, as compared with that of normal wild-type mice. These experimental results fit surprisingly closely with those of mathematical modelling (Fig. 4A) according to which, in the absence of creatine, the workload (the MgATPases, due to their inhibition by accumulating MgADP) and the rate of oxygen consumption are decreased by a factor of 4–5 (Vendelin et al. 2000). This model, however, does not yet include the AK and glycolytic systems and does not account for the adaptation phenomena in the CK knockout mice, which may increase the work capacity of the muscles.
Thus, effective cardiac work and fine metabolic regulation of respiration and energy fluxes need the organized and interconnected energy transfer and metabolic signalling systems. Direct transfer of ATP and ADP between mitochondria and different cellular compartments is not able to fulfil this important task efficiently.
Concluding remarks and future directions
The paradox of two apparently opposite conclusions concerning the role of calcium in regulating mitochondrial respiration that were reached in two different areas of bioenergetic research, i.e. the mitochondrial calcium cycle and the Frank-Starling mechanism, can thus be resolved by structural and quantitative analysis of cellular energy transfer networks as described in this review. The data reviewed indicate the critical importance of two interrelated systems regulating mitochondrial respiration and energy fluxes in the cells (Fig. 6): (1) the structurally organized enzymatic modules and networks of the CK–AK–glycolytic systems communicating flux changes in non-equilibrium steady state, and (2) the secondary system based on cellular and mitochondrial calcium cycles, which adjusts the capacity of substrate oxidation and energy-transducing processes to meet increasing cellular energy demands. Moreover, the coupled creatine kinase, adenylate kinase and glycolytic reactions by communicating signals to the KATP channel, a sarcolemmal metabolic sensor, provide fine-tuned regulation of excitation–contraction and thus the calcium cycle within a cell. Such integration of energetic and ion signalling systems provides the basis for ‘metabolic pacing’, synchronizing electrical and mechanical activities with energy supply processes, which is fundamental for optimal function of the heart. The integrated scheme described in Fig. 6 provides a good example of the problems of molecular system biology applied to the heart – a new and rapidly developing area of scientific research.
In cardiac cells, the first system explains quantitatively the metabolic aspect of the classical Frank-Starling law of regulation of cardiac function and respiration under conditions of metabolic stability and unchanged calcium transients. In agreement with this conclusion, recent studies have shown that also in skeletal muscle the metabolic signals arising from the contractile sites play a more substantial role than cytoplasmic calcium in the signalling pathway to oxidative phosphorylation (Kindig et al. 2005b). The second system explains adrenergic modulation of cardiac cell function and energetics under stress (Berridge et al. 2003). By regulating the sarcolemmal metabolic sensor – KATP channels, the CK–AK–glycolytic network affects the excitation–contraction process and thus the calcium cycle of the cell (Fig. 6). Both systems may be activated simultaneously, as it is observed in the case of positive inotropy induced by β-adrenergic agents, when Frank-Starling curves are shifted upward (Starling & Visscher, 1926; Opie, 1998). These two more-or-less independent systems regulating cellular energetics have already been described separately by mathematical models (Vendelin et al. 2000; Saucerman et al. 2003); their integration into quantitative models of whole cell function and metabolism is an interesting and challenging perspective of molecular system biology. Structural, functional, genomic and computational analysis of enzymatic clusters and networks is yet another future challenge in cellular energetics. One of the most intriguing questions among them is that of the nature of local restrictions of the diffusion of adenine nucleotides in a highly structured cytosol in spite of the relatively high rates of their diffusion in the intracellular bulk water phase (de Graaf et al. 2000). These diffusion restrictions result in compartmentation of adenine nucleotides and kinetic and thermodynamic inefficiency of energy-dependent processes, which may explain the necessity of energy transfer and metabolic signalling networks. Future developments to verify predictions made by theoretical considerations and by increasingly complex mathematical models should include new experimental methods allowing detection of small changes in the transduced metabolic signals (Cr, ADP, AMP, Pi) in critical cellular compartments, and new means of independent manipulation of energetic signals. This would provide a broader and more molecular understanding of regulation of cellular energetics and metabolic signalling, a rapidly growing area in cellular system biology.
Acknowledgments
This work was supported by INSERM, France, and by grants from the Estonian Science Foundation (Nos 5515 and 6142 to V.S.), the Swiss National Science Foundation and the Swiss Heart Foundation, and EU program LSHM-CT-2004-005272 “EXGENESIS” (to T.W. and U.S.), the National Institutes of Health (HL64822, HL07111), Marriott Program for Heart Disease Research, Marriott Foundation and Miami Heart Research Institute (A.T. and P.D.). M.V. is on leave from Department of Mechanics and Applied Mathematics Institute of Cybernetics, Tallinn Technical University, Tallinn, Estonia. We thank Mayis Aliev (Cardiology Research Center, Moscow) for contributing Fig. 4C and D. We are grateful to Dr David C. Turner, Syracuse University, USA and MD/PhD student Susan Chung, Mayo Clinic, USA for their help with critically reading and editing the manuscript, and to Dr Andrei Kuznetsov, Medical University Innsbruck, Austria, for his helpful critical remarks. Due to space limitations, as well as restriction of cited references, we apologize to all those colleagues and researchers in the field, whose work is not directly cited here, although they significantly contributed through their work and discussions to this synopsis.
References
- Abraham MR, Selivanov VA, Hodgson DM, Pucar D, Zingman LV, Wieringa B, Dzeja PP, Alekseev AE, Terzic A. Coupling of cell energetics with membrane metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J Biol Chem. 2002;277:24427–24434. doi: 10.1074/jbc.M201777200. [DOI] [PubMed] [Google Scholar]
- Alekseev AE, Hodgson DM, Karger AB, Park S, Zingman LV, Terzic A. ATP-sensitive K+ channel channel/enzyme multimer: metabolic gating in the heart. J Mol Cell Cardiol. 2005;38:895–905. doi: 10.1016/j.yjmcc.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev MK, Saks VA. Compartmentalized energy transfer in cardiomyocytes: use of mathematical modeling for analysis of in vivo regulation of respiration. Biophys J. 1997;73:428–445. doi: 10.1016/S0006-3495(97)78082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrienko T, Kuznetsov AV, Kaambre T, Usson Y, Orosco A, Appaix F, Tiivel T, Sikk P, Vendelin M, Margreiter R, Saks VA. Metabolic consequences of functional complexes of mitochondria, myofibrils and sarcoplasmic reticulum in muscle cells. J Exp Biol. 2003;206:2059–2072. doi: 10.1242/jeb.00242. [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol. 2002;34:1259–1271. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bessman SP, Carpenter CL. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- Bianchi K, Rimessi A, Prandini A, Szabadkai G, Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim Biophys Acta. 2004;1742:119–131. doi: 10.1016/j.bbamcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O'Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittl JA, Ingwall JS. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem. 1985;260:3512–3517. [PubMed] [Google Scholar]
- Bose S, French S, Evans FJ, Joubert F, Balaban RS. Metabolic network control of oxidative phosphorylation: multiple roles of inorganic phosphate. J Biol Chem. 2003;278:39155–39165. doi: 10.1074/jbc.M306409200. [DOI] [PubMed] [Google Scholar]
- Brini M. Ca2+ signalling in mitochondria: mechanism and role in physiology and pathology. Cell Calcium. 2003;34:399–405. doi: 10.1016/s0143-4160(03)00145-3. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Historical review: mitochondria and calcium: ups and downs of an unusual relationship. Trends Biochem Sci. 2003;28:175–181. doi: 10.1016/S0968-0004(03)00053-7. [DOI] [PubMed] [Google Scholar]
- Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins TJ, Berridge MJ, Lipp P, Bootman MD. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J. 2002;21:1616–1627. doi: 10.1093/emboj/21.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombini M. VDAC: the channel at the interface between mitochondria and the cytosol. Mol Cell Biochem. 2004;256–257:107–115. doi: 10.1023/b:mcbi.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- Cortassa S, Aon MA, Marban E, Winslow RL, O'Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys J. 2003;84:2734–2755. doi: 10.1016/S0006-3495(03)75079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Wieringa B, Westerblad H. Is the creatine kinase responsible for fatigue? Studies of isolated skeletal muscle deficient in creatine kinase. FASEB J. 2000;14:982–990. doi: 10.1096/fasebj.14.7.982. [DOI] [PubMed] [Google Scholar]
- de Graaf RA, van Kranenburg A, Nicolay K. In vivo 31P-NMR diffusion spectroscopy of ATP and phosphocreatine in rat skeletal muscle. Biophys J. 2000;78:1657–1664. doi: 10.1016/S0006-3495(00)76717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groof AJ, Oerlemans FT, Jost CR, Wieringa B. Changes in glycolytic network and mitochondrial design in creatine kinase-deficient muscles. Muscle Nerve. 2001;24:1188–1196. doi: 10.1002/mus.1131. [DOI] [PubMed] [Google Scholar]
- de Meis L, Inesi G. ATP synthesis by sarcoplasmic reticulum ATPase following Ca2+, pH, temperature, and water activity jumps. J Biol Chem. 1982;257:1289–1294. [PubMed] [Google Scholar]
- Denton RM, Richards DA, Chin JG. Calcium ions and the regulation of NAD+ linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J. 1978;176:899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar-Chowdhury P, Harrell MD, Han SY, Jankowska D, Parachuru L, Morrissey A, Srivastava S, Liu W, Malester B, Yoshida H, Coetzee WA. The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose phosphate isomerase and pyruvate kinase are components of the KATP channel macromolecular complex and regulate its function. J Biol Chem. 2005;280:38464–38470. doi: 10.1074/jbc.M508744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder M, Walzel B, Speer O, Schlattner U, Wallimann T. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem. 2003;278:17760–17766. doi: 10.1074/jbc.M208705200. [DOI] [PubMed] [Google Scholar]
- Dos Santos P, Aliev MK, Diolez P, Duclos F, Besse P, Bonoron-Adele S, Sikk P, Canioni P, Saks VA. Metabolic control of contractile performance in isolated perfused rat heart. Analysis of experimental data by reaction: diffusion mathematical model. J Mol Cell Cardiol. 2000;32:1703–1734. doi: 10.1006/jmcc.2000.1207. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Bortolon R, Perez-Terzic C, Holmuhamedov EL, Terzic A. Energetic communication between mitochondria and nucleus directed by catalyzed phosphotransfer. Proc Natl Acad Sci U S A. 2002;99:10156–10161. doi: 10.1073/pnas.152259999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzeja PP, Terzic A. Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. Faseb J. 1998;12:523–529. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J Exp Biol. 2003;206:2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Terzic A. Mitochondrial-nucleus energetic communication: role of phosphotransfer networks in processing cellular information. In: Gibson G, Dienel G, editors. Handbook of Neurochemistry and Molecular Neurobiology: Neural Energy Utilization. New York: Kluwer; 2005. [Google Scholar]
- Dzeja PP, Terzic A, Wieringa B. Phosphotransfer dynamics in skeletal muscle from creatine kinase gene-deleted mice. Mol Cell Biochem. 2004;256–257:13–27. doi: 10.1023/b:mcbi.0000009856.23646.38. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Vitkevicius KT, Redfield MM, Burnett JC, Terzic A. Adenylate kinase-catalyzed phosphotransfer in the myocardium: increased contribution in heart failure. Circ Res. 1999;84:1137–1143. doi: 10.1161/01.res.84.10.1137. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Zeleznikar RJ, Goldberg ND. Adenylate kinase: kinetic behavior in intact cells indicates it is integral to multiple cellular processes. Mol Cell Biochem. 1998;184:169–182. [PubMed] [Google Scholar]
- Ellington W. Evolution and physiological roles of phosphagen systems. Annu Rev Physiol. 2001;63:289–325. doi: 10.1146/annurev.physiol.63.1.289. [DOI] [PubMed] [Google Scholar]
- Evans CL, Matsuoka Y. The effects of various mechanical conditions on gaseous metabolism and efficiency of the mammalian heart. J Physiol. 1915;49:379–405. doi: 10.1113/jphysiol.1915.sp001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foell JD, Balijepalli RC, Delisle BP, Yunker AM, Robia SL, Walker JW, McEnery MW, January CT, Kamp TJ. Molecular heterogeneity of calcium channel beta-subunits in canine and human heart: evidence for differential subcellular localization. Physiol Genomics. 2004;17:183–200. doi: 10.1152/physiolgenomics.00207.2003. [DOI] [PubMed] [Google Scholar]
- Frank O. Zur Dynamik des Herzmuskels. Z Biologie. 1885;32:370–447. [Google Scholar]
- From AH, Petein MA, Michurski SP, Zimmer SD, Ugurbil K. 31P-NMR studies of respiratory regulation in the intact myocardium. FEBS Lett. 1986;206:257–261. doi: 10.1016/0014-5793(86)80992-9. [DOI] [PubMed] [Google Scholar]
- Fuchs F, Smith SH. Calcium, cross-bridges, and the Frank-Starling relationship. News Physiol Sci. 2001;16:5–10. doi: 10.1152/physiologyonline.2001.16.1.5. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Fujita H, Fujita T, Ishiwata S. Regulatory roles of MgADP and calcium in tension development of skinned cardiac muscle. J Muscle Res Cell Motil. 1998;19:909–921. doi: 10.1023/a:1005437517287. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Kajiwara H, Ishiwata S, Kurihara S. Effects of MgADP on length dependence of tension generation in skinned rat cardiac muscle. Circ Res. 2000;86:E1–E6. doi: 10.1161/01.res.86.1.e1. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Sasaki D, Ishiwata S, Kurihara S. Length dependence of tension generation in rat skinned cardiac muscle: role of titin in the Frank-Starling mechanism of the heart. Circulation. 2001;104:1639–1645. doi: 10.1161/hc3901.095898. [DOI] [PubMed] [Google Scholar]
- Gard JK, Kichura GM, Ackerman JJ, Eisenberg JD, Billadello JJ, Sobel BE, Gross RW. Quantitative 31P nuclear magnetic resonance analysis of metabolite concentrations in Langendorff-perfused rabbit hearts. Biophys J. 1985;48:803–813. doi: 10.1016/S0006-3495(85)83839-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlid KD. Physiology of mitochondria. In: Sperelakis N, editor. Cell Physiology Sourcebook a Molecular Approach. 3. San Diego-New York-London: Academic Press; 2001. pp. 139–151. [Google Scholar]
- Gellerich FN, Schlame M, Bohnensack R, Kunz W. Dynamic compartmentation of adenine nucleotides in the mitochondrial intermembrane space of rat-heart mitochondria. Biochim Biophys Acta. 1987;890:117–126. doi: 10.1016/0005-2728(87)90012-0. [DOI] [PubMed] [Google Scholar]
- Gibbs CL. Cardiac energetics. Physiol Rev. 1978;58:174–254. doi: 10.1152/physrev.1978.58.1.174. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Regnier M, Homsher E. Skeletal and cardiac muscle contractile activation: tropomyosin ‘rocks and rolls’. News Physiol Sci. 2001;16:49–55. [PubMed] [Google Scholar]
- Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- Hansford RG, Zorov D. Role of mitochondrial calcium transport in the control of substrate oxidation. Mol Cell Biochem. 1998;184:359–369. [PubMed] [Google Scholar]
- Hibberd MG, Jewell BR. Calcium- and length-dependent force production in rat ventricular muscle. J Physiol. 1982;329:527–540. doi: 10.1113/jphysiol.1982.sp014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW. Intracellular convection, homeostasis and metabolic regulation. J Exp Biol. 2003;206:2001–2009. doi: 10.1242/jeb.00402. [DOI] [PubMed] [Google Scholar]
- Hodgson DM, Zingman LV, Kane GC, Perez-Terzic C, Bienengraeber M, Ozcan C, Gumina RJ, Pucar D, O'Coclain F, Mann DL, Alekseev AE, Terzic A. Cellular remodeling in heart failure disrupts KATP channel-dependent stress tolerance. EMBO J. 2003;22:1732–1742. doi: 10.1093/emboj/cdg192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmuhamedov EL, Ozcan C, Jahangir A, Terzic A. Restoration of Ca2+-inhibited oxidative phosphorylation in cardiac mitochondria by mitochondrial Ca2+ unloading. Mol Cell Biochem. 2001;220:135–140. doi: 10.1023/a:1010894427373. [DOI] [PubMed] [Google Scholar]
- Hornemann T, Stolz M, Wallimann T. Isoenzyme-specific interaction of muscle-type creatine kinase with the sarcomeric M-line is mediated by NH(2)-terminal lysine charge-clamps. J Cell Biol. 2000;149:1225–1234. doi: 10.1083/jcb.149.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingwall JS. ATP and the Heart. Dordrecht-Boston-London: Kluwer Academic Publishers; 2002. pp. 1–244. [Google Scholar]
- Ingwall JS. Transgenesis and cardiac energetics: new insights into cardiac metabolism. J Mol Cell Cardiol. 2004;37:613–623. doi: 10.1016/j.yjmcc.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Jacobson J, Duchen MR. Interplay between mitochondria and cellular calcium signalling. Mol Cell Biochem. 2004;256–257:209–218. doi: 10.1023/b:mcbi.0000009869.29827.df. [DOI] [PubMed] [Google Scholar]
- Jacobus WE, Lehninger AL. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. J Biol Chem. 1973;248:4803–4810. [PubMed] [Google Scholar]
- Jacobus WE, Moreadith RW, Vandegaer KM. Mitochondrial respiratory control. Evidence against the regulation of respiration by extramitochondrial phosphorylation potentials or by [ATP]/[ADP] ratios. J Biol Chem. 1982;257:2397–2402. [PubMed] [Google Scholar]
- Jafri MS, Dudycha SJ, O'Rourke B. Cardiac energy metabolism: models of cellular respiration. Annu Rev Biomed Eng. 2001;3:57–81. doi: 10.1146/annurev.bioeng.3.1.57. [DOI] [PubMed] [Google Scholar]
- Janssen E, de Groof A, Wijers M, Fransen J, Dzeja PP, Terzic A, Wieringa B. Adenylate kinase 1 deficiency induces molecular and structural adaptations to support muscle energy metabolism. J Biol Chem. 2003a;278:12937–12945. doi: 10.1074/jbc.M211465200. [DOI] [PubMed] [Google Scholar]
- Janssen E, Dzeja PP, Oerlemans F, Simonetti AW, Heerschap A, de Haan A, Rush PS, Terjung RR, Wieringa B, Terzic A. Adenylate kinase 1 gene deletion disrupts muscle energetic economy despite metabolic rearrangement. EMBO J. 2000;19:6371–6381. doi: 10.1093/emboj/19.23.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen E, Terzic A, Wieringa B, Dzeja PP. Impaired intracellular energetic communication in muscles from creatine kinase and adenylate kinase (M-CK/AK1) double knock-out mice. J Biol Chem. 2003b;278:30441–30449. doi: 10.1074/jbc.M303150200. [DOI] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert F, Hoerter JA, Mazet JL. Discrimination of cardiac subcellular creatine kinase fluxes by NMR spectroscopy: a new method of analysis. Biophys J. 2001;81:2995–3004. doi: 10.1016/S0006-3495(01)75940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert F, Mateo P, Gillet B, Beloeil JC, Mazet JL, Hoerter JA. CK flux or direct ATP transfer: versatility of energy transfer pathways evidenced by NMR in the perfused heart. Mol Cell Biochem. 2004;256–257:43–58. doi: 10.1023/b:mcbi.0000009858.41434.fc. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kane GC, Behfar A, Yamada S, Perez-Terzic C, O'Cochlain F, Reyes S, Dzeja PP, Miki T, Seino S, Terzic A. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53:S169–S175. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapelko VI, Kupriyanov VV, Novikova NA, Lakomkin VL, Steinschneider A, Severina M, Veksler VI, Saks VA. The cardiac contractile failure induced by chronic creatine and phosphocreatine deficiency. J Mol Cell Cardiol. 1988;20:465–479. doi: 10.1016/s0022-2828(88)80074-9. [DOI] [PubMed] [Google Scholar]
- Karpen JW, Rich TC. Resolution of cAMP signals in three-dimensional microdomains using novel, real-time sensors. Proc West Pharmacol Soc. 2004;47:1–5. [PubMed] [Google Scholar]
- Katz AM. Ernest Henry Starling, his predecessors, and the ‘Law of the Heart’. Circulation. 2002;106:2986–2992. doi: 10.1161/01.cir.0000040594.96123.55. [DOI] [PubMed] [Google Scholar]
- Kentish JC, Wrzosek A. Changes in force and cytosolic Ca2+ concentration after length changes in isolated rat ventricular trabeculae. J Physiol. 1998;506:431–444. doi: 10.1111/j.1469-7793.1998.431bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindig CA, Howlett RA, Stary CM, Walsh B, Hogan MC. Effects of acute creatine kinase inhibition on metabolism and tension development in isolated single myocytes. J Appl Physiol. 2005a;98:541–549. doi: 10.1152/japplphysiol.00354.2004. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Stary CM, Hogan MC. Effect of dissociating cytosolic calcium and metabolic rate on intracellular PO2 kinetics in single frog myocytes. J Physiol. 2005b;562:527–534. doi: 10.1113/jphysiol.2004.074922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley-Hickman PB, Sako EY, Mohanakrishnan P, Robitaille PM, From AH, Foker JE, Ugurbil K. 31P NMR studies of ATP synthesis and hydrolysis kinetics in the intact myocardium. Biochemistry. 1987;26:7501–7510. doi: 10.1021/bi00397a045. [DOI] [PubMed] [Google Scholar]
- Kitano H. Systems biology: a brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- Kupriyanov VV, Ya Steinschneider A, Ruuge EK, Kapel'ko VI, Yu Zueva M, Lakomkin VL, Smirnov VN, Saks VA. Regulation of energy flux through the creatine kinase reaction in vitro and in perfused rat heart. 31P-NMR studies. Biochim Biophys Acta. 1984;805:319–331. doi: 10.1016/0167-4889(84)90014-4. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Usson Y, Leverve X, Margreiter R. Subcellular heterogeneity of mitochondrial function and dysfunction: evidence obtained by confocal imaging. Mol Cell Biochem. 2004;256–257:359–365. doi: 10.1023/b:mcbi.0000009881.01943.68. [DOI] [PubMed] [Google Scholar]
- Landesberg A. End-systolic pressure-volume relationship and intracellular control of contraction. Am J Physiol. 1996;270:H338–H349. doi: 10.1152/ajpheart.1996.270.1.H338. [DOI] [PubMed] [Google Scholar]
- Landesberg A, Sideman S. Regulation of energy consumption in cardiac muscle: analysis of isometric contractions. Am J Physiol. 1999;276:H998–H1011. doi: 10.1152/ajpheart.1999.276.3.H998. [DOI] [PubMed] [Google Scholar]
- Lange S, Auerbach D, McLoughlin P, Perriard E, Schafer BW, Perriard JC, Ehler E. Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J Cell Sci. 2002;115:4925–4936. doi: 10.1242/jcs.00181. [DOI] [PubMed] [Google Scholar]
- Lemeshko SV, Lemeshko VV. Model of the outer membrane potential generation by the inner membrane of mitochondria. Biophys J. 2000;82:684–692. doi: 10.1016/S0006-3495(02)75431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J Cell Sci. 2004;117:6327–6337. doi: 10.1242/jcs.01559. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the ‘phosphocreatine shuttle’. Am J Physiol. 1984;246:C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Millman BM. The filament lattice of striated muscle. Physiol Rev. 1998;78:359–391. doi: 10.1152/physrev.1998.78.2.359. [DOI] [PubMed] [Google Scholar]
- Mitchell P. The Ninth Sir Hans Krebs Lecture. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems. Eur J Biochem. 1979;95:1–20. doi: 10.1111/j.1432-1033.1979.tb12934.x. [DOI] [PubMed] [Google Scholar]
- Mizuno J, Araki J, Mohri S, Minami H, Doi Y, Fujinaka W, Miyaji K, Kiyooka T, Oshima Y, Iribe G, Hirakawa M, Suga H. Frank-Starling mechanism retains recirculation fraction of myocardial Ca2+ in the beating heart. Jpn J Physiol. 2001;51:733–743. doi: 10.2170/jjphysiol.51.733. [DOI] [PubMed] [Google Scholar]
- Momken I, Lechene P, Koulmann N, Fortin D, Mateo P, Hoerter J, Doan BT, Bigard X, Veksler V, Ventura-Clapier RF. Impaired voluntary running capacity of creatine kinase-deficient mice. J Physiol. 2005;565:951–964. doi: 10.1113/jphysiol.2005.086397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Spindler M, Hu K, Bauer L, Ritter O, Nordbeck P, Quaschning T, Hiller KH, Wallis J, Ertl G, Bauer WR, Neubauer S. Creatine kinase knockout mice show left ventricular hypertrophy and dilatation, but unaltered remodeling post-myocardial infarction. Cardiovasc Res. 2005;65:419–427. doi: 10.1016/j.cardiores.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD. Creatine kinase system in failing and nonfailing human myocardium. Circulation. 1996;94:1894–1901. doi: 10.1161/01.cir.94.8.1894. [DOI] [PubMed] [Google Scholar]
- Neely JR, Denton RM, England PJ, Randle PJ. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem J. 1972;128:147–159. doi: 10.1042/bj1280147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Schlattner U, Wallimann T. A molecular approach to the concerted action of kinases involved in energy homoeostasis. Biochem Soc Trans. 2003;31:169–174. doi: 10.1042/bst0310169. [DOI] [PubMed] [Google Scholar]
- Nicholls D, Ferguson SJ. Bioenergetics. London-New York: Academic Press; 2002. [Google Scholar]
- Noble D. Modeling the heart – from genes to cells to the whole organ. Science. 2002;295:1678–1682. doi: 10.1126/science.1069881. [DOI] [PubMed] [Google Scholar]
- Noma A, Shibasaki T. Membrane current through adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985;363:463–480. doi: 10.1113/jphysiol.1985.sp015722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie LH. Physiology, from Cell to Circulation. Philadelphia: Lippincott-Raven Publishers; 1998. The Heart; pp. 43–63. [Google Scholar]
- Patterson SW, Piper H, Starling EH. The regulation of the heartbeat. J Physiol. 1914;48:357–379. [Google Scholar]
- Pucar D, Bast P, Gumina RJ, Lim L, Drahl C, Juranic N, Macura S, Janssen E, Wieringa B, Terzic A, Dzeja PP. Adenylate kinase AK1 knockout heart: energetics and functional performance under ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2002;283:H776–H782. doi: 10.1152/ajpheart.00116.2002. [DOI] [PubMed] [Google Scholar]
- Pucar D, Dzeja PP, Bast P, Gumina RJ, Drahl C, Lim L, Juranic N, Macura S, Terzic A. Mapping hypoxia-induced bioenergetic rearrangements and metabolic signaling by 18O-assisted 31P NMR and 1H NMR spectroscopy. Mol Cell Biochem. 2004;256–257:281–289. doi: 10.1023/b:mcbi.0000009875.30308.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucar D, Dzeja PP, Bast P, Juranic N, Macura S, Terzic A. Cellular energetics in the preconditioned state: protective role for phosphotransfer reactions captured by 18O-assisted 31P NMR. J Biol Chem. 2001;276:44812–44819. doi: 10.1074/jbc.M104425200. [DOI] [PubMed] [Google Scholar]
- Rajashree R, Koster JC, Markova KP, Nichols CG, Hofmann PA. Contractility and ischemic response of hearts from transgenic mice with altered sarcolemmal KATP channels. Am J Physiol. 2002;283:H584–H590. doi: 10.1152/ajpheart.00107.2002. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Bernardi P, Pozzan T. Mitochondria as all-round players of the calcium game. J Physiol. 2000;529:37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JM, Wang Y, Kerrick WG, Kawai R, Cheung HC. Activation of striated muscle: nearest-neighbor regulatory-unit and cross-bridge influence on myofilament kinetics. J Mol Biol. 2002;322:1065–1088. doi: 10.1016/s0022-2836(02)00855-0. [DOI] [PubMed] [Google Scholar]
- Rossi AM, Eppenberger HM, Volpe P, Cotrufo R, Wallimann T. Muscle-type MM creatine kinase is specifically bound to sarcoplasmic reticulum and can support Ca2+ uptake and regulate local ATP/ADP ratios. J Biol Chem. 1990;265:5258–5266. [PubMed] [Google Scholar]
- Rube DA, van der Bliek AM. Mitochondrial morphology is dynamic and varied. Mol Cell Biochem. 2004;256–257:331–339. doi: 10.1023/b:mcbi.0000009879.01256.f6. [DOI] [PubMed] [Google Scholar]
- Saks VA, Aliev MK. Is there the creatine kinase equilibrium in working heart cells. Biochem Biophys Res Commun. 1996;227:360–367. doi: 10.1006/bbrc.1996.1513. [DOI] [PubMed] [Google Scholar]
- Saks VA, Kaambre T, Sikk P, Eimre M, Orlova E, Paju K, Piirsoo A, Appaix F, Kay L, Regitz-Zagrosek V, Fleck E, Seppet E. Intracellular energetic units in red muscle cells. Biochem J. 2001;356:643–657. doi: 10.1042/0264-6021:3560643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks VA, Khuchua ZA, Vasilyeva EV, Belikova O, Kuznetsov AV. Metabolic compartmentation and substrate channelling in muscle cells. Role of coupled creatine kinases in in vivo regulation of cellular respiration – a synthesis. Mol Cell Biochem. 1994;133–134:155–192. doi: 10.1007/BF01267954. [DOI] [PubMed] [Google Scholar]
- Saks VA, Kongas O, Vendelin M, Kay L. Role of the creatine/phosphocreatine system in the regulation of mitochondrial respiration. Acta Physiol Scand. 2000;168:635–641. doi: 10.1046/j.1365-201x.2000.00715.x. [DOI] [PubMed] [Google Scholar]
- Saks VA, Kuznetsov AV, Vendelin M, Guerrero K, Kay L, Seppet EK. Functional coupling as a basic mechanism of feedback regulation of cardiac energy metabolism. Mol Cell Biochem. 2004;256–257:185–199. doi: 10.1023/b:mcbi.0000009868.92189.fb. [DOI] [PubMed] [Google Scholar]
- Saks VA, Rosenshtraukh LV, Undrovinas AI, Smirnov VN, Chazov EI. Studies of energy transport in heart cells. Intracellular creatine content as a regulatory factor of frog heart energetics and force of contraction. Biochem Med. 1976;16:21–36. doi: 10.1016/0006-2944(76)90005-3. [DOI] [PubMed] [Google Scholar]
- Saks V, Vendelin M, Aliev MK, Kekelidze T, Engelbrecht J. Handbook of Neurochemistry and Molecular Neurobiology: Neural Energy Utilization. New York: Kluwer; 2005. Mechanisms and modeling of energy transfer between and among intracellular compartments. [Google Scholar]
- Sasaki N, Sato T, Marban E, O'Rourke B. ATP consumption by uncoupled mitochondria activates sarcolemmal KATP channels in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2001;280:H1882–H1888. doi: 10.1152/ajpheart.2001.280.4.H1882. [DOI] [PubMed] [Google Scholar]
- Sata M, Sugiura S, Yamashita H, Momomura S, Serizawa T. Coupling between myosin ATPase cycle and creatinine kinase cycle facilitates cardiac actomyosin sliding in vitro. A clue to mechanical dysfunction during myocardial ischemia. Circulation. 1996;93:310–317. doi: 10.1161/01.cir.93.2.310. [DOI] [PubMed] [Google Scholar]
- Saucerman JJ, Brunton LL, Michailova AP, McCulloch AD. Modeling beta-adrenergic control of cardiac myocyte contractility in silico. J Biol Chem. 2003;278:47997–48003. doi: 10.1074/jbc.M308362200. [DOI] [PubMed] [Google Scholar]
- Schlattner U, Dolder M, Wallimann T, Tokarska-Schlattner M. Mitochondrial creatine kinase and mitochondrial outer membrane porin show a direct interaction that is modulated by calcium. J Biol Chem. 2001;276:48027–48030. doi: 10.1074/jbc.M106524200. [DOI] [PubMed] [Google Scholar]
- Schlattner U, Forstner M, Eder M, Stachowiak O, Fritz-Wolf K, Wallimann T. Functional aspects of the X-ray structure of mitochondrial creatine kinase: a molecular physiology approach. Mol Cell Biochem. 1998;184:125–140. [PubMed] [Google Scholar]
- Schlattner U, Gehring F, Vernoux N, Tokarska-Schlattner M, Neumann D, Marcillat O, Vial C, Wallimann T. C-terminal lysines determine phospholipid interaction of sarcomeric mitochondrial creatine kinase. J Biol Chem. 2004;279:24334–24342. doi: 10.1074/jbc.M314158200. [DOI] [PubMed] [Google Scholar]
- Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta. 2005;1762:164–180. doi: 10.1016/j.bbadis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Schlattner U, Wallimann T. Metabolite channeling: creatine kinase microcompartments. In: Lennarz WJ, Lane MD, editors. Encyclopedia of Biological Chemistry. New York, USA: Academic Press; 2004. pp. 646–651. [Google Scholar]
- Schlattner U, Wallimann T. Molecular structure and function of mitochondrial creatine kinases. In: Varski U, editor. Creatine Kinase – Biochemistry, Physiology, Structure and Function. New York: Science Nova, Science Publishers; 2005. in press. [Google Scholar]
- Schroedinger E. The Physical Aspect of the Living Cell. London: The Folio Society; 2000. What Is Life. [Google Scholar]
- Selivanov VA, Alekseev AE, Hodgson DM, Dzeja PP, Terzic A. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol Cell Biochem. 2004;256–257:243–256. doi: 10.1023/b:mcbi.0000009872.35940.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppet EK, Kaambre T, Sikk P, Tiivel T, Vija H, Tonkonogi M, Sahlin K, Kay L, Appaix F, Braun U, Eimre M, Saks VA. Functional complexes of mitochondria with Ca,MgATPases of myofibrils and sarcoplasmic reticulum in muscle cells. Biochim Biophys Acta. 2001;1504:379–395. doi: 10.1016/s0005-2728(00)00269-3. [DOI] [PubMed] [Google Scholar]
- Shimizu J, Todaka K, Burkhoff D. Load dependence of ventricular performance explained by model of calcium–myofilament interactions. Am J Physiol Heart Circ Physiol. 2002;282:H1081–H1091. doi: 10.1152/ajpheart.00498.2001. [DOI] [PubMed] [Google Scholar]
- Spindler M, Meyer K, Stromer H, Leupold A, Boehm E, Wagner H, Neubauer S. Creatine kinase-deficient hearts exhibit increased susceptibility to ischemia-reperfusion injury and impaired calcium homeostasis. Am J Physiol Heart Circ Physiol. 2004;287:H1039–H1045. doi: 10.1152/ajpheart.01016.2003. [DOI] [PubMed] [Google Scholar]
- Srere PA. Macromolecular interactions: tracing the roots. Trends Biochem Sci. 2000;25:150–153. doi: 10.1016/s0968-0004(00)01550-4. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- Starling EH, Visscher MB. The regulation of the energy output of the heart. J Physiol. 1926;62:243–261. doi: 10.1113/jphysiol.1927.sp002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeghs K, Oerlemans F, de Haan A, Heerschap A, Verdoodt L, de Bie M, Ruitenbeek W, Benders A, Jost C, van Deursen J, Tullson P, Terjung R, Jap P, Jacob W, Pette D, Wieringa B. Cytoarchitectural and metabolic adaptations in muscles with mitochondrial and cytosolic creatine kinase deficiencies. Mol Cell Biochem. 1998;184:183–194. [PubMed] [Google Scholar]
- Suga H. Ventricular energetics. Physiol Rev. 1990;70:247–277. doi: 10.1152/physrev.1990.70.2.247. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H, Wilson CR, Razeghi P, Sharma S. Metabolic energetics and genetics in the heart. Ann N Y Acad Sci. 2005;1047:208–218. doi: 10.1196/annals.1341.019. [DOI] [PubMed] [Google Scholar]
- Tarasov A, Dusonchet J, Ashcroft F. Metabolic regulation of the pancreatic beta-cell ATP-sensitive K+ channel: a pas de deux. Diabetes. 2004;53(Suppl. 3):S113–S122. doi: 10.2337/diabetes.53.suppl_3.s113. [DOI] [PubMed] [Google Scholar]
- ten Hove M, Chan S, Lygate C, Monfared M, Boehm E, Hulbert K, Watkins H, Clarke K, Neubauer S. Mechanisms of creatine depletion in chronically failing rat heart. J Mol Cell Cardiol. 2005a;38:309–313. doi: 10.1016/j.yjmcc.2004.11.016. [DOI] [PubMed] [Google Scholar]
- ten Hove M, Lygate CA, Fischer A, Schneider JE, Sang AE, Hulbert K, Sebag-Montefiore L, Watkins H, Clarke K, Isbrandt D, Wallis J, Neubauer S. Reduced inotropic reserve and increased susceptibility to cardiac ischemia/reperfusion injury in phosphocreatine-deficient guanidinoacetate-N-methyltransferase-knockout mice. Circulation. 2005b;111:2477–2485. doi: 10.1161/01.CIR.0000165147.99592.01. [DOI] [PubMed] [Google Scholar]
- Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mV̇o2, NADH, and light scattering. J Biol Chem. 2001;276:2586–2599. doi: 10.1074/jbc.M002923200. [DOI] [PubMed] [Google Scholar]
- Unitt JF, Schrader J, Brunotte F, Radda GK, Seymour AM. Determination of free creatine and phosphocreatine concentrations in the isolated perfused rat heart by 1H- and 31P-NMR. Biochim Biophys Acta. 1992;1133:115–120. doi: 10.1016/0167-4889(92)90057-i. [DOI] [PubMed] [Google Scholar]
- Vance W, Arkin A, Ross J. Determination of causal connectivities of species in reaction networks. Proc Natl Acad Sci U S A. 2002;99:5816–5821. doi: 10.1073/pnas.022049699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen J, Heerschap A, Oerlemans F, Ruitenbeek W, Jap P, ter Laak H, Wieringa B. Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell. 1993;74:621–631. doi: 10.1016/0092-8674(93)90510-w. [DOI] [PubMed] [Google Scholar]
- van Deursen J, Ruitenbeek W, Heerschap A, Jap P, ter Laak H, Wieringa B. Creatine kinase (CK) in skeletal muscle energy metabolism: a study of mouse mutants with graded reduction in muscle CK expression. Proc Natl Acad Sci U S A. 1994;91:9091–9095. doi: 10.1073/pnas.91.19.9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendelin M, Beraud N, Guerrero K, Andrienko T, Kuznetsov AV, Olivares J, Kay L, Saks VA. Mitochondrial regular arrangement in muscle cells: a ‘crystal-like’ pattern. Am J Physiol Cell Physiol. 2005;288:C757–C767. doi: 10.1152/ajpcell.00281.2004. [DOI] [PubMed] [Google Scholar]
- Vendelin M, Bovendeerd PH, Engelbrecht J, Arts T. Optimizing ventricular fibers: uniform strain or stress, but not ATP consumption, leads to high efficiency. Am J Physiol Heart Circ Physiol. 2002;283:H1072–H1081. doi: 10.1152/ajpheart.00874.2001. [DOI] [PubMed] [Google Scholar]
- Vendelin M, Eimre M, Seppet E, Peet N, Andrienko T, Lemba M, Engelbrecht J, Seppet EK, Saks VA. Intracellular diffusion of adenosine phosphates is locally restricted in cardiac muscle. Mol Cell Biochem. 2004a;256–257:229–241. doi: 10.1023/b:mcbi.0000009871.04141.64. [DOI] [PubMed] [Google Scholar]
- Vendelin M, Kongas O, Saks V. Regulation of mitochondrial respiration in heart cells analyzed by reaction-diffusion model of energy transfer. Am J Physiol Cell Physiol. 2000;278:C747–C764. doi: 10.1152/ajpcell.2000.278.4.C747. [DOI] [PubMed] [Google Scholar]
- Vendelin M, Lemba M, Saks VA. Analysis of functional coupling: mitochondrial creatine kinase and adenine nucleotide translocase. Biophys J. 2004b;87:696–713. doi: 10.1529/biophysj.103.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol. 2004;555:1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R, Vassort G. The hypodynamic state of the frog heart. Further evidence for a phosphocreatine-creatine pathway. J Physiol (Paris) 1980;76:583–589. [PubMed] [Google Scholar]
- Vignais PV. Molecular and physiological aspects of adenine nucleotide transport in mitochondria. Biochim Biophys Acta. 1976;456:1–38. doi: 10.1016/0304-4173(76)90007-0. [DOI] [PubMed] [Google Scholar]
- Vignais P. La Biologie Des Origins a Nos Jours. Grenoble: EDP Science & Grenoble Science; 2005. [Google Scholar]
- Wallimann T. 31P-NMR-measured creatine kinase reaction flux in muscle: a caveat. J Muscle Res Cell Motil. 1996;17:177–181. doi: 10.1007/BF00124240. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SQ, Wei C, Zhao G, Brochet DX, Shen J, Song LS, Wang W, Yang D, Cheng H. Imaging microdomain Ca2+ in muscle cells. Circ Res. 2004;94:1011–1022. doi: 10.1161/01.RES.0000125883.68447.A1. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Venkatesh N. Metabolic regulation of cardiac ATP-sensitive K+ channels. Cardiovasc Drugs Ther. 1993;7(Suppl. 3):499–505. doi: 10.1007/BF00877614. [DOI] [PubMed] [Google Scholar]
- Westerhoff HV, Van Dam K. Thermodynamics and Control of Biological Free-Energy Transduction. Amsterdam-New York-Oxford: Elsevier; 1987. [Google Scholar]
- Williamson JR, Ford C, Illingworth J, Safer B. Coordination of citric acid cycle activity with electron transport flux. Circ Res. 1976;38:I39–I51. [PubMed] [Google Scholar]
- Wilson AJ, Jabr RI, Clapp LH. Calcium modulation of vascular smooth muscle ATP-sensitive K+ channels: role of protein phosphatase-2B. Circ Res. 2000;87:1019–1025. doi: 10.1161/01.res.87.11.1019. [DOI] [PubMed] [Google Scholar]
- Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- Yaffe MP. The machinery of mitochondrial inheritance and behavior. Science. 1999;283:1493–1497. doi: 10.1126/science.283.5407.1493. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Sata M, Sugiura S, Momomura S, Serizawa T, Iizuka M. ADP inhibits the sliding velocity of fluorescent actin filaments on cardiac and skeletal myosins. Circ Res. 1994;74:1027–1033. doi: 10.1161/01.res.74.6.1027. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- Zoll J, Ponsot E, Doutreleau S, Mettauer B, Piquard F, Mazzucotelli JP, Diemunsch P, Geny B. Acute myocardial ischaemia induces specific alterations of ventricular mitochondrial function in experimental pigs. Acta Physiol Scand. 2005;185:25–32. doi: 10.1111/j.1365-201X.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov DB, Kobrinsky E, Juhaszova M, Sollott SJ. Examining intracellular organelle function using fluorescent probes: from animalcules to quantum dots. Circ Res. 2004;95:239–252. doi: 10.1161/01.RES.0000137875.42385.8e. [DOI] [PubMed] [Google Scholar]