Abstract
Partial renal ablation leads to progressive renal insufficiency and is a model of chronic renal failure from diverse causes. We find that mice develop functional and morphologic characteristics of chronic renal failure after partial renal ablation, including glomerular sclerosis, systemic hypertension, and reduced glomerular filtration. However, we now report that littermates with a homozygous deletion of the gene for the cyclin-dependent kinase inhibitor, p21WAF1/CIP1, do not develop chronic renal failure after ablation. The markedly different reactions of the p21(+/+) and p21(−/−) animals was not because of differences in glomerular number or degree of renal growth but rather because of the presence or absence of a normal p21 gene. Although the reaction to the stress of renal ablation is both hyperplastic and hypertrophic in the presence of a functional p21 gene, it would appear that the absence of the p21 gene may induce a more hyperplastic reaction because proliferating-cell nuclear antigen expression, a marker of cell-cycle progression, in the renal epithelium of the remnant kidney was more than five times greater in the p21(−/−) mice than in the p21(+/+) animals. Because p21 is a potent inhibitor of the cell cycle, we speculate that p21 regulates the balance between hyperplasia and hypertrophy after renal ablation. We propose that this change in response inhibits the development of chronic renal failure. These studies suggest that controlling p21 function may ameliorate or even prevent progressive end-stage renal disease.
The removal of substantial amounts of renal tissue is followed by a progressive decline in renal function (1, 2). Glomerular hypertrophy occurs early in response to this ablation and is accompanied by short-term increases in glomerular filtration (3, 4). These structural and functional adaptations to loss of excretory function are thought to be maladaptive and to influence the progression to end-stage renal disease. Progression is initially seen as localized increases in mesangial matrix that then leads to global glomerular sclerosis and is usually associated with systemic hypertension, which has been speculated to accelerate its course. Although the early glomerular hypertrophy and hyperfunction, especially the glomerular hypertension that determines it, have been invoked as predeterminants of the later destructive effects of renal ablation, there is no established causal link between these events and the progressive nature of the renal disease.
Acute short-term stress in the kidney provokes molecular responses that involve the expression of several genes, including the cyclin-dependent kinase inhibitor p21 (5). p21 plays a critical role in processes by which nuclear events subsequent to environmental stress are regulated. It is induced to very high levels by oxidative stress (6) and DNA damage (7). The p21 protein (8) acts as an inhibitor of cyclin-dependent kinase activity (9) and effectively stops cell-cycle progression (8, 9). It is overexpressed in many cells undergoing senescence (10) or terminal differentiation (11, 12). The expression of p21 after short-term chemotoxic renal stress is rapid, and that expression under these circumstances played a protective role (13). We speculated that chronic, long-term stress would provoke sustained expression of p21 and that such expression could influence renal function and morphology. To test this hypothesis, we used renal ablation, a model of progressive renal insufficiency, as a chronic stress. Two groups of mice were compared: one in which the p21 gene was deleted and their wild-type littermates. We report that in the absence of a functional p21 gene, renal ablation did not result in progressive renal failure, which in the p21(+/+) mice included the development of glomerulosclerosis and systemic hypertension.
METHODS
Animal Preparation. Mice (strain 129/Sv) carrying a deletion of a large portion of the p21 gene in which neither p21 mRNA nor p21 protein is expressed (14) were obtained from Philip Leder (Harvard Medical School, Boston). Mice homozygous for the p21 deletion were selected from the offspring of heterozygous matings by using Southern blotting of tail DNA as described (14). Wild-type p21(+/+) littermates were used as controls for a normal p21 gene. The animals were housed at the Animal Research Center at the University of Texas Medical Branch at Galveston. Food and water were supplied ad libitum. Body weights were determined at the start of the protocol, at the time of surgery, and at the time of sacrifice.
Renal ablation was created by two-step nephrectomy (15) by using 6- to 8-week-old male mice. At the first stage of the procedure, the right kidney was decapsulated, and the upper and lower poles were resected under anesthesia with pentobarbital sodium (50 mg/kg) i.p. Bleeding was prevented by using a thrombin solution (3,000 units/ml, 0.9% NaCl). One week later, a total left nephrectomy was performed under anesthesia as described above. Renal function, kidney morphology, morphometry, and mean arterial blood pressure were studied at various times thereafter.
Clearance and Direct Systolic Blood Pressure Measurements.
Mice were anesthetized, as above, and placed on a heated surgical table to maintain body temperature between 37 and 38°C. Polyethylene catheters were placed in the trachea, bladder, both femoral arteries, and left jugular vein. The mean arterial blood pressure was obtained via the left femoral artery by using a strain-gauge transducer (Gould, Cleveland). The animals were infused with 0.9% sodium chloride solution via the left external jugular vein at a rate of 0.5% body weight per hour by using a constant-infusion syringe pump (model 355, Sage Instruments, Boston). The infusion solution contained enough [3H]methoxyinulin (American Radiolabeled Chemicals, St. Louis) to deliver 10 μCi/hour. After a 60-minute equilibration period, urine was collected under mineral oil for three 30-minute clearance determinations. Blood was drawn in heparinized microhematocrit tubes from the right femoral artery at the beginning and end of the clearance period to determine hematocrit and 3H activity. 3H activity in urine and plasma was determined in a liquid scintillation counter (LKB Wallace 1211 RackBeta), and the glomerular filtration rate (GFR) was calculated (see below).
Kidney Morphology and Morphometry.
At the time of sacrifice, kidney remnants were freed from the surrounding tissues, weighed, cut in half, fixed in 4% neutral buffered formaldehyde, and processed for light microscopy by paraffin embedding. Sections (5 μm) were stained with hematoxylin/eosin, periodic acid/Schiff reagent, or trichrome.
Morphological Studies.
Three to five animals at various time points were used for morphological studies. By using periodic acid/Schiff reagent-stained sections, at least 300 glomeruli were evaluated by light microscopy. The percentage of each glomerulus exhibiting mesangial expansion or glomerulosclerosis was determined by point counting (4) at ×400 by using an eyepiece reticle (SO75963, Nikon) Focal glomerulosclerosis was graded as to percent of glomerular area sclerotic by using the following criteria: minimal (1–25%), moderate (26–50%), and severe (51–84%). When ≥85% of glomerular area was sclerotic, the glomerulus was classified as globally sclerotic.
Glomerular Morphometry.
To determine glomerular hypertrophy, we measured mean glomerular volume (μm3) based on point counting (16–18) according to the following formula:
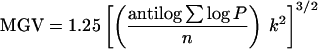 |
where P = number of points falling on each glomerular tuft profile, k = distance between the points in micrometers, and n = number of glomeruli counted. Glomeruli showing global sclerosis were excluded.
Quantitation of Glomerular Numbers per Kidney.
The number of glomeruli per kidney was determined by using the method described by MacKay et al. (19).
Immunohistochemistry.
Proliferating-cell nuclear antigen (PCNA) was detected by using a mouse mAb (Santa Cruz Biotechnology) and the ABC Elite Vectastain kit (Vector Laboratories) according to manufacturers instructions.
In Situ Hybridization.
In situ localization of p21 mRNA on kidney sections was performed as described (5).
Calculations.
GFR was calculated as Cinulin (ml/min) = U/P [3H] × Vu (ml/min). Percent nephrectomy was calculated as % nephrectomy = (RKremoved + LKadj) × 100/2 × LKadj, where RKremoved was the amount (mg) of the right kidney removed in the first operation and LKadj was the weight (mg) of the left kidney removed in the second operation 7 days later, adjusted for hypertrophy between the first and second operation. The adjustment is calculated by multiplying the weight of the left kidney at the time of removal by the average kidney weight per body weight of untreated animals divided by the average kidney weight per body weight of day-7 left kidneys. Percent hypertrophy was calculated as % hypertrophy = [(RKfinal − RKintact)/RKintact] × 100, where RKfinal was the weight (mg) of right kidney at sacrifice, and RKintact is LKadj − RKremoved.
Statistical Analysis.
Results are presented as mean ± SE. Differences between means were evaluated by using Student’s t test for unpaired data. P < 0.05 was considered statistically significant.
RESULTS
Body Weight and Renal Parameters Before Ablation.
Body weight, kidney weight, glomerular number and volume, and renal function in untreated p21(+/+) and p21(−/−) mice are given in Table 1. There were no phenotypic differences between the two groups of mice, although the untreated p21(−/−) animals were about 15% (P < 0.001) larger than those in the p21(+/+) group. Size increases have also been reported in mice lacking the p27 cyclin-dependent kinase inhibitor genes (20–23). However, kidney weight per gram body weight, total glomerular number, and mean glomerular volume were not different between the two genotypes. Similarly, the two-kidney glomerular filtration rate, expressed as Cinulin of the untreated animals, were not different.
Table 1.
Physical parameters in untreated mice: Body weight, kidney weight, glomerular number, glomerular volume, and GFR
| Body weight, g | Kidney weight, mg/g body weight | Glomeruli per kidney, no. | Mean glomerular volume × 10−5, μm3 | Cinulin, ml/min | |
|---|---|---|---|---|---|
| p21(+/+) | 24.35 ± 2.68 | 5.938 ± 0.656 | 12,583 ± 681 | 1.92 ± 0.58 | 1.088 ± 0.072 |
| p21(−/−) | 28.47 ± 4.18 | 5.720 ± 0.607 | 12,091 ± 555 | 1.74 ± 0.14 | 1.050 ± 0.076 |
| P value | <0.001 | NS | NS | NS | NS |
Values are means ± SD. NS, not significant.
Body Weight, Degree of Ablation, Remnant Hypertrophy, and Mean Glomerular Volume After Ablation.
Weight gain in renal ablated mice throughout the 14- to 16-week period of observation was not significantly different between the two groups, either in absolute terms (2.3 ± 0.8 g vs. 4.3 ± 1.1 g; +/+ vs. −/− groups, respectively; n = 11 in each group) or relative to initial body weight (10.2 ± 3.5% vs. 15.9 ± 4.3%; +/+ vs. −/− groups, respectively). The degree of renal ablation was determined for each genotype. Approximately 2/3 of the normal renal mass was removed after the two operations, and there was no significant difference between the groups. The percent nephrectomy in the p21(+/+) and p21(−/−) groups was 68.8 ± 3.6% and 68.3 ± 3.1% (P = 0.619), respectively. Furthermore, the degree of hypertrophy and the mean glomerular volume after ablation (Table 2) were not significantly different between the groups.
Table 2.
Percent hypertrophy and mean glomerular volume after renal ablation
| Weeks | Hypertrophy
|
Mean glomerular volume
|
||||
|---|---|---|---|---|---|---|
| p21(+/+) | p21(−/−) | t test | p21(+/+) | p21(−/−) | t test | |
| 0 | NA | NA | NA | 1.92 ± 0.58 | 1.74 ± 0.14 | NS |
| 2–4 | 66.9 ± 28.6 | 83.9 ± 85.7 | NS | 1.93 ± 0.32 | 2.58 ± 0.71 | NS |
| 6–8 | 86.8 ± 41.5 | 138.5 ± 55.8 | NS | 2.71 ± 0.33 | 2.99 ± 0.34 | NS |
| 10–12 | 137.1 ± 88.7 | 141.4 ± 31.3 | NS | 3.52 ± 0.21 | 3.34 ± 0.37 | NS |
| 14–16 | 145.0 ± 37.0 | 135.9 ± 42.1 | NS | 2.87 ± 0.50 | 3.17 ± 0.38 | NS |
Values are mean ± SD. NS, not significant; NA, not applicable.
Renal Function After Ablation.
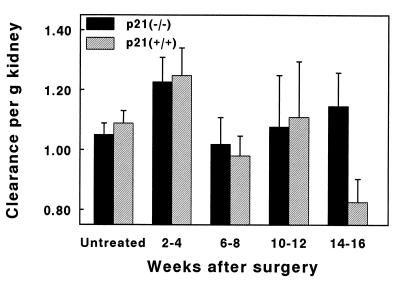
GFR increased to the same extent 2–4 weeks after ablation in both groups (Fig. 1). GFR was similar in both groups until the 14th–16th week after ablation when it fell in the wild-type animals but remained unchanged from previous values in the p21(−/−) group. The GFR at this time point was significantly different between the two groups (P < 0.05).
Figure 1.
Renal function after ablation. Clearance of inulin (ml per minute) was calculated per gram kidney in mice from both genotypes. Statistically significant differences were only noted between the two populations at 14–16 weeks after ablation (P = 0.04). Values represent mean ± SE.
Mean Arterial Pressure.
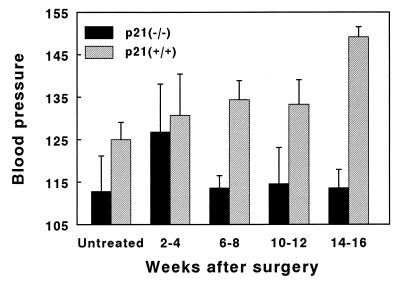
Mean arterial pressure was not significantly different between the untreated groups of animals (Fig. 2). After partial renal ablation, arterial pressure increased initially in both groups of animals and increased further in the p21(+/+) mice so that by the 14th–16th week, the average mean systolic pressure reached 150.7 ± 6.7 mmHg (mean ± SD). By contrast, mean systolic blood pressure in the p21(−/−) mice returned toward normal and remained there throughout the 16-week period of observation (113.8 ± 17.7 after 16 weeks versus 112.8 ± 16.7 in untreated mice).
Figure 2.
Mean arterial pressure. Mean systolic blood pressure was obtained by catheterizing the left femoral artery. Statistically significant differences between the two populations was noted as early as 6–8 weeks after ablation (P = 0.005), which increased by 14–16 weeks after ablation (P = 0.00002). Values represent mean ± SE.
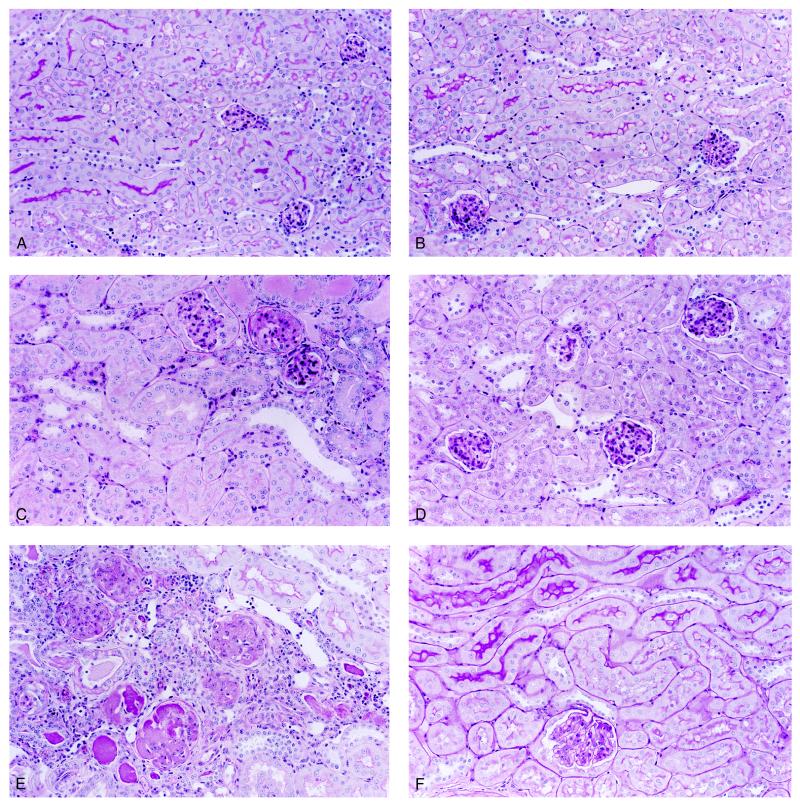
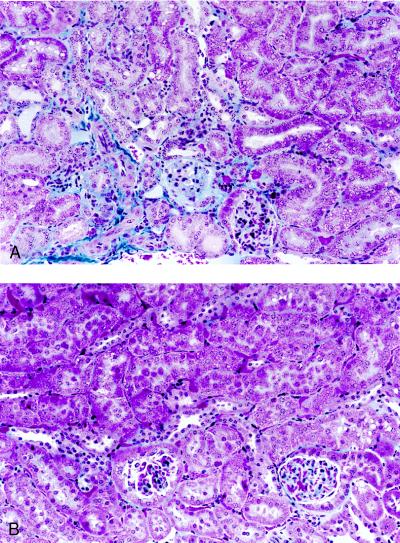
Morphology. Light microscopic study revealed a marked difference of histologic changes between the two groups of mice. Representative micrographs are shown in Figs. 3 and 4; the changes were quantified in Table 3. Kidney sections from untreated mice were morphologically indistinguishable (Fig. 3A and B). Mesangial expansion and mild focal glomerulosclerosis was observed in ≈70% of glomeruli in the p21(+/+) mice 4 weeks after ablation (Table 3). Beginning at 6–8 weeks, these mice developed severe focal and global glomerulosclerosis (Figs. 3C vs. D and 4A, Table 3). All of the p21(+/+) mice studied developed glomerulosclerosis accompanied by interstitial fibrosis and round cell infiltration by 14–16 weeks postablation (Fig. 3E, Table 3). In contrast, p21(−/−) mice never developed glomerulosclerosis nor interstitial changes even 26 weeks after renal ablation (Figs. 3F and 4B), although mesangial expansion was seen occasionally.
Figure 3.
Histologic changes in remnant kidney after ablation. Representative sections from either untreated (A and B), 8 weeks (C and D), 16 weeks (E), or 26 weeks (F) after ablation of wild-type (A, C, and E) or p21(−/−) (B, D, and F) mice. Magnification, ×390. Sections were stained with periodic acid/Schiff reagent.
Figure 4.
Detection of interstitial fibrosis by using trichrome stain in remnant kidney after ablation. Representative sections from either 6 weeks (A) or 26 weeks (B) after ablation of wild-type (A) or p21(−/−) (B) mice. Magnification, ×390.
Table 3.
Development of glomerulosclerosis in p21(+/+) mice
| Weeks | Glomerulosclerosis
|
||||
|---|---|---|---|---|---|
| None | Minimal | Moderate | Severe | Global | |
| 4 | 30.7 ± 0.9 | 65.8 ± 1.6 | 2.8 ± 1.7 | 0.8 ± 0.7 | 0 |
| 6–8 | 27.8 ± 5.1 | 41.0 ± 2.7 | 22.2 ± 3.5 | 4.9 ± 1.8 | 4.2 ± 3.5 |
| 10–12 | 15.3 ± 3.2 | 38.2 ± 7.8 | 25.2 ± 3.5 | 10.2 ± 3.3 | 11.1 ± 6.8 |
| 14–16 | 23.6 ± 2.5 | 22.4 ± 4.4 | 36.5 ± 4.1 | 9.5 ± 1.9 | 8.1 ± 3.9 |
Percent glomeruli in each category (±SE) as defined in Materials and Methods.
The percentages of glomerulosclerosis in the p21(+/+) mice at various times after ablation are quantified in Table 3. It can be seen that they developed a progressive increase in glomerular sclerosis. The p21(−/−) mice do not develop glomerulosclerosis throughout the period of observation and were omitted from Table 3.
Expression of p21 in the Remnant Kidney.
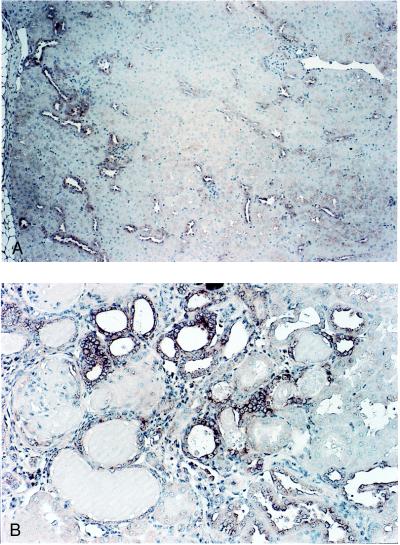
In situ hybridization (Fig. 5) for p21 mRNA identified the cells of the cortical thick ascending limbs and distal convoluted tubules as the principal site of p21 expression 4 weeks after ablation (Fig. 5A). At later times, p21 was also expressed in the epithelium of tubules (primarily dilated and collapsed) and glomeruli within or adjacent to sclerotic areas of the remnant kidney (Fig. 5B).
Figure 5.
In situ hybridization for localization of p21 mRNA in remnant kidney cells after partial renal ablation. Hybridization of an antisense p21 probe to RNA in cells of remnant kidney 4 weeks (A) and 14 weeks (B) after ablation. Magnification, ×390.
Cell Cycle Analysis.
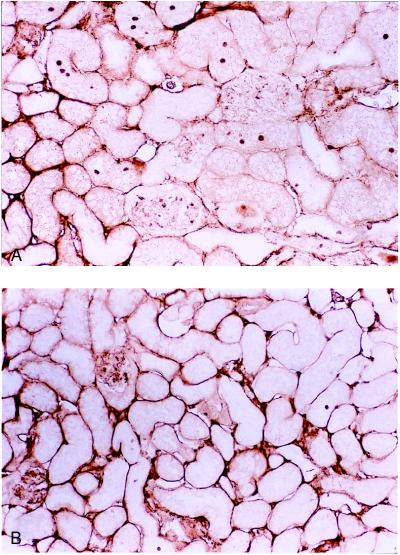
Nuclear PCNA, a marker for cells in the S phase of the cell cycle, was found in many cells of the remnant kidney in the p21(−/−) mice 2 weeks after surgery (Fig. 6A). The positive nuclei were primarily localized in the proximal convoluted tubules and occasionally in the glomeruli and distal convoluted tubules. By contrast, few cell nuclei were stained in the p21(+/+) remnant kidney (Fig. 6B). This difference in PCNA staining was quantified in nuclei from p21 (−/−) mice (18.64 ± 0.73 per mm2) and p21(+/+) mice (3.50 ± 0.65 per mm2) and was highly significant (P = 0.00006). At later time points, PCNA was greatly diminished in both animals (data not shown).
Figure 6.
Cell cycle analysis in remnant kidney cells after partial renal ablation. Immunodetection of nuclear PCNA localization 2 weeks after ablation in kidney sections from p21(−/−) (A) and wild-type (B) mice. Magnification, ×390.
DISCUSSION
These studies show that mice lacking a p21 gene are resistant to the functional and morphologic consequences of partial renal ablation. Not only is the resistance manifested locally in the surgically impaired remnant organ, but it is also evident systemically in the lack of increased arterial pressure. In considering the reasons for this resistance, we evaluated several parameters speculated to be early determinants of the long-term outcome of renal ablation. Severe protein restriction can partially ameliorate the development of glomerulosclerosis after partial renal ablation (24). However, weight gains in the two groups of animals were not significantly different, and the p21(−/−) mice even experienced slightly elevated gains, both relative and absolute. Reduced glomerular number is thought to be an etiologic link in the progressive nature of renal disease (25, 26). The p21(+/+) and p21(−/−) animals had similar numbers of glomeruli at the outset of the experiments (Table 1), and the degree of renal ablation was the same for each group. Thus, the loss of renal excretory function was equally applied to both groups. The increase in glomerular filtration that occurs in response to renal ablation, also thought to be an early determinant of the progression (4), occurred to the same extent in the p21(−/−) animals as it did in the wild type (Fig. 1). Glomerular hypertrophy, which has an independent role in the progression of renal ablation models of experimental renal disease (27), occurred to the same extent in both groups as well (Table 2).
Taken together, these observations are most consistent with a critical role of the p21 gene product in the functional and morphologic consequences subsequent to the stress of renal ablation, including the development of glomerular sclerosis and hypertension. The data also show that hypertension does not develop without the development of renal damage. We speculate that this resistance is critically linked to the prominent role the p21 protein plays in regulating the cell cycle. The growth of the kidney after renal ablation is a consequence of hyperplasia and hypertrophy of the glomerular and epithelial compartments of the kidney (28, 29). However, hypertrophy may be, in the long term, a maladaptive response to the loss of functional renal tissue (4, 27, 30). In the absence of the p21 gene, the growth response of the kidney after partial ablation is relatively more hyperplastic than hypertrophic. Consistent with this notion is a >5-fold increase in PCNA protein expression in p21(−/−) animals compared with the wild-type animals undergoing the response to renal ablation. By achieving growth after renal ablation by increasing the relative contribution of hyperplasia, the workload of the kidney is better accommodated. This proposal, first expressed by Goss (31), assumes that when an organ accommodates increases in work by hypertrophy rather than hyperplasia, it is at a serious physiologic disadvantage and is more likely to undergo regression of structure and function. Confirmation of this hypothesis must await a detailed description of the differences in the balance between hypertrophy and hyperplasia in the two groups of mice and, more specifically, the sites at which these differences are apparent. Regardless of its precise role, it is clear that p21 is a critical sensor of the stress of renal mass reduction. This model should be useful in identifying the mechanism of how this response to renal ablation is maladaptive. The studies also suggest that manipulation of p21 gene expression could be a target for the treatment of progressive renal failure.
Acknowledgments
We thank Philip Leder (Harvard Medical School) for providing several heterozygous mice carrying the p21 gene deletion and for providing a probe for screening and Bert Vogelstein (Johns Hopkins Oncology Center) for cloned mouse p21 cDNA. We also thank Thomas Andreoli (University of Arkansas) for his comments on the manuscript. The authors are supported in part by a grant from the National Institutes of Health (R01 DK54471).
ABBREVIATIONS
- PCNA
proliferating-cell nuclear antigen
- GFR
glomerular filtration rate
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
A Commentary on this article begins on page 10551.
References
- 1.Chanutin A, Ferris E B., Jr Arch Intern Med. 1932;49:767–787. [Google Scholar]
- 2.Morrison A B. Lab Invest. 1962;11:321–332. [PubMed] [Google Scholar]
- 3.Faraj A H, Morley A R. APMIS. 1992;100:1097–1105. doi: 10.1111/j.1699-0463.1992.tb04046.x. [DOI] [PubMed] [Google Scholar]
- 4.Daniels B S, Hostetter T H. Am J Physiol. 1990;258:F1409–F1416. doi: 10.1152/ajprenal.1990.258.5.F1409. [DOI] [PubMed] [Google Scholar]
- 5.Megyesi J, Udvarhelyi N, Safirstein R L, Price P M. Am J Physiol. 1996;271:F1211–F1216. doi: 10.1152/ajprenal.1996.271.6.F1211. [DOI] [PubMed] [Google Scholar]
- 6.Gorospe M, Martindale J L, Sheikh M S, Fornace A J, Jr, Holbrook N J. Mol Cell Differ. 1996;4:47–65. [Google Scholar]
- 7.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 8.Xiong Y, Zhang H, Beach D. Cell. 1992;71:505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- 9.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 10.Noda A, Ning Y, Venable S F, Pereira-Smith O M, Smith J R. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 11.Steinman R A, Hoffman B, Iro A, Guillouf C, Liebermann D A, el-Houseini M E. Oncogene. 1994;9:3389–3396. [PubMed] [Google Scholar]
- 12.Jiang H, Lin J, Su Z Z, Collart F R, Huberman E, Fisher P B. Oncogene. 1994;9:3397–3406. [PubMed] [Google Scholar]
- 13.Megyesi J, Safirstein R L, Price P M. J Clin Invest. 1997;101:777–782. doi: 10.1172/JCI1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 15.Hamamori Y, Samal B, Tian J, Kedes L. J Clin Invest. 1995;95:1808–1813. doi: 10.1172/JCI117859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nath K A, Salahudeen A K. J Clin Invest. 1990;86:1179–1192. doi: 10.1172/JCI114824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirose K, Østerby R, Nozawa M, Gundersen H J G. Kidney Int. 1982;21:689–695. doi: 10.1038/ki.1982.82. [DOI] [PubMed] [Google Scholar]
- 18.Bilous R W, Mauer S M, Sutherland D E R, Steffes M W. Diabetes. 1989;38:1142–1147. doi: 10.2337/diab.38.9.1142. [DOI] [PubMed] [Google Scholar]
- 19.MacKay K, Striker L J, Pinkert C A, Brinster R L, Striker G E. Kidney Int. 1987;32:827–837. doi: 10.1038/ki.1987.283. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh D Y, Nakayama K-i. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 21.Kiyokawa H, Kineman R D, Manova-Todorova K O, Soares V C, Hoffman E S, Ono M, Khanam D, Hayday A C, Frohman L A, Koff A. Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 22.Fero M L, Rivkin M, Tasch M, Porter P, Carow C E, Firpo E, Polyak K, Tsai L-H, Broudy V, Perlmutter R M, et al. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 23.Franklin D S, Godfrey V L, Lee H, Kovalev G I, Schoonhoven R, Chen-Kiang S, Su L, Xiong Y. Genes Dev. 1998;12:2899–2911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenner B M, Meyer T W, Hostetter T H. N Engl J Med. 1982;307:652–660. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- 25.Brenner B M, Garcia D L, Anderson S. Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 26.Fetterman G H, Habib R. Am J Clin Path. 1969;52:199–207. [Google Scholar]
- 27.Yoshida Y, Fogo A, Ichikawa I. Kidney Int. 1989;35:654–660. doi: 10.1038/ki.1989.35. [DOI] [PubMed] [Google Scholar]
- 28.Terzi F, Ticozzi C, Burtin M, Motel V, Beaufils H, Laouari D, Assael B M, Kleinknecht C. Am J Physiol. 1995;268:F793–F801. doi: 10.1152/ajprenal.1995.268.5.F793. [DOI] [PubMed] [Google Scholar]
- 29.Floege J, Burns M W, Alpers C E, Yoshimura A, Pritzl P, Gordon K, Seifert R A, Bowen-Pope D F, Couser W G, Johnson R J. Kidney Int. 1992;41:297–309. doi: 10.1038/ki.1992.42. [DOI] [PubMed] [Google Scholar]
- 30.Fries J W U, Sandstrom D J, Meyer T W, Rennke H G. Lab Invest. 1989;60:205–218. [PubMed] [Google Scholar]
- 31.Goss R J. Science. 1966;153:1615–1620. doi: 10.1126/science.153.3744.1615. [DOI] [PubMed] [Google Scholar]