Abstract
Bacterial communities associated with marine algae are often dominated by members of the Roseobacter clade, and in the present study, we describe Roseobacter phenotypes that may provide this group of bacteria with selective advantages when colonizing this niche. Nine of 14 members of the Roseobacter clade, of which half were isolated from cultures of the dinoflagellate Pfiesteria piscicida, produced antibacterial compounds. Many non-Roseobacter marine bacteria were inhibited by sterile filtered supernatants of Silicibacter sp. TM1040 and Phaeobacter (formerly Roseobacter) strain 27-4, which had the highest production of antibacterial compound. In contrast, Roseobacter strains were susceptible only when exposed to concentrated compound. The production of antibacterial compound was influenced by the growth conditions, as production was most pronounced when bacteria were grown in liquid medium under static conditions. Under these conditions, Silicibacter sp. TM1040 cells attached to one another, forming rosettes, as has previously been reported for Phaeobacter 27-4. A spontaneous Phaeobacter 27-4 mutant unable to form rosettes was also defective in biofilm formation and the production of antibacterial compound, indicating a possible link between these phenotypes. Rosette formation was observed in 8 of 14 Roseobacter clade strains examined and was very pronounced under static growth in 5 of these strains. Attachment to surfaces and biofilm formation at the air-liquid interface by these five strains was greatly facilitated by growth conditions that favored rosette formation, and rosette-forming strains were 13 to 30 times more efficient in attaching to glass compared to strains under conditions where rosette formation was not pronounced. We hypothesize that the ability to produce antibacterial compounds that principally inhibit non-Roseobacter species, combined with an enhancement in biofilm formation, may give members of the Roseobacter clade a selective advantage and help to explain the dominance of members of this clade in association with marine algal microbiota.
Bacteria belonging to the α-Proteobacteria are widely distributed in marine waters and are the dominant bacterial group in surface bacterioplankton communities (13, 30, 40). However the different α-proteobacterial groups are not equally distributed between the different marine niches, as members of the Roseobacter clade dominate among marine alga-associated bacteria (1, 9, 15, 21, 31, 47), whereas members of the SAR11 (Pelagibacter ubique) cluster dominate the total bacterioplankton community (13, 30). Understanding how the bacteria interact with other bacteria and with their algal hosts is important, since these interactions can affect the growth and physiology of the algae (1, 18), particularly in the production or modification of algal toxins (12). The dominance of the Roseobacter clade in bacterial communities associated with algae could indicate that there are specific phenotypic traits that provide these bacteria with a selective advantage in colonizing algae.
Bacteria belonging to the Roseobacter clade are rapid colonizers of surfaces (11), and they have been called aggressive colonizers, as a Phaeobacter gallaeciensis (reclassified from Roseobacter gallaeciensis [26]) invaded and dispersed preestablished biofilm of marine bacteria on the marine plant Ulva australis (34). This ability to colonize and form a biofilm may be one of the factors that allows these bacteria to colonize alga. It is not known why bacteria from the Roseobacter clade are so efficient in colonizing, but both attachment and biofilm formation have recently been linked to the ability of a Phaeobacter strain (27-4) to grow in a multicellular rosette shape (7, 8). This rosette shape has previously been reported for members of the Roseobacter clade (37, 43). The rosette mode of growth has also been described for Ruegeria atlantica, a member of the Roseobacter clade isolated from the toxic dinoflagellate Alexandrium catenella (2), but it is not known whether this phenotype facilitates the attachment and biofilm formation of that bacterium.
Several studies have found that members of the Roseobacter clade inhibit other bacteria (6, 8, 38), and this may contribute to their dominance among alga-associated bacteria. The production of antibacterial compound by Phaeobacter strain 27-4 was influenced by culture conditions and was detected only when the bacteria were grown in liquid nutrient medium under static conditions, which also facilitated rosette and biofilm formation (7, 8). Indeed particle-associated members of the Roseobacter clade are 13 times more likely to produce antimicrobial compounds than are free-living members (24). Furthermore, while growing in a biofilm, a member of the Roseobacter clade was able to prevent the growth of other bacteria on surfaces (33, 34).
Marine aggregates are characterized by high bacterial densities (108 to 109 bacteria/ml) that are 1,000- to 10,000-fold higher than the surrounding water (22, 32, 39) and comparable to bacterial densities (106 to 109 cells/ml) observed in algal cultures (31). At high cell densities, bacteria may use quorum sensing (QS) to regulate the production of extracellular compounds (46). QS signals have been detected in Roseobacter species (7, 16, 45), and since the production of antibacterial compounds in some bacteria is controlled by QS (3, 48), it has been suggested as a regulatory mechanism for Roseobacter production of antibacterial compounds (8, 44).
One may hypothesize that the dominance of members of the Roseobacter clade among alga-associated bacteria is influenced by specific phenotypes, such as an ability to attach and form biofilms or by the production of antibacterial compounds. The present study was undertaken to determine whether biofilm formation and the production of antibacterial compounds are common phenotypes found in members of the Roseobacter clade, especially strains associated with dinoflagellates (e.g., Pfiesteria piscicida). In addition, we address whether such behaviors occur under specific growth conditions, e.g., static or shaken cultures, and whether they occur when specific morphotypes, such as rosettes, are prevalent.
MATERIALS AND METHODS
Bacterial strains and media.
Thirteen stains of α-Proteobacteria belonging to the Roseobacter clade, all isolated from marine environments, most from dinoflagellate cultures of Pfiesteria piscicida, were tested in the present study (Table 1). Furthermore, the non-α-Proteobacteria marine bacteria Halomonas sp., Pseudomonas elongata, “Spongiobacter nickelotolerans,” Shewanella sp., Vibrio cholerae (four environmental strains and two clinical strains), Vibrio coralliilyticus, Vibrio fortis, Vibrio mediterranei, Vibrio harveyi, Vibrio shiloi (see Table 3), and Mycobacterium marinum were tested for their susceptibilities to active compounds produced by Silicibacter sp. strain TM1040 and Phaeobacter strain 27-4. Phaeobacter strain 27-4 isolated from turbot larval rearing units was included as a control strain exhibiting inhibitory activity to many γ-Proteobacteria and the ability to form rosettes and biofilms (8, 20). This strain was originally named Roseobacter and clustered close to the Roseobacter gallaciensis group (6). Recently, this group has been reclassified as Phaeobacter gallaciensis (26) and we have used this naming throughout the study. All strains were stored at −80°C in 30% glycerol. Strains were maintained on marine agar (Difco Marine Agar 2216), and liquid broth cultures were prepared in marine broth (MB; Difco Marine Broth 2216) incubated at 25°C under static or shaking (200 rpm) conditions in the dark, as previously described (8).
TABLE 1.
Rosette formation, attachment ability, and motility of bacteria belonging to the Roseobacter clade used in this studya
| Bacterial strain | Isolated from: | Formation of single cells or rosettes | Attachment ability | Motility | Reference |
|---|---|---|---|---|---|
| Sulfitobacter strain EE36 | Coastal seawater | Rosettesb | + | + | |
| Roseovarius strain ISM | Coastal seawater | Single cells | − | − | 14 |
| Sulfitobacter strain 1921 | P. piscicida | Rosettesb | + | + | |
| Silicibacter pomeroyi DSS-3 | Coastal seawater | Rosettes | − | + | 14 |
| Sulfitobacter strain SE62 | Coastal seawater | Rosettes | − | + | |
| Roseobacter litoralis 49566 | Seawater | Single cells | − | − | 41 |
| Roseobacter algicola 51442 | Prorocentrum lima | Rosettes | − | + | 23 |
| Roseobacter denitrificans 33942 | Seawater | Single cells | − | + | 41 |
| Silicibacter sp. strain TM1040 | P. piscicida | Rosettesb | + | + | 27 |
| Roseobacter sp. strain TM1039 | P. piscicida | Rosettesb | + | + | 27 |
| Roseovarius sp. strain TM1042 | P. piscicida | Single cells | − | − | 27 |
| Roseovarius sp. strain TM1035 | P. piscicida | Single cells | − | − | 27 |
| Roseobacter sp. strain TM1038 | P. piscicida | Single cells | − | − | 27 |
| Phaeobacter strain 27-4 | Turbot rearing facility | Rosettesb | + | + | 20 |
+, possessed the indicated phenotype; −, did not possess the indicated phenotype.
Rosette formation was pronounced in statically grown cultures.
TABLE 3.
Inhibition of marine bacteria on marine agar by raw sterile filtered supernatants of Silicibacter sp. TM1040 and Phaeobacter 27-4
| Species | Code | Role | Size (mm) of inhibition zone for:a
|
Sourceb | |
|---|---|---|---|---|---|
| TM1040 | 27-4 | ||||
| Vibrio anguillarum | 90-11-287 | Fish pathogen | 17 | 17 | |
| Pseudomonas elongata | Marine bacterium | 11 | 12 | 1 | |
| Spongiobacter nikelotolerans | Marine bacterium | 32 | 24 | 1 | |
| Vibrio cholerae | C25 | Clinical strain | 23 | 21 | 2 |
| Vibrio cholerae | C42 | Clinical strain | 36 | 28 | 2 |
| Vibrio cholerae | 1 | Environmental strain | 17 | 19 | 2 |
| Vibrio cholerae | 184 | Environmental strain | 21 | 20 | 2 |
| Vibrio cholerae | 4 | Environmental strain | 27 | 20 | 2 |
| Vibrio cholerae | 5 | Environmental strain | 30 | 23 | 2 |
| Vibrio coralliilyticus | Coral pathogen | 14 | 14 | ||
| Vibrio shiloi | Coral pathogen | 14 | 14 | ||
| Halomonas spp. | Marine bacterium | 17 | 18 | 1 | |
| Shewanella spp. | Marine bacterium | ni | ni | 1 | |
| Vibrio harveyi | Marine bacterium | ni | ni | 1 | |
| Vibrio fortis | Marine bacterium | ni | ni | 1 | |
| Vibrio mediterranei | Marine bacterium | ni | ni | 1 | |
ni, no inhibition.
The sources of the strains were the laboratories of Russell T. Hill (1) and Rita R. Colwell (2), Center of Marine Biotechnology, University of Maryland Biotechnology Institute, as indicated.
Attachment assay.
Glass coverslips (Corning; catalog no. 2865-22) were dipped into bacterial cultures grown under either static or shaking conditions and left for 5 s. After removal, nonattached and poorly attached bacteria were removed by placing the glass coverslip on a sterile absorbent paper. The coverslips were incubated at 60°C for 30 min to fix the bacteria. Bacterial cells attached to the coverslip were stained with 0.1% crystal violet for 15 min at room temperature, and unbound dye was removed by rinsing the glass coverslip with 1× phosphate-buffered saline. The coverslips were immersed in 2 ml 33% acetic acid to solubilize the dye bound to the biofilm, which was measured using a Beckman DU640 spectrophotometer at 590 nm.
Cell morphology, motility, and biofilm at air-liquid interphase.
Bacterial motility, rosette formation, and biofilm formation at the air-liquid interphase in shaken and statically grown cultures were assessed by phase-contrast microscopy using an Olympus BX60 microscope. Images were captured using a Qicam Fast 1394 digital camera (QImaging, Burnaby, British Columbia, Canada) and Volocity, version 3.5.1, computer software (Improvision, Coventry, England). Motility was also measured in both shaken and statically grown cultures by using marine motility agar (Difco Marine Broth 2216 supplemented with 3 g Bacto agar per liter) (28).
Assessment of antibacterial activity using a well diffusion assay.
Sterile filtered (0.22 μm, Millipore) supernatants from outgrown bacterial cultures as well as ethyl acetate extracts of the cultures were tested for antibacterial activity in a well diffusion assay using Vibrio anguillarum strain 90-11-287 (serotype O1 strain), as previously described (20). This species of γ-Proteobacteria was chosen as a target organism because of its high sensitivity to the antibacterial compound of Phaeobacter strain 27-4 (J. Bruhn, D. Hougaard, and L. Gram, personal observation). Sixty microliters of each sample was added to a well in an agar cast with V. anguillarum 90-11-287. Plates were incubated at 20°C for 24 h, whereupon inhibitory activity was detected as a zone of clearing in the turbid agar around the wells containing antibacterial activity (positive samples). The diameter of the clearing zones was measured to obtain a semiquantitative determination of the concentration of the antibacterial compound.
To investigate the spectrum of bacterial species inhibited by the antibacterial compounds from Silicibacter sp. strain TM1040 and Phaeobacter strain 27-4, marine bacteria (see Table 3) were grown to stationary phase in marine broth and 100 μl of a 10-fold dilution of each culture spread onto the surface of a petri dish containing marine agar. Wells were punched in the agar, supernatants from statically grown cultures of Silicibacter sp. strain TM1040 and Phaeobacter strain 27-4 were then added, and the cultures were incubated for 1 to 3 days until a lawn of the target bacteria was visible. As described earlier, a zone of clearing in the bacterial lawn around wells was used to indicate that the target bacteria were sensitive either to the Silicibacter sp. strain TM1040 or the Phaeobacter strain 27-4 supernatants.
Screening for AHLs.
Bacterial strains (Table 1) were grown in MB at 25°C for 4 days, and 20 ml each of the resulting cultures was extracted with 20 ml ethyl acetate (containing 0.5% formic acid). The ethyl acetate fraction was evaporated under airflow to dryness, reconstituted in 1 ml acidified ethyl acetate, and stored at −20°C. The detection of acylated homoserine lactone (AHL) compounds was carried out with the two AHL monitor strains, Agrobacterium tumefaciens NT1 (pZLR4) (10) and Chromobacterium violaceum CV026 (42), by using a well diffusion assay (35).
Pigment production.
The 14 bacterial species were grown in MB as static or shaken cultures for 4 days at 25°C. The amount of extracellular pigment contained in 0.22-μm-filtered supernatants was determined by measuring absorbance at 398 nm (8).
Dependence of pigment and antibacterial compound production on bacterial culture conditions.
Roseobacter strain 27-4 and Silicibacter sp. strain TM1040 were inoculated in MB at an initial density of 5 × 102 CFU/ml and incubated at 25°C under shaking and static conditions. Growth, as measured by CFU per milliliter, was followed by spread plating on marine agar (incubated at 25°C for 3 days) and enumeration of the resulting colonies. Pigment production and antibacterial activity were assessed as described above.
RESULTS
Attachment of Roseobacter clade strains during different growth conditions.
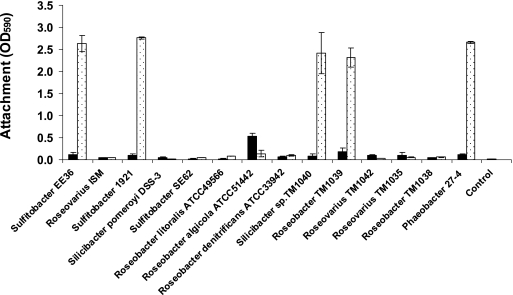
Fourteen members of the Roseobacter clade (Table 1) were tested for their abilities to attach to a surface, and the influence of preculture conditions (static or shaken) on attachment was determined. Static cultures of Roseobacter sp. strain TM1039, Silicibacter sp. strain TM1040, Sulfitobacter strain EE36, Sulfitobacter strain 1921, and Phaeobacter strain 27-4 attached very efficiently to the surfaces (Fig. 1). Preculture conditions influenced the attachment of these strains, and cells grown under static culture conditions attached 13 to 30 times more readily than did their shaken-culture counterparts, even though the shaken cultures had approximately 10-fold-higher cell densities (Table 2). The attachment of the remaining nine strains was low, and only small differences between shaken and statically grown cultures were noted, suggesting that this is a species- or strain-specific trait and not a clade-specific attribute.
FIG. 1.
Attachment of Roseobacter clade bacteria from statically grown (dotted bars) or shaken cultures (black bars) to glass cover slides measured by crystal violet at OD590. The bars represent averages of duplicate determinations, and error bars represent standard deviations. Glass cover slides dipped into sterile medium served as a control for both static and shaking cultures.
TABLE 2.
Detection of antibacterial compound, pigment and acylated homoserine lactones from Roseobacter clade strains depending on growth conditionsa
| Bacterial strain | Growth condition | Inhibition zone (mm) against V. anguillarum for:
|
Pigment production at OD398 | AHL induction in A. tumefaciens zone (mm) | Log CFU | |
|---|---|---|---|---|---|---|
| Sterile filtered culture | Ethyl acetate extract | |||||
| Sulfitobacter strain EE36 | Shaken | ni | ni | 0.00 | ni | 8.78 |
| Static | ni | 13 | 0.02 | ni | 8.70 | |
| Roseovarius strain ISM | Shaken | ni | ni | 0.00 | ni | 9.70 |
| Static | ni | 16 | 0.00 | 20 | 9.00 | |
| Sulfitobacter strain 1921 | Shaken | ni | ni | 0.00 | ni | 9.95 |
| Static | ni | ni | 0.00 | ni | 8.60 | |
| Silicibacter pomeroyi DSS-3 | Shaken | ni | 15 | 0.00 | 18 | 7.69 |
| Static | ni | ni | 0.00 | 25 | 7.00 | |
| Sulfitobacter strain SE62 | Shaken | ni | ni | 0.00 | ni | 9.60 |
| Static | ni | ni | 0.00 | ni | 8.48 | |
| Roseobacter litoralis ATCC 49566 | Shaken | ni | ni | 0.00 | ni | 7.30 |
| Static | ni | ni | 0.00 | ni | 8.78 | |
| Roseobacter algicola ATCC 51442 | Shaken | ni | ni | 0.00 | ni | 9.00 |
| Static | ni | ni | 0.01 | ni | 8.30 | |
| Roseobacter denitrificans ATCC 33942 | Shaken | ni | ni | 0.00 | ni | 8.48 |
| Static | ni | ni | 0.02 | ni | 9.00 | |
| Silicibacter sp. strain TM1040 | Shaken | 16 | 22 | 0.22 | ni | 9.30 |
| Static | 27 | 35 | 1.81 | ni | 8.48 | |
| Roseobacter sp. strain TM1039 | Shaken | ni | ni | 0.00 | ni | 9.70 |
| Static | ni | 13 | 0.02 | ni | 8.90 | |
| Roseovarius sp. strain TM1042 | Shaken | ni | ni | 0.00 | 36 | 9.30 |
| Static | ni | 13 | 0.00 | 38 | 8.60 | |
| Roseovarius sp. strain TM1035 | Shaken | ni | ni | 0.00 | 37 | 9.48 |
| Static | ni | 13 | 0.00 | 36 | 9.00 | |
| Roseobacter sp. strain TM1038 | Shaken | ni | ni | 0.00 | ni | 9.70 |
| Static | ni | 16 | 0.00 | ni | 8.90 | |
| Phaeobacter strain 27-4 | Shaken | ni | ni | 0.01 | tr | 9.90 |
| Static | 27 | 35 | 1.46 | 42 | 8.48 | |
Antibacterial activity assessed against Vibrio anguillarum; ni, no inhibition or no induction; tr, trace.
Cell morphology, biofilm formation, and motility of Roseobacter clade strains.
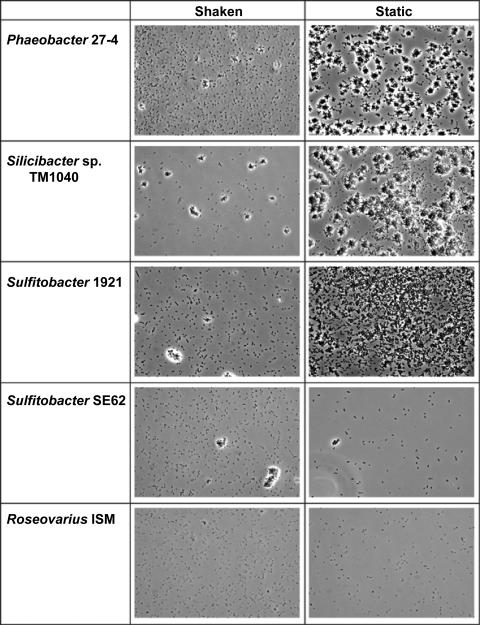
It has been suggested that the formation of rosettes of cells, which is seen under static growth of Phaeobacter strain 27-4, affects the attachment and biofilm formation of the bacterium (7). Several of the strains included in the present study were capable of growing as rosettes; however, the degrees to which this occurred differed markedly (Fig. 2; see the supplemental material). Rosettes were prominently formed in Roseobacter sp. strain TM1039, Silicibacter sp. strain TM1040, Sulfitobacter strain EE36, and Sulfitobacter strain 1921 in static culture, a condition that also resulted in maximal bacterial attachment (Fig. 1) and biofilm formation at the air-liquid interphase. Rosettes were also observed in Silicibacter pomeroyi DSS-3, Sulfitobacter strain SE62, and Roseobacter algicola ATCC 51442, albeit at a much lower level with a corresponding lower attachment (Table 1 and Fig. 2; see the supplemental material). The formations of rosettes in cultures of Silicibacter sp. strain TM1040 and Phaeobacter strain 27-4 were very similar and differed somewhat from what was observed with the other strains (Fig. 2; see the supplemental material). For example, Roseobacter denitrificans ATCC 33942 and Roseobacter litoralis ATCC 49566 produced an aggregate when grown as shaken cultures, not under static culture conditions, and also had a reduced CFU per milliliter in the shaken cultures compared to those of the static cultures (Table 2). However, these aggregates did not facilitate biofilm formation at the air-liquid interface and did not attach to the glass slide (Fig. 1).
FIG. 2.
Cell morphology of five Roseobacter clade strains grown under static or shaking conditions. Phase-contrast light microscopy (magnification, ×400) was used to observe morphology and biofilm formation at the air-liquid interphase. Shown are Phaeobacter strain 27-4, Silicibacter sp. strain TM1040, Sulfitobacter strain 1921 with high attachment capacity from static cultures (Fig. 1), Sulfitobacter strain SE62 forming few rosettes without having high attachment, and Roseovarius strain ISM without rosette formation and with low attachment capacity from static cultures (Fig. 1).
Nine of the 14 Roseobacter species were motile, as determined by their outward movement in semisolid motility agar. Two strains, the R. denitrificans and R. algicola strains, failed to show overt movement in motility agar, but light microscopy of these cells revealed that they swam (Table 1). Eight of the nine motile strains were capable of forming rosettes, with the exception of the Roseobacter denitrificans strain, which did not form rosettes yet was motile.
Production of antibacterial compounds.
Ethyl acetate extracts from 9 of the 14 strains inhibited the growth of V. anguillarum (Table 2). Seven of these nine strains caused inhibition only when extracts were prepared from Roseobacter species grown in static culture conditions. Antibacterial activity was observed from both static and shaken cultures of Silicibacter sp. strain TM1040; however, the inhibition zones from static cultures were much larger than those from shaken cultures, despite a higher cell density in the shaken culture. In one strain (S. pomeroyi DSS-3), inhibitory activity was seen only in extracts obtained from the shaken culture.
In contrast to the inhibitory activity of ethyl acetate extracts, antibacterial activity, as measured by the zone of clearing in V. anguillarum well diffusion assays, was detectable only in filtered culture medium supernatants obtained from Phaeobacter strain 27-4 and Silicibacter sp. strain TM1040 (Table 2). This may be due to an increase in the concentration of the antibacterial compound during ethyl acetate extraction.
Detection of AHLs.
This set of strains was also tested for the production of QS molecules, under the hypothesis that the production of antibacterial compound could be linked to the production of AHLs. AHLs were detected in five strains, all of which had antibacterial activity, but not in any of the noninhibitory strains. Ethyl acetate extracts from both shaken and statically grown cultures of Roseovarius sp. strain TM1035, Roseovarius sp. strain TM1042, and S. pomeroyi DSS-3 induced a response from A. tumefaciens NT1(pZLR4) (Table 2), whereas only the static cultures of Roseovarius sp. strain TM1035 and Roseovarius sp. strain TM1042 had detectable antibacterial activity. Only statically grown Roseovarius strain ISM and Phaeobacter strain 27-4 induced Agrobacterium tumefaciens NT1(pZLR4), and none of the extracts induced C. violaceum CV026.
Pigment production.
The amount of a dark brown pigment produced by Phaeobacter strain 27-4 is correlated with the production of the antibacterial compound tropodithietic acid (8). Pigment production was therefore measured from all strains grown under both static and shaken conditions. Of the 14 strains, Phaeobacter strain 27-4 and Silicibacter sp. strain TM1040 were the only 2 strains that formed visible pigment, although trace amounts of the pigment were detectable in 3 additional strains (Table 2). These experiments corroborated our earlier work (8) showing that Phaeobacter strain 27-4 produced pigment only when grown under static conditions and demonstrated that Silicibacter sp. strain TM1040 also produced a brownish/black pigment when grown under static conditions. Pigment was also measurable in Silicibacter sp. strain TM1040 when grown under shaking conditions. However, the amount of pigment was much larger (2- to 10-fold) in the static culture compared to that in the shaken culture, despite a 10-fold-lower cell density in the former. As observed previously in cultures of Phaeobacter strain 27-4, the production of pigment by Silicibacter sp. strain TM1040 started at the air-liquid interface in the static culture.
Bacterial attachment, biofilm formation, and antibacterial activity in spontaneous nonpigmented mutants of Phaeobacter strain 27-4 and Silicibacter sp. strain TM1040.
On rare occasions, colorless colonies appeared spontaneously in both Silicibacter sp. strain TM1040 and Phaeobacter strain 27-4. Although the nature of this change is not known, these nonpigmented mutants are stable, as the strains do not regain pigment production upon repeated attempts and prolonged culturing. One colorless mutant from each strain was isolated and tested for the ability to attach to surfaces, form rosettes and biofilm, and produce pigment and antibacterial activity. A nonpigmented mutant of Silicibacter sp. strain TM1040 lost its ability to produce pigment and antibacterial compounds under both shaken and static culture conditions. Under static conditions, these bacteria produced rosettes and attached similarly to the parental strain. On the other hand, a nonpigmented mutant of Phaeobacter strain 27-4 produced trace amounts of pigment (the optical density at 398 nm [OD398] was 0.1 compared to an OD398 of 1.4 produced by its parental strain) and also produced only a trace amount of the antibacterial compound when grown under static culture conditions, whereas cells grown in shaken cultures were devoid of antibacterial activity similar to that of the parental strain. The morphologies of the nonpigmented Phaeobacter strain 27-4 cells grown under static conditions differed from that of the parent strain, as the nonpigmented mutant strain lost its ability to produce rosettes, did not attached to glass slides, and did not form a biofilm at the air-liquid interface.
Correlation between pigment and antibacterial activity in Phaeobacter strain 27-4 and Silicibacter sp. strain TM1040.
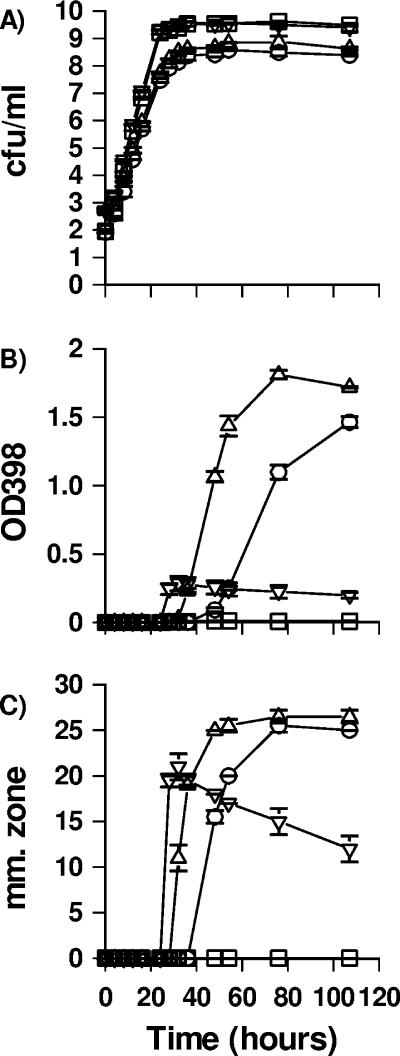
Phaeobacter strain 27-4 and Silicibacter sp. strain TM1040 were grown under static or shaking culture conditions in MB to further elucidate the co-occurrence of pigment and antibacterial activity. Under shaking conditions, the cell densities increased for both strains from 1 × 103 to 5 × 109 CFU/ml within 24 h, whereas the static cultures required 36 h to reach a density of 5 × 108 CFU/ml (Fig. 3). Pigment was detectable after 24 h in the shaken culture of Silicibacter sp. strain TM1040 and reached a constant level of an OD398 of 0.2 after 36 h. In the shaken culture of Phaeobacter strain 27-4, the pigment was detectable after 36 h at an OD398 of approximately 0.01 and remained at this level. The amount of pigment was significantly greater in both strains under static culture conditions, reaching a maximum of OD398 of ca. 1.5. Pigment, however, was observed at different times as it was detected after 32 h in static cultures of Silicibacter sp. strain TM1040 but after 48 h in Phaeobacter strain 27-4 cultures, even though the strains had a similar growth rates. Antibacterial inhibition zones coappeared at the same time that the pigment was detected for both strains grown under static conditions, reaching a maximum of ca. 25 mm. Inhibition zones from shaken cultures of Silicibacter sp. strain TM1040 appeared after 32 h with a zone of ca. 21 mm; thereafter, the zone decreased. Shaken cultures of Phaeobacter strain 27-4 did not inhibit V. anguillarum. These results indicate a clear correlation between pigment and antibacterial compound production for both Silicibacter sp. strain TM1040 and Phaeobacter strain 27-4 grown under static conditions.
FIG. 3.
Influence of culture conditions on the growth, production of pigment, and antibacterial activity of Phaeobacter strain 27-4 and Silicibacter sp. strain TM1040 in MB at 25°C. The data represent the mean of duplicate cultures (error bars represent standard deviations). (A) Growth was measured by plate counts. □, shaken culture of Phaeobacter strain 27-4; ○, static culture of Phaeobacter strain 27-4; ▿, shaken culture of Silicibacter sp. strain TM1040; ▵, static culture of Silicibacter sp. strain TM1040. (B) Pigment formation, as measured by absorbance at OD398. (C) Antibacterial activity represented by the zone of inhibition (in millimeters), as determined by using the well diffusion assay with V. anguillarum.
Susceptibility of Roseobacter strains to the antibacterial compound produced by Silicibacter sp. strain TM1040 and Phaeobacter strain 27-4.
None of the Roseobacter clade strains tested (Table 1) were sensitive to filtered culture supernatants containing the antibacterial activity produced by either Silicibacter sp. strain TM1040 or Phaeobacter strain 27-4. This is in contrast to the non-Roseobacter marine species tested, many of which were sensitive to the filtered supernatant. V. anguillarum 90-11-287, Pseudomonas elongate, “Spongiobacter nickelotolerans,” environmental and clinical strains of Vibrio cholerae, V. coralliilyticus, V. shiloi, and a Halomonas sp. (all members of the γ-Proteobacteria) were sensitive to the compound(s) (Table 3). Strains of Shewanella, V. harveyi, V. fortis, and V. mediterranei were each resistant to the antibacterial activity, showing no zone of inhibition. This pattern of inhibition changed when ethyl acetate extracts were used instead of filtered supernatants. In this case, both Roseobacter clade bacteria and non-Roseobacter species were impacted when ethyl acetate extracts of statically grown cultures of either Silicibacter sp. strain TM1040 and Phaeobacter strain 27-4 were used. Ethyl acetate extracts also inhibited Mycobacterium marinum, an acid-fast bacterium and important fish and human pathogen.
DISCUSSION
Bacteria belonging to the α-Proteobacteria, and especially members of the Roseobacter clade of the α-Proteobacteria, are widely distributed in marine waters and dominate the microbiota on phytoplankton (1, 9, 15, 21, 30, 40, 47). The reasons for this dominance are not known, but 9 of 14 Roseobacter species produced antibacterial compounds, as has previously been reported for several strains belonging to the Roseobacter clade (6, 8, 38), and antagonistic interactions between the bacteria in algal communities may partly explain the dominance of the Roseobacter species in this niche.
Cell-free culture supernatants from Phaeobacter inhibens T5 inhibited marine flavobacteria and actinobacteria, whereas bacteria belonging to the α- and γ-Proteobacteria were mostly unaffected (6). Similarly, we found that none of the Roseobacter species showed susceptibility to the filtered culture supernatant from either Silicibacter sp. strain TM1040 or Phaeobacter strain 27-4, whereas most non-Roseobacter species were inhibited. However concentrated antibacterial compound produced by Silicibacter sp. strain TM1040 or Phaeobacter strain 27-4 inhibited both Roseobacter species and non-Roseobacter species, a finding that is correlated with the observation that both α- and γ-Proteobacteria were inhibited by the pure antibacterial compound of strain T5 (tropodithietic acid) (6). Hence, many members of the Roseobacter clade produce active inhibitory compounds that are more active against non-Roseobacter species than Roseobacter species themselves.
The antibacterial activity of Phaeobacter strain 27-4 and the closely related P. inhibens T5 is due to the production of tropodithietic acid, which itself is correlated to the synthesis of a brown pigment (6, 8). Silicibacter sp. strain TM1040 also produced an antibacterial compound which is correlated to the occurrence of a similar brown pigment. Culture conditions affected the production of both pigment and antibacterial activity in Phaeobacter strain 27-4 and Silicibacter sp. strain TM 1040 and both had the highest production levels under static conditions (Fig. 3). Based on these findings, we are currently working on the hypothesis that the antibacterial compound produced by Silicibacter sp. strain TM1040 is tropodithietic acid. Tropodithietic acid contains two sulfur atoms, which is interesting since the Roseobacter clade has been linked to sulfur cycling in the sea, specifically by the ability of these bacteria to metabolize alga- and dinoflagellate-produced dimethylsulfoniopropionate (DMSP) (29). Silicibacter sp. strain TM1040 utilizes DMSP through the demethylation pathway (27), and sulfur from DMSP may be used in the synthesis of tropodithietic acid.
The production of antibacterial activity by Phaeobacter strain 27-4 co-occurred with the formation of rosettes as well as with bacterial attachment and biofilm formation (7, 8). In the present study, we found that a spontaneously occurring mutant of Phaeobacter strain 27-4 lacking the dark extracellular pigment simultaneously lost the ability to produce the antibacterial compound and failed to form biofilms, suggesting that there is a link between these phenotypes. For members of the Roseobacter clade, attached bacteria are 13 times more likely to produce antimicrobial compounds relative to their free-living counterparts, whereas the frequencies of antibacterial strains are similar in particle-attached and free-living bacteria for most non-Roseobacter marine species (24). Five of the Roseobacter species analyzed in the present study displayed proficient attachment capability when grown under static culture conditions, and four of these showed antibacterial activity at the same time. In contrast, a nonpigmented, antibacterial-activity-negative mutant of Silicibacter sp. strain TM1040 retained its biofilm-forming ability, demonstrating that the production of the antibacterial compound is not necessarily linked to biofilm formation in all Roseobacter species. The molecular mechanisms underlying these differences are under active investigation in our laboratories.
Bacteria of the Roseobacter clade are some of the most rapid colonizers of surfaces in the coastal environments (11), and we hypothesize that rosette formation may be an attachment phenotype. Although several strains were capable of growing in rosette formations, five strains had more pronounced rosette formations under static growth conditions and this result coincided with these five strains being the most efficient in attaching to surfaces and producing biofilms at the air-liquid interface.
The ability to produce an antibacterial compound that is more active against non-Roseobacter species during the formation of a biofilm may provide the Roseobacter clade bacteria a strong adaptive and selective advantage in establishing interactions with their algal and dinoflagellate hosts. The production of an antibacterial compound has indeed been demonstrated to have a role in the colonization of Ulva australis by Pseudoalteromonas tunicata (34). Furthermore, antagonistic interactions are common in particle-associated marine bacteria (24, 25) and studies have shown that antagonistically attached marine bacteria directly inhibited the colonization of particles by V. cholerae (25). A strain of Phaeobacter gallaeciensis was an aggressive colonizer of the alga Ulva australis since it was able to invade and disperse preestablished biofilms of other marine bacteria (34), and the strain was able to prevent the growth of other non-Roseobacter bacteria on surfaces while growing in a biofilm (33). On the other hand, the production of antibacterial compounds may not always result in a selective advantage during the colonization of particles, since others have demonstrated that an antibiotic-producing strain had no inhibitory effect on the attachment of two marine bacteria compared to that of a mutant unable to produce antibiotic (17). Grossart et al. (17) also found that the bacterial growth rate was the most important parameter controlling the long-term bacterial population density on agar particles. Taken as a whole, these results suggest that the outcome of interactions, whether antagonistic or mutualistic, between particle-associated bacteria is complex and multifaceted.
V. coralliilyticus and V. shiloi are important coral pathogens causing coral bleaching (4, 36), and both were inhibited by Silicibacter sp. strain TM1040 and Phaeobacter strain 27-4. The coral polyp is protected by a mucus layer that is populated by α-Proteobacteria group bacteria (5), and bacteria taxonomically related to Silicibacter sp. strain TM1040 are associated with corals (9). One may therefore hypothesize that Roseobacter species play a role in preventing coral bleaching, and, indeed, antibacterial activity in coral extracts which inhibit members of the Vibrionaceae has been detected (19), supporting this hypothesis.
The production of antibacterial compounds in some bacterial species is controlled by AHL in a QS-dependent manner (3, 48). AHL compounds can be isolated from several members of the Roseobacter clade (8, 16, 45), and it has been suggested that AHLs control the production of antibacterial compounds in Phaeobacter strain 27-4 (8). In the present study, five strains induced A. tumefaciens NT1(pZLR4), whereas none induced C. violaceum CV026, which could indicate that primarily long-chained AHLs were produced, in agreement with a recent study (45). AHLs were detected only in strains that produced antibacterial activity; however, there is not a direct correlation between these phenotypes since antibacterial activity was also detected in strains that did not induce the AHL monitor strains. Silicibacter sp. strain TM1040 produced antibacterial compound only at cell densities where QS is expected to occur; however, AHL molecules were not detected from this bacterium, and no LuxI homologs are found in the genome of this bacterium (R. Belas, personal observation). The bacteria in which we did not find AHLs may, in principle, produce other QS molecules that may be detected by other methods, but based on the current data, we find no evidence linking the production of antibacterial compounds to QS in the Roseobacter clade.
In conclusion, phenotypes for the production of antibacterial compounds, rosette formation, attachment ability, and biofilm formation were detected in several Roseobacter strains. Culture conditions influenced both antibacterial activity and bacterial attachment and biofilm formation, as these phenotypes were expressed under almost only static culture conditions. The degree to which the organisms grew as rosettes was linked to attachment and biofilm formation. Collectively, these phenotypes may facilitate the colonization of dinoflagellates, algae, and marine particles by Roseobacter clade bacteria.
Supplementary Material
Acknowledgments
We thank Allen Place, Feng Chen, Russell Hill, and Anwar Huq for providing dinoflagellate, environmental, and bacterial samples, respectively.
The research in the Belas laboratory is supported through grants from the National Science Foundation (MCB0446001) and from the Maryland Sea Grant Program Development Fund (Rp/DIS-153). The Ph.D. study of J. B. Bruhn was conducted in connection with the research network SCOFDA (Sustainable Control of Fish Diseases in Aquaculture) supported by the Danish Agricultural and Veterinary Research Council and the Danish Ministry of Food, Agriculture and Fisheries.
Footnotes
Published ahead of print on 10 November 2006.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alavi, M., T. Miller, K. Erlandson, R. Schneider, and R. Belas. 2001. Bacterial community associated with Pfiesteria-like dinoflagellate cultures. Environ. Microbiol. 3:380-396. [DOI] [PubMed] [Google Scholar]
- 2.Amaro, A. M., M. S. Fuentes, S. R. Ogalde, J. A. Venegas, and B. A. Suarez-Isla. 2005. Identification and characterization of potentially algal-lytic marine bacteria strongly associated with the toxic dinoflagellate Alexandrium catenella. J. Eucaryot. Microbiol. 52:191-200. [DOI] [PubMed] [Google Scholar]
- 3.Bainton, N. J., P. Steadm, S. R. Chhabra, B. W. Bycroft, G. P. C. Salmond, G. S. A. B. Stewart, and P. Williams. 1992. N-(3-Oxohexanoyl)-l-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem. J. 288:997-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Haim, Y., M. Zicherman-Keren, and E. Rosenberg. 2003. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl. Environ. Microbiol. 69:4236-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourne, D. G., and C. B. Munn. 2005. Diversity of bacteria associated with the coral Pocillopora damicornis from the Great Barrier Reef. Environ. Microbiol. 7:1162-1174. [DOI] [PubMed] [Google Scholar]
- 6.Brinkhoff, T., G. Bach, T. Heidorn, L. F. Liang, A. Schlingloff, and M. Simon. 2004. Antibiotic production by a Roseobacter clade-affiliated species from the German Wadden Sea and its antagonistic effects on indigenous isolates. Appl. Environ. Microbiol. 70:2560-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruhn, J. B., J. A. J. Haagensen, D. Bagge-Ravn, and L. Gram. 2006. Culture conditions of Roseobacter strain 27-4 affect its attachment and biofilm formation as quantified by real-time PCR. Appl. Environ. Microbiol. 72:3011-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruhn, J. B., K. F. Nielsen, M. Hjelm, M. Hansen, J. Bresciani, S. Schulz, and L. Gram. 2005. Ecology, inhibitory activity, and morphogenesis of a marine antagonistic bacterium belonging to the Roseobacter clade. Appl. Environ. Microbiol. 71:7263-7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchan, A., J. M. Gonzalez, and M. A. Moran. 2005. Overview of the marine Roseobacter lineage. Appl. Environ. Microbiol. 71:5665-5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cha, C., P. Gao, Y. C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 11.Dang, H. Y., and C. R. Lovell. 2000. Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes. Appl. Environ. Microbiol. 66:467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallacher, S., and E. A. Smith. 1999. Bacteria and paralytic shellfish toxins. Protist 150:245-255. [DOI] [PubMed] [Google Scholar]
- 13.Giovannoni, S. J., and U. Stingl. 2005. Molecular diversity and ecology of microbial plankton. Nature 437:343-348. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez, J. M., J. S. Covert, W. B. Whitman, J. R. Henriksen, F. Mayer, B. Scharf, R. Schmitt, A. Buchan, J. A. Fuhrman, R. P. Kiene, and M. A. Moran. 2003. Silicibacter pomeroyi sp. nov. and Roseovarius nubinhibens sp. nov., dimethylsulfoniopropionate-demethylating bacteria from marine environments. Int. J. Syst. Bacteriol. 53:1261-1269. [DOI] [PubMed] [Google Scholar]
- 15.González, J. M., R. Simo, R. Massana, J. S. Covert, E. O. Casamayor, C. Pedros-Alio, and M. A. Moran. 2000. Bacterial community structure associated with a dimethylsulfoniopropionate-producing North Atlantic algal bloom. Appl. Environ. Microbiol. 66:4237-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gram, L., H. P. Grossart, A. Schlingloff, and T. Kiorboe. 2002. Possible quorum sensing in marine snow bacteria: production of acylated homoserine lactones by Roseobacter strains isolated from marine snow. Appl. Environ. Microbiol. 68:4111-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossart, H. P., T. Kiorboe, K. Tang, and H. Ploug. 2003. Bacterial colonization of particles: growth and interactions. Appl. Environ. Microbiol. 69:3500-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossart, H. P., F. Levold, M. Allgaier, M. Simon, and T. Brinkhoff. 2005. Marine diatom species harbour distinct bacterial communities. Environ. Microbiol. 7:860-873. [DOI] [PubMed] [Google Scholar]
- 19.Harder, T., S. C. K. Lau, S. Dobretsov, T. K. Fang, and P. Y. Qian. 2003. A distinctive epibiotic bacterial community on the soft coral Dendronephthya sp. and antibacterial activity of coral tissue extracts suggest a chemical mechanism against bacterial epibiosis. FEMS Microb. Ecol. 43:337-347. [DOI] [PubMed] [Google Scholar]
- 20.Hjelm, M., Ø. Bergh, A. Riaza, J. Nielsen, J. Melchiorsen, S. Jensen, H. Duncan, P. Ahrens, H. Birkbech, and L. Gram. 2004. Selection and identification of autochthonous potential probiotic bacteria from turbot larvae (Scophthalmus maximus) rearing units. Syst. Appl. Microbiol. 27:360-371. [DOI] [PubMed] [Google Scholar]
- 21.Jasti, S., M. E. Sieracki, N. J. Poulton, M. W. Giewat, and J. N. Rooney-Varga. 2005. Phylogenetic diversity and specificity of bacteria closely associated with Alexandrium spp. and other phytoplankton. Appl. Environ. Microbiol. 71:3483-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiorboe, T. 2000. Colonization of marine snow aggregates by invertebrate zooplankton: abundance, scaling, and possible role. Limnol. Oceanogr. 45:479-484. [Google Scholar]
- 23.Lafay, B., R. Ruimy, C. R. Detraubenberg, V. Breittmayer, M. J. Gauthier, and R. Christen. 1995. Roseobacter algicola sp. nov., a new marine bacterium isolated from the phycosphere of the toxin-producing dinoflagellate Prorocentrum lima. Int. J. Syst. Bacteriol. 45:290-296. [DOI] [PubMed] [Google Scholar]
- 24.Long, R. A., and F. Azam. 2001. Antagonistic interactions among marine pelagic bacteria. Appl. Environ. Microbiol. 67:4975-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long, R. A., D. C. Rowley, E. Zamora, J. Y. Liu, D. H. Bartlett, and F. Azam. 2005. Antagonistic interactions among marine bacteria impede the proliferation of Vibrio cholerae. Appl. Environ. Microbiol. 71:8531-8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martens, T., T. Heidorn, R. Pukall, M. Simon, B. J. Tindall, and T. Brinkhoff. 2006. Reclassification of Roseobacter gallaeciensis Ruiz-Ponte et al. 1998 as Phaeobacter gallaeciensis gen. nov., comb. nov., description of Phaeobacter inhibens sp. nov., reclassification of Ruegeria algicola (Lafay et al. 1995) Uchino et al. 1999 as Marinovum algicola gen. nov., comb. nov., and emended descriptions of the genera Roseobacter, Ruegeria and Leisingera. Int. J. Syst. Evol. Microbiol. 56:1293-1304. [DOI] [PubMed] [Google Scholar]
- 27.Miller, T. R., and R. Belas. 2004. Dimethylsulfoniopropionate metabolism by Pfiesteria-associated Roseobacter spp. Appl. Environ. Microbiol. 70:3383-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, T. R., K. Hnilicka, A. Dziedzic, P. Desplats, and R. Belas. 2004. Chemotaxis of Silicibacter sp. strain TM1040 toward dinoflagellate products. Appl. Environ. Microbiol. 70:4692-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moran, M. A., J. M. Gonzalez, and R. P. Kiene. 2003. Linking a bacterial taxon to sulfur cycling in the sea: studies of the marine Roseobacter group. Geomicrobiol. J. 20:375-388. [Google Scholar]
- 30.Morris, R. M., M. S. Rappe, S. A. Connon, K. L. Vergin, W. A. Siebold, C. A. Carlson, and S. J. Giovannoni. 2002. SAR11 clade dominates ocean surface bacterioplankton communities. Nature 420:806-810. [DOI] [PubMed] [Google Scholar]
- 31.Nicolas, J. L., S. Corre, and J. C. Cochard. 2004. Bacterial population association with phytoplankton cultured in a bivalve hatchery. Microb. Ecol. 48:400-413. [DOI] [PubMed] [Google Scholar]
- 32.Ploug, H., and H. P. Grossart. 2000. Bacterial growth and grazing on diatom aggregates: respiratory carbon turnover as a function of aggregate size and sinking velocity. Limnol. Oceanogr. 45:1467-1475. [Google Scholar]
- 33.Rao, D., J. S. Webb, and S. Kjelleberg. 2005. Competitive interactions in mixed-species biofilms containing the marine bacterium Pseudoalteromonas tunicata. Appl. Environ. Microbiol. 71:1729-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao, D., J. S. Webb, and S. Kjelleberg. 2006. Microbial colonization and competition on the marine alga Ulva australis. Appl. Environ. Microbiol. 72:5547-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravn, L., A. B. Christensen, S. Molin, M. Givskov, and L. Gram. 2001. Methods for detecting acylated homoserine lactones produced by gram-negative bacteria and their application in studies of AHL-production kinetics. J. Microbiol. Methods 44:239-251. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg, E., and L. Falkovitz. 2004. The Vibrio shiloi/Oculina patagonica model system of coral bleaching. Annu. Rev. Microbiol. 58:143-159. [DOI] [PubMed] [Google Scholar]
- 37.Ruger, H. J., and M. G. Hofle. 1992. Marine star-shaped-aggregate-forming bacteria: Agrobacterium atlanticum sp. nov.; Agrobacterium meteori sp. nov.; Agrobacterium ferrugineum sp. nov., nom. rev.; Agrobacterium gelatinovorum sp. nov., nom. rev.; and Agrobacterium stellulatum sp. nov., nom. rev. Int. J. Syst. Bacteriol. 42:133-143. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz-Ponte, C., V. Cilia, C. Lambert, and J. L. Nicolas. 1998. Roseobacter gallaeciensis sp. nov., a new marine bacterium isolated from rearings and collectors of the scallop Pecten maximus. Int. J. Syst. Bacteriol. 48:537-542. [DOI] [PubMed] [Google Scholar]
- 39.Schweitzer, B., I. Huber, R. Amann, W. Ludwig, and M. Simon. 2001. α- and β-Proteobacteria control the consumption and release of amino acids on lake snow aggregates. Appl. Environ. Microbiol. 67:632-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selje, N., M. Simon, and T. Brinkhoff. 2004. A newly discovered Roseobacter cluster in temperate and polar oceans. Nature 427:445-448. [DOI] [PubMed] [Google Scholar]
- 41.Shiba, T. 1991. Roseobacter litoralis gen. nov., sp. nov., and Roseobacter denitrificans sp. nov., aerobic pink-pigmented bacteria which contain bacteriochlorophyll a. Syst. Appl. Microbiol. 14:140-145. [Google Scholar]
- 42.Throup, J. P., M. Camara, G. S. Briggs, M. K. Winson, S. R. Chhabra, B. W. Bycroft, P. Williams, and G. S. A. B. Stewart. 1995. Characterization of the Yenl/Yenr locus from Yersinia enterocolitica mediating the synthesis of 2 N-acylhomoserine lactone signal molecules. Mol. Microbiol. 17:345-356. [DOI] [PubMed] [Google Scholar]
- 43.Uchino, Y., A. Hirata, A. Yokota, and J. Sugiyama. 1998. Reclassification of marine Agrobacterium species: proposals of Stappia stellulata gen. nov., comb. nov., Stappia aggregata sp. nov., nom. rev., Ruegeria atlantica gen. nov., comb. nov., Ruegeria gelatinovora comb. nov., Ruegeria algicola comb. nov., and Ahrensia kieliense gen. nov., sp. nov., nom. rev. J. Gen. Appl. Microbiol. 44:201-210. [DOI] [PubMed] [Google Scholar]
- 44.Wagner-Döbler, I., and H. Biebl. 2006. Environmental biology of the marine Roseobacter lineage. Annu. Rev. Microbiol. 60:255-280. [DOI] [PubMed] [Google Scholar]
- 45.Wagner-Döbler, I., V. Thiel, L. Eberl, M. Allgaier, A. Bodor, S. Meyer, S. Ebner, A. Hennig, R. Pukall, and S. Schulz. 2005. Discovery of complex mixtures of novel long-chain quorum sensing signals in free-living and host-associated marine alphaproteobacteria. Chembiochem 6:2195-2206. [DOI] [PubMed] [Google Scholar]
- 46.Whitehead, N. A., A. M. L. Barnard, H. Slater, N. J. L. Simpson, and G. P. C. Salmond. 2001. Quorum-sensing in gram-negative bacteria. FEMS Microbiol. Rev. 25:365-404. [DOI] [PubMed] [Google Scholar]
- 47.Wichels, A., C. Hummert, M. Elbrachter, B. Luckas, C. Schutt, and G. Gerdts. 2004. Bacterial diversity in toxic Alexandrium tamarense blooms off the Orkney Isles and the Firth of Forth. Helgol. Mar. Res. 58:93-103. [Google Scholar]
- 48.Wood, D. W., and L. S. Pierson III. 1996. The phzI gene of Pseudomonas aureofaciens 30-84 is responsible for the production of a diffusible signal required for phenazine antibiotic production. Gene 168:49-53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.