Abstract
2,6-Dichlorobenzamide (BAM), a persistent metabolite from the herbicide 2,6-dichlorobenzonitrile (dichlobenil), is the pesticide residue most frequently detected in Danish groundwater. A BAM-mineralizing bacterial community was enriched from dichlobenil-treated soil sampled from the courtyard of a former plant nursery. A BAM-mineralizing bacterium (designated strain MSH1) was cultivated and identified by 16S rRNA gene sequencing and fatty acid analysis as being closely related to members of the genus Aminobacter, including the only cultured BAM degrader, Aminobacter sp. strain ASI1. Strain MSH1 mineralized 15 to 64% of the added [ring-U-14C]BAM to 14CO2 with BAM at initial concentrations in the range of 7.9 nM to 263.1 μM provided as the sole carbon, nitrogen, and energy source. A quantitative enzyme-linked immunoassay analysis with antibodies against BAM revealed residue concentrations of 0.35 to 18.05 nM BAM following incubation for 10 days, corresponding to a BAM depletion of 95.6 to 99.9%. In contrast to the Aminobacter sp. strain ASI1, strain MSH1 also mineralized the herbicide itself along with several metabolites, including ortho-chlorobenzonitrile, ortho-chlorobenzoic acid, and benzonitrile, making it the first known dichlobenil-mineralizing bacterium. Aminobacter type strains not previously exposed to dichlobenil or BAM were capable of degrading nonchlorinated structural analogs. Combined, these results suggest that closely related Aminobacter strains may have a selective advantage in BAM-contaminated environments, since they are able to use this metabolite or structurally related compounds as a carbon and nitrogen source.
Each year, millions of tons of xenobiotic compounds are applied globally as pesticides in agricultural production and in consolidated urban areas, along railways and roads and within farmyards. As an outcome of this extensive environmental input, natural water in rivers, lakes, and aquifers has been contaminated with trace amounts of pesticide residues. In Denmark, where more than 99% of the drinking water originates from groundwater, the detection of pesticide residues above the European Commission (EC) threshold limit of 0.1 μg liter−1 has resulted in the costly closure of numerous groundwater abstraction wells (29). Not only are the pesticides themselves monitored, but selected stable metabolites are also included, and often these are detected more frequently than the pesticide itself (3). The most commonly encountered pesticide residue in Danish groundwater is 2,6-dichlorobenzamide (BAM). BAM is a metabolite produced from partial degradation of the benzonitrile herbicide 2,6-dichlorobenzonitrile (dichlobenil) (1, 9, 16) and is often highly persistent in the environment. Dichlobenil is a broad-spectrum herbicide mostly used on nonagricultural areas, as well as in plant nurseries and fruit orchards. This herbicide was banned for use in Denmark in 1997, but BAM is still the main pesticide residue in Danish groundwater, with 19.7% of the abstraction wells analyzed in 2003 having detectable BAM concentrations and 8.1% of the wells containing BAM concentrations exceeding the EC threshold limit of 0.1 μg liter−1 for drinking water (3). Similar results were reported in 2003 in a monitoring program from Sweden (13), and BAM has additionally been detected in groundwater in The Netherlands, Germany, and Italy (18, 42, 44).
A potential for partial degradation of the herbicide dichlobenil to BAM has been measured in various soils and subsurface sediments, with estimated half-lives ranging from 106 to 2,079 days (6, 8, 16, 41). Also, 2,6-dichlorobenzoic acid, another known metabolite from dichlobenil, has been measured in dichlobenil-treated soils (8, 24) and in groundwater samples (3, 18). Two additional metabolites, ortho-chlorobenzamide and ortho-chlorobenzoic acid, have been detected in laboratory experiments with dichlobenil-treated soils (8). The metabolite BAM appears to be much more persistent than dichlobenil itself, and several studies have reported no apparent degradation of BAM in soils (1, 6, 8, 16, 40, 41), aquifer sediments (2, 6, 36, 37), or bacterial isolates (9, 43). Little is therefore known about the environmental degradation of BAM.
We are interested in using degradative bacteria for remediation of groundwater contaminated with low concentrations of BAM, and we therefore recently initiated a large-scale screening of dichlobenil-treated areas to locate soils capable of rapid mineralization of BAM (10, 25). These efforts have pinpointed 6 soils that have a potential for mineralization of [ring-U-14C]BAM to 14CO2, out of a total of 79 samples screened for mineralization activity, obtained from 39 different Danish locations previously exposed to dichlobenil. One of the soils with a potential for BAM mineralization but with no evident dichlobenil mineralization was recently used to enrich and isolate a BAM-mineralizing bacterium identified as an Aminobacter isolate, designated strain ASI1 (25). A second soil sampled from the courtyard of a former plant nursery located above a BAM-contaminated aquifer had a unique ability to mineralize both BAM and [ring-U-14C]dichlobenil to 14CO2 (25), and we later succeeded in obtaining stable BAM-mineralizing enrichment cultures (10), but isolation of degradative bacteria was not achieved. In this study we revived one of these enrichment cultures and isolated a BAM- and dichlobenil-mineralizing Aminobacter sp. strain (designated strain MSH1). This strain is closely related to our BAM-mineralizing Aminobacter sp. strain ASI1, and these two strains along with selected Aminobacter type strains were compared with regard to their taxonomy, degradative capacity toward dichlobenil and related compounds, and ability to degrade and mineralize low concentrations of BAM and dichlobenil.
MATERIALS AND METHODS
Dichlobenil and metabolites.
Analytical-grade dichlobenil (CAS RN 001194-65-6) (99.5% purity; 18 mg liter−1 water solubility at 20°C), BAM (CAS RN 002008-58-4) (95.5% purity; 2,730 mg liter−1 water solubility at 23°C), 2,6-dichlorobenzoic acid (CAS RN 000050-30-6) (99.5% purity; 14,100 mg liter−1 water solubility at 20°C), benzonitrile (CAS RN 000100-47-0) (99.5% purity; water solubility, 2,000 mg liter−1 at 25°C), and benzoic acid (CAS RN 000065-85-0) (99.5% purity; water solubility, 3,400 mg liter−1 at 25°C) were all purchased from Ehrenstorfer (Augsburg, Germany). Ortho-chlorobenzonitrile (CAS RN 000873-32-5) (99% purity; water solubility unknown), ortho-chlorobenzamide (CAS RN 000609-66-5) (97% purity; water solubility unknown), and ortho-chlorobenzoic acid (CAS RN 000118-91-2) (98% purity; water solubility, 2,090 mg liter−1 at 25°C) were acquired from ABCR GmbH & Co. (Karlsruhe, Germany). Benzamide (CAS RN 027208-38-4) (99.3% purity; water solubility unknown) was obtained from Acros Amresco ICN Biomedicals (Irvine, CA). [ring-U-14C]dichlobenil (28.3 mCi mmol−1) ([14C]dichlobenil) and [ring-U-14C]BAM (25.2 mCi mmol−1) ([14C]BAM) were purchased from International Izotop (Budapest, Hungary). Radiochemical purities better than 95% were verified for the [14C]dichlobenil and [14C]BAM in our laboratory by a thin-layer-chromatography-based assay. Both these 14C tracers are labeled in the ring structure, which enables us to conclude that mineralization is occurring whenever more than 5% of the initially added 14C tracer is recovered as 14CO2.
Enrichment and isolation of a BAM-mineralizing bacterium.
A BAM-mineralizing enrichment culture, designated 2-MS-V, derived from dichlobenil-treated soil sampled from the courtyard of a former plant nursery located above a BAM-contaminated aquifer near Hvidovre in Denmark (10), served as the inoculum for the current enrichment procedure. A 50% glycerol stock solution of enrichment culture 2-MS-V was removed from storage at −80°C and washed twice in sterile phosphate buffer before inoculation into sterilized 100-ml glass flasks containing 25.0 ml of an autoclaved mineral salt solution (MS) (26). BAM and [14C]BAM were added from a 5,000-mg liter−1 stock solution in high-performance liquid chromatography (HPLC)-grade dimethyl sulfoxide (Merck, Darmstadt, Germany) directly to the medium, providing 263.12 μM BAM (50 mg liter−1) as the sole source of carbon, nitrogen, and energy and 10,000-dpm [14C]BAM as a tracer for detection of 14CO2 production. [14C]BAM mineralization to 14CO2 was followed as described below. When the mineralization of BAM was completed, a 10% volume was transferred to fresh BAM-containing MS medium to a final volume of 25.0 ml. After 10 subculturings of the enrichment culture over a period of approximately 500 days, plating on R2A (Difco Laboratories, Detroit, MI) was performed. The plates were incubated for up to 1 month at 20°C, and dominant colonies were picked, streaked for purity, and screened for BAM degradation in MS medium.
Characterization of strains MSH1 and ASI1.
One isolate, designated strain MSH1, and the recently isolated BAM-degrading Aminobacter sp. strain ASI1 (25) were both characterized and identified by Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany (DSMZ) by analysis of the cellular fatty acids and different physiological tests. Additionally, almost-complete 16S rRNA gene sequences were determined for strains MSH1 (1,463 bp sequenced) and ASI1 (1,485 bp sequenced) within the current study. Genomic DNA extraction, PCR amplification of the 16S rRNA gene, and purification of the PCR products were performed as described previously (19). Sequence data were compared to 16S rRNA gene sequences deposited in the GenBank database. Sequences from closely related type strains were obtained and manually aligned using the AE2 alignment editor (14). Strains MSH1 and ASI1 were also tested for resistance to the antibiotics ampicillin, chloramphenicol, kanamycin, penicillin G, streptomycin, rifampin, nalidixin, and tetracycline in 1/10-strength Luria-Bertani medium (Difco Laboratories, Detroit, MI). All antibiotics were purchased from ICN Biomedicals, Inc. (Aurora, OH).
Bacterial strains and cultivation conditions.
Three type strains from the genus Aminobacter, A. aminovorans DSM 7048T, A. aganoensis DSM 7051T, and A. niigataensis DSM 7050T (38), were obtained from DSMZ and tested for degradation of dichlobenil, its metabolites, and related structural analogs. These were cultured in MS medium supplemented with 5.0 g liter−1 proteose peptone no. 3 (Difco Laboratories) and 3.0 g liter−1 yeast extract (Difco Laboratories) (MS-C) prior to the degradation experiments. The strains MSH1 and ASI1 were either pregrown in MS with 25 to 50 mg liter−1 BAM (132.5 to 263.1 μM) or washed from R2A plates prior to the degradation and mineralization experiments.
Degradation and mineralization experiments.
Mineralization of [14C]BAM in MS was followed by insertion of a 10-ml glass tube containing 2.0 ml 0.5 M NaOH to trap 14CO2 into the 100-ml culture flasks following inoculation. The final volume of liquid medium in the culture flasks was 25 ml. The NaOH was replaced at regular intervals in a laminar flow bench, mixed with 10 ml Wallac OptiPhase HiSafe 3 scintillation cocktail (Turku, Finland), and counted for 10 min in a Wallac 1409 liquid scintillation counter. Correction for evaporation of [14C]dichlobenil into the NaOH trap was necessary due to its high vapor pressure (31) potentially leading to an overestimation of the extent of mineralization. Therefore, 2.2 ml 0.5 M NaOH served as the base trap in the dichlobenil mineralization experiments, with 1.0 ml used for measuring the total 14C trapped in the NaOH trap by scintillation counting and another 1.0 ml used for estimating the amount of [14C]dichlobenil evaporated into the trap. The trapped [14C]dichlobenil was quantified by acidifying the 1.0 ml base solution with 1.5 ml 1 M HCl, allowing the 14CO2 to evaporate during incubation for 1 h followed by scintillation counting as described above. The dichlobenil mineralization was calculated by subtracting the evaporated [14C]dichlobenil from the total 14C amount measured. Mineralization experiments with BAM and dichlobenil in different initial concentrations were prepared by adding 250 μl of different stock solution strengths containing a mixture of 14C and unlabeled compound in dimethyl sulfoxide. The lowest concentrations tested were 7.9 nM BAM and 8.7 nM dichlobenil, corresponding to the addition of only 14C-labeled compound equal to approximately 10,000 dpm per flask. The highest dichlobenil concentration tested, 290.7 μM, was above the level of solubility of dichlobenil, and crystals were visible at the initiation of the experiment.
Degradation of dichlobenil, BAM, and seven other dichlobenil metabolites and structural analogs by the Aminobacter strains was tested in 5.0 ml MS medium supplemented with 50.0 μM of each compound individually. MS medium (4.9 ml) was inoculated with 0.1 ml of a washed cell suspension having an optical density at 600 nm of 0.5, and subsamples were analyzed by the HPLC method developed by Holtze et al. (8) following incubation for 14 days. The quantification was performed using a Hewlett-Packard series 1050 HPLC system equipped with a UV detector (Phenomenex, Chire, United Kingdom) as described by Holtze et al. (8). Prior to the HPLC analysis, 750-μl subsamples of the medium were filtered through a 0.2-μm polytetrafluoroethylene membrane 17-mm syringe filter (Titan Filtration Systems; Sun SRI, Wilmington, NC), and the last 250 μl was collected for analysis. The HPLC detection limits for the nine compounds were <1 μM (0.035 to 0.159 mg liter−1) by direct HPLC analysis of the filtered liquid medium (8). The medium samples for HPLC measurements were stored at −15°C for up to 2 weeks before analysis. All degradation experiments were conducted at 20°C in the dark, and sterile controls were included.
Specific enzyme-linked immunosorbent assay (ELISA) for quantification of residual BAM concentrations.
A highly specific and sensitive ELISA assay developed by Bruun et al. (4) for detection of BAM in groundwater samples was used for quantifying the residual BAM concentrations in subsamples of the culture medium following the mineralization experiments. This ELISA assay has a detection limit of 0.02 μg liter−1, equal to 0.105 nM BAM, and is very specific, with only negligible cross-reactions with dichlobenil and BAM metabolites, as well as structural analogs (4). Medium samples for ELISA were stored at −15°C for up to 2 weeks before analysis.
Nucleotide sequence accession numbers.
The 16S rRNA sequences for Aminobacter sp. strain MSH1 and Aminobacter sp. strain ASI1 have been deposited in the GenBank database under accession numbers DQ401867 and DQ401866, respectively.
RESULTS
Isolation and characterization of a BAM-mineralizing bacterium.
A previously described BAM-mineralizing mixed bacterial enrichment culture was successfully revived and used for enrichment and isolation of the BAM-mineralizing bacterial strain MSH1. Isolate MSH1 is a gram-negative irregular motile rod with a width of 0.6 to 0.8 μm and a length of 2.0 to 3.5 μm after growth in nutrient-rich broth. It is oxidase, catalase, and urease positive. MSH1 did not hydrolyze esculin, gelatin, Tween 80, DNA, or starch. It gives negative results in tests for nitrate reduction and production of H2S. When grown on R2A agar, its colony morphology is similar to that of Aminobacter sp. strain ASI1, with white colonies having a characteristic reddish-brown center appearing after 4 to 5 days at 20°C. MSH1 used glucose, d-arabinose, mannose, maltose, N-acetyl-glucosamine, acetate, d-arabitol, d-fructose, fumarate, pyruvate, l-rhamnose, and d-sucrose as growth substrates. In contrast, no growth was detectable with l-arabinose, malate, adipate, citrate, caprate, gluconate, 2-OH-valerate, lactose, and lactulose. Both MSH1 and ASI1 were insensitive to the antibiotic streptomycin (50 mg liter−1) but sensitive to ampicillin, chloramphenicol, kanamycin, penicillin G, rifampin, nalidixin, and tetracycline. It was not possible to distinguish MSH1 from Aminobacter sp. strain ASI1 based on the different physiological tests mentioned above. Additionally, whole-cell fatty-acid profiles were prepared for isolate MSH1 and compared to previously published data for Aminobacter sp. strain ASI1 (25). For both strains, the dominant fatty acid was 18:1ω7c, with 75.0% in MSH1 and 67.8% in ASI1. The second-most-dominant fatty acid in MSH was 16:0, with 8.3% in comparison to 7.2% in ASI1. In contrast, 11-methyl-18:1ω7c was the second-most-dominating fatty acid in ASI1 with 10.6%, while MSH1 had only 1.2% of this fatty acid.
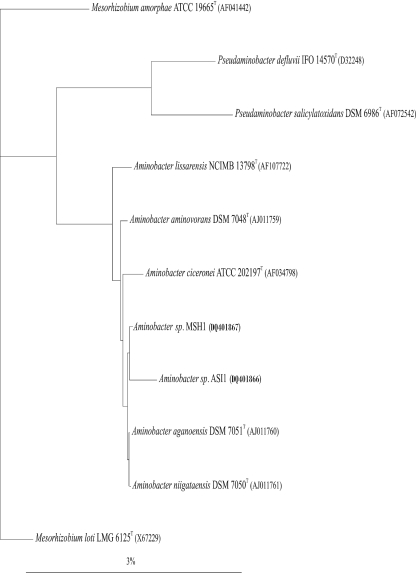
The 1,463-bp 16S rRNA gene sequence obtained from isolate MSH1 is identical to the 16S rRNA gene sequences from A. aganoensis DSM 7051T and A. niigataensis DSM 7050T. It has 99.5 to 99.8% similarity to the three remaining members of the genus Aminobacter: A. aminovorans, A. lissarensis, and A. ciceronei. Similarities of 96.8 to 97.8% are apparent with different type strains from the genera Pseudaminobacter and Mesorhizobium. A 99.6% similarity to the 1,485-bp 16S rRNA gene sequence obtained from the recently described BAM-mineralizing Aminobacter sp. strain ASI1 was observed. A phylogenetic tree showing the affiliation of strains MSH1 and ASI1 with closely related type strains is presented in Fig. 1.
FIG. 1.
Phylogenetic affiliations of 16S rRNA gene sequences derived from strains ASI1 and MSH1 and closely related α-proteobacteria type strains. The phylogenetic tree was constructed using a neighbor-joining algorithm with Jukes-Cantor distance correction. The root of the tree was determined by including the 16S rRNA gene sequence of Mesorhizobium loti in the analysis. The accession number for each sequence is given in parentheses. The scale bar indicates 3 nucleotide substitutions per 100 nucleotides.
Mineralization of BAM and dichlobenil by strains MSH1 and ASI1.
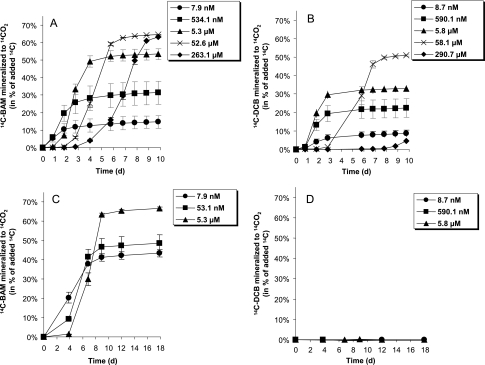
Strain MSH1 was tested for mineralization of BAM in concentrations ranging from 7.9 nM to 263.1 μM (Fig. 2A). With the lowest concentration, 14.7% ± 3.7% of the added [14C]BAM was mineralized to 14CO2 within 10 days. Increasing the BAM concentration to 534.1 nM gave a greater extent of mineralization, with 31.4% ± 6.6% measured as 14CO2 within the same period. In contrast, a longer lag phase before the onset of rapid mineralization was measured with the three highest BAM concentrations of 5.3 to 263.1 μM, and at the end of the experiment, 53.4% to 64.4% was mineralized (Fig. 2A). Generally, a similar pattern was observed with the mineralization of dichlobenil, with the lowest concentrations having the smallest extent of mineralization (Fig. 2B). However, compared to the mineralization of BAM, dichlobenil mineralization was slow and occurred to a small extent within a comparable concentration range. The highest dichlobenil mineralization of 50.9% ± 0.4% was observed with a concentration of 58.1 μM within the 10-day experiment. Dichlobenil at the highest concentration of 290.7 μM was mineralized to the smallest extent during the 10-day experiment (Fig. 2B), and it took 20 additional days of incubation before this dichlobenil concentration was mineralized to approximately 50% 14CO2 (data not shown). This might reflect an inhibition of the degradative activity by strain MSH1 with high dichlobenil concentrations. Minor evaporation of [14C]dichlobenil into the base trap was measured during the 10-day experiment, with a maximum of 2.5% ± 1.0% detected in the flask initiated with 5.8 μM dichlobenil. Aminobacter sp. strain ASI1 was tested for mineralization with the three lowest concentrations of BAM and dichlobenil (Fig. 2C and D). ASI1 mineralized BAM to a greater extent than MSH1, with 43.5 to 66.6% of the added BAM mineralized to 14CO2 within 10 days (Fig. 2C), and ASI1, in contrast to strain MSH1, had no degradative activity towards dichlobenil (Fig. 2D; Table 1). Evaporation of dichlobenil into the base trap was quantified to a maximum of 2.4% (±0.1%) of the initially added [14C]dichlobenil during the 18-day experiment with ASI1.
FIG. 2.
Mineralization of [14C]BAM to 14CO2 by Aminobacter sp. strain MSH1 (A) or Aminobacter sp. strain ASI1 (C) and mineralization of [14C]DCB to 14CO2 by Aminobacter sp. strain MSH1 (B) or Aminobacter sp. strain ASI1 (D). Initial cell density was 2 × 106 to 4 × 106 ml−1. The data are mean values (n = 3). The bars indicate standard deviations, and some are smaller than the symbols.
TABLE 1.
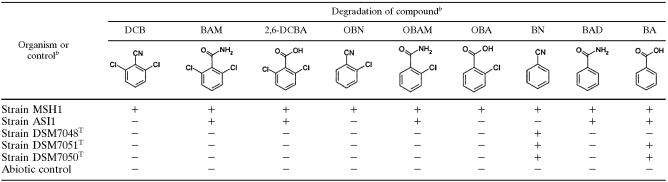
Degradation of dichlobenil and eight potential metabolites by Aminobacter strainsa
Aminobacter sp. strain MSH1, Aminobacter sp. strain ASI1, Aminobacter aminovorans DSM 7048T, Aminobacter aganoensis DSM 7051T, and Aminobacter niigataensis DSM 7050T were used. Degradation was determined by high-performance liquid chromatography analysis of the liquid medium following 14 days of incubation.
Abbreviations: DCB, dichlobenil; BAM, 2,6-dichlorobenzamide; 2,6-DCBA, 2,6-dichlorobenzoic acid; OBN, ortho-chlorobenzonitrile; OBAM, ortho-chlorobenzamide; OBA, ortho-chlorobenzoic acid; BN, benzonitrile; BAD, benzamide; BA, benzoic acid. All compounds were added in concentrations of 50 μM. +, degradation; −, no degradation.
Growth and yield studies with strains MSH1 and ASI1.
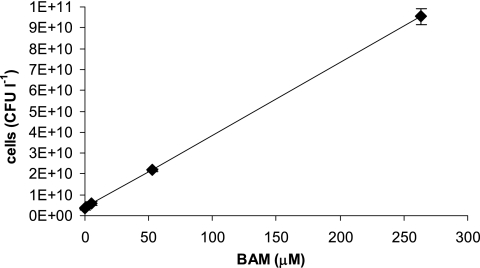
Strain MSH1 used BAM as well as dichlobenil as sources of carbon, nitrogen, and energy for growth, and the cell density after mineralization of different BAM concentrations estimated by plating onto R2A is presented in Fig. 3. Initial cell densities were 2 × 106 to 4 × 106 ml−1 for both strains and in all experiments, and after mineralization of 263.1 μM BAM, the highest tested concentration, 9.6 × 1010 ± 0.4 × 1010 cells of MSH1 per liter culture medium, was measured. In comparison, 3.4 × 109 ± 0.6 × 109 cells of MSH1 per liter culture medium was measured with the lowest tested BAM concentration (7.9 nM) possibly representing smaller cell sizes (17). Strain ASI1 showed minor growth, with cell densities of 1.8 × 108 ± 0.4 × 108, 2.4 × 108 ± 1.2 × 108, and 3.1 × 108 ± 0.8 × 108 ASI cells per liter culture medium measured following the mineralization of 7.9 nM, 534.1 nM, or 5.3 μM BAM, respectively. This growth was not clearly distinct from the background growth in MS medium. It is, however, possible to produce dense and flocculated ASI1 cell cultures with higher BAM concentrations and extended periods of incubation (data not shown).
FIG. 3.
Cell densities of Aminobacter sp. strain MSH1 after mineralization of different concentrations of BAM provided as the sole source of carbon, nitrogen, and energy (7.9 nM, 534.1 nM, 5.3 μM, 52.6 μM, or 263.1 μM; see Fig. 2A) measured as CFU following plating on R2A. The data are mean values (n = 3). The bars indicate standard deviations. Some bars are smaller than the symbols.
Residual BAM concentrations following BAM and dichlobenil mineralization by strains MSH1 and ASI1.
A quantitative ELISA assay was used to measure residual BAM concentrations following the mineralization experiments with strains MSH1 and ASI1 presented in Fig. 2A and C. Residue concentrations of 0.22 to 18.05 nM BAM were measured following mineralization of initial BAM concentrations from 7.9 nM to 263.1 μM by MSH1 and ASI1, corresponding to a depletion of BAM in the range of 95.6% to 99.9% relative to the initial concentration (Table 2). With a 99.9% BAM depletion, the most efficient degradation was apparent with strain MSH1 and initial BAM concentrations from 5.3 μM BAM and above. At the BAM concentrations below 5.3 μM, the two strains were highly similar in their degradation efficiencies, with remaining residues in concentrations of 0.35 to 0.41 nM BAM. This is in contrast to the different extents of [14C]BAM mineralization observed for the two strains by measurement of 14CO2 production (Fig. 2A and C).
TABLE 2.
Residual BAM concentrations determined by a quantitative ELISA analysis following BAM mineralization experiments with Aminobacter sp. strain MSH1 or Aminobacter sp. strain ASI1
| Strain | Initial concn of BAM | Residual concn of BAM (nM)a | BAM depletion (%) |
|---|---|---|---|
| MSH1 | 0.0 | 0.0 | |
| 7.9 nM | 0.35 (±0.03) | 95.60 (±0.40) | |
| 534.1 nM | 0.35 (±0.11) | 99.93 (±0.02) | |
| 5.3 μM | 0.70 (±0.08) | 99.99 (±0.00) | |
| 52.6 μM | 7.54 (±0.61) | 99.99 (±0.00) | |
| 263.1 μM | 18.05 (±0.82) | 99.99 (±0.00) | |
| ASI1 | 0.0 | 0.0 | |
| 7.9 nM | 0.22 (±0.04) | 97.27 (±0.50) | |
| 534.1 nM | 0.41 (±0.27) | 99.92 (±0.05) | |
| 5.3 μM | 3.47 (±0.60) | 99.93 (±0.01) | |
| 52.6 μM | NDb | ND | |
| 263.1 μM | ND | ND |
Means and standard deviations (given in parentheses) of residual BAM concentrations in triplicate flasks following the 10-day (strain MSH1) or 18-day (strain ASI1) BAM mineralization presented in Fig. 2. Initial cell density was (2 to 4) × 106 ml−1.
ND, Not determined.
Following the mineralization of dichlobenil by MSH1 (Fig. 2B), subsamples of the liquid medium were analyzed using the ELISA approach. No BAM was detected below an initial dichlobenil concentration of 5.9 μM (Table 3). Above this initial dichlobenil concentration, only 0.004 to 0.006% of the initially added herbicide was detected as BAM, corresponding to detections of 0.22 to 3.34 nM of BAM after the 10-day mineralization assay.
TABLE 3.
BAM concentrations determined by a quantitative ELISA analysis following dichlobenil mineralization experiments with Aminobacter sp. strain MSH1
| Initial concn of DCB | Concn of BAM (nM)a | Amt of DCB accumulated as BAM (%b) |
|---|---|---|
| 0.0 | 0.00 | 0.000 |
| 8.7 nM | 0.00 | 0.000 |
| 590.1 nM | 0.00 | 0.000 |
| 5.8 μM | 0.22 (±0.06) | 0.004 (±0.001) |
| 58.1 μM | 3.34 (±0.60) | 0.006 (±0.001) |
Means and standard deviations (given in parentheses) of BAM concentrations in triplicate flasks after the 10-day DCB mineralization presented in Fig. 2B. Initial cell density was 4 × 106 ml−1.
Expressed as a percentage of added DCB.
Substrate range for Aminobacter spp.
The results of testing of our BAM-mineralizing Aminobacter isolates and the closely related Aminobacter type strains for degradative capacity towards dichlobenil, BAM, and several of their potential metabolites and structural analogs are summarized in Table 1. Strain MSH1 is the most versatile among the tested strains and degrades all tested compounds. In contrast, strain ASI1 degrades only BAM, 2,6-dichlorobenzoic acid, ortho-chlorobenzamide, benzamide, and benzoic acid (Table 1). Surprisingly, however, A. aminovorans DSM 7048T, A. aganoensis DSM 7051T, and A. niigataensis DSM 7050T have a native ability to degrade benzonitrile (Table 1), with a corresponding accumulation of a compound tentatively identified as benzamide by comparing its HPLC retention time with that of an authentic standard (data not shown). The only other metabolite detected within the screening experiment summarized in Table 1 was a compound produced by degradation of ortho-chlorobenzamide by strain ASI1 with an HPLC retention time similar to that of ortho-chlorobenzoic acid. Both A. aganoensis DSM 7051T and A. niigataensis DSM 7050T degraded benzoic acid, separating them from A. aminovorans DSM 7048T, which had no degradative activity towards this compound (Table 1).
DISCUSSION
Following a screening of samples obtained from different dichlobenil-treated locations in Denmark, we have obtained a collection of soils with indigenous microorganisms capable of mineralizing the groundwater contaminant BAM. This metabolite was previously considered persistent in the environment. Within this screening, we also located one apparently unique soil, sampled from the courtyard of a former plant nursery, able to perform a rapid and extensive mineralization of dichlobenil itself. This soil served as the basis for the BAM-grown enrichment culture revived and used to isolate Aminobacter sp. strain MSH1 within the current study. This isolate reflects the degradation capacity of the original soil and mineralizes both BAM and the mother compound dichlobenil. Similarly, the recently isolated Aminobacter sp. strain ASI1 mirrors the degradation capacity of its native soil, performing only BAM mineralization, and has no capacity to degrade dichlobenil. However, the ability to degrade dichlobenil to BAM seems to be widespread, since even bacteria without any previous contact with the herbicide are capable of performing this initial degradation step (9), possibly explaining the much more frequent detection of BAM as a water contaminant in comparison to dichlobenil. Furthermore, dichlobenil is moderately to strongly sorbed to soils, opposite BAM, which has a much lower soil sorption and higher water solubility (5), which suggests that dichlobenil could act as a soil-sorbed reservoir slowly releasing BAM to the soil water. The fact that our previous screening located only one soil performing extensive dichlobenil mineralization, in contrast to several soils performing rapid BAM mineralization, likewise suggests that the high soil sorption of dichlobenil restricts its availability to the soil microorganisms, as shown for other pesticide residues (e.g., see reference 11), whereby the microbial populations within a dichlobenil-treated area are more exposed to BAM than to dichlobenil itself. Microbial adaptation to biodegradation of various persistent pesticides as a consequence of prolonged exposure has been described before (e.g., see references 7 and 36), and the same phenomenon seems like a plausible explanation for the occurrence of BAM-mineralizing bacteria in dichlobenil-treated soils.
Besides the difference in the metabolic versatility of our two Aminobacter isolates, they seem to be highly taxonomically similar. The α-proteobacteria genus Aminobacter was proposed by Urakami et al. (38) and originally consisted of three species, A. aminovorans, A. aganoensis, and A. niigataensis, and only recently have two new species, A. lissarensis and A. ciceronei, been included (15). Members of this genus appear to be widely distributed in terrestrial environments (15, 21, 32, 33), and several isolates have been enriched and cultivated based on their ability to use different xenobiotics as growth substrates, including the pesticides atrazine and carbofuran (15, 21, 32, 33). Other Aminobacter strains have been isolated based on the ability to utilize methyl halides as carbon and energy sources (15) or nitriloacetate as a carbon, nitrogen, and energy source (12), suggesting that the members of this genus are very metabolically versatile. Besides being closely related to each other, our two BAM-mineralizing strains are also highly similar to all five Aminobacter species (Fig. 1). 16S rRNA gene sequencing and alignment suggest that strains ASI1 and MSH1 belong to either A. aganoensis or A. niigataensis. However, neither the fatty acid analysis nor the phenotypic characterizations are sufficient to assign the isolates to one of these species with certainty.
The fact that such highly similar Aminobacter strains can be enriched and isolated from geographically different soils when BAM is provided as the sole source of carbon, nitrogen, and energy seems remarkable. Interestingly, similar results have been presented by Topp et al. (32) in a study where 14 indistinguishable atrazine degraders were isolated from different French and Canadian agricultural soils. These strains were originally reported to be members of the genus Pseudaminobacter (32), but 1 representative for the 14 isolates, strain C147, was recently assigned to the genus Aminobacter as the new species A. ciceronei (15). Topp et al. (32) suggested that enrichment of Aminobacter strains from the different atrazine-treated soils may be due to a native ability of their isolates to utilize alkyl amines, which allowed the usage of atrazine as a source of nitrogen and carbon when the atzABC genes are acquired. Three Aminobacter type strains closely related to our BAM-mineralizing isolates, A. aminovorans, A. aganoensis, and A. niigataensis, all appeared to harbor nitrile hydratase activity, degrading benzonitrile to benzamide. Additionally, A. aganoensis and A. niigataensis were both capable of degrading benzoic acid (Table 1). These compounds are nonchlorinated structural analogs of dichlobenil and the dichlobenil and BAM metabolite 2,6-dichlorobenzoic acid, which indicates that these Aminobacter strains naturally may develop the entire degradation pathway upon acquiring or evolving genes encoding enzymes involved in the initial steps of the mineralization pathway. One defining feature of members of the genus Aminobacter is that they are facultative methylotrophs capable of utilizing methyl amines as growth substrates (38). According to this, utilization of the side chain of BAM may also be part of the explanation for the proliferation of the BAM-mineralizing Aminobacter strains in our study. Besides using the nitrogen and possibly also the carbon from the side chain of BAM, both strains ASI1 and MSH1 were capable of mineralizing the ring structure with a maximum of 60 to 70% 14C-ring carbon metabolized to 14CO2. The remaining 14C may serve as a carbon source, whereby it is incorporated into the biomass, as known for other pesticide-mineralizing isolates (27, 28). The occurrence of 14C-labeled metabolites not measured with our HPLC method cannot be excluded, however.
Many studies with environmental samples (e.g., see references 22, 30, and 34) and isolated microorganisms (22, 39) have reported threshold concentrations for biodegradation of xenobiotics below which no extensive degradative activity has been detected. The phenomenon is often explained by lack of microbial growth below a critical substrate concentration being in the micro- to nanomolar range (e.g., see references 23, 34, and 35). Such thresholds therefore appear as an obvious explanation for the persistence of biodegradable pesticide residues at trace concentrations in water resources. Some observations, however, suggest that bacteria adapted to processing low concentrations of xenobiotic compounds can be isolated (20, 35). Both Aminobacter sp. strain MSH1 and strain ASI1 appeared to be very efficient at degrading and mineralizing ecologically relevant concentrations of BAM. The EC threshold limit of 0.1 μg liter−1 for pesticide residues in drinking water corresponds to 0.53 nM BAM, and both isolates were capable of removing BAM to concentrations below this threshold limit when provided with initial BAM concentrations of 7.9 nM and 534.1 nM (Fig. 2; Table 2). The presence of metabolites from partial degradation of BAM cannot be excluded, however, since the ELISA assay exclusively targets BAM (4). There might be a threshold for BAM degradation at about 0.2 to 0.4 nM, since both isolates appear to have BAM residues in this concentration range in the liquid medium following the mineralization experiments (Table 2). Further studies are needed, however, to address the possibility of degradation to even lower residual concentrations given prolonged incubation periods and following additional optimization of the cultivation process. Measurements of the BAM concentrations following the dichlobenil mineralization experiment with strain MSH1 revealed no BAM or only minor amounts of BAM (Table 3), which could suggest that the strain is very efficient in degrading dichlobenil. Based on the specificity of the ELISA approach, however, it cannot be excluded that other metabolites, as well as dichlobenil itself, still are present in the liquid medium.
In conclusion, this is the first time that a dichlobenil-mineralizing bacterium has been isolated and characterized. There is a considerable interest in developing efficient remediation methods for BAM-contaminated water resources, and our results demonstrate that this new isolate, Aminobacter sp. strain MSH1, is a promising candidate for use in bioremediation processes aimed at cleaning natural waters polluted with low concentrations of BAM or dichlobenil. The high degree of similarity to the recently isolated BAM-mineralizing Aminobacter strain ASI1 and the fact that closely related Aminobacter type strains are capable of degrading nonchlorinated structural analogs add to knowledge of the metabolic capacities of this genus and suggest that this closely related group of Aminobacter spp. could have a selective advantage in BAM-contaminated environments.
Acknowledgments
This work was supported by the Danish Technical Research Council, talent grant 26-04-0051 (funding for S.R.S.), and the PESTICON Project, grant 274-05-0399, financed by the Danish Research Council for Technology and Production Sciences (funding for M.S.H.).
We thank Pia B. Jacobsen (GEUS) and Spire M. Kiersgaard (GEUS) for skillful technical assistance and Patricia Simpson for excellent help during the writing of the manuscript. Additionally, we appreciate the help from C. Spröer (DSMZ) and S. Chen (The Questor Centre and School of Biology and Biochemistry, The Queen's University of Belfast, United Kingdom) in the taxonomic analysis of strains MSH1 and ASI1.
Footnotes
Published ahead of print on 17 November 2006.
REFERENCES
- 1.Beynon, K. I., and A. N. Wright. 1972. The fates of the herbicides chlorthiamid and dichlobenil in relation to residues in crops, soils and animals. Residue rev. 43:23-53. [Google Scholar]
- 2.Broholm, M. M., K. Rügge, N. Tuxen, A. L. Højberg, H. Mosbæk, and P. L. Bjerg. 2001. Fate of herbicides in a shallow aerobic aquifer: a continuous field injection experiment (Vejen, Denmark). Water Resour. Res. 37:3163-3176. [Google Scholar]
- 3.Brüsch, W. 2004. Pesticider og nedbrydningsprodukter, p. 33-40. In L. F. Jørgensen (ed.), Groundwater monitoring 2003. Geological Survey of Denmark and Greenland, Copenhagen, Denmark.
- 4.Bruun, L., C. Koch, B. Pedersen, M. H. Jakobsen, and J. Aamand. 2000. A quantitative enzyme-linked immunoassay for the detection of 2,6-dichlorobenzamide (BAM), a degradation product of the herbicide dichlobenil. J. Immunol. Methods 240:133-142. [DOI] [PubMed] [Google Scholar]
- 5.Clausen, L., F. Larsen, and H.-J. Albrechtsen. 2004. Sorption of the herbicide dichlobenil and the metabolite 2,6-dichlorobenzamide on soils and aquifer sediments. Environ. Sci. Technol. 38:4510-4518. [DOI] [PubMed] [Google Scholar]
- 6.Clausen, L., N. P. Arildskov, F. Larsen, J. Aamand, and H.-J. Albrechtsen. 2007. Degradation of the herbicide dichlobenil and its metabolite BAM in soil and subsurface. J. Contam. Hydrol. 89:157-173. [DOI] [PubMed]
- 7.de Lipthay, J. R., N. Tuxen, K. Johnsen, L. H. Hansen, H.-J. Albrechtsen, P. L. Bjerg, and J. Aamand. 2003. In situ exposure to low herbicide concentrations affects microbial population composition and catabolic gene frequency in an aerobic shallow aquifer. Appl. Environ. Microbiol. 69:461-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holtze, M. S., H. C. B. Hansen, R. K. Juhler, J. Sørensen, and J. Aamand. Microbial degradation pathways of the herbicide dichlobenil in soils with different history of dichlobenil-exposure. Environ. Pollut., in press. [DOI] [PubMed]
- 9.Holtze, M. S., J. Sørensen, H. C. B. Hansen, and J. Aamand. 2006. Transformation of the herbicide 2,6-dichlorobenzonitrile to the persistent metabolites 2,6-dichlorobenzamide (BAM) by soil bacteria known to harbour nitrile hydratase and nitrilase. Biodegradation 17:503-510. [DOI] [PubMed] [Google Scholar]
- 10.Holtze, M. S., S. R. Sørensen, J. Sørensen, H. C. B. Hansen, and J. Aamand. 2007. Biostimulation and enrichment of 2,6-dichlorobenzamide-mineralising soil bacterial communities from dichlobenil-exposed soil. Soil Biol. Biochem. 39:216-223.
- 11.Johannesen, H., S. R. Sørensen, and J. Aamand. 2003. Mineralization of soil-aged isoproturon and isoproturon metabolites by Sphingomonas sp. SRS2. J. Environ. Qual. 32:1250-1257. [DOI] [PubMed] [Google Scholar]
- 12.Kämpfer, P., A. Neef, M. S. Salkinoja-Salonen, and H. J. Busse. 2002. Chelatobacter heintzii (Auling et al. 1993) is a later subjective synonym of Aminobacter aminovorans (Urakami et al. 1992). Int. J. Syst. Evol. Microbiol. 52:835-839. [DOI] [PubMed] [Google Scholar]
- 13.Kreuger, J., H. Holmberg, H. Kylin, and B. Ulén. 2003. Bekämpningsmedel i vatten från typområden, åar och i nederbörd under 2002. Ekohydrologi 77, Rapport 2003:12. Swedish University of Agricultural Sciences, Uppsala, Sweden.
- 14.Maidak, B. L., J. R. Cole, C. T. Parker, Jr., G. M. Garrity, N. Larsen, B. Li, T. G. Lilburn, M. J. McCaughey, G. J. Olsen, R. Overbeek, S. Pramanik, T. M. Schmidt, J. M. Tiedje, and C. R. Woese. 1999. A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res. 27:171-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald, I. R., P. Kämpfer, E. Topp, K. L. Warner, M. J. Cox, T. L. C. Hancock, L. G. Miller, M. J. Larkin, V. Ducrocq, C. Coulter, D. B. Harper, J. C. Murrell, and R. S. Oremland. 2005. Aminobacter ciceronei sp. nov and Aminobacter lissarensis sp. nov., isolated from various terrestrial environments. Int. J. Syst. Evol. Microbiol. 55:1827-1831. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery, M., T. C. Yu, and V. H. Freed. 1972. Kinetics of dichlobenil degradation in soil. Weed Res. 12:31-36. [Google Scholar]
- 17.Morita, R. Y. 1982. Starvation-survival of heterotrophs in the marine environment. Adv. Microbial. Ecol. 6:171-197. [Google Scholar]
- 18.Porazzi, E., M. Pardo Martinez, R. Fanelli, and E. Benfenati. 2005. GC-MS analysis of dichlobenil and its metabolites in groundwater. Talanta 68:146-154. [DOI] [PubMed] [Google Scholar]
- 19.Rainey, F. A., N. Ward-Rainey, R. M. Kroppenstedt, and E. Stackebrandt. 1996. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage; proposal of Nocardiopsaceae fam. nov. Int. J. Sys. Bacteriol. 46:1088-1092. [DOI] [PubMed] [Google Scholar]
- 20.Rapp, P., and K. N. Timmis. 1999. Degradation of chlorobenzenes at nanomolar concentrations by Burkholderia sp. strain PS14 in liquid cultures and in soil. Appl. Environ. Microbiol. 65:2547-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rousseaux, S., A. Hartmann, and G. Soulas. 2001. Isolation and characterization of new Gram-negative and Gram-positive atrazine degrading bacteria from different French soils. FEMS Microbiol. Ecol. 36:211-222. [DOI] [PubMed] [Google Scholar]
- 22.Rubin, H. H., R. V. Subba-Rao, and M. Alexander. 1982. Rates of mineralization of trace concentrations of aromatic compounds in lake water and sewage samples. Appl. Environ. Microbiol. 43:1133-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos, P. M., J. M. Blatny, I. D. Bartolo, S. Valla, and E. Zennaro. 2000. Physiological analysis of the expression of the styrene degradation gene cluster in Pseudomonas fluorescens ST. Appl. Environ. Microbiol. 66:1305-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheets, T. J., C. I. Harris, and J. W. Smith. 1968. Persistence of dichlobenil and SD-7961 in soil. Weed Sci. 16:245-249. [Google Scholar]
- 25.Simonsen, A., M. S. Holtze, S. R. Sørensen, S. J. Sørensen, and J. Aamand. 2006. Mineralisation of 2,6-dichlorobenzamide (BAM) in dichlobenil-exposed soils and isolation of a BAM-mineralising Aminobacter sp. Environ. Pollut. 144:289-295. [DOI] [PubMed] [Google Scholar]
- 26.Sørensen, S. R., and J. Aamand. 2003. Rapid mineralization of the herbicide isoproturon in soil from a previously treated Danish agricultural field. Pest Manag. Sci. 59:1118-1124. [DOI] [PubMed] [Google Scholar]
- 27.Sørensen, S. R., J. Rasmussen, C. S. Jacobsen, O. S. Jacobsen, R. K. Juhler, and J. Aamand. 2005. Elucidating the key member of a linuron-mineralizing bacterial community by PCR and RT-PCR denaturing gradient gel electrophoresis 16S rRNA gene fingerprinting and cultivation. Appl. Environ. Microbiol. 71:4144-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sørensen, S. R., Z. Ronen, and J. Aamand. 2001. Isolation from agricultural soil and characterization of a Sphingomonas sp. able to mineralize the phenylurea herbicide isoproturon. Appl. Environ. Microbiol. 67:5403-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stockmarr, J. 2005. Groundwater quality monitoring in Denmark. Geol. Surv. Den. Greenl. Bull. 7:33-36. [Google Scholar]
- 30.Subba-Rao, R. V., H. E. Rubin, and M. Alexander. 1982. Kinetics and extent of mineralization of organic trace levels in freshwater and sewage. Appl. Environ. Microbiol. 43:1139-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomlin, S. D. S. 1997. The pesticide manual, 11th ed. Bracknell, Berks, United Kingdom.
- 32.Topp, E., H. Zhu, S. M. Nour, S. Houot, M. Lewis, and D. Cuppels. 2000. Characterization of an atrazine-degrading Pseudoaminobacter sp. isolated from Canadian and French agricultural soils. Appl. Environ. Microbiol. 66:2773-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topp, E., R. S. Hanson, D. B. Ringelberg, D. C. White, and R. Wheatcroft. 1993. Isolation and characterization of an N-methylcarbamate insecticide-degrading methylotrophic bacterium. Appl. Environ. Microbiol. 59:3339-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toräng, L., N. Nyholm, and H.-J. Albrechtsen. 2003. Shifts in biodegradation kinetics of the herbicides MCPP and 2,4-D at low concentrations in aerobic aquifer materials. Environ. Sci. Technol. 37:3095-3103. [DOI] [PubMed] [Google Scholar]
- 35.Tros, M. E., G. Schraa, and A. J. B. Zehnder. 1996. Transformation of low concentrations of 3-chlorobenzoate by Pseudomonas sp. strain B13: kinetics and residual concentrations. Appl. Environ. Microbiol. 62:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuxen, N., J. R. de Lipthay, H.-J. Albrechtsen, J. Aamand, and P. L. Bjerg. 2002. Effect of exposure history on microbial herbicide degradation in an aerobic aquifer affected by a point source. Environ. Sci. Technol. 36:2205-2212. [DOI] [PubMed] [Google Scholar]
- 37.Tuxen, N., P. L. Tüchsen, K. Rügge, H.-J. Albrechtsen, and P. L. Bjerg. 2000. Fate of seven pesticides in an aerobic aquifer studied in column experiments. Chemosphere 41:1485-1494. [DOI] [PubMed] [Google Scholar]
- 38.Urakami, T., H. Araki, H. Oyanagi, K.-I. Suzuki, and K. Komagata. 1992. Transfer of Pseudomonas aminovorans (den Dooren de Jong 1926) to Aminobacter gen. nov. as Aminobacter aminovorans comb. nov. and description of Aminobacter aganoensis sp. nov. and Aminobacter niigataensis sp. nov. Int. J. Syst. Bacteriol. 42:84-92. [Google Scholar]
- 39.van der Meer, J. R., W. Roelofsen, G. Schraa, and A. J. B. Zehnder. 1987. Degradation of low concentrations of dichlorobenzenes and 1,2,4-trichloro-benzene by Pseudomonas sp. strain P51 in nonsterile soil columns. FEMS Microbiol. Ecol. 45:333-341. [Google Scholar]
- 40.Verloop, A. 1972. Fate of the herbicide dichlobenil in plants and soil in relation to its biological activity. Residue Rev. 43:55-103. [Google Scholar]
- 41.Verloop, A., and W. B. Nimmo. 1970. Metabolism of dichlobenil in sandy soil. Weed Res. 10:65-70. [Google Scholar]
- 42.Versteegh, J. F. M., and J. D. te Biesebeek. 2003. De Kwaliteit van het drinkwater in Nederland, in 2001. Rijksinstituut voor Volksgezondheid en Milieu (RIVM). RIVM-rapportnr. 703719003. http://www.rivm.nl/bibliotheek/rapporten/703719003.pdf.
- 43.Vosáhlová, J., L. Pavlú, J. Vosáhlo, and V. Brenner. 1997. Degradation of bromoxynil, ioxynil, dichlobenil and their mixtures by Agrobacterium radiobacter 8/4. Pestic. Sci. 49:303-306. [Google Scholar]
- 44.Wolter, R., S. Rosenbaum, and S. Hannappel. 2001. The German groundwater monitoring network. Proc. Monit. Taylor Made III, p. 277-282. http://www.mtm-conference.nl/mtm3/docs/Wolterea2001.pdf.