Abstract
Dietary soy intake in man is proposed to provide cardiovascular protection, but it is not established whether this property is attributable to the soy protein per se or to associated dietary isoflavones. This investigation aimed to establish whether the dietary isoflavones in soy protein affect cardiovascular function. Ten days prior to mating, male and female Wistar rats were habituated to either a soy based isoflavone rich diet (plasma concentration 1.87 μmol l−1 isoflavones) or the same diet after isoflavone elution (plasma isoflavone not detectable). Offspring were weaned onto and maintained on the same diet as their dam and sire for 6 months. Blood pressure, and constrictor and dilator responses in the aorta and mesenteric resistance arteries were assessed at 3 and 6 months of age. There was no effect of isoflavone removal from the diet on blood pressure, heart rate, aortic function or mesenteric artery contractile function, at either 3 or 6 months of age. Resistance mesenteric arteries from 6-month-old female rats fed the isoflavone rich diet demonstrated a modest increase in arterial distensibility compared with those fed the depleted diet, and mesenteric arteries from male and female rats fed the isoflavone rich diet showed increased sensitivity to acetylcholine. In summary, the isoflavone content of soy protein has no influence on blood pressure in healthy rats fed a diet based on soy protein, but influences small artery function.
Phytooestrogens are naturally formed environmental oestrogens that are synthesized from phenylpropanoids and simple phenols and have a similar structure to oestradiol. Soy protein is rich in phytooestrogens, notably the isoflavones genistein and daidzein (Lissin & Cooke, 2000). Epidemiological evidence suggests that populations that consume soy products as a dietary staple have a lower incidence of cardiovascular disease than those in which soy protein consumption is minimal (Erdman, 2000), and this has led to the suggestion that isoflavones may be beneficial for cardiovascular health. Indeed, dose dependency between soy food intake and risk of coronary heart disease has been reported in 40- to 70-year-old women in China (Zhang et al. 2003), although population based studies of this nature cannot prove causality and may be confounded by other dietary influences. Moreover, recent studies have suggested that soy protein per se rather than dietary isoflavones may have biological effects that could reduce cardiovascular risk (Fukui et al. 2004; Adams et al. 2005).
Studies in experimental animals in which cardiovascular responses to genistein and daidzein have been assessed are broadly supportive of a protective role of isoflavones. Daidzein has been shown to improve aortic function through enhancement of the nitric oxide synthase pathway in male rats (Sobey et al. 2004; Woodman et al. 2004), and in female ovariectomized rats genistein leads to improved acetylcholine-mediated relaxation (Squadrito et al. 2000). The relevance of these studies to dietary exposure throughout life is limited as isoflavones were administered by subcutaneous injection and for a relatively short period of time (1–4 weeks). However, a recent report from our group suggested that soy protein consumption throughout life substantially reduced blood pressure and improved endothelial function and antioxidant gene expression in 12- to 16-month-old male rats (Mahn et al. 2005).
In the present study, we aimed to establish whether the cardiovascular benefits of soy protein are attributable to the soy associated isoflavones by evaluating cardiovascular function in animals fed throughout life an isoflavone rich soy protein diet or the same diet in which the soy was replaced with isoflavone-depleted soy protein. Blood pressure, heart rate, and aortic and resistance artery function were assessed in animals at 3 and 6 months of age. Plasma lipids and isoflavones were also evaluated.
Methods
Animal husbandry and experimental diets
Female and male Wistar rats (100–120 days of age; n = 10 per group, Harlan UK) were fed for 10 days prior to mating and throughout pregnancy and lactation either a balanced diet containing soy protein rich in naturally occurring isoflavones (260 mg kg−1) or the same diet in which the soy was replaced with soy protein from which the isoflavones had been previously eluted (40 mg kg−1; Table 1). The concentrations of isoflavones in the high isoflavone diet were designed to mimic those in soy protein-based rodent chow (RM3, Special Diets Services, Witham, UK). Soy protein isolates were obtained from The Solae Co. (St Louis, MO, USA) and diets prepared by Special Diets Services. At birth, litters were reduced to six to eight pups and, when possible, to equal numbers of females and males. Offspring were fed the same diet as the dam and sire. Food intake and animal weights were recorded daily during pregnancy and weaning, then weekly from 22 to 180 days of age. Animals were housed with same-sex littermates (maximum 4 animals per cage) in a controlled environment (21°C, 12 h light–dark cycle, 50% humidity) and food and water provided ad libitum. All animal care guidelines and animal procedures followed were licensed under the UK Home Office Animal (Scientific Procedures) Act 1986.
Table 1.
Dietary constituents of experimental diets, plasma isoflavone concentrations, body weight and food intake
| Constituents | Diet rich in isoflavones | Diet poor in isoflavones | ||
|---|---|---|---|---|
| Genistein all forms* (mg kg−1) | 170 | 30 | ||
| Daidzein all forms* (mg kg−1) | 90 | 10 | ||
| Genistein aglycone* (mg kg−1) | 110 | 20 | ||
| Daidzein aglycone* (mg kg−1) | 60 | 10 | ||
| Lipids (total,% total weight) | 3.46 | 3.47 | ||
| Saturated fatty acids (% total weight) | 0.72 | 0.72 | ||
| Monounsaturated fatty acids (% total weight) | 1.17 | 1.18 | ||
| Polyunsaturated fatty acids (% total weight) | 1.57 | 1.57 | ||
| Protein (% total weight) | 23.41 | 23.52 | ||
| Fibre (% total weight) | 3.55 | 3.55 | ||
| Carbohydrate (total) (% total weight) | 40.03 | 40.03 | ||
| Simple sugars (% total weight) | 5.39 | 5.39 |
| Diet rich in isoflavones | Diet poor in isoflavones | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Plasma isoflavone concentration (6 months) | ||||
| Genistein (μmol l−1) | 0.57 ± 0.1 | — | ND | — |
| Daidzein (μmol l−1) | 0.30 ± 0.06 | — | ND | — |
| Equol (μmol l−1) | 1.00 ± 0.08 | — | ND | — |
| Maternal body weight (g) day 21 of pregnancy | — | 348 ± 14 | — | 323 ± 25 |
| Offspring body weight (g), 6 months | 428 ± 19 | 253 ± 4 | 460 ± 9 | 261 ± 7 |
| Offspring food intake 6 month (g day−1) | 21.7 ± 0 | 15.9 ± 0.6 | 22.7 ± 1.6 | 16.3 ± 1.7 |
Evaluated independently by NP Analytical (St Louis, MO, USA). All other constituents were evaluated by SDS. ND = not detected. Data are presented as the mean ±s.e.m. of 7–10 animals per group.
Radiotelemetry recording of blood pressure, heart rate and activity
Blood pressure, heart rate and activity were assessed by radiotelemetry (Dataquest IV, Data Sciences International, Arden Hills, MN, USA; Anderson et al. 1999) in the same animals subsequently used for study of isolated artery function (see below). Three- and six-month-old littermates were administered buprenorphine (0.1 mg kg−1 subcutaneous, Alstoe Animal Health, York, UK) before surgery under anaesthesia by inhalation (4% isofluorane). The radiotelemetry probe was implanted in the abdominal aorta and transfixed to the abdominal wall of the peritoneal cavity (Khan et al. 2003). On recovery, rats were housed in individual cages. After a 1 week recovery period, heart rate, systolic and diastolic blood pressure, and activity were recorded for 10 s every 5 min for 1 week.
Assessment of oestrus cycle
Conscious female offspring were subject to a vaginal smear daily (between 09.00 and 11.00 h) over 14 consecutive days prior to surgery. Vaginal smearing showed that at 3 and 6 months of age, all female rats demonstrated a normal 4 day cycle. In order to avoid disturbance of the animals during blood pressure and heart rate recording, the stage of the oestrus cycle was assessed indirectly from remotely recorded activity data, as it is recognized that female Wistar rats are more active during proestrus than in other stages of the oestrus cycle (Takezawa et al. 1994).
Vascular function
Vascular function was assessed in 3- and 6-month-old offspring. One male and one female from each litter were studied. Animals were killed by a rising concentration of CO2 after an overnight fast. Blood samples for determination of fasting concentrations of total cholesterol (Unimate Chol), triglycerides (Unimate Trig, Roche Diagnostic Systems) and high density lipoproteins (HDL; Alpha Laboratories, Eastleigh, UK) were obtained by cardiac puncture, centrifuged (4000 r.p.m., 15 min at 4°C) and plasma stored at −70°C. Third order mesenteric arteries and the thoracic aorta were placed in ice-cold physiological salt solution (PSS; mmol l−1: NaCl 119, CaCl2 2.5, KCl 4.7, MgSO4 7H2O 1.17, NaHCO3 25, KH2PO4, 1.18, EDTA, 0.025, d-(+)-glucose 6) and dissected clean of fat and connective tissue.
Mesenteric small artery function
Third-order mesenteric arteries were mounted on a pressure myograph (Living Systems International, Burlington, VT, USA; Halpern et al. 1984). Changes in diameter to incremental pressure steps were measured (20 mmHg steps) over the range 20–100 mmHg at 2 min intervals. Following re-equilibration (15 min, 60 mmHg), arterial responses to the same pressure range were assessed, but in the absence of active tension (Ca2+ free PSS, EGTA, 1 mmol l−1, 15 min).
Myogenic tone and passive distensibility were calculated as:
 |
 |
Where x lies between 20 and 100 mmHg in 20 mmHg incremental steps.
Third-order branches of the mesenteric artery were mounted on a small vessel myograph (J. P. Trading, Aarhus, Denmark; Mulvany & Halpern, 1977). Concentration–response curves to noradrenaline (NA; 0.1–10 μmol l−1), endothelin-1 (0.1–30 nmol l−1), and acetylcholine (1 nmol l−1–10 μmol l−1) and aqueous nitric oxide (NO; 0.1 nmol l−1–0.1 mmol l−1) in NA preconstricted vessels (approximately 80% maximal tension) were determined.
Aorta function
Two transverse rings of the thoracic aorta, 2 mm in length were mounted in a tissue bath (Model 700MO, Danish Myotech, Aarhus, Denmark) maintained in PSS (95% O2–5% CO2, 37°C) and a force of 5 mN (1 g force) was applied (Armitage et al. 2005). Concentration responses were determined to phenylephrine (3 nmol l−1–10 μmol l−1), and to acetylcholine (3 nmol l−1–10 μmol l−1) and aqueous NO (0.3 μmol l−1–10μmol l−1) after preconstriction with phenylephrine (80% maximum force). The passive diameter–force relationship was determined over three cumulative stretches of 2 min duration (500, 200 and 50 μm) following equilibration in Ca2+ free PSS (containing EGTA, 1 mmol l−1).
Plasma isoflavone concentrations
Blood samples were obtained from non-fasted littermates of animals used for vascular protocols. After killing by rising CO2, samples were obtained by cardiac puncture and centrifuged (4000 r.p.m., 15 min, 4°C). Plasma was acidified with acetic acid (final pH 4.9) and stored (−80°C).
Samples were incubated for 18 h (37°C) with 1000 units of β-glucuronidase and 45 units of sulphatase (Sigma, France), mixed with methanol (4 volumes, 200 mmol l−1) and centrifuged (12 500 g, 5 min). A calibration curve was produced by spiking control samples of human plasma with known concentrations of each isoflavone. HPLC was performed using a two pump system (for high pressure gradient, Model 580, ESA, Chelmsford, MA, USA). The mobile phase consisted of 30 mmol l−1 NaH3PO4 buffer (pH 3) containing 20% acetonitrile (A) and 40% acetonitrile (B). Separation was achieved using a gradient elution (0.5 ml min−1; 0–15 min 100% A–100% B; 15–19 min 100% B; 19–25 min 100% A). Detection was performed using an eight-channel CoulArray detector (model 5600, Eurosep, Cergy, France) with potentials set at 200, 280, 450, 550, 600, 650, 700, 750 mV (Pd as reference).
Statistical analysis
Radio-telemetric data were assessed over 1 week and reported as the mean value over 12 h night (active) and day (inactive) periods. Day and night averages were analysed by repeated measures analysis of variance (RM ANOVA). Relaxation was calculated as a percentage of preconstrictor tone. Statistical analysis of comparisons across the entire concentration–response were assessed by RM ANOVA. pEC50 was calculated by fitting raw data to sigmoidal logistic curve (Prism; GraphPad Software Inc., San Diego, CA, USA) and analysed by ANOVA.
Vaginal smear data was assessed by χ-square test. All other data were assessed by ANOVA. Statistical analyses were performed using StatView 5.0.1 (SAS Institute, Cary, NC, USA). Where no significant difference of sex was found, data for males and females were combined. A P-value of < 0.05 was considered statistically significant.
Results
Weights, food consumption, offspring plasma isoflavone and lipid concentrations
Dietary isoflavones did not affect maternal body weight during pregnancy. Offspring body weight was unaffected by diet. Daidzein, equol and genistein were detected in plasma from male rats fed an isoflavone rich diet. Equol (metabolite of daidzein) was predominant, followed by genistein and daidzein. Isoflavones were not detectable in plasma from rats fed the low isoflavone diet (Table 1). There was no effect of sex or diet on plasma concentrations of total or HDL cholesterol or triglycerides in 3- or 6-month-old offspring (Table 2).
Table 2.
Plasma lipid concentrations
| High isoflavone diet | Low isoflavone diet | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| 3 month | ||||
| Total cholesterol (mmol l−1) | 1.92 ± 0.12 | 1.97 ± 0.14 | 1.82 ± 0.16 | 1.83 ± 0.12 |
| HDL (mmol l−1) | 1.04 ± 0.06 | 1.25 ± 0.11 | 1.03 ± 0.07 | 1.06 ± 0.71 |
| Triglycerides (mmol l−1) | 0.97 ± 0.07 | 0.97 ± 0.13 | 1.2 ± 0.19 | 1.19 ± 0.19 |
| 6 month | ||||
| Total cholesterol (mmol l−1) | 1.74 ± 0.21 | 1.87 ± 0.08 | 1.97 ± 0.11 | 1.82 ± 0.15 |
| HDL (mmol l−1) | 0.99 ± 0.14 | 1.23 ± 0.07 | 1.06 ± 0.10 | 1.08 ± 0.09 |
| Triglycerides (mmol l−1) | 1.11 ± 0.19 | 0.94 ± 0.09 | 1.29 ± 0.15 | 1.11 ± 0.17 |
HDL = high density lipoproteins. Data are presented as the mean ±s.e.m. of 6–10 animals per group.
Blood pressure, heart rate and activity
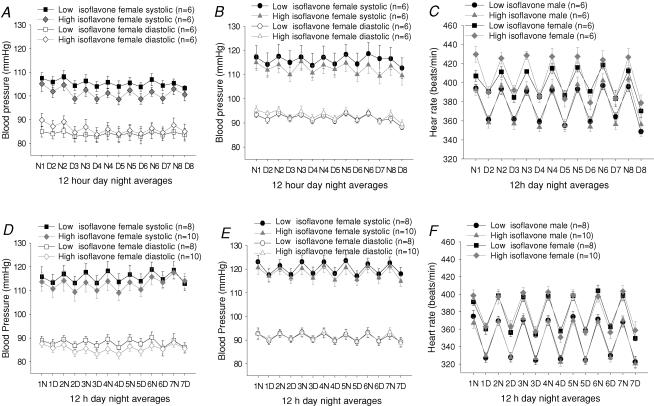
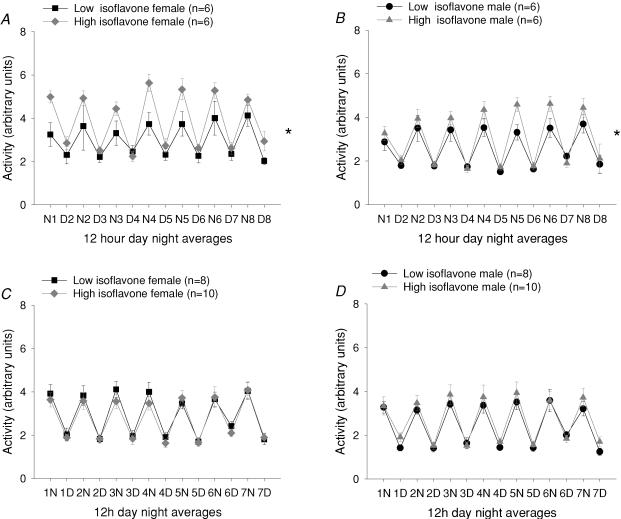
No significant effect of isoflavone consumption on blood pressure or heart rate was observed in 3- or 6-month-old rats (Fig. 1). At 3 but not 6 months male and female rats eating the isoflavone rich diet were significantly more active during the dark period (activity 3.53 ± 0.5 versus 4.23 ± 0.44 arbitrary units (a.u.), low versus high isoflavone fed males, and 3.69 ± 0.62 versus 5.33 ± 0.33 a.u. low versus high isoflavone fed females; n = 6 all groups, P < 0.05, RM ANOVA). Isoflavone consumption did not affect activity during the light (inactive) phase (Fig. 2).
Figure 1. Systolic and diastolic blood pressure in female (A) and male (B) rats at 3 months and in female (D) and male (E) rats at 6 months fed either a high or low isoflavone diet, and heart rate in female and male rats at 3 (C) and 6 (F) months fed a high or low isoflavone diet.
Data are presented as the mean ±s.e.m. of 12 h night (N) and day (D) averages.
Figure 2. Activity in female (A) and male (B) rats at 3 months and female (C) and male (D) rats at 6 months fed either a high or low isoflavone diet.
At three months, male and female rats fed the high isoflavone diet had increased night-time activity compared with rats fed a low isoflavone diet (*P < 0.05 high isoflavone versus low isoflavone fed rats). Data are presented as the mean ±s.e.m. of 12 h night (N) and day (D) averages.
Oestrus cycle
All female rats demonstrated a 4 day cycle, with no difference in the length of oestrus cycle between rats fed a high (n = 10) compared with low (n = 8) isoflavone diet for either 3 or 6 months. There was no difference in the number of rats in oestrus during the period of radiotelemetry recording in 3- or 6-month-old rats (data not shown).
Vascular function
Mesenteric artery function
There was no effect of either diet or sex on mesenteric artery diameter at either 3 or 6 months of age (mesenteric artery diameters (μm) at 3 months: high isoflavone: females 264 ± 13, males 284 ± 17; low isoflavone: females 274 ± 12, males 279 ± 14; 6 months: high isoflavone: females 289 ± 17, males 303 ± 15; low isoflavone: females 279 ± 10, males 309 ± 9; P > 0.05, ANOVA).
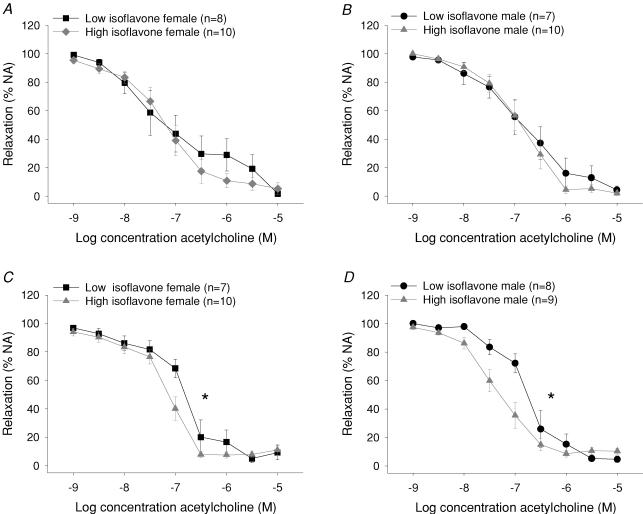
Acetylcholine-mediated, endothelium-dependent relaxation was not different between the two groups at 3 months (Fig. 3A and B), but after 6 months male and female animals fed the isoflavone rich diet demonstrated increased sensitivity to acetylcholine (males, pEC50 high isoflavone 7.34 ± 0.14, n = 9 versus low isoflavone 6.73 ± 0.17, n = 8; female 7.24 ± 0.08, n = 10 versus 6.81 ± 0.16, n = 7, P < 0.05; ANOVA; Fig. 3C and D). The high isoflavone diet had no significant effect on endothelium-independent relaxation to aqueous NO at 3 or 6 months (Table 3). Neither were responses to noradrenaline or endothelin-1 at 3 or 6 months significantly different between groups (Table 3).
Figure 3. Concentration–response curves in mesenteric arteries to acetylcholine in female (A) and male (B) rats at 3 months of age and female (C) and male (D) rats at 6 months fed either a high or low isoflavone diet.
At 6 months male and female rats fed the high isoflavone diet demonstrated an increased sensitivity to acetylcholine compared with low isoflavone fed rats (*P < 0.05 high isoflavone versus low isoflavone fed rats). Data are presented as the mean ±s.e.m. Relaxation was calculated as percentage noradrenaline (NA)-induced tension.
Table 3.
Contractile and dilator function of mesenteric arteries from male and female high and low isoflavone fed rats
| 3 month | 6 month | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| High isoflavone diet | Low isoflavone diet | High isoflavone diet | Low isoflavone diet | ||||||
| Female | Male | Female | Male | Female | Male | Female | Male | ||
| ACh | pEC50 (−log10m) | 7.12 ± 0.17 | 6.93 ± 0.15 | 6.78 ± 0.56 | 6.72 ± 0.36 | 7.24 ± 0.08 | 7.34 ± 0.14 | 6.81 ± 0.16* | 6.73 ± 0.17† |
| Max (%) | 89.01 ± 6.83 | 97.87 ± 1.68 | 87.40 ± 9.46 | 97.87 ± 10.06 | 88.91 ± 3.81 | 83.55 ± 7.45 | 84.80 ± 7.45 | 95.34 ± 2.29 | |
| NO | pEC50 (−log10m) | 6.01 ± 0.08 | 5.92 ± 0.13 | 5.98 ± 0.11 | 5.77 ± 0.71 | 5.50 ± 0.20 | 4.91 ± 0.21‡ | 5.72 ± 0.16 | 4.97 ± 0.17‡ |
| Max (%) | 96.91 ± 3.09 | 99.65 ± 0.35 | 98.44 ± 1.18 | 96.12 ± 2.17 | 92.25 ± 3.56 | 93.17 ± 6.25 | 93.25 ± 4.26 | 92.07 ± 3.3 | |
| NA | pEC50 (−log10m) | 6.24 ± 0.17 | 6.12 ± 0.12 | 6.14 ± 0.19 | 5.99 ± 0.04 | 5.74 ± 0.14 | 6.14 ± 0.15‡ | 5.90 ± 0.12 | 6.06 ± 0.12‡ |
| Max (mN) | 2.69 ± 0.24 | 3.44 ± 0.36 | 2.65 ± 0.21 | 3.02 ± 0.22 | 3.17 ± 0.24 | 4.04 ± 0.18 ‡ | 2.90 ± 0.28 | 3.70 ± 0.25 ‡ | |
| ET-1 | pEC50 (−log10m) | 7.33 ± 0.04 | 7.24 ± 0.07 | 7.20 ± 0.12 | 7.21 ± 0.02 | 7.11 ± 0.12 | 7.30 ± 0.12‡ | 7.26 ± 0.18 | 7.27 ± 0.07‡ |
| Max (mN) | 3.33 ± 0.36 | 3.68 ± 0.31 | 3.74 ± 0.21 | 3.77 ± 0.17 | 3.24 ± 0.31 | 3.38 ± 0.14 | 3.38 ± 0.14 | 3.92 ± 0.25 | |
Data are presented as the mean ±s.e.m. of 6–10 animals per group.
P < 0.05 low isoflavone females compared with high isoflavone females, ANOVA.
P < 0.05 low isoflavone males compared with high isoflavone males.
P < 0.05 males (both groups) compared with females (both groups), ANOVA.
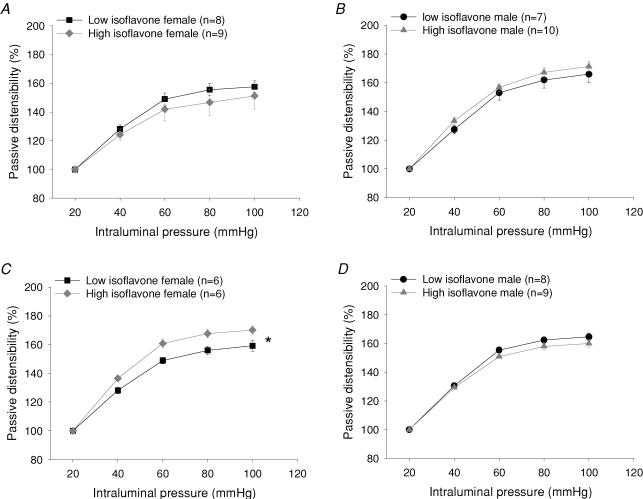
Vascular distensibility (passive increase in diameter as a function of pressure) was unaffected by diet in 3-month-old males and females, but at 6 months, mesenteric arteries from females (but not males) fed the high isoflavone diet were significantly more distensible than arteries of the low isoflavone fed females (Fig. 4). There was no significant effect of diet or sex on wall thickness across the pressure range measured (data not shown).
Figure 4. Passive distensibility of mesenteric arteries from female (A) and male (B) rats at 3 months and female (C) and male (D) rats as 6 months fed either a high or low isoflavone diet.
At 6 months female rats fed the high isoflavone diet showed increased arterial distensibility compared with female rats fed the low isoflavone diet (*P < 0.05 high isoflavone versus low isoflavone fed rats). Data are presented as the mean ±s.e.m.
Aortic function
There was no significant difference in the concentration–response curve (P > 0.05, RM ANOVA) or the pEC50 to phenylephrine, acetylcholine or NO in the aorta of the two dietary groups at either 3 or 6 months. Neither was passive stretch-induced aortic tension different between the two groups at 3 or 6 months (Table 4).
Table 4.
Contractile and dilator function of aortas from male and female high and low isoflavone fed rats
| 3 month | 6 month | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| High isoflavone diet | Low isoflavone diet | High isoflavone diet | Low isoflavone diet | ||||||
| Female | Male | Female | Male | Female | Male | Female | Male | ||
| ACh | pEC50 (−log10m) | 6.08 ± 0.14 | 6.08 ± 0.14 | 6.23 ± 0.09 | 6.31 ± 0.11 | 6.52 ± 0.16 | 6.38 ± 0.10 | 6.63 ± 0.09 | 6.36 ± 0.12 |
| Max (%) | 80.56 ± 0.14 | 83.09 ± 0.14 | 84.28 ± 0.09 | 80.73 ± 0.11 | 85.52 ± 3.43 | 80.74 ± 2.22 | 87.45 ± 2.22 | 75.37 ± 7.58 | |
| NO | pEC50 (−log10m) | 5.56 ± 0.20 | 4.98 ± 0.132 | 5.54 ± 0.21 | 5.18 ± 0.08 | 5.25 ± 0.08 | 4.95 ± 0.11 | 5.10 ± 0.26 | 5.07 ± 0.08 |
| Max (%) | 99.50 ± 1.54 | 98.90 ± 0.78 | 95.72 ± 3.29 | 99.22 ± 0.61 | 96.32 ± 3.45 | 97.68 ± 0.96 | 97.48 ± 1.60 | 99.17 ± 0.3 | |
| PE | pEC50 (−log10m) | 5.67 ± 0.27 | 5.67 ± 0.242 | 5.57 ± 0.24 | 5.49 ± 0.26 | 5.81 ± 0.05 | 5.74 ± 0.08 | 5.71 ± 0.11 | 5.8 ± 0.12 |
| Max (% K+) | 118.24 ± 13.43 | 121.44 ± 13.43 | 106.16 ± 6.54 | 121.44 ± 4.52 | 107.38 ± 13.28 | 105.57 ± 7.64 | 103.44 ± 12.26 | 119.09 ± 7.8 | |
| Stretch | Max (mN) | 20.92 ± 1.42 | 18.78 ± 0.95 | 19.53 ± 0.93 | 21.21 ± 1.47 | 16.91 ± 0.66 | 18.45 ± 0.84 | 18.55 ± 1.11 | 18.21 ± 0.86 |
Maximum relaxation (Max (%)) to acetylcholine and nitric oxide is presented as percentage phenylephrine-induced contraction. Maximum constriction to phenylephrine is presented as percentage high potassium (K+)-induced contraction (Max (%K+)). Data are presented as the mean ±s.e.m. of 6–10 animals per group.
Discussion
This study has shown for the first time that the isoflavone component of dietary soy protein (principally genistein, daidzein and its metabolite equol) induces modest functional changes in small arteries from rats fed a balanced diet containing soy protein from conception to 6 months of age. The lack of a significant effect on blood pressure, heart rate or conduit artery function in 6-month-old rats of both sexes does not support a major role for soy-derived isoflavones in modulation of cardiovascular function in healthy adult rats. Dietary soy isoflavones may, however, afford cardioprotection during the course of ageing and in vascular disease (Mahn et al. 2005; Trujillo et al. 2005).
In contrast to many previous studies, the design of this study discounts any potential cardiovascular effects due to differences in the experimental and control diets, other than in the isoflavone content. Male and female rats fed soy protein naturally rich in isoflavones showed an increment in endothelium-dependent dilatation in small mesenteric arteries in comparison to animals fed an identical diet that was exclusively depleted of soy isoflavones. In addition, female rats fed the isoflavone rich diet compared with the isoflavone depleted diet demonstrated a modest increase in resistance artery distensibility. This, to our knowledge, is the first demonstration that dietary isoflavones influence the distensibility and endothelium-dependent dilatation of resistance arteries. These functional changes, although modest, could be associated with reduction in cardiovascular risk as they occurred in the presence of plasma genistein concentrations comparable to those reported in Japanese men consuming a standard soy rich Japanese diet (Adlercreutz et al. 1993).
There was no effect of the soy isoflavones on blood pressure or heart rate in adult male and female rats at both ages studied. Previous studies of Chinese women (mean age 50 years) and Japanese men (mean age 50 years) have shown an inverse association between soy protein consumption and blood pressure (Nagata et al. 2003; Yang et al. 2005; He et al. 2005). The present study would suggest that these associations may be unrelated to the isoflavone content of the soy diet and that soy protein itself may have some hypotensive benefit. To our knowledge there has been no report to suggest that soy protein per se may have hypotensive properties, and associations in the human populations could relate to other cardioprotective life style factors associated with dietary soy intake. However, a recent investigation in young Sprague-Dawley rats found that cholesterol lowering effects of a soy protein isolate were partly explicable on the basis of the protein component rather than the isoflavone content (Fukui et al. 2004). There were no differences in fasting plasma concentrations of plasma lipids between 3- and 6-month-old rats on the two different soy based diets in the present study, which would add support to this and both studies concur with an earlier report showing a lack of effect of genistein or daidzein on total cholesterol or triglycerides in Sprague-Dawley rats (Banz et al. 2004).
The lack of an effect of the isoflavone-depleted soy diet on blood pressure is consistent with some but not all reports of experimental dietary soy or isoflavone supplementation. No effect on blood pressure in adult female ovariectomized Sprague-Dawley rats was observed in animals receiving 0.2 mg kg−1 of genistein (subcutaneous injection) for 4 weeks (Squadrito et al. 2000) compared with controls. Isoflavones have been implicated in reducing the mean arterial pressure in adult ovariectomized spontaneously hypertensive rats fed a soy based diet compared with a casein based diet for 8 weeks whereas no effect was seen in normotensive ovariectomized animals (Martin et al. 2001). Our recent study of 16-month-old Wistar male rats fed a soy protein diet showed lower systolic and diastolic blood pressure and lower heart rates compared with rats fed a fish meal based diet (Mahn et al. 2005). At 16 months of age the average systolic blood pressure in the Wistar rat is approximately 150 mmHg, whilst the 6-month-old rats studied here had a systolic blood pressure of approximately 120 mmHg. Although the present data argue against a role for soy-derived isoflavones in lowering the blood pressure in mature healthy adult rats, a hypotensive effect in much older relatively hypertensive animals or genetically hypertensive rats cannot be ruled out. This is supported by the demonstration that oxidative stress and elevated blood pressure in the 16 month male rats was significantly decreased upon refeeding these animals a soy protein diet rich in genistein and daidzein (Mahn et al. 2005) and by a recent report showing that isoflavones diminish oxidative stress and augment NO production in spontaneously hypertensive rats (Park et al. 2005).
There was a modest effect of isoflavone elution from the soy protein on endothelial function as assessed by acetylcholine-induced relaxation in the small mesenteric arteries. Although of physiological relevance, the magnitude was small compared with, for example, the beneficial effect of exercise (Xiang et al. 2005) or the deleterious effect of a high fat diet (Naderali et al. 2001). As no differences were observed in endothelium-independent relaxation (aqueous NO), it is unlikely that this resulted from greater NO smooth muscle sensitivity, but more probably was associated with greater NO bio-availability. Increased NO synthesis has been implicated in Sprague-Dawley rats treated subcutaneously with daidzein (Sobey et al. 2004; Woodman et al. 2004) and our recent study in Wistar rats demonstrated up-regulation of liver eNOS mRNA in rats fed a soy diet for 16 months compared with those fed a fish meal diet (Mahn et al. 2005). Isoflavones may also increase NO bioavailability by enhancement of antioxidant capacity (Mahn et al. 2005). However, endothelium-derived hyperpolarizing factor (EDHF) is also very likely to play a role, since in Sprague-Dawley rats EDHF accounts for 60–70% of acetylcholine mediated relaxation in small mesenteric arteries (Taylor et al. 2004), whereas acetylcholine mediated relaxation in aorta is almost exclusively due to NO (Woodman et al. 2000). Preferential increment of EDHF could therefore explain the lack of effect of isoflavone depletion in the aorta. Indeed in our previous study, aged Wistar male rats fed the soy-free diet compared with the soy-rich diet were found to have a blunting of the residual relaxation to acetylcholine in small mesenteric arteries treated with L-NAME (G. A. Knock, K. Mahn, G. E. Mann, J. P. Ward & P. I. Aaronson, unpublished observations), strongly indicative of a permissive role for isoflavones in EDHF-mediated relaxation.
The divergence from the other reports which have shown enhancement by isoflavones of aortic endothelial function (Squadrito et al. 2000; Yamaguchi et al. 2001; Sobey et al. 2004; Woodman et al. 2004; Mahn et al. 2005) could potentially be explained by the dose of isoflavones used (Yamaguchi et al. 2001) or the route of administration, particularly since subcutaneous injection, used in several studies (Sobey et al. 2004; Woodman et al. 2004) leads to greater plasma concentrations than if given orally (Mallis et al. 2003). Duration of administration could also play a role, as we observed enhancement of acetylcholine-induced relaxation in aorta in rats after 1 year on a soy rich diet compared with animals fed a non-soy protein based diet (Mahn et al. 2005). No study in healthy young animals has addressed the possibility that soy protein per se may enhance acetylcholine-induced relaxation, and this remains to be elucidated.
Six-month-old female, but not male, rats fed the isoflavone rich soy diet demonstrated a modest increase in arterial distensibility. Healthy males and females (Teede et al. 2003) and postmenopausal women (Nestel et al. 1997; Nestel et al. 1999) demonstrate improved arterial compliance after consumption of either the genistein and daidzein precursors biochanin and formononetin (80 mg day−1 for 6 weeks) or isoflavones (40–80 mg day−1), but the mechanism is unknown. We found no difference in wall thickness during the pressure response curve, hence inward or outward hypertrophy were unlikely to be contributors. It is of relevance that oestrogens improve mesenteric arterial compliance in female Sprague-Dawley rats, and that this has been attributed to arterial remodelling mediated via MMP2 (Zhang et al. 2000).
Consumption of a diet rich in isoflavones did not effect the oestrus cycle in the female rats. Endothelial dependent dilatation, heart rate and blood pressure vary with the phase of the oestrus cycle (Takezawa et al. 1994; Liu et al. 2001). As female rats had a standard 4 day cycle and the number of rats at each stage of the cycle was evenly distributed between the two dietary groups, the observed changes in vascular function and activity were unlikely to have been influenced by the phase of oestrus. However, in rats fed either isoflavone deplete or replete diets there were notable sex differences in blood pressure, heart rate and vascular function presumably attributable to endogenous oestrogens and suggesting a predominant role of the endogenous hormones.
Three-month-old rats fed the isoflavone rich diet demonstrated increased physical activity. Ovariectomised rats and mice demonstrate reduced activity compared with oestrogen treated ovariectomised animals suggesting oestrogens increase physical activity (Morgan et al. 2004), which may be mediated by ERα (Ogawa et al. 2003). However, genistein and daidzein are preferential ERβ agonists, but equol, a daidzein metabolite, has relatively greater ERα activity (Morito et al. 2001). In the present study plasma concentrations of equol were 2- to 3-fold higher than genistein and daidzein in rats fed the soy isoflavone rich diet. Equol is the product of intestinal bacterial metabolism of daidzein; however, equol is not produced in all healthy adults in response to dietary soy (Setchell et al. 2002). Thus, any effects of equol may only be observed in individuals capable of metabolizing dietary isoflavones to equol. The lack of difference in activity at 6 months may be reflective of a general decline in activity with age. Consumption of a high isoflavone diet did not alter appetite or growth trajectory, which concurs with a study of similar dietary design (Fukui et al. 2004).
Elution of isoflavones from the soy protein was achieved by alcohol-washing the soy protein isolate, and others have shown that the process of alcohol washing results in the elution of other alcohol soluble compounds, such as saponins, and to alteration in the physical characteristics of the protein (Friedman & Brandon, 2001; Gianazza et al. 2003). However, to the best of our knowledge no studies have shown that consumption of alcohol-washed soy protein compared with non-washed soy protein results in altered vascular function.
This is one of the first studies to examine with appropriately controlled diets any putative cardiovascular benefits of dietary isoflavones. Importantly, our results do not indicate any effect on blood pressure or conduit artery function in mature 6-month-old adult rats of either sex. The modest effect of elevated plasma levels of genistein, daidzein and equol on acetylcholine-induced relaxation and arterial distensibility of mesenteric resistance arteries in vitro highlights a potentially physiologically beneficial role for soy-derived isoflavones on small artery function. We hypothesize that this may be exaggerated as animals age and vascular function deteriorates.
Acknowledgments
We acknowledge Special Diets Services (SDS; Essex, UK) for preparation of the diets and Solae (St Louis, MO, USA) for the kind donation of the soy protein isolate. We also thank C. Manach, INRA de Clermont-ferrand, St Genes-Campanile, France for measurement of the plasma isoflavones. We gratefully acknowledge the support of Tommy's the Baby Charity, UK.
References
- Adams MR, Golden DL, Williams JK, Franke AA, Register TC, Kaplan JR. Soy protein containing isoflavones reduces the size of atherosclerotic plaques without affecting coronary artery reactivity in adult male monkeys. J Nutr. 2005;135:2852–2856. doi: 10.1093/jn/135.12.2852. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- Anderson NH, Devlin AM, Graham D, Morton JJ, Hamilton CA, Reid JL, Schork NJ, Dominiczak AF. Telemetry for cardiovascular monitoring in a pharmacological study: new approaches to data analysis. Hypertension. 1999;33:248–255. doi: 10.1161/01.hyp.33.1.248. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Lakasing L, Taylor PD, Balachandran AA, Jensen RI, Dekou V, Ashton N, Nyengaard JR, Poston L. Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J Physiol. 2005;565:171–184. doi: 10.1113/jphysiol.2005.084947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banz W, Hauck S, Gename B, Winters T, Bartke A. Soy isoflavones modify liver free radical scavenger systems and liver parameters in Sprague-Dawley rats. J Med Food. 2004;7:477–481. doi: 10.1089/jmf.2004.7.477. [DOI] [PubMed] [Google Scholar]
- Erdman JW., Jr AHA Science Advisory: Soy protein and cardiovascular disease: a statement for healthcare professionals from the Nutrition Committee of the AHA. Circulation. 2000;102:2555–2559. doi: 10.1161/01.cir.102.20.2555. [DOI] [PubMed] [Google Scholar]
- Friedman M, Brandon DL. Nutritional and health benefits of soy proteins. J Agric Food Chem. 2001;49:1069–1086. doi: 10.1021/jf0009246. [DOI] [PubMed] [Google Scholar]
- Fukui K, Tachibana N, Fukuda Y, Takamatsu K, Sugano M. Ethanol washing does not attenuate the hypocholesterolemic potential of soy protein. Nutrition. 2004;20:984–990. doi: 10.1016/j.nut.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Gianazza E, Eberini I, Arnoldi A, Wait R, Sirtori CR. A proteomic investigation of isolated soy proteins with variable effects in experimental and clinical studies. J Nutr. 2003;133:9–14. doi: 10.1093/jn/133.1.9. [DOI] [PubMed] [Google Scholar]
- Halpern W, Osol G, Coy GS. Mechanical behavior of pressurized in vitro prearteriolar vessels determined with a video system. Ann Biomed Eng. 1984;12:463–479. doi: 10.1007/BF02363917. [DOI] [PubMed] [Google Scholar]
- He J, Gu D, Wu X, Chen J, Duan X, Chen J, Whelton PK. Effect of soybean protein on blood pressure: a randomized, controlled trial. Ann Intern Med. 2005;143:1–9. doi: 10.7326/0003-4819-143-1-200507050-00004. [DOI] [PubMed] [Google Scholar]
- Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- Lissin LW, Cooke JP. Phytoestrogens and cardiovascular health. J Am Coll Cardiol. 2000;35:1403–1410. doi: 10.1016/s0735-1097(00)00590-8. [DOI] [PubMed] [Google Scholar]
- Liu MY, Hattori Y, Fukao M, Sato A, Sakuma I, Kanno M. Alterations in EDHF-mediated hyperpolarization and relaxation in mesenteric arteries of female rats in long-term deficiency of oestrogen and during oestrus cycle. Br J Pharmacol. 2001;132:1035–1046. doi: 10.1038/sj.bjp.0703899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahn K, Borras C, Knock GA, Taylor P, Khan IY, Sugden D, Poston L, Ward JP, Sharpe RM, Vina J, Aaronson PI, Mann GE. Dietary soy isoflavone-induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005;19:1755–1757. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- Mallis LM, Sarkahian AB, Harris HA, Zhang MY, McConnell OJ. Determination of rat oral bioavailability of soy-derived phytoestrogens using an automated on-column extraction procedure and electrospray tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2003;796:71–86. doi: 10.1016/j.jchromb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Martin DS, Breitkopf NP, Eyster KM, Williams JL. Dietary soy exerts an antihypertensive effect in spontaneously hypertensive female rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R553–R560. doi: 10.1152/ajpregu.2001.281.2.R553. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, Pfaff DW. Estrogens and non-reproductive behaviors related to activity and fear. Neurosci Biobehav Rev. 2004;28:55–63. doi: 10.1016/j.neubiorev.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24:351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Naderali EK, Pickavance LC, Wilding JP, Williams G. Diet-induced endothelial dysfunction in the rat is independent of the degree of increase in total body weight. Clin Sci (Lond) 2001;100:635–641. doi: 10.1042/cs1000635. [DOI] [PubMed] [Google Scholar]
- Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Association of blood pressure with intake of soy products and other food groups in Japanese men and women. Prev Med. 2003;36:692–697. doi: 10.1016/s0091-7435(03)00052-5. [DOI] [PubMed] [Google Scholar]
- Nestel PJ, Pomeroy S, Kay S, Komesaroff P, Behrsing J, Cameron JD, West L. Isoflavones from red clover improve systemic arterial compliance but not plasma lipids in menopausal women. J Clin Endocrinol Metab. 1999;84:895–898. doi: 10.1210/jcem.84.3.5561. [DOI] [PubMed] [Google Scholar]
- Nestel PJ, Yamashita T, Sasahara T, Pomeroy S, Dart A, Komesaroff P, Owen A, Abbey M. Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arterioscler Thromb Vasc Biol. 1997;17:3392–3398. doi: 10.1161/01.atv.17.12.3392. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144:230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- Park E, Shin JI, Park OJ, Kang MH. Soy isoflavone supplementation alleviates oxidative stress and improves systolic blood pressure in male spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo) 2005;51:254–259. doi: 10.3177/jnsv.51.254. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- Sobey CG, Weiler JM, Boujaoude M, Woodman OL. Effect of short-term phytoestrogen treatment in male rats on nitric oxide-mediated responses of carotid and cerebral arteries: comparison with 17beta-estradiol. J Pharmacol Exp Ther. 2004;310:135–140. doi: 10.1124/jpet.103.063255. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Altavilla D, Squadrito G, Saitta A, Cucinotta D, Minutoli L, Deodato B, Ferlito M, Campo GM, Bova A, Caputi AP. Genistein supplementation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovasc Res. 2000;45:454–462. doi: 10.1016/s0008-6363(99)00359-4. [DOI] [PubMed] [Google Scholar]
- Takezawa H, Hayashi H, Sano H, Saito H, Ebihara S. Circadian and estrous cycle-dependent variations in blood pressure and heart rate in female rats. Am J Physiol. 1994;267:R1250–R1256. doi: 10.1152/ajpregu.1994.267.5.R1250. [DOI] [PubMed] [Google Scholar]
- Taylor PD, Khan IY, Hanson MA, Poston L. Impaired EDHF-mediated vasodilatation in adult offspring of rats exposed to a fat-rich diet in pregnancy. J Physiol. 2004;558:943–951. doi: 10.1113/jphysiol.2002.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teede HJ, McGrath BP, DeSilva L, Cehun M, Fassoulakis A, Nestel PJ. Isoflavones reduce arterial stiffness: a placebo-controlled study in men and postmenopausal women. Arterioscler Thromb Vasc Biol. 2003;23:1066–1071. doi: 10.1161/01.ATV.0000072967.97296.4A. [DOI] [PubMed] [Google Scholar]
- Trujillo J, Ramirez V, Perez J, Torre-Villalvazo I, Torres N, Tovar AR, Munoz RM, Uribe N, Gamba G, Bobadilla NA. Renal protection by a soy diet in obese Zucker rats is associated with restoration of nitric oxide generation. Am J Physiol Renal Physiol. 2005;288:F108–F116. doi: 10.1152/ajprenal.00077.2004. [DOI] [PubMed] [Google Scholar]
- Woodman OL, Missen MA, Boujaoude M. Daidzein and 17 beta-estradiol enhance nitric oxide synthase activity associated with an increase in calmodulin and a decrease in caveolin-1. J Cardiovasc Pharmacol. 2004;44:155–163. doi: 10.1097/00005344-200408000-00003. [DOI] [PubMed] [Google Scholar]
- Woodman OL, Wongsawatkul O, Sobey CG. Contribution of nitric oxide, cyclic GMP and K+ channels to acetylcholine-induced dilatation of rat conduit and resistance arteries. Clin Exp Pharmacol Physiol. 2000;27:34–40. doi: 10.1046/j.1440-1681.2000.03199.x. [DOI] [PubMed] [Google Scholar]
- Xiang L, Naik J, Hester RL. Exercise-induced increase in skeletal muscle vasodilatory responses in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R987–R991. doi: 10.1152/ajpregu.00702.2004. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Honda H, Wakisaka C, Tohei A, Kogo H. Effects of phytoestrogens on acetylcholine- and isoprenaline-induced vasodilation in rat aorta. Jpn J Pharmacol. 2001;87:67–73. doi: 10.1254/jjp.87.67. [DOI] [PubMed] [Google Scholar]
- Yang G, Shu XO, Jin F, Zhang X, Li HL, Li Q, Gao YT, Zheng W. Longitudinal study of soy food intake and blood pressure among middle-aged and elderly Chinese women. Am J Clin Nutr. 2005;81:1012–1017. doi: 10.1093/ajcn/81.5.1012. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shu XO, Gao YT, Yang G, Li Q, Li H, Jin F, Zheng W. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 2003;133:2874–2878. doi: 10.1093/jn/133.9.2874. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Stewart KG, Davidge ST. Estrogen replacement reduces age-associated remodeling in rat mesenteric arteries. Hypertension. 2000;36:970–974. doi: 10.1161/01.hyp.36.6.970. [DOI] [PubMed] [Google Scholar]