Abstract
Localization of newly synthesized poly(3hydroxybutyrate) (PHB) granules was determined by confocal laser scanning fluorescence microscopy of Nile red-stained cells and by transmission electron microscopy (TEM). PHB granules of Nile red-stained living cells of Caryophanon latum at the early stages of PHB accumulation were frequently found at or close to the cytoplasmic membrane. TEM analysis of the same culture revealed electron-translucent globular structures resembling PHB granules that were nonrandomly distributed in the cell lumen but were frequently found at or close to the cytoplasmic membrane. Immunogold labeling using PHB-specific antiserum confirmed that the electron-translucent structures represented PHB granules. Electron microscopy examination of PHB granules after cell lysis revealed that PHB granules were often associated with membrane vesicles. Nonrandom localization of PHB granules was also found in Beijerinckia indica. Cells of this species harbored one PHB granule at each cell pole. Our results show that newly synthesized PHB granules often are close to or even in physical contact with the cytoplasmic membrane. Possible explanations for this unexpected finding and a hypothetical model of PHB granule formation in C. latum are discussed.
Poly(3-hydroxyalkanoates) (PHA) are the most widely distributed bacterial storage compounds of carbon and energy, with poly(3-hydroxybutyrate) (PHB) being the most prominent member. Biosynthesis and biodegradation of PHA, including the key enzymes of these processes, PHA synthases, PhaCs (PHA synthesis), and PHA depolymerases, PhaZs (PHA degradation), have been investigated by many research groups for about 3 decades (1, 2, 8, 17, 24). Despite considerable progress in understanding the function of individual proteins involved in PHA metabolism, the initiation of PHA synthesis is not understood very well. The literature contains hundreds of images showing PHB granules localized more or less randomly in the bacterial cell, and it has been assumed that PHB granules are cytoplasm-located subcellular structures. A short selection of mainly early reports is given in references 4, 10-12, and 15). However, these studies used cells at the end of growth and/or at the end of the PHB accumulation phase and are unlikely to be useful for prediction for the initiation site of PHB granule formation. It was only recently that PHB granule formation in vivo came into focus. While our laboratory found evidence for PHB granule formation in the cell periphery (7), Tian et al. noticed dark-stained so-called “mediation elements” localized in the center of Ralstonia eutropha cells at the onset of PHB accumulation and assumed that these structures represented the sites of PHB granule formation (26). The diameters of PHB granules in R. eutropha and most other PHB-accumulating bacteria are in the range of 0.2 to 0.5 μm. This size is only a little smaller than the average cell diameter of most bacterial species (0.4 to 1 μm), including R. eutropha. Conclusive determination of subcellular localization of early PHB granules by microscopy is therefore hardly possible in these bacteria. Consequently, in this study, we investigated PHB accumulation and subcellular localization in bacterial species that have significantly larger cell dimensions. Caryophanon latum is a gram-positive trichome-forming bacterium consisting of more or less globular cells 2 to 4 μm in diameter. The ability of C. latum to accumulate PHB is well documented (18, 27). In addition, we investigated Beijerinckia indica, an N2-fixing species with a known ability to accumulate PHB (3).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. latum (DSMZ 484) and B. indica (DSMZ 1715) were obtained from the Deutsche Sammlung für Mikroorganismen und Zellkulturen GmbH. C. latum strains L and H were gifts from D. Claus, Göttingen, Germany. C. latum strains had been isolated from fresh cow manure collected from meadows near Lenglern/Göttingen, Germany (strain L), and near Herberhausen/Göttingen, Germany (strain H). C. latum was grown in C. latum medium (www.dsmz.de). For induction of PHB formation, a 24-h preculture of C. latum medium without acetate was transferred (10 to 20 vol%) to fresh medium containing 1 to 2 g/liter acetate. PHB granules appeared within 2 h at 30°C. B. indica was grown in B. indica medium (www.dsmz.de) under N2-fixing conditions with glucose (0.5%) as the carbon source.
Fluorescence microscopy.
PHB granules in bacteria were visualized with the fluorescent dye Nile red (5, 23). Bacterial samples were taken at defined time-points and were immediately stained with Nile red by addition of 0.02 to 0.1 volumes of Nile red solution (0.1 to 1 μg/ml in acetone or ethanol). To obtain good staining in B. indica, addition of 0.5 volume Nile red solution was necessary. Bacteria were immobilized by placing 7 μl of bacterial cell suspension directly on a glass slide before 7 μl of 1% agarose (55°C) was added, and the slide was covered by a coverslip. Bacterial cells were imaged on a Leica TCS SP2 confocal laser scanning microscope with a ×100/1.4 HCX Plan Apo objective lens (Leica Microsystems, Germany). Nile red was excited with 543-nm light (green helium-neon laser). Image stacks were acquired according to sampling requirements for subsequent image processing and deconvolution, using Nyquist sampling with “optimized” numbers of optical sections according to Leica Confocal software. For better optical resolution and detection of sub-micrometer-size PHB granules and their localization relative to the cell membrane, the data sets were deconvolved with Huygens Essential software (Scientific Volume Imaging, Hilversum, The Netherlands). Estimated point spread functions were used. The data were then analyzed with Amira 3.1 software (Mercury Computer Systems, TGS Unit, Düsseldorf, Germany). Visualization of maximum intensity projections and volume rendering were used to analyze whether PHB granules were in contact with bacteria cell walls or not. Threshold settings and display-level settings were varied over a wide range to ensure that the ratio of membrane-connected versus free granules does not depend on visualization parameters.
Electron microscopy.
Samples were taken at times of interest, cooled on ice, and centrifuged at 5,000 × g. The pellet was resuspended in phosphate-buffered saline (PBS; 50 mM, pH 7.4) and then chemically fixed in PBS containing 0.2% (wt/vol) formaldehyde and 0.3% (vol/vol) glutardialdehyde for 1.5 h at 4°C. Embedding in Lowicryl K4M resin was performed as described previously (6, 19). Resin sections of about 80 nm in thickness were cut with glass knives. The sections were stained (3 min, 3% [wt/vol] phosphotungstic acid solution, pH 7.0). Electron microscopy was carried out with a Philips EM 301 instrument at calibrated magnifications and using IMAGO electron-sensitive films. Immunogold labeling was performed as described earlier (6). PHB-specific antibodies were a gift from O. Peoples, Metabolix, Inc., and were used in a 1,000-fold dilution. The antibodies had been generated against a synthetic 3-hydroxybutyrate octamer (D. Seebach) coupled to gelatin.
RESULTS AND DISCUSSION
LSFM of C. latum.
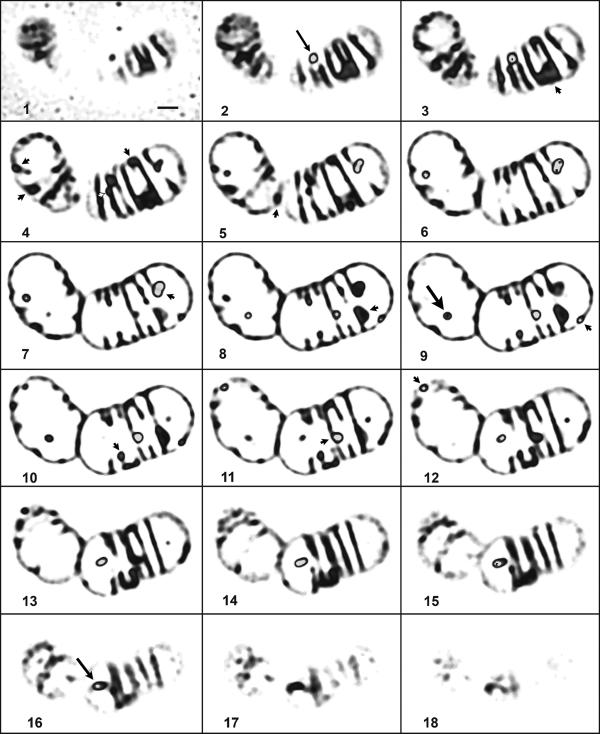
C. latum was grown in liquid medium for about 30 h at 30°C. At this stage of growth, the bacteria had reutilized almost all previously accumulated PHB and were free of visible PHB granules (data not shown). Volumes (0.1 to 0.2) of the culture were transferred to fresh C. latum liquid medium supplemented with 1 to 2 g/liter acetate and incubated at 30°C. Light microscopy inspection of the cells revealed that most trichomes harbored several PHB granules after 60 to 120 min of incubation. Similar results were obtained after addition of 1 to 2 g/liter acetate to an 18- to 24-h-old C. latum culture on C. latum medium (not shown). Gas chromatographic analysis revealed the presence of significant amounts of PHB only in cells that harbored fluorescent granules after Nile red staining (not shown). A sample of a C. latum culture was stained with Nile red after 90 min of incubation and immediately imaged by confocal laser scanning fluorescence microscopy (LSFM). Figure 1 shows a typical image of an optical section at the equatorial region of C. latum: one trichome is visible containing several septa, some of which are not closed. The observation of multiple septa in successive stages of development is typical for C. latum (13, 21, 28). Several Nile red-stained globular structures (PHB granules) are visible. Most of the PHB granules were localized at or close to the cytoplasmic membrane. Occasionally, PHB granules appeared to be localized in the center of the cells. Due to the thickness of the PHB granules (0.2 to 0.4 μm), it is possible that several of such granules were connected to the cytoplasmic membrane at the top or the bottom of the cell or to an open septum. To investigate the three-dimensional localization of the PHB granules, a series of ≈0.12-μm optical sections through C. latum trichomes was recorded. Figure 2 exemplarily shows the result for one trichome. Arrowheads indicate PHB granules close to the cytoplasmic membrane. Arrows indicate PHB granules in the upper or lower cuts, and these granules presumably are attached to the cytoplamic membrane at the top or bottom of the cell (Fig. 2, slices 2 and 16). It is evident that most of the PHB granules were localized at or very near the cytoplasmic membrane. Occasionally, free PHB granules were found localized in the cytoplasm (arrow in Fig. 2, slice 9) A three-dimensional reconstruction is given in the supplemental material.
FIG. 1.
Confocal laser scanning fluorescence microscopy of C. latum. Shown is a typical image of PHB granules in a C. latum trichome after Nile red staining (red color in gray). The granules were exclusively found close to the membrane of the bacterium (arrowheads). The image shows a volume rendering of three optical sections with 120-nm steps each, representing a slice of about 360 nm. Color levels above 200 (of the 255 levels of the 8-bit image) have been set to 255 to mark the granules (white). The scale bar represents 1 μm.
FIG. 2.
Confocal laser scanning fluorescence microscopy of C. latum. Shown is a series of optical sections through the same trichome as in Fig. 1. Each image is a volume rendering of two optical sections with a 120-nm step size, hence representing about 240 nm of the organism. The sections cover the whole volume of the cells. Color levels (in gray) above 200 (of the 255 levels of the 8-bit image) have been artificially stained in gray to mark the granules. The scale bar represents 1 μm. Arrowheads indicate PHB granules at or close to the cytoplasmic membrane. Arrows in slices 2 and 16 (thin arrows) indicate structures that are located close to the top or bottom layer of the cells. The big arrow in slice 9 indicates a PHB granule clearly detached from the membrane.
For statistical analysis, the subcellular localization of all PHB granules in six trichomes was determined. Forty-eight PHB granules out of 55 (87%) colocalized with the cytoplasmic membrane, while 4 granules (7%) appeared cytosolic, and 3 (5%) granules could not be addressed. In summary, most PHB granules in C. latum, at the early stage of formation, are located close to or are attached to the cytoplasmic membrane. The same observation was made in two other strains of C. latum (strain L and strain H [data not shown]). We conclude that the localization of PHB granules close to the cytoplasmic membrane shortly after induction of PHB accumulation is a general feature of C. latum.
TEM of C. latum.
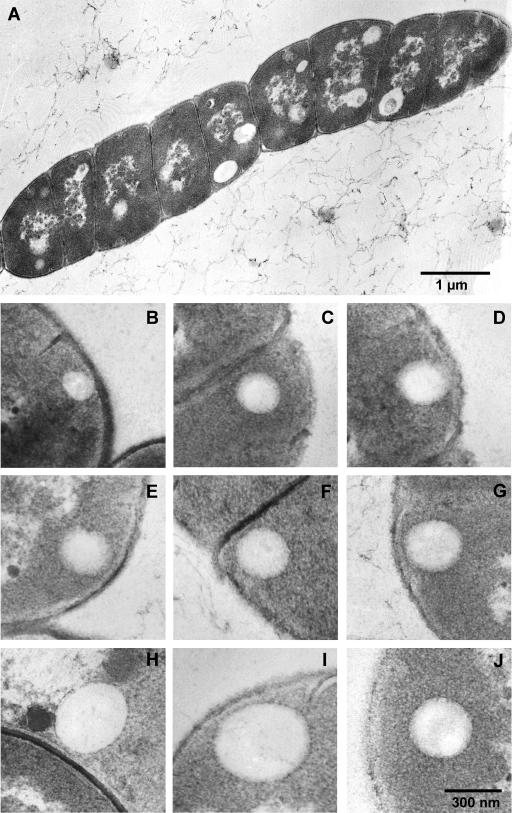
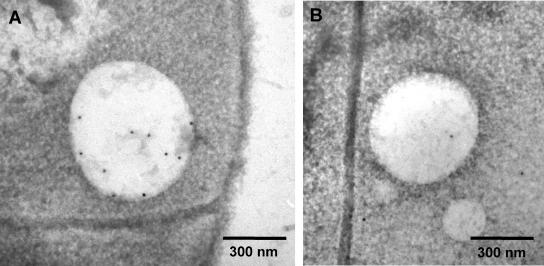
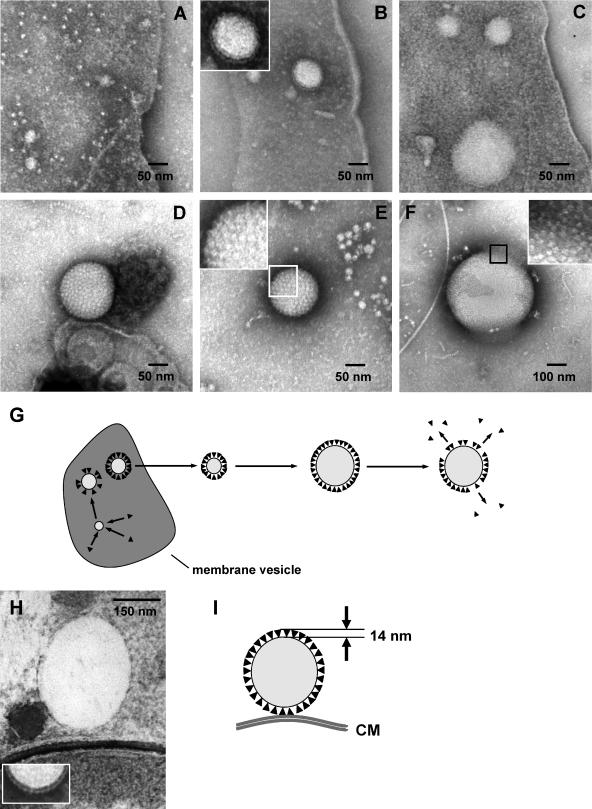
Inspired by the results shown above, we speculated that initiation of PHB granule formation in C. latum might happen in or at the cytoplasmic membrane. Since resolution of light microscopy is not high enough to distinguish between localization in, at, or very near the membrane, transmission electron microscopy (TEM) analysis was performed. We chose C. latum as an appropriate model organism since this bacterial species has large cell dimensions and synthesizes several relatively small PHB granules at a time. Figure 3A shows an ultrathin section through one trichome (nine cells) of C. latum 80 min after transfer to acetate medium. The trichome contains several globular structures that resemble PHB granules. To examine whether the observed globular structures represent PHB, immunogold staining using PHB-specific antibodies was performed. As shown in Fig. 4A, the globular structures were significantly labeled while a control, in which the primary antibody was omitted, showed negligible unspecific labeling (Fig. 4B). This result confirmed that the globular structures represent PHB granules and was in agreement with the finding that PHB granules appeared shortly after addition of acetate, a precursor metabolite of PHB. When the ultrathin section does not hit the center of a granule, but instead is a sagittal plane near the granular surface, or when the granule diameter is of comparable magnitude to the section thickness (≈80 nm), the granule appears light gray instead of bright (Fig. 3A, left cell, bottom). In these cases, the stained cytoplasm partially engulfs the globular cap (compare (22). For statistical analysis, the subcellular localization of all PHB granules in 50 cell sections was determined. From 283 granules, 82% colocalized with the cytoplasmic membrane, 12% appeared cytosolic, and 6% could not be addressed. In summary, most PHB granules in C. latum, at the early stage of formation, are located close to or are attached to the cytoplasmic membrane. In accordance with the results obtained by light microscopy, PHB granules were generally localized close to the cytoplasmic membrane. Only larger granules (≥300 nm in diameter) were clearly detached from the membrane. However, direct evidence for a coalescence of the PHB granule surface layer (4) with the cytoplasmic membrane was never observed. Figures 3B to J show a representative selection of PHB granules.
FIG. 3.
TEM analysis of C. latum: ultrathin sections of C. latum grown for 80 min on acetate medium. (A) Overview of a C. latum trichome. (B to J) Granules in immediate vicinity of the cytoplasmic membrane (B to I) and detached from the membrane (J). Note that the thickness of the PHB granule phasin layer is about 14 nm and that most PHB granules are not cut in the middle of the granule where the diameter would be maximal.
FIG. 4.
Immunolocalization of PHB in C. latum. (A) Anti-PHB antiserum. (B) PBS instead of antiserum (negative control).
TEM analysis of C. latum membrane vesicles.
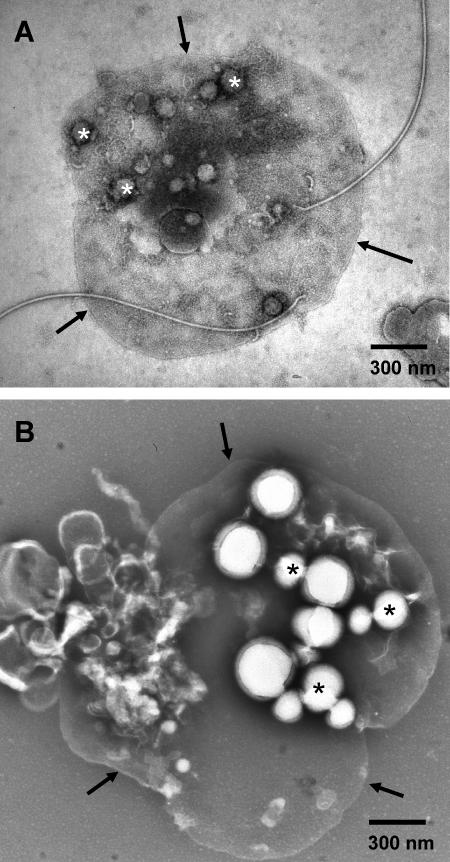
C. latum trichomes are very sensitive to mechanical disruption, as indicated by the large number of broken cells and by the appearance of membrane vesicles after centrifugation and resuspension. Preparations of membrane vesicles from almost PHB-free cells and from PHB-rich cells showed considerable differences: vesicles of PHB-depleted cells contained many globular structures that resembled small PHB granules. The diameter of these structures varied from 20 nm to about 100 nm. In Fig. 5A, three particles of this type are marked by asterisks. The marked structures are not vesicle blebs because they have a bright outline and are more irregularly shaped. PHB granules attached to membrane vesicles of cells that had been grown under PHB accumulation conditions were significantly larger, ranging from 70 to 300 nm in diameter (Fig. 5B). The larger granules appear much brighter than the small ones in Fig. 5A because they are less immersed in negative-staining salt, due to their size and the fact that they contain a hydrophobic compound. Our observation indicates an affinity of PHB granules to membranes. To investigate the time course of PHB granule formation, we looked at C. latum cells at different stages of growth. Figure 6A exemplarily shows small globular particles of 20 to 30 nm in diameter attached to membranes of cells that had been grown in the absence of acetate. Some of the frequently observed tiny structures about 10 nm in diameter may represent ATPase units, because they show the typical head-and-stalk morphology of the FoF1-ATPase. Occasionally, other globular structures (30 to 100 nm in diameter), resembling medium-sized PHB granules, were observed (data not shown). Figures 6B and C are representative for cells that had been exposed to PHB accumulation conditions (acetate medium) for 20 min. Globular structures with diameters ranging from approximately 30 to 150 nm (Fig. 6B and C) were found and probably represent PHB granules at early stages of growth. After longer incubation under PHB accumulation conditions, large PHB granules of various sizes were observed. Figures 6D to F show three individual PHB granules (ø 120 to 420 nm). Interestingly, the surface of the PHB granules is covered by a densely packed, paracrystalline-like layer of particles with a diameter of 8 nm in the front view (i.e., parallel to the particle surface). We assume that these particles represent phasin proteins that have been found at the surface of PHB in all PHB-accumulating bacteria analyzed so far (17). The granular surface layer was also observed at the early stages of granule formation (Fig. 6B and C). In several of the large PHB granules, the particles are sometimes detached from the PHB granule (Fig. 6F). In these cases, the surface is no longer covered with a densely packed array of particles. Some of the detached particles could be detected on the support film at some distance to the granule. The thickness of the observed surface layer of an isolated PHB granule after negative staining was determined to be 14 nm, suggesting that the phasin proteins are ellipsoid rather than globular (see inset in Fig. 6H). Taking this into consideration, the PHB granules shown in Fig. 6H, as well as those shown in Fig. 3B, E, F, H (same as in 6H), and I, seem attached to the inner side of the membrane because the minimum spacing between the PHB core and the cytoplasmic membrane is approximately 14 nm.
FIG. 5.
TEM analysis of C. latum: membrane vesicles in culture samples after negative staining. Some of the granules are marked by asterisks. Arrows point to the membrane boundaries of the vesicles. (A) Sample taken from an overnight culture grown on acetate-free medium. (B) Sample taken from a culture grown on acetate medium for 7 h.
FIG. 6.
Proposed model of formation and structure of granules. (A to F) Granules (C. latum) at different stages of development. Shown are PHB granules attached to membrane vesicles (A to C) and free PHB granules (D to F). Cells were grown on acetate-free medium (A) or on acetate medium for 20 min (B and C) or for 16 h (D to F). The inset in panel B indicates 14-nm particles on the surface of the enlarged PHB granule. The insets in panels E and F show enlarged views of the boxed areas. (G) Schematic drawing of granule development as implied by the observations described in panels A to F. An ultrathin section (see Fig. 3H) is compared with a negatively stained sample (H). The distance between a granule and the cytoplasmic membrane may be easily explained by the particle shell measuring 14 nm in thickness (I).
Discussion of LSFM and TEM data.
Our data show that in C. latum, PHB granules at the early stages of formation are located very close to the cytoplasmic membrane and/or are frequently attached to the inner cytoplasmic membrane via surface proteins of the PHB granules (presumably phasins). Binding of proteins to PHB granules and to other subcellular molecules, such as DNA (e.g., PhaR), has been described for R. eutropha and was shown to be important for regulation of PHB synthesis (16, 29). Our data do not indicate which of the different phasin proteins could be responsible for membrane attachment. The finding of a large number of regularly orientated proteins on the surface layer (Fig. 6D to F) does not exclude that other proteins could be responsible for the attachment. Recently, the presence of intracellularly localized dark-stained “mediation elements” serving as potential nucleation sites for PHB granules was described for R. eutropha (25, 26). These results differ from findings in this and previous studies (7, 14). However, despite this apparent difference, the data from all studies on PHB initiation indicate that formation of PHB granules does not occur randomly in the cytoplasm, as has been assumed for the last decades. Recently, convincing evidence was provided that the energetically not-favored linear organization of magnetosomes in the cytoplasm of magnetotactic bacteria was caused by attachment of magnetosomes via magnetosome-specific proteins (MamJ and MamK) to a cytoskeleton-like structure (9, 20). Apparently, the degree of subcellular organization in prokaryotes is underestimated.
Model of PHB granule formation.
Based on the results of our study, we propose the following model for PHB formation in C. latum (Fig. 6G). An initiation complex of PHB synthase and other components of unknown composition (phasins) binds to the inner surface of the cytoplasmic membrane or to proteins bound to the membrane. In the presence of sufficient substrate (3-hydroxybutyryl-coenzyme A), the nascent PHB granules quickly grow. Phasin proteins bind to the increasing surface of the growing PHB granule, probably via noncovalent interactions. Similar interactions might exist between phasin proteins and the cytoplasmic membrane, leading to the observed frequent localization of early PHB granules at or near the cytoplasmic membrane. During growth, the PHB granules occasionally detach from the membrane and become diffusible in the cytoplasm. At later stages of growth, cells reutilize (mobilize) accumulated PHB and phasin proteins partially detach from the surface of the decomposing PHB granule. This model is in agreement with the early finding of Shekhovtsov and Zharikova that lipid inclusions of C. latum attach and detach from the membranes during growth on solid medium (21).
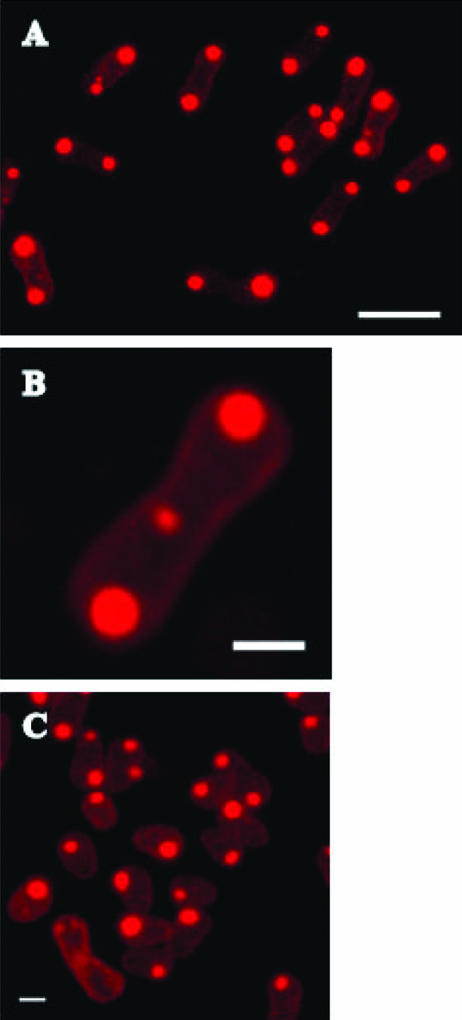
To exclude that the observed localization of PHB granules at or near the cytoplasmic membrane is a particularity of C. latum, the same experiment was performed with B. indica, a gram-negative, not trichome-forming bacterium with a known capability of PHB accumulation. Nile red staining and fluorescence microscopy investigation of glucose-grown cells of B. indica showed that almost all cells (≈98%) contained two PHB granules, each of which was localized at one end of the dumbbell-like cells (Fig. 7A). We never observed cells with more than three PHB granules. Occasionally, we found cells with one PHB granule at each of the two cell poles and one small granule in the middle of the cell (Fig. 7B). We assume that the cell in Fig. 7B is on the verge of cell division, suggesting that a new PHB granule can be formed at the site of septum formation. This could explain the fact that we frequently found cells with one large and one small PHB granule. Similar observations have been made in recombinant Escherichia coli expressing the PHB biosynthetic genes (7, 14). Older (resting) cells of B. indica tended to become ovoid and mostly harbored only one PHB granule (Fig. 7C). We suggest investigation of PHB granule formation in other bacteria of different taxa and with different types of PHB synthases and phasins to find out whether formation of PHB granules near the cytoplasmic membrane is a general feature or is a peculiarity of a few selected species.
FIG. 7.
Confocal laser scanning fluorescence microscopy of B. indica. Maximum projections of confocal stacks. A typical picture during growth is shown (24 h, overview) in panel A. The scale bar is 4 μm. Enlargement of one dumbbell-like cell harboring PHB granules at the two cell poles and one small PHB granule at the site of septum of the upcoming cell division is shown in panel B. The scale bar is 1 μm. Resting cells (48 h) became ellipsoid and often harbor only one PHB granule (C). The scale bar is 1 μm.
Supplementary Material
Acknowledgments
This work was supported by a grant of the Deutsche Forschungsgemeinschaft to D.J.
We thank Scientific Volume Imaging for generous support with respect to the deconvolution software package and O. Peoples (Metablix, Inc.) for supplying the PHB-specific antiserum.
Footnotes
Published ahead of print on 3 November 2006.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Abe, T., T. Kobayashi, and T. Saito. 2005. Properties of a novel intracellular poly(3-hydroxybutyrate) depolymerase with high specific activity (PhaZd) in Wautersia eutropha H16. J. Bacteriol. 187:6982-6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, A. J., and E. A. Dawes. 1990. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 54:450-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becking, J. 1992. The genus Beijerinckia, p. 2254-2267. In A. Balows, H. Trüper, M. Dworkin, W. Harder, and K. Schleifer (ed.), The prokaryotes, vol. III. Springer Verlag, New York, NY. [Google Scholar]
- 4.Boatman, E. S. 1964. Observations on the fine structure of spheroplasts of Rhodospirillum rubrum. J. Cell Biol. 20:297-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degelau, A., T. Scheper, J. Bailey, and C. Guske. 1995. Fluorometric measurements of poly-beta-hydroxybutyrate in Alcaligenes eutrophus by flow cytometry and spectrofluoromety. Appl. Microbiol. Biotechnol. 42:653-657. [Google Scholar]
- 6.Hoppert, M., and A. Holzenburg. 1998. Electron microscopy in microbiology. Bios Scientific Publishers, Oxford, United Kingdom.
- 7.Jendrossek, D. 2005. Fluorescence microscopical investigation of poly(3-hydroxybutyrate) granule formation in bacteria. Biomacromolecules 6:598-603. [DOI] [PubMed] [Google Scholar]
- 8.Jendrossek, D., and R. Handrick. 2002. Microbial degradation of polyhydroxyalkanoates. Annu. Rev. Microbiol. 56:403-432. [DOI] [PubMed] [Google Scholar]
- 9.Komeili, A., Z. Li, D. K. Newman, and G. J. Jensen. 2006. Magnetosomes are cell membrane invaginations organized by the actin-like protein MamK. Science 311:242-245. [DOI] [PubMed] [Google Scholar]
- 10.Lundgren, D. G., R. M. Pfister, and J. M. Merrick. 1964. Structure of poly-beta-hydroxybutyric acid granules. J. Gen. Microbiol. 34:441-446. [DOI] [PubMed] [Google Scholar]
- 11.Merrick, J. M., D. G. Lundgren, and R. M. Pfister. 1965. Morphological changes in poly-β-hydroxybutyrate granules associated with decreased susceptibility to enzymatic hydrolysis. J. Bacteriol. 89:234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page, W. J., R. Sherburne, D. D′Elia, and L. L. Graham. 1995. Poly(β-hydroxybutyrate) extrusion from pleomorphic cells of Azotobacter vinelandii UWD. Can. J. Microbiol. 41(Suppl. 1):22-31. [Google Scholar]
- 13.Peshkov, M., and B. Marek. 1972. Fine structure of Caryphanon latum and Caryophanon tenue PESHKOFF. Mikrobiologiya 41:1064-1067. [PubMed] [Google Scholar]
- 14.Peters, V., and B. H. Rehm. 2005. In vivo monitoring of PHA granule formation using GFP-labeled PHA synthases. FEMS Microbiol. Lett. 248:93-100. [DOI] [PubMed] [Google Scholar]
- 15.Pfister, R. M., and D. G. Lundgren. 1964. Electron microscopy of polyribosomes within Bacillus cereus. J. Bacteriol. 88:1119-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pötter, M., M. H. Madkour, F. Mayer, and A. Steinbüchel. 2002. Regulation of phasin expression and polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha H16. Microbiology 148:2413-2426. [DOI] [PubMed] [Google Scholar]
- 17.Pötter, M., and A. Steinbüchel. 2005. Poly(3-hydroxybutyrate) granule-associated proteins: impacts on poly(3-hydroxybutyrate) synthesis and degradation. Biomacromolecules 6:552-560. [DOI] [PubMed] [Google Scholar]
- 18.Provost, P., and R. Doetsch. 1962. An appraisal of Caryophanon latum. J. Gen. Microbiol. 28:547-557. [DOI] [PubMed] [Google Scholar]
- 19.Roth, J., M. Bendayan, E. Carlemalm, W. Villiger, and M. Garavito. 1981. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J. Histochem. Cytochem. 29:663-671. [DOI] [PubMed] [Google Scholar]
- 20.Scheffel, A., M. Gruska, D. Faivre, A. Linaroudis, J. M. Plitzko, and D. Schüler. 2006. An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nature 440:110-114. [DOI] [PubMed] [Google Scholar]
- 21.Shekhovtsov, V., and G. Zharikova. 1978. Cytomorphology of lipid inclusions of Caryphanon during its growth on a solid medium. Mikrobiologiya 47:733-738. [PubMed] [Google Scholar]
- 22.Shively, J. M. 1974. Inclusion bodies of prokaryotes. Annu. Rev. Microbiol. 28:167-187. [DOI] [PubMed] [Google Scholar]
- 23.Spiekermann, P., B. H. Rehm, R. Kalscheuer, D. Baumeister, and A. Steinbüchel. 1999. A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 171:73-80. [DOI] [PubMed] [Google Scholar]
- 24.Steinbüchel, A., and S. Hein. 2001. Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv. Biochem. Eng. Biotechnol. 71:81-123. [DOI] [PubMed] [Google Scholar]
- 25.Tian, J., A. He, A. G. Lawrence, P. Liu, N. Watson, A. J. Sinskey, and J. Stubbe. 2005. Analysis of transient polyhydroxybutyrate production in Wautersia eutropha H16 by quantitative Western analysis and transmission electron microscopy. J. Bacteriol. 187:3825-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian, J., A. J. Sinskey, and J. Stubbe. 2005. Kinetic studies of polyhydroxybutyrate granule formation in Wautesia eutropha H16 by transmission electron microscopy. J. Bacteriol. 187:3814-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trentini, W. 1978. Biology of the genus Caryophanon. Annu. Rev. Microbiol. 32:123-141. [DOI] [PubMed] [Google Scholar]
- 28.Trentini, W., and R. Murray. 1975. Ultrastructural effects of lysozymes on the cell wall of Caryophanon latum. Can. J. Microbiol. 21:164-172. [DOI] [PubMed] [Google Scholar]
- 29.York, G. M., J. Stubbe, and A. J. Sinskey. 2002. The Ralstonia eutropha PhaR protein couples synthesis of the PhaP phasin to the presence of polyhydroxybutyrate in cells and promotes polyhydroxybutyrate production. J. Bacteriol. 184:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.