Abstract
An in situ flow cytometric viability assay employing carboxyfluorescein diacetate and propidium iodide was used to identify Streptococcus macedonicus acid tolerance phenotypes. The logarithmic-phase acid tolerance response (L-ATR) was evident when cells were (i) left to autoacidify unbuffered medium, (ii) transiently exposed to nonlethal acidic pH, or (iii) systematically grown under suboptimal acidic conditions (acid habituation). Stationary-phase ATR was also detected; this phenotype was gradually degenerated while cells resided at this phase. Single-cell analysis of S. macedonicus during induction of L-ATR revealed heterogeneity in both the ability and the rate of tolerance acquisition within clonal populations. L-ATR was found to be partially dependent on de novo protein synthesis and compositional changes of the cell envelope. Interestingly, acid-habituated cells were interlaced in lengthier chains and exhibited an irregular pattern of active peptidoglycan biosynthesis sites when probed with BODIPY FL vancomycin. L-ATR caused cells to retain their membrane potential after lethal challenge, as judged by ratiometric analysis with oxonol [DiBAC4(3)]. Furthermore, F-ATPase was important during the induction of L-ATR, but in the case of a fully launched response, inhibition of F-ATPase affected acid resistance only partially. Activities of both F-ATPase and the glucose-specific phosphoenolpyruvate-dependent phosphotransferase system were increased after L-ATR induction, distinguishing S. macedonicus from oral streptococci. Finally, the in situ viability assessment was compared to medium-based recovery after single-cell sorting, revealing that the culturability of subpopulations with identical fluorescence characteristics is dependent on the treatments imposed to the cells prior to acid challenge.
The existence of common strategies employed by gram-positive bacteria, particularly lactic acid bacteria, in response to acid challenge has emerged only recently (14, 53). However, generalizations about specific mechanisms involved in such responses could prove premature, since it has been shown that these mechanisms can be species or even subspecies specific (14). A typical example relevant to this report is the current knowledge about streptococcal responses to acid stress. Linkages of acid production (acidogenicity) or tolerance (aciduricity) with pathogenicity have fueled recent interest in the basic physiology of oral streptococci (44). Today, Streptococcus mutans is probably the best-studied Streptococcus species in terms of acid stress physiology, but it is uncertain whether the behavior of this species is truly representative of the genus. For instance, a recent report on S. sobrinus revealed key differences from S. mutans concerning the involvement of F-ATPase and glucose-specific phosphoenolpyruvate-phosphotransferase system (PEP-PTS) activities in the manifestation of acid adaptation (35). Additionally, comparison of the recently completed genome sequence of S. thermophilus with those of other streptococci showed the inactivation or absence of genes involved in virulence, both of which were attributed to its adaptation to the milk environment (4). Therefore, more detailed studies of the acid stress physiology of food-related lactic acid streptococci are needed.
S. macedonicus was first isolated from traditional Greek Kasseri cheese (51), and since then it has been shown to participate in the fermenting floras of different traditional dairy products (10, 18, 43). S. macedonicus ACA-DC 198 exhibits antimicrobial activity against a number of important food spoilage and pathogenic bacteria, due to the production of a bacteriocin peptide named macedocin (23). In the quest for new biopreservatives, S. macedonicus is a promising candidate to be used as a protective culture (5). In parallel to practical application research, we wish to unravel the basic physiology of the bacterium in order to rationally incorporate it into processes.
We previously adapted an in situ viability assay for S. macedonicus, combining carboxyfluorescein diacetate (cFDA) and propidium iodide (PI) as viability markers (41). The cFDA-PI two-color flow cytometric assay has been applied for the study of a number of lactic acid bacterial species under thermal (52), bile salt (2), and ethanol (25) stress. The advantages of technologies that assess populations at the single-cell level over traditional culture-based techniques have been described clearly (9, 16, 48). Even though such approaches can reveal the heterogenic behavior of clonal populations—an important piece of information, especially for stress physiology (6)—their application is still far less than common. One of the major drawbacks in applying flow cytometry to bacterial populations is the natural clumping of cells (36). To overcome this problem, S. macedonicus cell chains were disaggregated by a mild sonication procedure prior to flow cytometric analysis, ensuring the assessment of the physiological status on a cell-by-cell basis, as we have described before (41).
The aim of this study was to assess the acid stress physiology of S. macedonicus by using dynamic cell staining techniques with different fluorochromes and flow cytometry. The main objective for implementing this approach was to determine the contribution of S. macedonicus intrapopulation heterogeneity to the overall behavior of the bacterial population. In a first step, we evaluated the ability of S. macedonicus to acquire acid tolerance (AT) after various treatments. In a second step, we attempted to reveal central cellular processes involved in the mechanism of acid tolerance. Finally, we wanted to correlate cells' fluorescence labeling status with culturability by examining medium-based recovery after single-cell sorting. Notably, this is one of very few studies concerning a food-related lactic Streptococcus species under acid stress conditions.
MATERIALS AND METHODS
Microorganism and growth conditions.
The gram-positive bacterium S. macedonicus strain ACA-DC 198 was isolated from naturally fermented Greek Kasseri cheese. The strain was grown at 37°C in MRS broth (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom) supplemented with 200 mM morpholinepropanesulfonic acid (MOPS) and adjusted to pH 7.0 with 5 N NaOH (MRS-MOPS). MOPS has been employed previously to maintain neutral pH in batch-type cultures for the study of acid tolerance (26, 45). In this medium, S. macedonicus exhibited better growth with the pH of the culture remaining above 6.8 until late logarithmic phase (data not shown) than in unbuffered MRS adjusted to pH 7.0 or in M17 medium. Stock cultures were maintained on Preserver beads (Technical Service Consultants, Heywood, Lancashire, United Kingdom) at −80°C.
AT phenotypes and acidic lethal challenge.
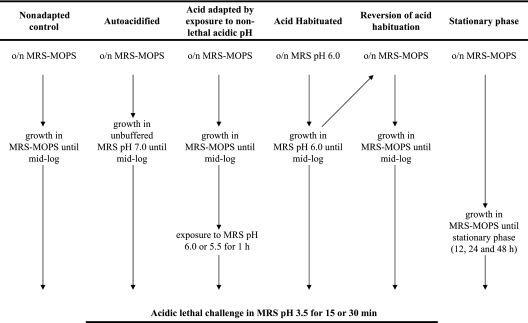
Different treatments of S. macedonicus were performed in order to reveal possible AT phenotypes (Fig. 1). For induction of the logarithmic-phase acid tolerance response (L-ATR), cells which were subcultured in MRS-MOPS were inoculated into fresh MRS-MOPS, grown until mid-log phase (approximately 109 cells; optical density [OD], ∼0.6), recovered by centrifugation, and transiently exposed in MRS, pH 6.0 or 5.5, for 1 h at 37°C. Alternatively, cells were inoculated in unbuffered MRS, pH 7.0, and left to progressively acidify the medium through glycolysis until mid-log phase (approximately 109 cells; OD, ∼0.6). For acid habituation, both subculture and final growth until mid-log phase were carried out in MRS, pH 6.0 (approximately 5 × 108 cells; OD, ∼0.5). Furthermore, acid-habituated cells were subcultured twice in MRS-MOPS, and mid-log-phase cells of the second subculture were assessed for possible retention of L-ATR. To investigate stationary-phase ATR, cells were obtained after an overnight subculture and final growth for 12, 24, and 48 h in MRS-MOPS (approximately 1010 cells; OD, ∼1.2).
FIG. 1.
Experimental design for the study of the S. macedonicus acid tolerance response. The sequence of subcultures and incubation conditions that led to nonadapted cells, autoacidified cells, acid-adapted cells (by transient exposure to nonlethal acidic pH), acid-habituated cells, cells reversed from acid habituation, and stationary-phase cells is presented schematically. o/n, overnight.
In all cases, untreated and treated cells were harvested by centrifugation and resuspended in MRS, pH 3.5 (adjusted with HCl). Samples of 100 μl were removed aseptically after 15 or 30 min (lethal challenge), instantly diluted in 900 μl of buffered peptone water, pH 7.2 (Merck, Darmstadt, Germany), to sharply stop the effect of the acidic environment, centrifuged, and resuspended in 100 μl phosphate-buffered saline (PBS), pH 7.0, for subsequent labeling with fluorochromes.
Treatment with inhibitors during induction of L-ATR.
Antibiotics (purchased from Sigma, St. Louis, MO) were used at the following final concentrations: 100 μg/ml chloramphenicol, 40 μg/ml actinomycin D, 40 μg/ml cerulenin, 10 μg/ml vancomycin, 10 μg/ml penicillin, and 0.2 or 0.4 mM N,N′-dicyclohexylcarbodiimide (DCCD) (see below). Typically, antibiotics were added to the culture 20 min prior to and for the 30 min of acid adaptation at pH 5.5. DCCD was added at a low concentration (0.2 mM) 20 min prior to and for the 60 min of acid adaptation at pH 5.5 or at a high concentration (0.4 mM) during only the last 40 min of acid adaptation performed under the same conditions. All samples were challenged at pH 3.5 for 15 min. Final concentrations of antibiotics were higher than the MICs since cells were exposed to the inhibitors for relatively short periods of time. In all cases, control unchallenged cultures were examined and it was ensured that none of the antibiotics exhibited any bactericidal effect on its own during the treatment.
F-ATPase and glucose-specific PEP-PTS assays.
F-ATPase and glucose-specific PEP-PTS activities were assayed in permeabilized cells following protocols previously described for other streptococcal species (21, 42). In detail, 40 ml of culture to be assayed was washed once and resuspended in 1.8 ml membrane buffer (75 mM Tris, pH 7.0, 10 mM MgSO4). Toluene was added to a concentration of 10% (vol/vol), and the suspension was vortexed for 30 s and subjected to two rounds of freeze-thawing. Cells were collected, resuspended in 1 ml membrane buffer, and aliquoted in 100-μl samples. F-ATPase activity was assayed in terms of the release of inorganic phosphate in 50 mM Tris-maleate buffer, pH 7.0, containing 10 mM MgSO4 and 50 μM ATP. Phosphate was assayed by the malachite green method, using a commercially available kit (R&D Systems, Inc., Minneapolis, MN). Glucose-specific PEP-PTS activity of permeabilized cells was assayed by the reaction of NADH in the presence of lactate dehydrogenase with pyruvate that was produced from PEP in response to the addition of glucose. Permeabilized cells were incubated in 100 mM Tris-maleate buffer, pH 7.0, 20 mM MgCl2, 1 mM NaF, 40 mM glucose, and 5 mM PEP for 30 min. Two hundred microliters of cleared supernatant at 0 and 30 min was removed and mixed with 300 μl of double-distilled water. Five hundred microliters of NADH solution (0.21 mg/ml NADH, 1.5 M Tris, pH 7.0, 0.021% NaN3) and 3 U of lactate dehydrogenase were added to the mixture. The utilization of NADH was recorded at 340 nm. The difference in the amounts of NADH between time zero and 30 min reflects the amount of glucose phosphorylated. In both F-ATPase and glucose-specific PEP-PTS assays, total protein was assayed by the Bradford method (Bio-Rad Laboratories, Inc., Munich, Germany).
Fluorescent probes.
cFDA, PI, and oxonol [DiBAC4(3)] were purchased from Sigma (St. Louis, MO), while BODIPY FL vancomycin was purchased from Molecular Probes Inc. (Eugene, OR).
In situ assessment of the viable status of S. macedonicus cells by cFDA-PI double staining.
Labeling of S. macedonicus with cFDA and PI has been described previously (41). Briefly, 107 to 108 cells in 500 μl PBS, pH 7.0, were probed with final concentrations of 25 μM cFDA and 5 μg/ml PI for 45 and 10 min, respectively, at 37°C. Cells were washed once to discard residual fluorochromes and resuspended in an equal volume of PBS, pH 7.0. Samples were further processed in a sonicator bath (Transsonic T460; Elma GmbH, Siengen, Germany) filled with water at room temperature. Sonication was performed for 3 min with 1-min intervals in order to disaggregate S. macedonicus chains into single-cell preparations. Untreated and heat-killed (80°C, 30 min) mid-log-phase cells were used as controls.
DiBAC4(3) staining for ratiometric analysis of MP.
Ratiometric analysis of membrane potential (MP) measurements was adapted from a protocol designed for bifidobacteria (2), since DiBAC4(3) fluorescence for S. macedonicus correlated linearly with side scatter intensity (data not shown). One milliliter of nonadapted or acid-adapted (at pH 5.5 for 30 min) mid-log-phase cells, after lethal challenge (at pH 3.5 for 15 min), was resuspended in an equal volume of PBS, pH 7.0, and probed with a 0.5 μΜ final concentration of DiBAC4(3) for 15 min at 37°C. Apart from live and dead cell controls, depolarized mid-log-phase cells prepared by the addition of 40 μg/ml gramicidin for the last 15 min of culture growth were analyzed.
Labeling of active cell wall biosynthesis sites with BODIPY FL vancomycin.
Nonadapted, acid-adapted (at pH 5.5 for 1 h), and acid-habituated S. macedonicus mid-log-phase cells (100 μl) were incubated for the last 15 min before being harvested with 1 μg/ml (final concentration) of BODIPY FL vancomycin mixed with an equal quantity of unlabeled vancomycin as previously described (15). After two washes in PBS, samples were either analyzed by flow cytometry or mounted onto glass slides and directly observed under an MRC 1024 confocal laser scanning microscope (CLSM) (Bio-Rad Laboratories, Inc., Hercules, CA) with the appropriate excitation and emission filters for BODIPY FL vancomycin. To verify that BODIPY FL vancomycin specifically stained active cell wall biosynthesis sites for S. macedonicus, the ability of the fluorochrome to label nondividing stationary-phase cells was also assessed.
Flow cytometry.
A FACSCalibur instrument (Becton Dickinson, San Jose, CA) was used for flow cytometric analysis. The instrument is equipped with an argon ion laser for the excitation of the fluorescent dyes, providing 15 mW at 488 nm. The filter setup was standard. All parameters were collected at logarithmic scale. cFDA, DiBAC4(3), and BODIPY FL vancomycin fluorescent emissions were detected in the FL1 channel (530 ± 15 nm), while PI emission was detected in the FL2 channel (584 ± 21 nm). FACSflow solution (Becton Dickinson) was used as sheath fluid. The flow rate and cell concentration of the samples were adjusted to keep acquisition lower than 500 cells/s in order to avoid coincidence. At least 10,000 cells were acquired for analysis. Data were collected with the CELLQuest program (version 3.1; Becton Dickinson) and further analyzed with the WinMDI program (version 2.8; Joseph Trotter, Salk Institute for Biological Studies, La Jolla, CA [available at http://facs.Scripps.edu/software.html]). Ratiometric analysis of green fluorescence versus side scatter of DiBAC4(3)-stained samples was performed with FCSPress software (Ray Hiks, Department of Medicine, University of Cambridge, Cambridge, United Kingdom). Mixtures of stained and unstained cells for both cFDA and PI served as controls for adjusting detectors and compensation settings for the two-color analysis.
Cell sorting.
Cell sorting was performed with an EPICS Elite flow cytometer (Beckman-Coulter, High Wycombe, United Kingdom) equipped with an Autoclone unit, using EPICS Elite flow cytometry workstation software, version 4.5. The instrument was operated using an air-cooled argon ion laser at 488 nm and 15 mW, with a confocal beam shaper (15- by 60-μm spot size) and a 76-μm sort-sense flow cell running at 15 lb/in2 sheath pressure and a droplet frequency of 38 kHz. Signals were discriminated based on their peak forward scatter signal and sorted based on a region of interest around their light scatter cluster, together with either red-only (PI), red and green (cFDA and PI), or green-only (cFDA) fluorescence. The discriminator was set just above instrument noise on peak narrow forward angle light scatter for a noise background of <50 events per second on a filtered sheath sample. Samples were sorted at <500 cells/s, with the coincidence abort set on, using a three-drop envelope for low coincidence and maximum recovery. Nonadapted and acid-adapted (at pH 5.5 for 30 min) S. macedonicus mid-log-phase cells were exposed to pH 3.5 for 15 min, stained with cFDA and PI, and analyzed by flow cytometry. From each subpopulation, at least 100 cells were sorted on specific points on MRS-MOPS, MRS (pH 6.0), MRS (pH 5.5), and 1% NaCl-MRS-MOPS agar plates, which then were incubated for 24 h at 37°C before colony counting. Cells were sorted from three independent experiments.
Statistical analysis.
All data presented are the results of at least three independent experiments. Differences in percentages of cFDA- or PI-positive subpopulations deriving from S. macedonicus cultures after acidic lethal challenge were detected by analysis of variance. Whenever necessary, data were transformed logarithmically to ensure the homogeneity of variance and the normality of distributions. In all cases, the nonparametric Kruskal-Wallis test was performed to confirm the analysis of variance results. Means of population percentages were compared by Tukey's test, with an alpha level of 0.05. Percentages of recovery of cFDA+ PI− and cFDA+ PI+ cells were analyzed by binomial comparison of proportions, with P values of <0.05 considered significant. All analyses were performed with Statgraphics Centurion software (version XV).
RESULTS
AT phenotypes of S. macedonicus as assessed by flow cytometry.
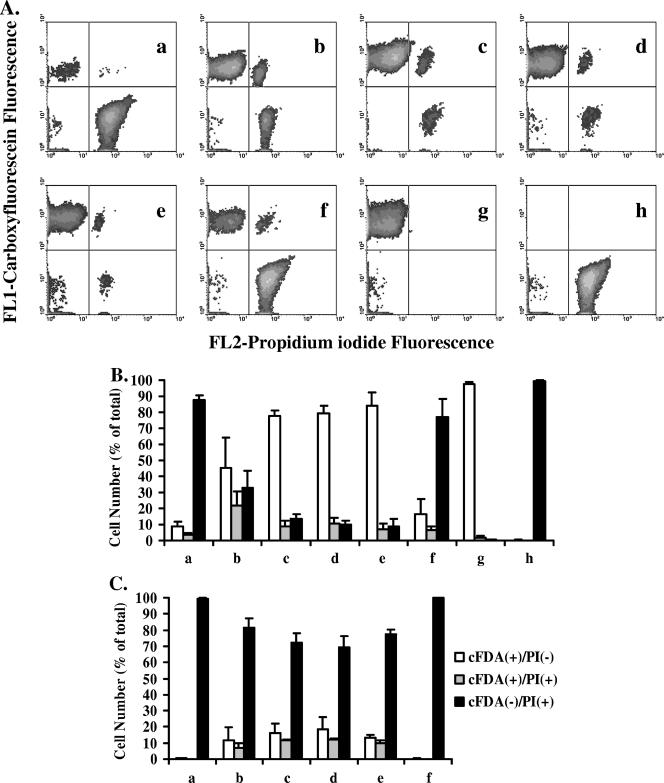
The ability of S. macedonicus to develop ATR was investigated. Control mid-log-phase cells grown in MRS-MOPS as well as mid-log-phase cells which were (i) transiently exposed to MRS, pH 6.0 or 5.5, for 1 h prior to lethal challenge, (ii) autoacidified in unbuffered MRS, pH 7.0 (pH 5.8 ± 0.2 at the time of sampling), or (iii) acid habituated (AH) in MRS, pH 6.0 (pH 5.3 ± 0.1 at the time of sampling), were exposed to MRS, pH 3.5, for 15 min. Unstressed and heat-killed mid-log-phase cells were always analyzed in parallel as additional controls. The differences in the patterns of fluorescent subpopulations after lethal challenge were compared in order to elucidate possible induction of L-ATR (Fig. 2A). AT would be expected to increase the percentage of the cFDA+ PI− (viable) and/or cFDA+ PI+ (injured) subpopulation, accompanied by a decrease of the percentage of the cFDA− PI+ (dead) subpopulation after treatment, in comparison to the control (25). Indeed, all treatments resulted in protection of S. macedonicus against lethal challenge (Tukey's test; α = 0.05) (Fig. 2B). L-ATR phenotypes acquired after transient exposure to nonlethal acidic pH (pH 6.0 or pH 5.5) and acid habituation resulted in the highest level of (but equal among them) protection, while autoacidification protected S. macedonicus cells to a lesser extent (Tukey's test; α = 0.05). Increasing the time of lethal challenge from 15 to 30 min revealed that all treatments protected S. macedonicus to the same extent (Tukey's test; α = 0.05) (Fig. 2C). Further exposure of the cells at pH 3.5 for 45 min resulted in all samples being almost 100% dead by cytometry (data not shown), indicating that none of the treatments could lead to any rescue of S. macedonicus, at least for the detection limits of the analysis. In order to investigate whether acid habituation of S. macedonicus in MRS, pH 6.0, led to the selection of acid-resistant mutants, cells of this culture were regrown in MRS-MOPS and challenged for 15, 30, and 45 min at pH 3.5. The results demonstrate that the acid resistance phenotype was reversed, since all three subpopulations for all time points were statistically indistinguishable from those of the untreated control (Fig. 2), suggesting an epigenetic nature for this phenotype (Tukey's test; α = 0.05).
FIG. 2.
(A) Flow cytometric analysis of S. macedonicus acid tolerance at mid-log phase. Nonadapted (a) or acid-adapted (b to f) cells, after exposure to pH 3.5 for 15 min, were labeled with cFDA and PI and analyzed by flow cytometry. Prior to acid challenge, bacteria were adapted either by autoacidification (b), transient exposure to pH 6.0 and 5.5 (c and d), or acid habituation at pH 6.0 (e). Acid habituation was also reversed after regrowth of habituated cells in MRS-MOPS (f). As additional controls, unstressed (g) and heat-killed (h) mid-log-phase cells were analyzed in parallel. (B) Percentages (means ± standard deviations) of fluorescent subpopulations of the samples presented in panel A. (C) Percentages (means plus standard deviations) of fluorescent subpopulations of samples challenged at pH 3.5 for 30 min in the same order as that presented in panel A.
The ability of S. macedonicus cells to mount a stationary-phase ATR was then assessed (Table 1). Unchallenged samples after 12, 24, and 48 h of continuous growth in MRS-MOPS exhibited a time-dependent tendency for decreased live and increased injured and dead subpopulations in comparison to unchallenged mid-log-phase cells (Tukey's test; α = 0.05). This indicates that stationary phase constitutes a lethal stress for cells on its own (throughout stationary phase, the buffering capacity of MRS-MOPS batch-type cultures was lost, and the pH was stabilized at 4.3 ± 0.1). Taking into account the decreased viability of the starting material, it was shown that stationary-phase cultures at 12 and 24 h were more resistant to acidic lethal challenge than were mid-log-phase cells. The resistance decreased along with the time cells resided in stationary phase, becoming completely abolished by 48 h.
TABLE 1.
Ability of stationary-phase S. macedonicus cells to resist acidic lethal challenge
| Growth phasea | Unchallenged cellsb
|
Challenged cellsb
|
% Viable cells after lethal challengec | Stationary-phase ATRd | ||||
|---|---|---|---|---|---|---|---|---|
| % Viable | % Injured | % Dead | % Viable | % Injured | % Dead | |||
| Mid-log | 97.7 ± 1.3 | 1.8 ± 1.2 | 0.5 ± 0.2 | 8.7 ± 2.9 | 3.7 ± 1.0 | 87.6 ± 2.7 | 9 | Control |
| Stationary (12 h) | 90.6 ± 5.6 | 1.9 ± 1.3 | 7.5 ± 6.4 | 86.1 ± 3.2 | 3.2 ± 2.3 | 10.7 ± 4.9 | 95 | + |
| Stationary (24 h) | 65.2 ± 3.6 | 6.1 ± 4.3 | 28.7 ± 6.0 | 16.2 ± 9.0 | 6.5 ± 4.4 | 77.4 ± 7.3 | 25 | + |
| Stationary (48 h) | 24.7 ± 4.0 | 9.4 ± 1.7 | 66.0 ± 5.9 | 0.9 ± 0.2 | 0.4 ± 0.2 | 98.8 ± 0.5 | 3 | − |
S. macedonicus cells grown in MRS-MOPS were harvested at mid-log phase or at stationary phase after 12, 24, or 48 h.
Percentages (means ± standard deviations) of viable, injured, and dead cells, corresponding to cFDA+ PI−, cFDA+ PI+, and cFDA− PI+ subpopulations, respectively, as determined by flow cytometry of cultures prior to (unchallenged) or after (challenged) exposure to MRS, pH 3.5, for 15 min.
Viable cells in the challenged culture after acidic lethal challenge, expressed as the percentage of viable cells in the unchallenged control culture.
Stationary-phase acid tolerance response in comparison to that of mid-log-phase cells (control). +, stationary-phase ATR detected; −, cells were more sensitive than the control.
Kinetics of L-ATR induced by transient exposure to nonlethal acidic environments.
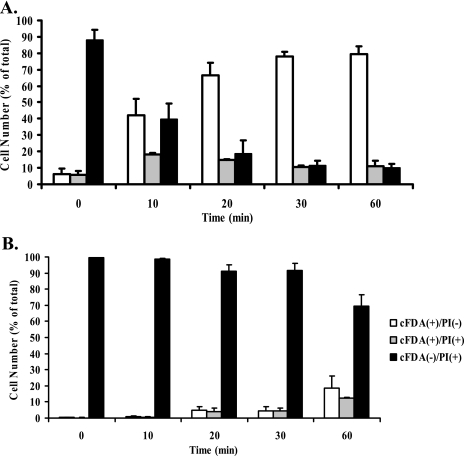
Mid-log-phase S. macedonicus cells grown in MRS-MOPS were exposed for increasing time periods to MRS, pH 5.5, and then challenged at pH 3.5 for 15 and 30 min. Multicolor flow cytometric analysis allowed the simultaneous monitoring of changes for all subpopulations under these conditions (Fig. 3A and B). After a 15-min lethal challenge, acid tolerance was evident within the first 10 min of acid adaptation and was fully induced at 20 min (Tukey's test; α = 0.05). The number of cFDA+ PI− cells stabilized to 79.3% ± 4.8% at 60 min of acid adaptation, while dead cells followed complementary reverse kinetics, leveling off at 20 min and reaching 9.8% ± 2.6% at 60 min. In contrast, injured cells (cFDA+ PI+) gave a peak value of 18.1% ± 0.9% at 10 min of exposure and then decreased, leveling off after 20 min and reaching 10.9% ± 3.4% at the end of the treatment. Increasing the time of lethal challenge to 30 min caused major changes in the kinetics of the subpopulations (Fig. 3B). Numbers of viable and injured cells gradually increased, exhibiting the highest values after 60 min of adaptation at pH 5.5 (18.4% ± 7.6% and 12.3% ± 0.6%, respectively). In accordance, dead cells gradually decreased, giving the lowest value at the end of acid adaptation (69.3% ± 7.1%).
FIG. 3.
Kinetics of acid adaptation of S. macedonicus. Mid-log-phase cells were adapted to pH 5.5 for 0 to 60 min and exposed to lethal challenge for 15 or 30 min (A and B, respectively). Percentages (means ± standard deviations) of fluorescent subpopulations, as determined by flow cytometry after cFDA-PI labeling, are presented.
L-ATR induction involves de novo protein synthesis and changes in the cell envelope of S. macedonicus.
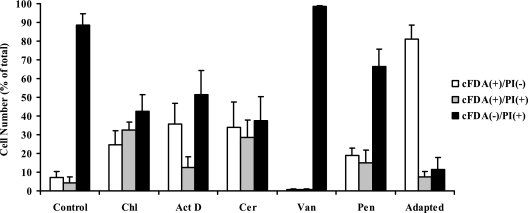
Various antibiotics were tested to elucidate probable mechanisms that underlie acid adaptation of S. macedonicus during transient exposure to nonlethal acidic pH. Chloramphenicol and actinomycin D were employed for the inhibition of de novo protein synthesis and induction of gene expression during treatment, respectively. Cerulenin was used to inhibit fatty acid biosynthesis, while vancomycin and penicillin were used to block newly synthesized cell wall assembly. None of the antibiotics exhibited any significant bactericidal effect at the concentrations used and during the course of the treatment, as mid-log-phase cells exposed to them remained >96% viable after cFDA-PI staining (data not shown). The presence of the inhibitors prior to and during the process of acid adaptation radically influenced the ability of S. macedonicus mid-log-phase cells to launch a full L-ATR, as judged by their aptitude for overcoming lethal challenge (Fig. 4). In detail, all antibiotics except for vancomycin resulted in partial inhibition of the L-ATR, as the increase of viable cells and decrease of dead cells in comparison to those of the nonadapted control did not match the magnitude of the corresponding changes observed for the adapted control (Tukey's test; α = 0.05). Most interestingly, partial inhibition of L-ATR was accompanied by a significant increase in injured cells in comparison to both nonadapted and adapted controls (Tukey's test; α = 0.05). Vancomycin was the only inhibitor that completely abolished L-ATR and rendered cells more susceptible to lethal challenge in comparison to the nonadapted control, since the numbers of both viable and injured cells decreased, while the number of dead cells marginally increased (Tukey's test; α = 0.05). Vancomycin has been shown to inhibit cell wall biosynthesis by being incorporated into nascent peptidoglycan chains (15). The commercial availability of the fluorescent derivative BODIPY FL vancomycin allowed us to assess possible changes in the cell wall formation profile due to acid adaptation. Flow cytometric analysis and CLSM did not reveal any obvious differences between nonadapted and acid-adapted (at pH 5.5) populations (Fig. 5A and B). Interestingly, though, acid-habituated (at pH 6.0) cells exhibited an almost 1.5-fold increase of the mean side scatter intensity and a >2.5-fold increase of the mean fluorescence intensity in comparison to nonadapted cells (Fig. 5A). Microscopic examination demonstrated that acid habituation resulted in the formation of long cell chains coinciding with the increase of the side scatter intensity, while peptidoglycan synthesis occurred at nearly every division septum and cell equator (Fig. 5B). Nongrowing stationary-phase cells could not be labeled with BODIPY FL vancomycin, verifying its specificity for active sites of cell wall biosynthesis for S. macedonicus (Fig. 5A).
FIG. 4.
Effects of inhibitors on the induction of acid tolerance in S. macedonicus. Prior to acidic lethal challenge at pH 3.5 for 15 min, mid-log-phase cells were either left unadapted (control) or acid adapted at pH 5.5 for 30 min in the presence of chloramphenicol (Chl), actinomycin D (Act D), cerulenin (Cer), vancomycin (Van), or penicillin (Pen) or in the absence of any inhibitor (adapted). Percentages (means ± standard deviations) of fluorescent subpopulations, as determined by flow cytometry after cFDA-PI labeling, are presented.
FIG. 5.
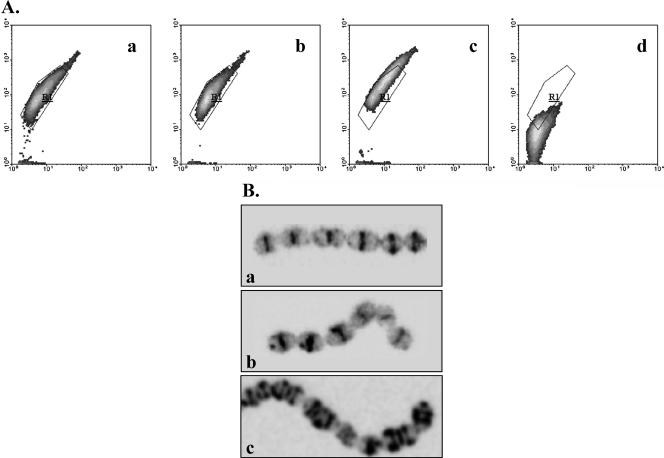
(A) Flow cytometric analysis of active cell wall biosynthesis sites of nonadapted (a), acid-adapted (at pH 5.5 for 1 h) (b), acid-habituated (c), and stationary-phase (d) S. macedonicus cells labeled with BODIPY FL vancomycin. Results are presented as density plots of green fluorescence versus side scatter. Region R1 was drawn to enclose the core of nonadapted cells' distribution. (B) CLSM photographs of cells treated as described above (a to c). It should be noted that stationary-phase cells were negative for BODIPY FL vancomycin labeling under CLSM.
L-ATR leads to retention of MP of S. macedonicus cells after lethal challenge.
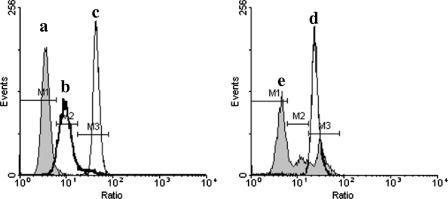
DiBAC4(3) ratiometric analysis was employed to compare the MP of nonadapted and acid-adapted cells after acidic lethal challenge. The log ratio of green fluorescence to side scatter enhanced the resolution of live, depolarized, and dead cells (Fig. 6) compared to that we previously reported (41), since this type of analysis corrects for DiBAC4(3) fluorescence variations due to cell size heterogeneity (2) or cell clumping (38). The membrane potential of nonadapted cells was completely dissipated after lethal challenge, giving a single peak of the ratio parameter with a mean intensity higher than that of depolarized cells and similar to that of heat-killed cells (Fig. 6, peak d). In contrast, acid-adapted cells were separated into three subpopulations after lethal challenge (Fig. 6, peak e). The majority of the cells retained normal membrane potential (55.8%), while the other two groups coincided with either the depolarized or the heat-killed population (19.0% and 25.2%, respectively). These results indicate that induction of L-ATR enables cells to resist the abolishment of membrane potential caused by lethal challenge.
FIG. 6.
Ratiometric analysis of membrane potential changes in S. macedonicus cells after acidic lethal challenge with DiBAC4(3). Untreated (a), depolarized (b), and heat-killed (c) mid-log-phase S. macedonicus cells served as controls. Nonadapted (d) or acid-adapted (at pH 5.5 for 30 min) (e) mid-log-phase cells were exposed to pH 3.5 for 15 min. All samples were labeled with DiBAC4(3) and analyzed by flow cytometry. Results are presented as single-parameter histograms of the log ratios of green fluorescence to side scatter. M1, M2, and M3 were defined by the untreated, depolarized, and heat-killed cells' distributions, respectively.
Involvement of F-ATPase and glucose-specific PEP-PTS activities in L-ATR of S. macedonicus.
DCCD was employed as an inhibitor of F-ATPase. Mid-log-phase S. macedonicus cells were exposed for 1 h to pH 5.5, and DCCD was added either at 0.2 mM 20 min prior to and during the 1 h of acid adaptation or at 0.4 mM for only the last 40 min of treatment (Fig. 7). Inhibition of F-ATPase activity throughout acid adaptation resulted in abolishment of L-ATR, as lethal challenge resulted in >99% dead cells. In contrast, adapting S. macedonicus cells for 20 min before inhibiting F-ATPase activity (for the last 40 min at pH 5.5) allowed a partial L-ATR. DCCD had no effect on the viability of control unchallenged cultures (data not shown). Additionally, since DCCD causes covalent modification of F-ATPase (28), it should be presumed that inhibition persisted throughout lethal challenge. All of these data indicate an important role for F-ATPase during the induction of L-ATR, but its activity appears to be dispensable to a degree once L-ATR has been launched fully (i.e., after exposure for 20 min at pH 5.5).
FIG. 7.
Effect of DCCD on the induction of acid tolerance in S. macedonicus. Prior to lethal challenge, cells were nonadapted (a), acid adapted at pH 5.5 for 60 min, with DCCD being added either 20 min prior to and during the 60 min of acid adaptation (b) or only during the last 40 min of the treatment (c), and acid adapted under the same conditions in the absence of DCCD (d). Percentages (means ± standard deviations) of fluorescent subpopulations, as determined by flow cytometry after cFDA-PI labeling, are presented.
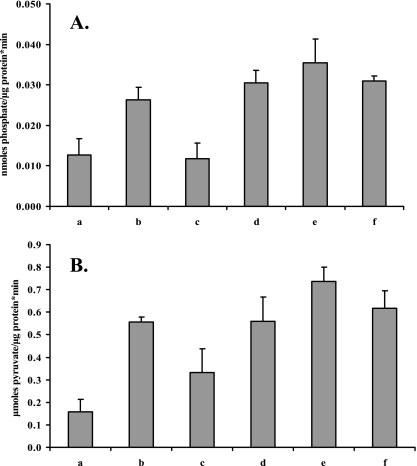
The activities of F-ATPase and the glucose-specific PEP-PTS were further assessed in permeabilized S. macedonicus cells (Fig. 8A and B, respectively). F-ATPase activity of acid-adapted cells (by transient exposure to pH 5.5) and AH mid-log-phase cells increased 2.6-fold compared to that of nonadapted cells, while for autoacidified cells there was a lesser, 2-fold increase (Tukey's test; α = 0.05). Glucose-specific PEP-PTS activity of S. macedonicus increased to the same extent for all three AT phenotypes (>3.6-fold). Acid-adapted cells in the presence of chloramphenicol had the same F-ATPase and PEP-PTS activities as control cells. While it has been reported that cell membrane fatty acid composition influences both F-ATPase and PEP-PTS activities (21), in our case a marginal, not statistically significant decrease of both enzymatic activities was observed when cells were treated with cerulenin during acid adaptation.
FIG. 8.
F-ATPase (A) and glucose-specific PEP-PTS (B) activities of S. macedonicus mid-log-phase cells that were nonadapted (a), autoacidified (b), acid adapted at pH 5.5 for 30 min in the presence of chloramphenicol (c) or cerulenin (d) or in the absence of inhibitors (e), and acid habituated (f).
Single-cell sorting of S. macedonicus subpopulations according to their cFDA-PI fluorescence status.
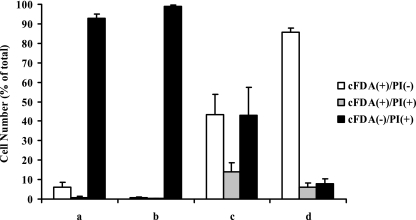
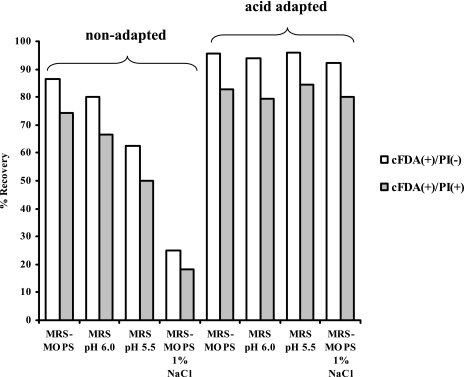
In our previous work on acid-stressed cells of S. macedonicus (41), we already found that the cFDA-positive cells detected after acid challenge showed recovery when sorted onto conventional agar plates. In order to investigate the effect of acid adaptation on the culturability of S. macedonicus cFDA-PI fluorescent subpopulations, nonadapted and transiently acid-exposed (to pH 5.5 for 30 min) mid-log-phase cells were challenged at pH 3.5 for 15 min, labeled with the fluorochromes, and sorted onto both optimal and suboptimal media. Unchallenged control mid-log-phase cells showed recovery above 96% in all different medium types. This indicated that neither sonication treatment nor sorting had any serious impact on the cells' culturability and that the suboptimal media used only delayed growth (data not shown). Additionally, sorted cFDA− PI+ cells were unable to recover in all cases (data not shown), as previously reported (41). Systematically, cFDA+ PI− cells exhibited a higher capacity to form colonies than did the corresponding cFDA+ PI+ cells (binomial comparison of proportions; P < 0.05) (Fig. 9). Most importantly, shifting the pH of the sorting medium to 6.0 or 5.5 caused a drastic decrease in the recovery of both cFDA+ PI− and cFDA+ PI+ cells derived from nonadapted samples. In contrast, the recovery of the two subpopulations for acid-adapted cells was not considerably influenced under such conditions. Furthermore, sorting cFDA+ PI− and cFDA+ PI+ cells onto MRS-MOPS agar plates supplemented with 1% NaCl revealed that the observed differences in the recovery of these two subpopulations between nonadapted and acid-adapted samples were not solely dependent on the pH of the sorting medium. In summary, these results show that subpopulations identical in fluorescence characteristics may differ significantly in their potential to recover.
FIG. 9.
Percentages of recovery of sorted cFDA+ PI− and cFDA+ PI+ cells derived from nonadapted and acid-adapted populations after acid challenge at pH 3.5 for 15 min. Medium-based recovery of sorted cells was examined on MRS-MOPS, MRS (pH 6.0 and 5.5), and MRS-MOPS-1% NaCl agar plates. It should be noted that cFDA− PI+ cells exhibited no recovery in all cases (data not shown).
DISCUSSION
In this study, we employed flow cytometry along with different fluorescence-based assays to characterize the acid stress responses of S. macedonicus. Our data clearly show that all three manipulations imposed on mid-log-phase S. macedonicus cells resulted in induction of L-ATR. In detail, transient exposure to nonlethal acidic pHs (pH 6.0 and pH 5.5) and continuous growth at suboptimal pH 6.0, i.e., acid habituation (first described for Escherichia coli [24]) of mid-log-phase cells, prior to lethal challenge, resulted in equal protection, making these phenotypes indistinguishable in terms of their survival. Interestingly, the acidifying capacity of S. macedonicus also stimulated a protective response. This type of L-ATR was weaker than those in response to the other treatments and could probably be ascribed to physiological changes similar to those described for Lactococcus lactis autoacidified cells (19, 46). Stationary-phase ATR was also verified, constituting a growth phase resistance pattern for S. macedonicus. The response decayed progressively along with the time that the cells resided at stationary phase. Apparently, prolonged exposure to pH 4.3 (i.e., the pH value at stationary phase) caused the degeneration of nongrowing S. macedonicus cells, making them more susceptible to low pH. This was also evident by the increasing numbers of both injured and dead cells exhibited by the control stationary-phase cultures. Similar findings have been reported for S. mutans (47) and L. lactis (37). Our batch-type culture system cannot rule out the compensation of low pH for the induction of the observed stationary-phase ATR, which has been shown to be essential in the case of Lactobacillus acidophilus (32). All the same, S. mutans and L. lactis grown at constant pH have also been reported to exhibit stationary-phase ATR (1, 49).
Subsequently, flow cytometric analysis of acid-stressed S. macedonicus cells following acid adaptation revealed the heterogenic behavior of the bacterium, similar to that of Oenococcus oeni adapted to ethanol stress (25). We demonstrated not only that S. macedonicus cells differ in the ability to induce L-ATR but that there are also cell-to-cell deviations in the rate of this induction. Such an individualized response of a population to an external stimulus (in this case, exposure to sublethal pH) has been linked to asynchronous cell cycle progression, differences in cell physiological status, and stochastic variations (6, 9).
We then employed different inhibitors to elucidate the possible involvement of central cellular mechanisms in the manifestation of acid tolerance of mid-log-phase S. macedonicus cells. L-ATR was partially inhibited by both chloramphenicol and actinomycin D, suggesting that part of the acid resistance phenotype is dependent on de novo protein synthesis that seems to be the result of a genetically programmed response. Indeed, chloramphenicol has been shown to inhibit induction of L-ATR in L. lactis (46), Listeria monocytogenes (39), and Lactobacillus sanfranciscensis (17). In addition, acid-adapted oral streptococci are enriched in membrane long-chain monounsaturated fatty acids in order to decrease proton membrane permeability (20-22). Inhibition of fatty acid biosynthesis of S. macedonicus by cerulenin caused a decrease of L-ATR to some extent. Furthermore, cell wall formation proved to be important for the induction of L-ATR, as vancomycin and penicillin led to severe inhibition of resistance to lethal challenge. Previous work with mutant strains of S. mutans, L. monocytogenes, and Enterococcus faecalis have also indicated that proper cell wall biosynthesis is necessary for acid resistance (8, 12, 50). Further investigation with fluorescent vancomycin that labeled sites of nascent peptidoglycan biosynthesis revealed that acid-habituated cells exhibited an abnormal pattern of active cell wall biosynthesis sites, which correspond to recent or forthcoming division sites (15). This observation may indicate severe perturbations of cell cycle progression due to continuous growth at low pH and is in agreement with the fact that the FtsZ and FtsA proteins (which belong to the cell division machinery and are located at septum formation sites) were found to be up-regulated when S. mutans was grown at pH 5.2 (54). The increase in the length of cell chains of AH cells could be attributed to impairment of enzymatic activities that are necessary for cell segregation after division due to low pH, similar to those described for S. mutans, S. pneumoniae, and S. thermophilus (7, 11, 31). It should also be mentioned that the increase of the length of cell chains due to acid habituation may be an advertent adaptation response, as it has been shown that dechained S. mutans cells were less aciduric than chained ones (27) and that chains can give growth advantages to S. thermophilus under aerobic conditions (34).
Since acid-challenged mid-log-phase S. macedonicus cells suffer severe cell membrane damage and since acid adaptation causes changes to the cell membrane composition in an attempt to reduce the extensive proton influx (22), the perturbation of cell MP was examined as a possible consequence of acid stress. After acid challenge, nonadapted cells exhibited completely dissipated MP. Significantly, under the same conditions, the majority of acid-adapted cells retained normal MP. This difference clearly indicates that physiological mechanisms induced during acid adaptation actively increase the ability of cells to maintain ion gradients across the cell membrane during acid challenge. Since the MP of streptococcal cells is mainly attributed to the proton-translocating F-ATPase (29) and since it has been reported that several streptococci, as well as L. lactis, cope with high extracellular proton concentrations by increased F-ATPase activity (3, 40), we investigated the involvement of this enzymatic system in L-ATR of S. macedonicus. Our findings strongly suggest that F-ATPase activity is important during the induction of L-ATR, probably in order to maintain the proper pH gradient (ΔpH) necessary for the function of key cellular components needed for the induction of the response. However, once L-ATR is fully induced, F-ATPase activity is only partially required, since other physiological changes that already have occurred may aid the survival of cells during lethal challenge (i.e., newly synthesized proteins, changes in the cell envelope, etc.). In favor of this reasoning, nonadapted mid-log-phase L. monocytogenes cells were found to be more susceptible to acid killing after DCCD treatment than were acid-adapted cells (13). It was further determined that both F-ATPase and glucose-specific PEP-PTS activities increased in all three logarithmic-phase AT phenotypes in comparison to those in nonadapted cells. The increase of F-ATPase activity in autoacidified cells was lower than that for the other two phenotypes and probably reflects the decreased acid tolerance of this phenotype. The up-regulation of both enzymatic systems in S. macedonicus was due to de novo protein synthesis, similar to the case for other streptococci (30, 33, 35, 44). In addition, a fabM deletion mutant of S. mutans which is unable to produce unsaturated fatty acids was sensitive to low pH and exhibited significantly increased F-ATPase and glucose-specific PEP-PTS activities (21). In the case of S. macedonicus, inhibition of fatty acid biosynthesis by cerulenin did not influence the increased activities of F-ATPase and glucose-specific PEP-PTS observed during acid adaptation. Up-regulation of the glucose-specific PEP-PTS under acid adaptation conditions may be necessary for the increased glucose intake required to compensate for the depletion of ATP pools caused by enhanced F-ATPase activity (35). To the best of our knowledge, S. macedonicus is the first Streptococcus species reported to induce both enzymatic activities during acid adaptation. This feature demonstrates that S. macedonicus responds differently to acidic conditions than do the majority of oral streptococci, which typically up-regulate F-ATPase and down-regulate the glucose-specific PEP-PTS (44), or S. sobrinus, which up-regulates only the glucose-specific PEP-PTS and retains a stable F-ATPase level (35).
Previous sorting experiments attempted to establish a direct correlation between the fluorescence status of stressed bacteria after cFDA-PI labeling and culturability, i.e., cFDA+ PI−, cFDA+ PI+, and cFDA− PI+ events have been reported to correspond to viable, injured, and dead cells, respectively (2, 25). The reduced recovery of the sorted cFDA+ PI+ cells compared to that of cFDA+ PI− S. macedonicus cells on optimal medium is in accordance with these findings. However, sorting cFDA+ PI− and cFDA+ PI+ cells of nonadapted acid-stressed S. macedonicus samples onto suboptimal media demonstrated that cells referred to as injured by culture-based techniques (i.e., cells that are able to replicate under optimal conditions but unable to replicate under suboptimal, nonlethal conditions) exist in both populations. In contrast, recovery of these two fluorescent subpopulations derived from acid-adapted samples after acid challenge remained more or less unaffected by the different recovery conditions, thus precluding the existence of injured cells in culture-based terms in both subpopulations. This leads us to the conclusion that classical injury, as detected by the ability of a cell to grow under adverse conditions, may not be reflected fully by cFDA-PI labeling. Finally, while S. macedonicus cells can be separated into three discrete stages of activity and permeability by cFDA-PI labeling after lethal challenge, our findings strongly indicate that the recovery potential of any given fluorescent subpopulation may be influenced by the physiological status of the cells prior to lethal challenge. This was evident because the regrowth patterns of nonadapted and acid-adapted cells were substantially different.
Here we elucidated traits of the hitherto unknown acid stress physiology of S. macedonicus. Multiparameter flow cytometry combined with single-cell sorting revealed effectively diverse physiological changes that S. macedonicus underwent during acquisition of acid tolerance. Our findings should facilitate a better understanding of the physiology of food-related lactic streptococci that will allow their rational utilization in industrial processes. We will continue to refine this approach, along with molecular techniques, such as RNA arbitrarily primed PCR, to further establish possible differences among the different AT phenotypes we described and to determine their relevance to the food environment.
Acknowledgments
The present work was cofinanced by the European Social Fund and national resources EPEAEK and YPEPTH.
We thank G. Voutsinas (Institute of Biology, NCSR Demokritos, Athens, Greece) for providing the space to perform microbiological work in his laboratory as well as I. Nes (Norwegian University of Life Sciences, Ås, Norway) and G. Jan (INRA, Rennes, France) for critical reading of the manuscript.
Footnotes
Published ahead of print on 10 November 2006.
REFERENCES
- 1.Alemayehu, D., E. O'Sullivan, and S. Condon. 2000. Changes in acid tolerance of Lactococcus lactis during growth at constant pH. Int. J. Food Microbiol. 55:215-221. [DOI] [PubMed] [Google Scholar]
- 2.Amor, K. B., P. Breeuwer, P. Verbaarschot, F. M. Rombouts, A. D. Akkermans, W. M. De Vos, and T. Abee. 2002. Multiparametric flow cytometry and cell sorting for the assessment of viable, injured, and dead bifidobacterium cells during bile salt stress. Appl. Environ. Microbiol. 68:5209-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender, G. R., S. V. Sutton, and R. E. Marquis. 1986. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect. Immun. 53:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolotin, A., B. Quinquis, P. Renault, A. Sorokin, S. D. Ehrlich, S. Kulakauskas, A. Lapidus, E. Goltsman, M. Mazur, G. D. Pusch, M. Fonstein, R. Overbeek, N. Kyprides, B. Purnelle, D. Prozzi, K. Ngui, D. Masuy, F. Hancy, S. Burteau, M. Boutry, J. Delcour, A. Goffeau, and P. Hols. 2004. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat. Biotechnol. 22:1554-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonn, D. 2003. New bacteriocin from Greek cheese. Lancet Infect. Dis. 3:61.12560176 [Google Scholar]
- 6.Booth, I. R. 2002. Stress and the single cell: intrapopulation diversity is a mechanism to ensure survival upon exposure to stress. Int. J. Food Microbiol. 78:19-30. [DOI] [PubMed] [Google Scholar]
- 7.Borges, F., S. Layec, A. Thibessard, A. Fernandez, B. Gintz, P. Hols, B. Decaris, and N. Leblond-Bourget. 2005. cse, a chimeric and variable gene, encodes an extracellular protein involved in cellular segregation in Streptococcus thermophilus. J. Bacteriol. 187:2737-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd, D. A., D. G. Cvitkovitch, A. S. Bleiweis, M. Y. Kiriukhin, D. V. Debabov, F. C. Neuhaus, and I. R. Hamilton. 2000. Defects in d-alanyl-lipoteichoic acid synthesis in Streptococcus mutans result in acid sensitivity. J. Bacteriol. 182:6055-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brehm-Stecher, B. F., and E. A. Johnson. 2004. Single-cell microbiology: tools, technologies, and applications. Microbiol. Mol. Biol. Rev. 68:538-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callon, C., L. Millet, and M. C. Montel. 2004. Diversity of lactic acid bacteria isolated from AOC Salers cheese. J. Dairy Res. 71:231-244. [DOI] [PubMed] [Google Scholar]
- 11.Chatfield, C. H., H. Koo, and R. G. Quivey, Jr. 2005. The putative autolysin regulator LytR in Streptococcus mutans plays a role in cell division and is growth-phase regulated. Microbiology 151:625-631. [DOI] [PubMed] [Google Scholar]
- 12.Cotter, P. D., N. Emerson, C. G. Gahan, and C. Hill. 1999. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J. Bacteriol. 181:6840-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotter, P. D., C. G. Gahan, and C. Hill. 2000. Analysis of the role of the Listeria monocytogenes F0F1-ATPase operon in the acid tolerance response. Int. J. Food Microbiol. 60:137-146. [DOI] [PubMed] [Google Scholar]
- 14.Cotter, P. D., and C. Hill. 2003. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67:429-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel, R. A., and J. Errington. 2003. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell 113:767-776. [DOI] [PubMed] [Google Scholar]
- 16.Davey, H. M., and D. B. Kell. 1996. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analyses. Microbiol. Rev. 60:641-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Angelis, M., L. Bini, V. Pallini, P. S. Cocconcelli, and M. Gobbetti. 2001. The acid-stress response in Lactobacillus sanfranciscensis CB1. Microbiology 147:1863-1873. [DOI] [PubMed] [Google Scholar]
- 18.Diaz-Ruiz, G., J. P. Guyot, F. Ruiz-Teran, J. Morlon-Guyot, and C. Wacher. 2003. Microbial and physiological characterization of weakly amylolytic but fast-growing lactic acid bacteria: a functional role in supporting microbial diversity in pozol, a Mexican fermented maize beverage. Appl. Environ. Microbiol. 69:4367-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Even, S., N. D. Lindley, P. Loubiere, and M. Cocaign-Bousquet. 2002. Dynamic response of catabolic pathways to autoacidification in Lactococcus lactis: transcript profiling and stability in relation to metabolic and energetic constraints. Mol. Microbiol. 45:1143-1152. [DOI] [PubMed] [Google Scholar]
- 20.Fozo, E. M., J. K. Kajfasz, and R. G. Quivey, Jr. 2004. Low pH-induced membrane fatty acid alterations in oral bacteria. FEMS Microbiol. Lett. 238:291-295. [DOI] [PubMed] [Google Scholar]
- 21.Fozo, E. M., and R. G. Quivey, Jr. 2004. The fabM gene product of Streptococcus mutans is responsible for the synthesis of monounsaturated fatty acids and is necessary for survival at low pH. J. Bacteriol. 186:4152-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fozo, E. M., and R. G. Quivey, Jr. 2004. Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments. Appl. Environ. Microbiol. 70:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgalaki, M. D., E. Van Den Berghe, D. Kritikos, B. Devreese, J. Van Beeumen, G. Kalantzopoulos, L. De Vuyst, and E. Tsakalidou. 2002. Macedocin, a food-grade lantibiotic produced by Streptococcus macedonicus ACA-DC 198. Appl. Environ. Microbiol. 68:5891-5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodson, M., and R. Rowbury. 1989. Habituation to normal lethal acidity by prior growth of Escherichia coli at a sublethal pH value. Lett. Appl. Microbiol. 8:77-79. [Google Scholar]
- 25.Graca da Silveira, M., M. Vitoria San Romao, M. C. Loureiro-Dias, F. M. Rombouts, and T. Abee. 2002. Flow cytometric assessment of membrane integrity of ethanol-stressed Oenococcus oeni cells. Appl. Environ. Microbiol. 68:6087-6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanna, M. N., R. J. Ferguson, Y. H. Li, and D. G. Cvitkovitch. 2001. uvrA is an acid-inducible gene involved in the adaptive response to low pH in Streptococcus mutans. J. Bacteriol. 183:5964-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iacono, V. J., T. P. Byrnes, I. T. Crawford, B. L. Grossbard, J. J. Pollock, and B. J. MacKay. 1985. Lysozyme-mediated de-chaining of Streptococcus mutans and its antibacterial significance in an acidic environment. J. Dent. Res. 64:48-53. [DOI] [PubMed] [Google Scholar]
- 28.Kakinuma, Y. 1998. Inorganic cation transport and energy transduction in Enterococcus hirae and other streptococci. Microbiol. Mol. Biol. Rev. 62:1021-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konings, W. N. 2002. The cell membrane and the struggle for life of lactic acid bacteria. Antonie Leeuwenhoek 82:3-27. [PubMed] [Google Scholar]
- 30.Kuhnert, W. L., and R. G. Quivey, Jr. 2003. Genetic and biochemical characterization of the F-ATPase operon from Streptococcus sanguis 10904. J. Bacteriol. 185:1525-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez, R., M. P. Gonzalez, E. Garcia, J. L. Garcia, and P. Garcia. 2000. Biological roles of two new murein hydrolases of Streptococcus pneumoniae representing examples of module shuffling. Res. Microbiol. 151:437-443. [DOI] [PubMed] [Google Scholar]
- 32.Lorca, G. L., and G. F. Valdez. 2001. A low-pH-inducible, stationary-phase acid tolerance response in Lactobacillus acidophilus CRL 639. Curr. Microbiol. 42:21-25. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Galiano, A. J., M. J. Ferrandiz, and A. G. de la Campa. 2001. The promoter of the operon encoding the F0F1 ATPase of Streptococcus pneumoniae is inducible by pH. Mol. Microbiol. 41:1327-1338. [DOI] [PubMed] [Google Scholar]
- 34.Mercier, C., E. Domakova, J. Tremblay, and S. Kulakauskas. 2000. Effects of a muramidase on a mixed bacterial community. FEMS Microbiol. Lett. 187:47-52. [DOI] [PubMed] [Google Scholar]
- 35.Nascimento, M. M., J. A. Lemos, J. Abranches, R. B. Goncalves, and R. A. Burne. 2004. Adaptive acid tolerance response of Streptococcus sobrinus. J. Bacteriol. 186:6383-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nebe-von-Caron, G., P. J. Stephens, C. J. Hewitt, J. R. Powell, and R. A. Badley. 2000. Analysis of bacterial function by multi-colour fluorescence flow cytometry and single cell sorting. J. Microbiol. Methods 42:97-114. [DOI] [PubMed] [Google Scholar]
- 37.Niven, G. W., and F. Mulholland. 1998. Cell membrane integrity and lysis in Lactococcus lactis: the detection of a population of permeable cells in post-logarithmic phase cultures. J. Appl. Microbiol. 84:90-96. [DOI] [PubMed] [Google Scholar]
- 38.Novo, D., N. G. Perlmutter, R. H. Hunt, and H. M. Shapiro. 1999. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry 35:55-63. [DOI] [PubMed] [Google Scholar]
- 39.O'Driscoll, B., C. G. Gahan, and C. Hill. 1996. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl. Environ. Microbiol. 62:1693-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Sullivan, E., and S. Condon. 1999. Relationship between acid tolerance, cytoplasmic pH, and ATP and H+-ATPase levels in chemostat cultures of Lactococcus lactis. Appl. Environ. Microbiol. 65:2287-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papadimitriou, K., H. Pratsinis, G. Nebe-von-Caron, D. Kletsas, and E. Tsakalidou. 2006. Rapid assessment of the physiological status of Streptococcus macedonicus by flow cytometry and fluorescence probes. Int. J. Food Microbiol. 111:197-205. [DOI] [PubMed] [Google Scholar]
- 42.Phan, T. N., P. T. Nguyen, J. Abranches, and R. E. Marquis. 2002. Fluoride and organic weak acids as respiration inhibitors for oral streptococci in acidified environments. Oral Microbiol. Immunol. 17:119-124. [DOI] [PubMed] [Google Scholar]
- 43.Poznanski, E., A. Cavazza, F. Cappa, and P. S. Cocconcelli. 2004. Indigenous raw milk microbiota influences the bacterial development in traditional cheese from an alpine natural park. Int. J. Food Microbiol. 92:141-151. [DOI] [PubMed] [Google Scholar]
- 44.Quivey, R. G., W. L. Kuhnert, and K. Hahn. 2001. Genetics of acid adaptation in oral streptococci. Crit. Rev. Oral Biol. Med. 12:301-314. [DOI] [PubMed] [Google Scholar]
- 45.Rallu, F., A. Gruss, S. D. Ehrlich, and E. Maguin. 2000. Acid- and multistress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol. Microbiol. 35:517-528. [DOI] [PubMed] [Google Scholar]
- 46.Rallu, F., A. Gruss, and E. Maguin. 1996. Lactococcus lactis and stress. Antonie Leeuwenhoek 70:243-251. [DOI] [PubMed] [Google Scholar]
- 47.Renye, J. A., Jr., P. J. Piggot, L. Daneo-Moore, and B. A. Buttaro. 2004. Persistence of Streptococcus mutans in stationary-phase batch cultures and biofilms. Appl. Environ. Microbiol. 70:6181-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shapiro, H. M. 2000. Microbial analysis at the single-cell level: tasks and techniques. J. Microbiol. Methods 42:3-16. [DOI] [PubMed] [Google Scholar]
- 49.Svensater, G., O. Bjornsson, and I. R. Hamilton. 2001. Effect of carbon starvation and proteolytic activity on stationary-phase acid tolerance of Streptococcus mutans. Microbiology 147:2971-2979. [DOI] [PubMed] [Google Scholar]
- 50.Teng, F., L. Wang, K. V. Singh, B. E. Murray, and G. M. Weinstock. 2002. Involvement of PhoP-PhoS homologs in Enterococcus faecalis virulence. Infect. Immun. 70:1991-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsakalidou, E., E. Zoidou, B. Pot, L. Wassill, W. Ludwig, L. A. Devriese, G. Kalantzopoulos, K. H. Schleifer, and K. Kersters. 1998. Identification of streptococci from Greek Kasseri cheese and description of Streptococcus macedonicus sp. nov. Int. J. Syst. Bacteriol. 48:519-527. [DOI] [PubMed] [Google Scholar]
- 52.Ueckert, J., P. Breeuwer, T. Abee, P. Stephens, G. N. von Caron, and P. F. ter Steeg. 1995. Flow cytometry applications in physiological study and detection of foodborne microorganisms. Int. J. Food Microbiol. 28:317-326. [DOI] [PubMed] [Google Scholar]
- 53.van de Guchte, M., P. Serror, C. Chervaux, T. Smokvina, S. D. Ehrlich, and E. Maguin. 2002. Stress responses in lactic acid bacteria. Antonie Leeuwenhoek 82:187-216. [PubMed] [Google Scholar]
- 54.Wilkins, J. C., K. A. Homer, and D. Beighton. 2002. Analysis of Streptococcus mutans proteins modulated by culture under acidic conditions. Appl. Environ. Microbiol. 68:2382-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]