Abstract
We investigated the functional roles of circulating and locally produced angiotensin II (Ang II) in fasting and postprandial adipose tissue blood flow (ATBF) regulation and examined the interaction between Ang II and nitric oxide (NO) in ATBF regulation. Local effects of the pharmacological agents (or contralateral saline) on ATBF, measured with 133Xe wash-out, were assessed using the recently developed microinfusion technique. Fasting and postprandial (75 g glucose challenge) ATBF regulation was investigated in nine lean healthy subjects (age, 29 ± 3 years; BMI, 23.4 ± 0.7 kg m−2) using local Ang II stimulation, Ang II type 1 (AT1) receptor blockade, and angiotensin-converting enzyme (ACE) inhibition. Furthermore, NO synthase (NOS) blockade alone and in combination with AT1 receptor blockade was used to examine the interaction between Ang II and NO. Ang II induced a dose-dependent decrease in ATBF (10−9m: −16%, P = 0.04; 10−7m: −33%, P < 0.01; 10−5m: −53% P < 0.01). Fasting ATBF was not affected by ACE inhibition, but was increased by ∼55% (P < 0.01) by AT1 receptor blockade. NOS blockade induced a ∼30% (P = 0.001) decrease in fasting ATBF. Combined AT1 receptor and NOS blockade increased ATBF by ∼40% (P = 0.003). ACE inhibition and AT1 receptor blockade did not affect the postprandial increase in ATBF. We therefore conclude that circulating Ang II is a major regulator of fasting ATBF, and a major proportion of the Ang II-induced decrease in ATBF is NO independent. Locally produced Ang II does not appear to regulate ATBF. Ang II appears to have no major effect on the postprandial enhancement of ATBF.
Tissue-specific regulation of blood flow in tissues such as skeletal muscle, liver and adipose tissue is needed to meet the local metabolic and physiological demands under varying conditions. Previous studies have clearly shown that adipose tissue blood flow (ATBF) is increased after glucose intake (Bulow et al. 1987) or the ingestion of a mixed meal (Coppack et al. 1992), whereas fat intake alone does not evoke a blood flow response (Evans et al. 1999). The ATBF response to nutrient intake may be of great importance in the regulation of metabolism by facilitating signalling between adipose tissue and other tissues, such as skeletal muscle and liver (Frayn et al. 2003). For example, it has been shown that the extraction of plasma triacylglycerol is elevated with increasing ATBF (Samra et al. 1996b).
Both fasting ATBF (Blaak et al. 1995; Summers et al. 1996; Jansson et al. 1998) and ATBF responsiveness to nutrients (Summers et al. 1996; Jansson et al. 1998) are reduced in obesity, and this impairment is associated with insulin resistance (Jansson et al. 1998; Karpe et al. 2002b). Although insulin itself does not seem to be the actual stimulus for this nutrient-related elevation of ATBF (Karpe et al. 2002a), it has been demonstrated that the postprandial enhancement of ATBF coincides with an increased plasma insulin concentration and suppression of circulating non-esterified fatty acids (NEFA) (Coppack et al. 1992). There is abundant evidence that β-adrenergic stimulation elevates ATBF (Blaak et al. 1995; Samra et al. 1996a; Millet et al. 1998; Schiffelers et al. 2003) and it has recently been shown that a major proportion of the postprandial enhancement of ATBF results from local β-adrenergic stimulation (Ardilouze et al. 2004a), which may be secondary to the postprandial increase in insulin concentrations that might activate the sympathetic nervous system (Karpe et al. 2002a). Furthermore, nitric oxide (NO) seems to be involved in fasting ATBF regulation (Ardilouze et al. 2004a).
There is evidence for a local angiotensin (Ang) II generating system in adipose tissue (Dzau, 1988; Unger & Gohlke, 1990; Phillips et al. 1993; Danser, 1996; Harte et al. 2005), implying that the vasoactive component Ang II may be produced in adipose tissue. Local Ang II stimulation using the microdialysis technique decreased fasting nutritive blood flow and inhibited lipolysis in abdominal subcutaneous adipose tissue and skeletal muscle in normal-weight and obese subjects (Goossens et al. 2004). However, the functional importance of locally produced Ang II in adipose tissue and circulating Ang II and their role in postprandial ATBF regulation have never been assessed. In addition, there is evidence that Ang II increases oxidative stress and interacts with NO function, leading to endothelial dysfunction (de Gasparo, 2002). This suggests that there could be an interaction between Ang II and NO in the Ang II-induced effect on ATBF.
Therefore, we used a pharmacological approach, using local Ang II stimulation, Ang II type 1 (AT1) receptor blockade, and ACE inhibition, to investigate whether locally produced and circulating Ang II decrease ATBF both under fasting and postprandial conditions. We also examined the contribution of NO to the Ang II-induced effect on ATBF using NO synthase (NOS) blockade. To accomplish this, the recently developed microinfusion technique was used, which makes quantitative assessment of the local effects of vasoactive compounds on ATBF possible (Karpe et al. 2002a). With the model used in the present study, we are to our knowledge the first to determine the relative contribution of locally produced Ang II in adipose tissue and circulating Ang II that reaches adipose tissue to an Ang II-induced effect in this tissue.
Methods
Subjects
Nine lean healthy non-smoking volunteers (5 female) free of any medication participated in the studies. Subject characteristics are summarized in Table 1. The microinfusion system allows for local administration of up to two pharmacological agents simultaneously in the same subject. Most participants participated in three of the four experiments using different protocols. Subject characteristics and distribution of the sexes did not differ between experiments. The Oxfordshire Clinical Research Ethics Committee approved the studies, which conformed to the Declaration of Helsinki, and all subjects gave written informed consent before participating in the studies.
Table 1.
Subject characteristics
| Parameter | Mean ±s.e.m. |
|---|---|
| Sex (male/female) | 4/5 |
| Age (years) | 30 ± 3 |
| Weight (kg) | 70.1 ± 4.1 |
| Height (m) | 1.72 ± 0.04 |
| BMI (kg m−2) | 23.4 ± 0.7 |
| Body fat (%) | 22.7 ± 2.2 |
| Waist (cm) | 79.0 ± 2.1 |
| Waist/hip ratio | 0.82 ± 0.02 |
| SBP (mmHg) | 112 ± 4 |
| DBP (mmHg) | 67 ± 1 |
| Fasting glucose (mmol l−1) | 4.9 ± 0.1 |
| Fasting insulin (mU l−1) | 9.5 ± 0.5 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Study design
Subjects were asked to refrain from drinking alcohol and doing strenuous exercise for a period of 24 h before the study. Subjects came to the Clinical Research Unit by car or bus in the morning after an overnight fast and were studied at rest. On arrival, height, weight, waist and hip circumferences were measured. Body composition was determined using bioelectrical impedance (Bodystat-1500, Bodystat Ltd, Isle of Man, UK).
Small catheters were inserted into the abdominal subcutaneous adipose tissue (see ‘Microinfusion protocol’). Resting blood pressure was recorded after 15 min rest and at intervals throughout the study. A 20-gauge cannula was inserted retrogradely into a superficial dorsal hand vein. This vein was heated in a hot box for at least 20 min to obtain arterialized venous blood. Arterialized blood samples were taken at different time points, depending on the type of experiment.
Microinfusion protocol
The microinfusion technique offers the unique possibility of monitoring local ATBF using the 133xenon (133Xe) wash-out technique (Samra et al. 1995) and to manipulate local ATBF at the same time by local administration of a pharmacological agent (Karpe et al. 2002a). Right and left sides of the abdomen were studied simultaneously on each subject to allow direct comparison of the effects of the vasoactive compound on one site with the contralateral control site (saline infusion, 9 g l−1 NaCl). Importantly, the vasoactive compound was administered at the same level on the abdomen as the saline control site, as it has been previously shown that ATBF is greater at the upper level compared to the lower level of the abdomen (Simonsen et al. 2003; Ardilouze et al. 2004b), but is not different between the right and the left sides at either level (Ardilouze et al. 2004b). To achieve this, small catheters (6 mm long, internal diameter 0.38 mm, outer diameter 1.5 mm, dead-space volume 60 μl) (Quick-set infusion set, MiniMed, Applied Medical Technology Ltd, Cambridge, UK) were inserted 6–8 cm on either side of the midline of the abdomen into the abdominal subcutaneous adipose tissue, and a saline infusion was started at 2 μl min−1 (CMA100 pumps, CMA microdialysis Ltd, Sunderland, UK). After 20 min recovery, 133Xe (total dose of 2.0 MBq per study) was injected at each site through the port in the hub of the catheter, and a γ-counter probe (Oakfield Instruments, Eynsham, UK) was placed over the infusion device. The half-life of 133Xe (5.25 days) allows measurements of local ATBF throughout the entire study after a single injection of 133Xe. Subsequently, the catheter was perfused with saline for 1 min at 60 μl min−1 to wash the dead space, and for a further 40–60 min at 2 μl min−1 to allow for equilibration of 133Xe. Importantly, it can be estimated that tissue concentrations of the agents that are administered are approximately 1000-fold lower than the concentrations in the infusate. Using the present methodology (infusing agents at an infusion rate of 2 μl min−1 in a presumed volume of 1 cm3), it would take several hours to achieve tissue concentrations that are comparable with the concentrations in the infusate. In addition, there is a rapid turnover of the fluid phase due to ATBF, which further dilutes the tissue concentrations.
Ang II dose–response experiments
The effect of angiotensin II (Ang II: 10−9, 10−7, 10−5m) on ATBF was investigated in four subjects (Fig. 1A). At time zero, the saline was switched at either the left or the right side (chosen at random), by disconnection at the hub of the infusion set, to Ang II (h-Angiotensin II, Clinalfa, CH), while the infusion rate was maintained at 2 μl min−1. Each dose of Ang II was administered for 45 min starting with the lowest dose. The ATBF recording over the next 135 min assessed the effect of Ang II, while the saline infusion was continued on the contralateral site to examine whether there were any changes in ATBF during the experiment that could not be attributed to Ang II action. After two baseline arterialized blood samples had been taken at time points (t, in minutes) −20 (t–20) and t0, further samples were taken at t45, t90 and t135. Ang II was dissolved in saline and diluted to the appropriate concentrations just before the start of the experiment.
Figure 1. Time line diagrams of the different experiments.
Ang II dose–response experiments were performed to assess the effect of local Ang II stimulation on adipose tissue blood flow (ATBF) (A). In addition, the functional ATBF response to the ACE inhibitor enalaprilate (inhibition of local Ang II production) and the AT1 receptor blocker losartan (complete blockade of Ang II action) (B) was investigated under fasting and postprandial (after 75 g oral glucose at 60 min (t60)) conditions. To assess the contribution of NO action to the Ang II-induced effect on ATBF, the ATBF response to the NOS inhibitor l-NMMA (inhibition of local NO production) in combination with AT1 receptor blockade was compared to the ATBF response to NOS blockade alone (C). Thick arrows represent blood samples and blood pressure time points.
AT1 receptor blockade and ACE inhibition experiments
The effects of the ACE inhibitor enalaprilate (10−3m) and the specific AT1 receptor antagonist losartan (10−3m) were investigated in six subjects to examine the importance of locally produced and circulating Ang II in fasting and postprandial ATBF regulation (Fig. 1B). The reason for using a high dose of the ACE inhibitor was to block local Ang II generation. Similarly, a high dose of the AT1 receptor blocker was administered to block Ang II action.
At time zero, the saline was switched at either the left or the right side (chosen at random) to either the ACE inhibitor enalaprilate (Renitec, MSD, Haarlem, The Netherlands) or the AT1 receptor blocker losartan (Cozaar, MSD), while the infusion rate was maintained at 2 μl min−1. The ATBF recording over the next 60 min assessed the effects of ACE inhibition and AT1 receptor blockade, while the saline infusions were continued on the contralateral sites. At t60, 75 g glucose dissolved in 200 ml of lemon-flavoured water was ingested to stimulate ATBF, and infusions were continued for a further 120 min. After two baseline arterialized blood samples had been taken (at t–20 and t0), additional samples were taken at 30 min intervals. Losartan was dissolved in saline, sterile-filtered and diluted to the appropriate concentration just before the start of the experiment. Enalaprilate was provided as a sterile solution by the manufacturer.
Ang II–NO interaction experiments
To assess the contribution of NO action to the Ang II-induced effect on ATBF, the effects of the NO synthase (NOS) inhibitor NG-monomethyl-l-arginine (l-NMMA) alone (10−3m) and in combination with the AT1 receptor blocker losartan (10−3m) were investigated in five subjects (Fig. 1C). At time zero, the saline was switched at either the left or the right side (chosen at random) to the NOS inhibitor l-NMMA, while the infusion rate was maintained at 2 μl min−1. The ATBF recording over the next 60 min assessed the effect of NOS blockade, while the saline infusions were continued on the contralateral sites. At t60, one of the saline sites (chosen at random) was switched to the AT1 receptor blocker and one of the NOS blockade sites was switched to the AT1 receptor blocker in combination with the NOS inhibitor, and infusions were continued for a further 60 min to assess the interaction between Ang II and NO in ATBF regulation. After two baseline arterialized blood samples had been taken (at t–20 and t0), further samples were taken at t60 and t120. l-NMMA was dissolved in saline, sterile-filtered and diluted to the appropriate concentration just before the start of the experiment.
Biochemical measurements
Arterialized blood samples were immediately placed on ice and were centrifuged (1000 g) at 4°C for 10 min, and plasma was stored at −20°C until analysis. Plasma glucose (Simonsen et al. 2003) and non-esterified fatty acids (NEFA) concentrations (Wako NEFA C kit, Alpha Laboratories, Eastleigh, UK) were measured using an enzymatic method. Plasma insulin concentrations were determined by a double-antibody radioimmunoassay (Pharmacia and Upjohn, Milton Keynes, UK).
Calculations
133Xe counts were recorded continuously as 20 s readings, and blood flow was calculated as the mean of consecutive 10 min time periods, as previously described (Karpe et al. 2002a; Ardilouze et al. 2004a). The partition coefficient for 133Xe between adipose tissue and blood was assumed to have a value of 10 g ml−1 for all subjects (Yeh & Peterson, 1965).
The effect of Ang II was analysed by averaging the two consecutive time points at the end of each 45 min infusion period (t35 and t45, t80 and t90, and t125 and t135), when a steady state in ATBF was reached, after subtraction of baseline blood flow (t–20 to t0). It is important to recognize that we cannot be certain of the volume of distribution of the agents infused with respect to the depot of 133Xe and the exact tissue concentrations, because microinfusion of pharmacological agents creates drug concentration gradients in the tissue volume from which 133Xe clearance is recorded. This experimental problem implies that the estimates of the size of the effects of the pharmacological agents that are infused on ATBF should be regarded as qualitative or semi-quantitative. For the AT1 receptor blockade and ACE inhibition experiments, the stability of ATBF during the baseline period (t–20 to t0) and the 30 min period before glucose ingestion (pre-glucose period, t30 to t60) at the control (saline) sites was tested using Student's paired t test. Responses to pharmacological agents were evaluated within individuals by comparison with the saline control site at the same level on the abdomen. Relative changes in ATBF at the experimental site are presented as changes from baseline values after correction for corresponding changes in ATBF at the control site. The effects of local AT1 receptor blockade and ACE inhibition were analysed by averaging the three consecutive time points before glucose intake (t40, t50 and t60) after subtraction of baseline blood flow (t–20 to t0). Peak ATBF values were calculated as the mean of three consecutive time points, including the maximum ATBF, which resulted in the highest mean value within each subject (Karpe et al. 2002a). The effect of glucose intake on ATBF was analysed using the area under the curve (AUC) by the trapezoidal rule. AUCs were divided by the time over which they were calculated (120 min). To assess the contribution of NO action to the Ang II-induced effect on ATBF, the effect of combined AT1 receptor and NOS blockade was compared with that of AT1 receptor blockade alone by averaging the three consecutive time points at the end of each 60 min infusion period (t40, t50 and t60, and t100, t110 and t120). The reproducibility of the blood flow recordings was assessed by calculating coefficients of variation (CVs).
Statistics
Data are presented as mean ± standard error of the mean (s.e.m.). Overall effects of treatment compared with control were analysed using repeated-measures ANOVA to identify time effects, drug effects, and time and drug interactions. Changes in glucose, insulin and NEFA concentrations with time were assessed by repeated-measures ANOVA, using time and treatment as within-subject factors. Post hoc comparisons of drug effects and changes in biochemical data within subjects were done using Student's paired t tests. Calculations were done using SPSS 10.1 for Windows (Chicago, IL, USA). P < 0.05 was considered to be statistically significant.
Results
Systemic responses
Fasting plasma glucose (4.9 ± 0.1 mmol l−1), insulin (9.5 ± 0.5 mU l−1), and NEFA (551 ± 72 μmol l−1) concentrations were stable during the baseline period and the 30 min period before glucose ingestion (pre-glucose period) in the AT1 receptor blockade and ACE inhibition experiments and during the course of the Ang II dose–response and Ang II–NO interaction experiments. After ingestion of glucose in the AT1 receptor blockade and ACE inhibition experiments, plasma glucose and insulin concentrations were significantly increased, whereas plasma NEFA concentrations were significantly decreased. Concentrations and responses corresponded to those expected in healthy lean subjects and were comparable between the different experiments. Mean blood pressure was unchanged during the course of the experiments (data not shown).
Reproducibility of blood flow recordings
Baseline blood flow recordings were stable; there were no significant differences in ATBF between t–20 and t0 in the different experiments: mean values were 5.0 ± 0.4 and 5.1 ± 0.4 ml (100 g tissue)−1 min−1, respectively (CV, 5.8 ± 0.8%). Baseline ATBF was significantly correlated between right and left sides (Pearson's r = 0.89, P < 0.001 and r = 0.92, P < 0.001 for superior and inferior sides, respectively). The CVs, calculated from the paired data from left and right sides, were 18.8 ± 1.9% and 15.9 ± 4.7% for superior and inferior sides, respectively.
Effect of Ang II on ATBF
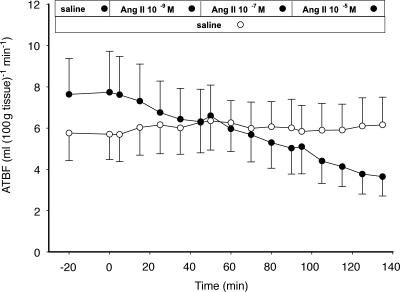
Ang II stimulation induced a dose-dependent decrease in ATBF (10−9m: −16%, P = 0.04; 10−7m: −33%, P < 0.01; 10−5m: −53%, P < 0.01 compared to baseline) (Fig. 2). The reduction in ATBF during Ang II stimulation was significantly different between the different Ang II concentrations that were administered (10−9mversus 10−7m, P = 0.03; 10−7mversus 10−5m, P < 0.01). As expected, saline infusion on the contralateral site had no effect on ATBF, indicating that the effect of local Ang II stimulation on ATBF cannot be attributed to systemic changes.
Figure 2. ATBF in response to local stimulation with Ang II.
Ang II was applied at three concentrations: 10−9m, 10−7m and 10−5m (•) (n = 4). ○, microinfusion of saline (control). Values are means ±s.e.m.
Effects of AT1 receptor blockade and ACE inhibition on ATBF
At the saline infusion (control) sites, ATBF was stable during the baseline period and the 30 min period before glucose ingestion (pre-glucose period) in the ACE inhibition experiment (baseline: 5.0 ± 1.1; pre-glucose: 4.9 ± 1.1 ml (100 g tissue)−1 min−1, not significant (n.s.)) and the AT1 receptor blockade experiment (baseline: 4.1 ± 1.2; pre-glucose: 4.1 ± 1.2 ml (100 g tissue)−1 min−1, n.s.) (Table 2). There were no significant differences in baseline ATBF between contralateral saline and treatment sites (all saline during baseline period). In response to glucose ingestion, the ATBF increase on the control (saline infusion) site was comparable in the ACE inhibition (42%, P < 0.01) and AT1 receptor blockade (50%, P < 0.01) experiments (Figs 3 and 4 and Table 2).
Table 2.
Baseline, pre-glucose and postprandial adipose tissue blood flow (ATBF) in response to ACE inhibition (enalaprilate) and AT1 receptor blockade (losartan)
| Baseline | Pre-glucose | Postprandial | |
|---|---|---|---|
| ACE inhibition | 4.6 ± 0.9 | 5.3 ± 1.1 | 7.1 ± 1.1* |
| Control | 5.0 ± 1.1 | 4.9 ± 1.1 | 7.0 ± 1.5* |
| AT1 receptor blockade | 3.6 ± 1.1 | 5.5 ± 1.2†‡ | 6.3 ± 1.2† |
| Control | 4.1 ± 1.2 | 4.1 ± 1.2 | 6.2 ± 1.5† |
Control indicates infusion of saline on the contralateral site in the same individual. The effect of ACE inhibition and AT1 receptor blockade on fasting adipose tissue blood flow (ATBF) was examined by comparison of ATBF during the pre-glucose period with baseline ATBF. The effect of treatments on postprandial ATBF (ingestion of an oral glucose load) was assessed by comparison with baseline ATBF. Data are presented as mean ±s.e.m., in ml (100 g tissue)−1 min−1.
P < 0.05 versus baseline
P < 0.01 versus baseline
P < 0.01 versus control.
Figure 3. ATBF in response to local ACE inhibition.
•, pharmacological inhibition of Ang II production by the ACE inhibitor enalaprilate (10−3m) (n = 6). ○, contralateral saline control. Oral glucose is given at t60 to stimulate ATBF. Values are means ±s.e.m.
Figure 4. ATBF in response to local AT1 receptor blockade.
•, pharmacological blockade of the AT1 receptor by losartan (10−3m) (n = 6). ○, contralateral saline control. Oral glucose is given at t60 to stimulate ATBF. Values are means ±s.e.m.
Fasting ATBF was not affected by local ACE inhibition (baseline: 4.6 ± 0.9; pre-glucose: 5.3 ± 1.1 ml (100 g tissue)−1 min−1, n.s.) (Fig. 3), but was increased by 53% during local AT1 receptor blockade (baseline: 3.6 ± 1.1; pre-glucose: 5.5 ± 1.2 ml (100 g tissue)−1 min−1, P < 0.01) (Fig. 4 and Table 2).
Local ACE inhibition and AT1 receptor blockade did not affect the postprandial enhancement of ATBF. The ATBF increase after glucose ingestion during ACE inhibition (baseline: 4.6 ± 0.9; postprandial: 7.1 ± 1.1 ml (100 g tissue)−1 min−1, P = 0.02) was not significantly different compared to the ATBF increase at the saline control site (baseline: 5.0 ± 1.1; postprandial: 7.0 ± 1.5 ml (100 g tissue)−1 min−1, P = 0.02). Likewise, the ATBF increase after the oral glucose load during AT1 receptor blockade (baseline: 3.6 ± 1.1; postprandial: 6.3 ± 1.2 ml (100 g tissue)−1 min−1, P < 0.01) was comparable with the ATBF increase at the saline control site (baseline: 4.1 ± 1.2; postprandial: 6.2 ± 1.5 ml (100 g tissue)−1 min−1, P < 0.01). This is further illustrated by comparable ATBF AUCs during local ACE inhibition (ACE inhibition: 5.8 ± 0.9; control: 5.8 ± 0.2 ml (100 g tissue)−1 min−1, n.s.) and AT1 receptor blockade (AT1 receptor blockade: 5.3 ± 1.1; control: 4.9 ± 1.3 ml (100 g tissue)−1 min−1, n.s.) compared to control.
Interaction between Ang II and NO in ATBF regulation
No significant differences in baseline ATBF were observed between control and treatment sites (all saline during baseline period). NOS blockade by l-NMMA induced a ∼30% decrease in fasting ATBF at both NOS blockade sites (t0 to t60) (both P = 0.001) (Fig. 5). At t60, one of the saline sites was switched to the AT1 receptor blocker losartan and one of the NOS blockade sites was switched to the NOS blocker l-NMMA in combination with the AT1 receptor blocker. Local AT1 receptor blockade increased fasting ATBF compared to contralateral saline infusion (60%versus 6%, P = 0.001, respectively), whereas combined AT1 receptor and NOS blockade elevated fasting ATBF compared to NOS blockade alone (39%versus−7%, P = 0.003, respectively). AT1 receptor blockade induced a more pronounced increase in ATBF compared to combined AT1 receptor and NOS blockade (60%versus 39%, P = 0.02, respectively). However, after correction for corresponding changes in ATBF at the control sites, the net increase in ATBF during local AT1 receptor blockade (control: saline site) was not significantly different from the net increase in ATBF during combined AT1 receptor and NOS blockade (control: NOS blockade site) (54%versus 46%, respectively).
Figure 5. ATBF in response to local AT1 receptor blockade alone and in combination with NOS blockade.
ATBF in response to local AT1 receptor blockade by losartan (10−3m) (•), contralateral saline control for AT1 receptor blockade (○), local NOS blockade by l-NMMA (10−3m) alone and in combination with AT1 receptor blockade (10−3m) (▾), and local NOS blockade (10−3m) (control for AT1 receptor blockade in combination with NOS blockade) (▿) (n = 5). Values are means ±s.e.m.
Discussion
We demonstrated that, using the recently developed microinfusion technique, Ang II acts as a potent vasoconstrictor in adipose tissue under fasting conditions, as shown by the marked decrease in ATBF when Ang II was infused locally. Local ACE inhibition (inhibition of local Ang II generation) showed that locally produced Ang II in adipose tissue does not appear to regulate ATBF, as no significant change in ATBF was observed. In contrast, AT1 receptor blockade (blockade of Ang II action) induced a marked increase in ATBF, indicating that circulating Ang II that reaches adipose tissue is a major regulator of fasting ATBF. Ang II does not appear to have great impact on the postprandial enhancement of ATBF. Finally, the Ang II–NO interaction experiments demonstrated that a major proportion of the Ang II-induced decrease of ATBF is NO independent. Biochemical parameters and blood pressure were unchanged during the course of the different experiments, which clearly indicates that there were no systemic effects of the pharmacological agents that were locally infused in adipose tissue.
The Ang II dose–response data show that local administration of Ang II to abdominal subcutaneous adipose tissue induced a dose-dependent decrease in fasting ATBF, which was sustained throughout the course of the experiment. In line with the well-known vasoconstrictive effect of Ang II in other tissues, this finding was not unexpected. The magnitude of the observed effect indicates that Ang II could be a major regulator of fasting ATBF in humans. These observations are in agreement with our previous findings, where we showed that Ang II reduced adipose and skeletal muscle tissue blood flow under fasting conditions using the microdialysis technique (Goossens et al. 2004).
There is evidence that Ang II may be produced locally in adipose tissue (Dzau, 1988; Unger & Gohlke, 1990; Phillips et al. 1993; Danser, 1996; Harte et al. 2005) and could play a role in obesity-related hypertension and insulin resistance (Goossens et al. 2003). It is not easy to investigate the actions of Ang II in individual tissues, but an elegant approach to examining such a question has recently been made using a cross-transplantation strategy and AT1A receptor-deficient mice. The equal contribution of Ang II actions in the kidney and in extrarenal tissues with regard to blood pressure regulation by the renin–angiotensin system (RAS) was demonstrated (Crowley et al. 2005). In addition, it is extremely difficult to disentangle the physiological relevance of locally produced and circulating Ang II in an individual tissue. With the model used in the present study, we are to our knowledge the first to determine the relative contributions of locally produced Ang II in adipose tissue and circulating Ang II that reaches adipose tissue to an Ang II-induced effect in this tissue, in this case the Ang II-induced effect on ATBF. To achieve this, we exposed the adipose tissue to selective AT1 receptor blockade and ACE inhibition. For reasons related to the methodology used, it was impossible to demonstrate that local Ang II production was indeed decreased after local ACE inhibition. However, it seems highly likely that substantial inhibition of local Ang II production was accomplished by local ACE inhibition, as > 95% blockade of Ang I-to-Ang II conversion has been demonstrated in the human forearm using the same ACE inhibitor and an even lower dose than was used in the present study (Saris et al. 2000). Similarly, a high dose of the AT1 receptor blocker was administered to substantially block Ang II action. We demonstrated that local AT1 receptor blockade markedly increased fasting ATBF, whereas fasting ATBF was not significantly increased during local ACE inhibition, suggesting that fasting ATBF is predominantly controlled by circulating Ang II concentrations, whereas locally produced Ang II does not appear to regulate ATBF. Because Ang II is produced by the adipocyte, the present data suggest that locally produced Ang II in adipose tissue acts as a paracrine hormone that does not reach the endothelium to induce vasoconstriction.
We cannot fully exclude the possibility that locally generated Ang II plays a minor role in ATBF regulation, because ACE-independent pathways of Ang II generation have been demonstrated in several tissues (Urata et al. 1996; Wolny et al. 1997; Hollenberg et al. 1998), and human studies indicate that such pathways could substantially contribute to total Ang II formation (Hollenberg, 2002). However, there is no evidence that non-ACE pathways play a role in Ang II formation in vivo in human adipose tissue. Secondly, ACE inhibition reduces the breakdown of bradykinin (Linz et al. 1995; Waeber & Brunner, 1996), and in vitro findings and animal studies suggest that this may lead to a vasodilatory response via the cGMP/NO pathway (Gohlke et al. 1998; Siragy & Carey, 1999; Tsutsumi et al. 1999). Based on the present findings, however, involvement of ACE inhibitor-induced stimulation of the bradykinin/cGMP/NO pathway in ATBF regulation seems unlikely.
There were several reasons why we wanted to address the question as to whether the Ang II-induced decrease in ATBF is dependent on interaction with NO. First, it has been demonstrated that NOS inhibition decreased fasting ATBF (Ardilouze et al. 2004a), suggesting that the balance between Ang II and NO stimulation may be an important determinant of the vascular tone and thus ATBF under fasting conditions. Secondly, it can be argued that the increase in ATBF during AT1 receptor blockade may be enhanced by increased production of NO. In this case, AT1 receptor blockade may increase Ang II action through the Ang II type 2 (AT2) receptor, and it has been suggested that this receptor may play a counter-regulatory role mediated via bradykinin and NOS against the pressor actions of Ang II (Gohlke et al. 1998; Siragy & Carey, 1999; Tsutsumi et al. 1999). Furthermore, Ang II increased through its AT1 receptor the activity of the superoxide-producing enzyme NADPH oxidase and thereby inactivated NO, leading to impaired endothelium-dependent vasodilatation (de Gasparo, 2002). In line with this, there is evidence for increased NO bioavailability and decreased oxidative stress after AT1 receptor blockade (de Gasparo, 2002). Therefore, both stimulation of the AT2 receptor, resulting in stimulation of the bradykinin/cGMP/NO pathway, and increased NO action due to inhibition of NADPH oxidase activity could be relevant in (pathophysiological) situations where AT1 receptor blockers are used. Our Ang II–NO interaction experiments clearly show that the major proportion of the increase in ATBF during AT1 receptor blockade is NO independent or, in other words, that the Ang II-induced decrease in ATBF is predominantly independent of NO action.
The postprandial enhancement of ATBF was not affected by either ACE inhibition or AT1 receptor blockade, suggesting that Ang II has no major effect on the regulation of ATBF after a meal. This is in accordance with previous investigations showing that postprandial ATBF is principally controlled by the β-adrenergic system (Simonsen et al. 1990; Ardilouze et al. 2004a). The ATBF response induced by a meal may have metabolic consequences. It has been shown that the extraction of plasma triacylglycerol is elevated with increasing ATBF (Samra et al. 1996b), and this may also be the case for glucose extraction. The present data show that Ang II is involved in fasting, but not postprandial ATBF regulation. However, because Ang II decreases the absolute level of fasting ATBF, this may result in a reduced absolute ATBF after a meal in conditions where RAS activity is increased. This could have pathophysiological implications in that postprandial hyperlipidaemia and hyperglycaemia may occur, which are well-known cardiovascular risk factors.
In conclusion, the present findings demonstrate that circulating Ang II is a major regulator of fasting ATBF, and that a major proportion of the Ang II-induced decrease of ATBF is NO independent. Locally produced Ang II does not appear to regulate ATBF. Furthermore, Ang II appears to have no major effect on the postprandial enhancement of ATBF.
Acknowledgments
G. Goossens was supported by a grant from The Netherlands Organization for Scientific Research (NWO). S. McQuaid is a Wellcome Trust Clinical Training Fellow. F. Karpe is a Wellcome Trust Senior Clinical Research Fellow. The research was supported by the Wellcome Trust.
References
- Ardilouze JL, Fielding BA, Currie JM, Frayn KN, Karpe F. Nitric oxide and beta-adrenergic stimulation are major regulators of preprandial and postprandial subcutaneous adipose tissue blood flow in humans. Circulation. 2004a;109:47–52. doi: 10.1161/01.CIR.0000105681.70455.73. [DOI] [PubMed] [Google Scholar]
- Ardilouze JL, Karpe F, Currie JM, Frayn KN, Fielding BA. Subcutaneous adipose tissue blood flow varies between superior and inferior levels of the anterior abdominal wall. Int J Obes Relat Metab Disord. 2004b;28:228–233. doi: 10.1038/sj.ijo.0802541. [DOI] [PubMed] [Google Scholar]
- Blaak EE, van Baak MA, Kemerink GJ, Pakbiers MT, Heidendal GA, Saris WH. Beta-adrenergic stimulation and abdominal subcutaneous fat blood flow in lean, obese, and reduced-obese subjects. Metabolism. 1995;44:183–187. doi: 10.1016/0026-0495(95)90262-7. [DOI] [PubMed] [Google Scholar]
- Bulow J, Astrup A, Christensen NJ, Kastrup J. Blood flow in skin, subcutaneous adipose tissue and skeletal muscle in the forearm of normal man during an oral glucose load. Acta Physiol Scand. 1987;130:657–661. doi: 10.1111/j.1748-1716.1987.tb08189.x. [DOI] [PubMed] [Google Scholar]
- Coppack SW, Evans RD, Fisher RM, Frayn KN, Gibbons GF, Humphreys SM, et al. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism. 1992;41:264–272. doi: 10.1016/0026-0495(92)90269-g. [DOI] [PubMed] [Google Scholar]
- Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danser AH. Local renin-angiotensin systems. Mol Cell Biochem. 1996;157:211–216. doi: 10.1007/BF00227900. [DOI] [PubMed] [Google Scholar]
- Dzau VJ. Circulating versus local renin-angiotensin system in cardiovascular homeostasis. Circulation. 1988;77:I4–I13. [PubMed] [Google Scholar]
- Evans K, Clark ML, Frayn KN. Effects of an oral and intravenous fat load on adipose tissue and forearm lipid metabolism. Am J Physiol. 1999;276:E241–E248. doi: 10.1152/ajpendo.1999.276.2.E241. [DOI] [PubMed] [Google Scholar]
- Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord. 2003;27:875–888. doi: 10.1038/sj.ijo.0802326. [DOI] [PubMed] [Google Scholar]
- de Gasparo M. Angiotensin II and nitric oxide interaction. Heart Fail Rev. 2002;7:347–358. doi: 10.1023/a:1020714518246. [DOI] [PubMed] [Google Scholar]
- Gohlke P, Pees C, Unger T. AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension. 1998;31:349–355. doi: 10.1161/01.hyp.31.1.349. [DOI] [PubMed] [Google Scholar]
- Goossens GH, Blaak EE, Saris WH, van Baak MA. Angiotensin II-induced effects on adipose and skeletal muscle tissue blood flow and lipolysis in normal-weight and obese subjects. J Clin Endocrinol Metab. 2004;89:2690–2696. doi: 10.1210/jc.2003-032053. [DOI] [PubMed] [Google Scholar]
- Goossens GH, Blaak EE, van Baak MA. Possible involvement of the adipose tissue renin-angiotensin system in the pathophysiology of obesity and obesity-related disorders. Obes Rev. 2003;4:43–55. doi: 10.1046/j.1467-789x.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- Harte A, McTernan P, Chetty R, Coppack S, Katz J, Smith S, et al. Insulin-mediated upregulation of the renin angiotensin system in human subcutaneous adipocytes is reduced by rosiglitazone. Circulation. 2005;111:1954–1961. doi: 10.1161/01.CIR.0000161954.17870.5D. [DOI] [PubMed] [Google Scholar]
- Hollenberg NK. AT1-receptor blockade and the kidney: importance of non-ACE pathways in health and disease. J Hum Hypertens. 2002;16(Suppl. 3):S59–S63. doi: 10.1038/sj.jhh.1001441. [DOI] [PubMed] [Google Scholar]
- Hollenberg NK, Fisher ND, Price DA. Pathways for angiotensin II generation in intact human tissue: evidence from comparative pharmacological interruption of the renin system. Hypertension. 1998;32:387–392. doi: 10.1161/01.hyp.32.3.387. [DOI] [PubMed] [Google Scholar]
- Jansson PA, Larsson A, Lonnroth PN. Relationship between blood pressure, metabolic variables and blood flow in obese subjects with or without non-insulin-dependent diabetes mellitus. Eur J Clin Invest. 1998;28:813–818. doi: 10.1046/j.1365-2362.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- Karpe F, Fielding BA, Ardilouze JL, Ilic V, Macdonald IA, Frayn KN. Effects of insulin on adipose tissue blood flow in man. J Physiol. 2002a;540:1087–1093. doi: 10.1113/jphysiol.2001.013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe F, Fielding BA, Ilic V, Macdonald IA, Summers LK, Frayn KN. Impaired postprandial adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes. 2002b;51:2467–2473. doi: 10.2337/diabetes.51.8.2467. [DOI] [PubMed] [Google Scholar]
- Linz W, Wiemer G, Gohlke P, Unger T, Scholkens BA. Contribution of kinins to the cardiovascular actions of angiotensin- converting enzyme inhibitors. Pharmacol Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- Millet L, Barbe P, Lafontan M, Berlan M, Galitzky J. Catecholamine effects on lipolysis and blood flow in human abdominal and femoral adipose tissue. J Appl Physiol. 1998;85:181–188. doi: 10.1152/jappl.1998.85.1.181. [DOI] [PubMed] [Google Scholar]
- Phillips MI, Speakman EA, Kimura B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regul Pept. 1993;43:1–20. doi: 10.1016/0167-0115(93)90403-u. [DOI] [PubMed] [Google Scholar]
- Samra JS, Frayn KN, Giddings JA, Clark ML, Macdonald IA. Modification and validation of a commercially available portable detector for measurement of adipose tissue blood flow. Clin Physiol. 1995;15:241–248. doi: 10.1111/j.1475-097x.1995.tb00515.x. [DOI] [PubMed] [Google Scholar]
- Samra JS, Simpson EJ, Clark ML, Forster CD, Humphreys SM, Macdonald IA, et al. Effects of adrenaline infusion on the interstitial environment of subcutaneous adipose tissue as studied by microdialysis. Clin Sci (Lond) 1996a;91:425–430. doi: 10.1042/cs0910425. [DOI] [PubMed] [Google Scholar]
- Samra JS, Simpson EJ, Clark ML, Forster CD, Humphreys SM, Macdonald IA, et al. Effects of epinephrine infusion on adipose tissue: interactions between blood flow and lipid metabolism. Am J Physiol. 1996b;271:E834–E839. doi: 10.1152/ajpendo.1996.271.5.E834. [DOI] [PubMed] [Google Scholar]
- Saris JJ, van Dijk MA, Kroon I, Schalekamp MA, Danser AH. Functional importance of angiotensin-converting enzyme-dependent in situ angiotensin II generation in the human forearm. Hypertension. 2000;35:764–768. doi: 10.1161/01.hyp.35.3.764. [DOI] [PubMed] [Google Scholar]
- Schiffelers SL, Akkermans JA, Saris WH, Blaak EE. Lipolytic and nutritive blood flow response to beta-adrenoceptor stimulation in situ in subcutaneous abdominal adipose tissue in obese men. Int J Obes Relat Metab Disord. 2003;27:227–231. doi: 10.1038/sj.ijo.802230. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Bulow J, Astrup A, Madsen J, Christensen NJ. Diet-induced changes in subcutaneous adipose tissue blood flow in man: effect of beta-adrenoceptor inhibition. Acta Physiol Scand. 1990;139:341–346. doi: 10.1111/j.1748-1716.1990.tb08932.x. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Enevoldsen LH, Bulow J. Determination of adipose tissue blood flow with local 133Xe clearance. Evaluation of a new labelling technique. Clin Physiol Funct Imaging. 2003;23:320–323. doi: 10.1046/j.1475-0961.2003.00509.x. [DOI] [PubMed] [Google Scholar]
- Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–1242. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- Summers LK, Samra JS, Humphreys SM, Morris RJ, Frayn KN. Subcutaneous abdominal adipose tissue blood flow: variation within and between subjects and relationship to obesity. Clin Sci (Lond) 1996;91:679–683. doi: 10.1042/cs0910679. [DOI] [PubMed] [Google Scholar]
- Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T, Gohlke P. Tissue renin-angiotensin systems in the heart and vasculature: possible involvement in the cardiovascular actions of converting enzyme inhibitors. Am J Cardiol. 1990;65:3I–10I. doi: 10.1016/0002-9149(90)90118-k. [DOI] [PubMed] [Google Scholar]
- Urata H, Nishimura H, Ganten D. Chymase-dependent angiotensin II forming systems in humans. Am J Hypertens. 1996;9:277–284. doi: 10.1016/0895-7061(95)00349-5. [DOI] [PubMed] [Google Scholar]
- Waeber B, Brunner HR. Cardiovascular hypertrophy: role of angiotensin II and bradykinin. J Cardiovasc Pharmacol. 1996;27:S36–S40. doi: 10.1097/00005344-199600002-00008. [DOI] [PubMed] [Google Scholar]
- Wolny A, Clozel JP, Rein J, Mory P, Vogt P, Turino M, et al. Functional and biochemical analysis of angiotensin II-forming pathways in the human heart. Circ Res. 1997;80:219–227. doi: 10.1161/01.res.80.2.219. [DOI] [PubMed] [Google Scholar]
- Yeh SY, Peterson RE. Solubility of krypton and xenon in blood, protein solutions, and tissue homogenates. J Appl Physiol. 1965;20:1041–1047. doi: 10.1152/jappl.1965.20.5.1041. [DOI] [PubMed] [Google Scholar]