Abstract
We report the homofermentative production of lactate in Escherichia coli strains containing mutations in the aceEF, pfl, poxB, and pps genes, which encode the pyruvate dehydrogenase complex, pyruvate formate lyase, pyruvate oxidase, and phosphoenolpyruvate synthase, respectively. The process uses a defined medium and two distinct fermentation phases: aerobic growth to an optical density of about 30, followed by nongrowth, anaerobic production. Strain YYC202 (aceEF pfl poxB pps) generated 90 g/liter lactate in 16 h during the anaerobic phase (with a yield of 0.95 g/g and a productivity of 5.6 g/liter · h). Ca(OH)2 was found to be superior to NaOH for pH control, and interestingly, significant succinate also accumulated (over 7 g/liter) despite the use of N2 for maintaining anaerobic conditions. Strain ALS961 (YYC202 ppc) prevented succinate accumulation, but growth was very poor. Strain ALS974 (YYC202 frdABCD) reduced succinate formation by 70% to less than 3 g/liter. 13C nuclear magnetic resonance analysis using uniformly labeled acetate demonstrated that succinate formation by ALS974 was biochemically derived from acetate in the medium. The absence of uniformly labeled succinate, however, demonstrated that glyoxylate did not reenter the tricarboxylic acid cycle via oxaloacetate. By minimizing the residual acetate at the time that the production phase commenced, the process with ALS974 achieved 138 g/liter lactate (1.55 M, 97% of the carbon products), with a yield of 0.99 g/g and a productivity of 6.3 g/liter · h during the anaerobic phase.
Lactic acid (lactate) and its derivatives have a wide range of applications in the food, pharmaceutical, leather, and textile industries (13, 27). Recently, polylactic acid has been developed as a renewable, biodegradable, and environmentally friendly polymer (16, 28). An advantage of a biological process over a chemical process for the production of lactate is the prospect of generating optically pure lactate (2, 26, 28), which is an important characteristic for many of its end uses (2, 10, 26).
Although several microorganisms can produce lactate by fermentation of glucose and other renewable resources (13), Escherichia coli has many advantages, including rapid growth and simple nutritional requirements. Moreover, the ease of genetic manipulation of E. coli makes possible metabolic engineering strategies for improving lactate accumulation in E. coli (6). Several E. coli strains have been studied for lactate production. For example, a pfl ldhA mutant (FBR11) containing a plasmid with the ldh gene from Streptococcus bovis (l-lactate dehydrogenase [l-LDH]) produced l-lactic acid anaerobically on complex media (11). Using glucose, a concentration of 73 g/liter was obtained with a yield of 0.93 g/g and a productivity of 2.3 g/liter · h. A small amount of succinate was also generated (0.9 to 2.2 g/liter). A pta ppc mutant (JP203) first grown aerobically in complex media to 10 g/liter dry cell weight (DCW) generated 62 g/liter of d-lactate under subsequent anaerobic conditions, with a yield of about 0.9 g/g and a volumetric productivity of 1.0 g/liter · h (6). Similarly, the ldhA mutant of JP203 containing pLS65 encoding Lactobacillus casei l-LDH was grown aerobically to 7.2 g/liter DCW and generated 45 g/liter l-lactate, with a 0.7 g/g yield and a productivity of 0.67 g/liter · h after an anaerobic switch (6). A pflB frdBC adhE ackA mutant (SZ63) grown in minimal media generated 48 g/liter d-lactate of 99% optical purity, close to the theoretical maximum yield of 1.0 g/g (33). After part of the chromosomal ldhA coding region was replaced with Pediococcus acidilactici ldhL, encoding l-LDH, the new strain (SZ85) generated 50 g/liter l-lactate at a 95% yield, with a productivity of 0.42 g/liter · h (34). Recent results indicate that some characteristics of E. coli compare favorably with those of lactic acid bacteria, which often attain yields surpassing 90% (13).
E. coli normally generates a mixture of reduced end products anaerobically from carbohydrates (7) as a result of reducing pyruvate to achieve a redox balance. Although lactate is one possible product, pyruvate is principally metabolized to acetyl-coenzyme A (CoA) by pyruvate formate lyase (PFL; anaerobic) or by the pyruvate dehydrogenase complex (aerobic). To maximize production of lactate, genes encoding these two enzymes could be removed. However, a consequence of preventing metabolic flux from pyruvate to acetyl-CoA is that acetyl-CoA must be derived from another carbon source, such as acetate. In addition to removing genes involved in acetyl-CoA synthesis from pyruvate, genes encoding other enzymes which compete with LDH for the substrate pyruvate also are candidates for deletion.
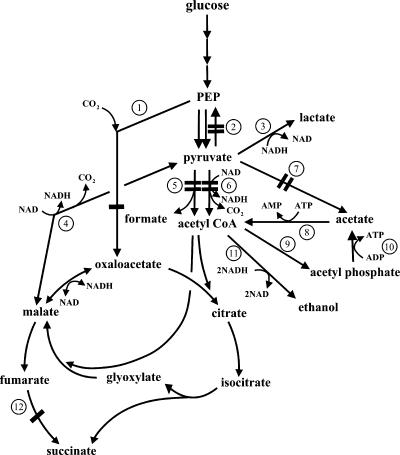
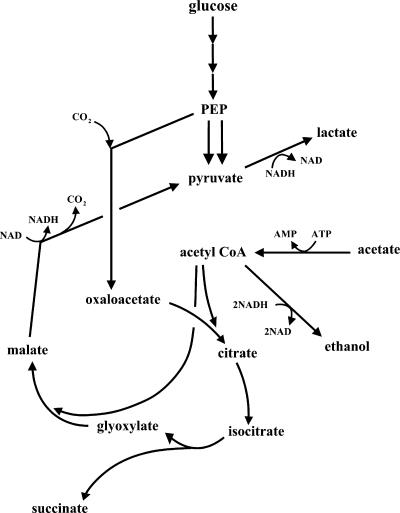
E. coli YYC202, with mutations in the pflB, aceEF, poxB, and pps genes, was previously used to produce 70 g/liter pyruvate aerobically (32) but also generated 26 g/liter lactate. The objective of the present study was to achieve high lactate yields and productivities by a two-phase (aerobic and then anaerobic) fermentation of YYC202 in a defined medium. Moreover, we sought to reduce the formation of the by-product succinate by removing the ppc or frdABCD genes, which encode phosphoenolpyruvate (PEP) carboxylase or fumarate reductase, respectively. Figure 1 shows the principal enzymes involved in the consumption of acetate and glucose relevant to the production of lactate. The simplicity of fermentation conditions and nutritional requirements is the advantage of this approach for lactate production.
FIG. 1.
Key enzymatic reactions in the production of lactate by Escherichia coli strains. Enzymes: 1, PEP carboxylase; 2, PEP synthase; 3, lactate dehydrogenase; 4, malic enzyme; 5, pyruvate formate lyase; 6, pyruvate dehydrogenase complex; 7, pyruvate oxidase; 8, acetyl-CoA synthetase; 9, phosphotransacetylase; 10, acetate kinase; 11, alcohol dehydrogenase; 12, fumarate reductase. Double lines indicate mutations present in YYC202. Single lines indicate additional mutations studied.
MATERIALS AND METHODS
Strains.
Escherichia coli YYC202 [DSM 14335, Hfr zbi::Tn10 poxB1 Δ(aceEF) rpsL pps-4 pfl-1] was generously provided by J. E. Cronan, Jr., University of Illinois. Strains ALS961 (YYC202 ppc) and ALS974 (YYC202 frdABCD) were constructed during this study.
To construct ALS961, the ppc gene, which encodes PEP carboxylase, was knocked out using the lambda Red recombination system (31). Primers which could amplify the chloramphenicol acetyltransferase gene and promoter from pACYC184 (5) bracketed by the first and last 50 bases of the ppc coding sequence were designed. The forward primer, 5′-ATGAACGAACAATATTCCGCATTGCGTAGTAATGTCAGTATGCTCGGCAATTGAGAAGCACACGGTCACA-3′, contained the first 50 bases of the ppc coding sequence, followed by bases 3601 to 3620 of pACYC184, while the reverse primer, 5′-TTAGCCGGTATTACGCATACCTGCCGCAATCCCGGCAATAGTGACCATTATACCTGTGACGGAAGATCAC-3′, contained the last 50 bases of the ppc coding sequence, followed by bases 400 to 419 of pACYC184. The bases homologous to those of pACYC184 are underlined in the primers. The two primers were used to amplify a 1,143-bp fragment from pACYC184 DNA using PCR with Pfu polymerase. The resulting DNA was gel isolated and electroporated into DY330 electrocompetent cells prepared as described previously (31). Camr colonies were then selected. The presence of the ppc::Cam knockout was confirmed by performing PCR with the following two primer pairs, which could amplify the ppc coding sequence. The forward primer, 5′-CGAACAATATTCCGCATTGCG-3′, contains bases 6 to 26 of the ppc gene, while the reverse primer, 5′-TATTACGCATACCTGCCGCAA-3′, contains bases 2624 to 2644 of the ppc gene. PCR amplification with these two primers yields a 2,639-bp fragment from the wild-type ppc gene and a 1,130-bp fragment from the Δppc::Cam knockout.
The frdABCD genes of the fumarase reductase complex were knocked out to construct ALS974 using the lambda Red recombination system. Primers which could amplify the chloramphenicol acetyltransferase gene and promoter from pACYC184 bracketed by the first 50 bases of the frdA gene and the last 50 bases of the frdD gene were designed. The forward primer, 5′-GTGCAAACCTTTCAAGCCGATCTTGCCATTGTAGGCGCCGGTGGCGCGGGTTGAGAAGCACACGGTCACA-3′, contains the first 50 bases of the frdA coding sequence, followed by bases 3601 to 3620 of pACYC184, while the reverse primer, 5′-TTAGATTGTAACGACACCAATCAGCGTGACAACTGTCAGGATAGCAGCCATACCTGTGACGGAAGATCAC-3′, contains the last 50 bases of the frdD coding sequence, followed by bases 400 to 419 of pACYC184 (bases homologous to those of pACYC184 are underlined). The two primers were used to amplify a 1,143-bp fragment from pACYC184 DNA using PCR with Pfu polymerase. The resulting DNA was gel isolated and electroporated into DY330 electrocompetent cells. Camr colonies were then selected. The presence of the ΔfrdABCD::Cam knockout was confirmed by performing PCR with the following two primer pairs, which could amplify the frdABCD genes. The forward primer, 5′-GTGCAAACCTTTCAAGCCGA-3′, contains the first 20 bases of the frdA gene, while the reverse primer, 5′-TGTAACGACACCAATCAGCG-3′, contains bases 335 to 354 of the frdD gene. PCR amplification with these two primers yielded a 3,306-bp fragment from the wild-type frdABCD gene and a 1,137-bp fragment from the ΔfrdABCD::Cam knockout.
Growth conditions.
For each bioreactor experiment, cells were first grown in a 250-ml shake flask containing 30 ml TYA medium for about 6 h, before 5 ml was transferred to 50 ml of SF medium in a 250-ml shake flask. After 10 h of growth, the contents of this shake flask were used to inoculate a fermentor containing GAM medium. All flasks were incubated at 37°C and 250 rpm (19-mm pitch). TYA medium contained (per liter) 10.0 g tryptone, 5.0 g NaCl, 1.0 g yeast extract, and 1.36 g Na(CH3COO) · 3H2O. SF medium contained (per liter) 10.0 g glucose, 2.3 g Na(CH3COO) · 3H2O, 5.66 g Na2HPO4 · 7H2O, 1.5 g KH2PO4, 0.25 g NaCl, 0.5 g NH4Cl, 0.1 g MgSO4 · 7H2O, 0.013 g CaCl2 · 2H2O, 0.02 g thiamine · HCl, and 0.5 g l-isoleucine. GAM medium contained (per liter: 20.0 g glucose, 11.52 g Na(CH3COO) · 3H2O, 1.5 g NaH2PO4 · H2O, 3.25 g KH2PO4, 3.275 g K2HPO4 · 3H2O, 0.2 g NH4Cl, 2.0 g (NH4)2SO4, 1.024 g MgSO4 · 7H2O, 0.01 g CaCl2 · 2H2O, 0.5 mg ZnSO4 · 7H2O, 0.25 mg CuCl2 · 2H2O, 2.5 mg MnSO4 · H2O, 1.75 mg CoCl2 · 6H2O, 0.12 mg H3BO3, 1.772 mg Al2(SO4)3, 0.5 mg Na2MoO4 · 2H2O, 18.29 mg FeSO4 · 7H2O, 0.02 g thiamine · HCl, and 0.75 g l-isoleucine.
Fed-batch fermentation.
Two-phase fed-batch experiments were carried out with a 2.5-liter bioreactor (Bioflow 2000; New Brunswick Scientific Co., Edison, NJ), initially containing 1.5 liters of medium. In the first phase, air and O2 were mixed as necessary at a 1.0-liter/min total flow rate and a 400-rpm agitation to maintain a dissolved oxygen concentration above 40% saturation. During this aerobic phase, the pH was controlled at 7.0 using 30% (wt/vol) NH4OH, and the temperature was controlled at 37°C. A solution of 400 g/liter glucose and 150 g/liter Na(CH3COO) · 3H2O was automatically fed to maintain the glucose concentration above 10 g/liter (YSI 7200 select glucose analyzer; YSI Inc., Yellow Springs, OH). After the cell concentration reached an optical density at 600 nm (OD600) of 30 (corresponding to about 11 g/liter DCW), an anaerobic phase was initiated. In this second phase, N2 was sparged into the fermentor at 0.15 liter/min, the pH was controlled at 7.0 using 20% (wt/vol) NaOH or 25% (wt/vol) Ca(OH)2, and the temperature was controlled at 37°C unless otherwise noted. During the anaerobic phase, 600 g/liter glucose was automatically fed to maintain glucose above 10 g/liter.
Several baffled shake flask studies using temperatures of 37°C and agitations of 250 rpm were completed to determine whether ALS961 grew in the presence of specific carbon sources. For these studies, cells were grown for 6 h in 250-ml shake flasks containing 30 ml TYA medium, and 2 ml was used to inoculate 20 ml GAM medium supplemented with either Casamino Acids (5 g/liter), a single amino acid (0.1 g/liter), citrate (0.1 g/liter), succinate (0.1 g/liter), or fumarate (0.1 g/liter). After 12 h of growth, 2 ml was again used to inoculate 20 ml of an identical medium, and cell growth was monitored for 24 h. Additionally, in separate experiments, glucose in GAM medium was replaced with 1 g/liter succinate or fumarate.
Analyses.
OD600 (UV-650 spectrophotometer; Beckman Instruments, San Jose, CA) was used to monitor cell growth, and this value was correlated with dry cell mass. The final concentrations of soluble organic compounds were determined by liquid chromatography (12). When Ca(OH)2 was used for pH control, a homogeneous sample was diluted by a factor of 10 to dissolve any calcium lactate prior to analysis. Thus, in these cases, the concentrations reported reflect the total amounts of lactate formed, both dissolved and precipitated.
13C NMR analysis.
In order to determine how acetate was being metabolized by the cells, one additional fed-batch experiment was conducted, during which approximately 1 g/liter of uniformly 13C-labeled acetate was added 1 h after the beginning of the anaerobic phase. Samples were centrifuged (twice at 10,000 × g for 10 min), and the supernatant was mixed with a 33% volume of deuterium dioxide (D2O) and filtered for nuclear magnetic resonance (NMR) analysis. Proton-decoupled 13C NMR spectra (300 NMR spectroscopy; Varian Inc., Palo Alto, CA) at 75.4 MHz were obtained with the following spectral parameters: 45° pulses, 18.1-kHz spectral width, and 4.5-s relaxation delay. Field stabilization was achieved by locking on the D2O frequency. 13C chemical shift assignments for glucose, acetate, lactate, pyruvate, succinate, and glyoxylate were determined by comparison with natural abundance standards. The ratio of peak area to labeled acetate concentration was used to calculate concentrations of other labeled compounds. The concentration of labeled acetate added was obtained from high-performance liquid chromatography. Concentrations of other labeled compounds were calculated using the following equation:
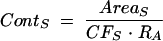 |
(1) |
where ContS is the labeled compound concentration, AreaS is the integral area of the peak corresponding to a labeled carbon atom of the compound, CFS is the correction factor obtained from standard spectra for the compound due to difference in relaxation time, and RA is the ratio of peak area to labeled-acetate concentration.
RESULTS
Production of lactate.
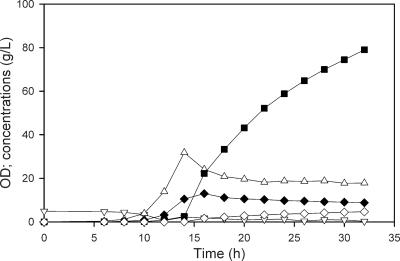
The two-phase fermentation of E. coli YYC202 involved an aerobic growth phase to an OD600 of about 30, followed by an anaerobic nongrowth, lactate production phase. The deletion of both pyruvate dehydrogenase complex genes (aceEF) and pfl in YYC202 necessitated the use of acetate in addition to glucose as a carbon source during the growth phase. Using NaOH during the anaerobic phase to control the pH at 7.0, 75.1 g/liter lactate was generated in 32 h (Fig. 2), for an overall process productivity of about 2.4 g/liter ·h (in replicate fermentations). Lactate was produced only during the 16-h anaerobic phase, and during this anaerobic phase alone, the average productivity was about 4.3 g/liter · h. However, the productivity decreased substantially during the course of the anaerobic phase, with a volumetric productivity of 9 g/liter · h at the onset of the anaerobic conditions, decreasing to about 2 g/liter · h. This decline was greater than could be accounted for merely by the dilution of the fermentor contents from about 1.5 liters to 2.4 liters. Interestingly, at the end of an experiment, 9 g/liter pyruvate and over 4 g/liter succinate remained despite the use of 100% N2 to maintain anaerobic conditions (Table 1) . During the anaerobic phase, the OD600 decreases merely because of the dilution effect and because the volume of individual cells decreases when cells are no longer growing.
FIG. 2.
Production of lactate during the two-phase fermentation of YYC202. Cells were grown aerobically to an OD600 of 30, with NH4OH for pH control, and then switched to anaerobic conditions (at approximately 14 h), with NaOH for pH control. After the initial 20 g/liter glucose was reduced to 10 g/liter, glucose was automatically fed to maintain a concentration above 10 g/liter. The figure shows OD600s (▵) and concentrations of lactate (▪), acetate (▿), pyruvate (♦), and succinate (⋄).
TABLE 1.
Comparison of E. coli strains for homolactate fermentationa
| Strain | Base | OD600d | Acetate (g/liter)d | Final concn (g/liter) for indicated agent
|
||||
|---|---|---|---|---|---|---|---|---|
| Lactate | Pyruvate | Acetate | Succinate | Ethanol | ||||
| YYC202 | NaOH | 32.3 | 1.4 | 75.1 | 9.1 | 0.1 | 4.4 | 0 |
| YYC202 | Ca(OH)2 | 22.9 | 5.1 | 90.0 | 2.6 | 1.8 | 7.3 | 0 |
| ALS961b | Ca(OH)2 | 4.5 | 7.2 | 14.1 | 7.1 | 7.0 | 0 | 0 |
| ALS974 | Ca(OH)2 | 26.0 | 5.1 | 92.0 | 2.1 | 0.3 | 2.6 | 0.6 |
| ALS974c | Ca(OH)2 | 30.6 | 5.0 | 138.1 | 1.5 | 0 | 2.3 | 0.3 |
Fermentor volumes were initially 1.5 liters. The two substrates glucose and acetate were fed until the OD600 reached about 30 during aerobic growth, and then 600 g/liter glucose was fed anaerobically to maintain 10 g/liter until the volume reached about 2.4 liters. Data represent means for two experimental runs.
Medium supplemented with 1.0 g/liter glutamate.
An initial volume of 1.0 liters and a more concentrated base were used.
At the end of the aerobic phase.
We next examined lactate production by YYC202 at a temperature of 40°C or using 10% H2 in N2 during the anaerobic phase. In both cases, we observed an insignificant change in lactate concentrations, yields, or productivities. A study using a pH of 6.0, however, resulted in a 50% decrease in final concentration and productivity, with a 20% reduction in lactate yield (data not shown). Therefore, for subsequent studies, we maintained the pH at 7.0 and the temperature at 37°C and used 100% N2 to maintain anaerobic conditions.
Selection of base for pH control.
The formation of lactate requires the use of a significant quantity of base to maintain the pH at 7.0. When NaOH was used in our initial experiments, the Na+ concentration by the end of the process was about 0.60 mol/liter. Since we observed a significant decrease in the rate of lactate formation during the course of the anaerobic phase, we completed two experiments to determine whether the Na+ ion contributed to this decline in productivity. Because Ca(OH)2 is used in some commercial lactate fermentations (8), the first experiment that we conducted used Ca(OH)2 instead of NaOH as the base for pH control (Fig. 3). With this base, the volumetric lactate production rate remained high throughout the course of the anaerobic production phase (about 5 to 8 g/liter · h, with an average of 5.6 g/liter · h), resulting in a final lactate concentration of 90.0 g/liter (Table 1). Since the reduction in lactate productivity over the course of the anaerobic phase can largely be attributed to a dilution of fermentor contents (i.e., addition of base and glucose solution to a nongrowing culture), this result suggests that the presence of Ca2+ did not reduce the rate of lactate production. Note that OD600 cannot be measured accurately after the addition of a slurry of Ca(OH)2.
FIG. 3.
Production of lactate during the two-phase fermentation of YYC202. Cells were grown aerobically to an OD600 of 30, with NH4OH for pH control, and then switched to anaerobic conditions (at approximately 14 h), with 25% Ca(OH)2 for pH control. After the initial 20 g/liter glucose was reduced to 10 g/liter, glucose was automatically fed to maintain a concentration above 10 g/liter. The figure shows OD600s (▵) and concentrations of lactate (▪), acetate (▿), pyruvate (♦), and succinate (⋄).
To confirm whether the Na+ was reducing lactate productivity, we conducted a second experiment in which the pH was controlled with a mixture of NaOH and Na2SO4, thereby adding more Na+ than necessary merely to control pH. In this case, the final concentration of lactate achieved was 41 g/liter, at which point lactate production essentially ceased (data not shown).
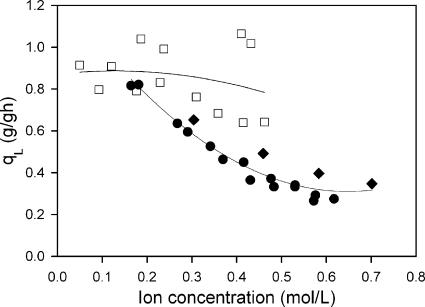
In order to quantify the relationship between Na+, Ca2+, and the rate of lactate production, we next calculated the specific productivity using the lactate concentrations observed during the course of the anaerobic phase and the cell mass concentration at the time of transition between the two phases (Fig. 4). The consistent decline in specific productivity with increasing Na+ regardless of how that ion was added (i.e., NaOH and/or Na2SO4) demonstrates an inhibition of this ion toward lactate formation to a much greater extent than that observed with Ca2+. This inhibition in lactate formation occurs in the absence of any cell growth.
FIG. 4.
The effect of ion concentrations on the specific rate of lactate production over the course of the anaerobic production of lactate. The figure shows specific rate of lactate production (g/g DCW · h) in the presence of Na+ (•) and Ca2+ (□).
Effect of a ppc knockout.
Despite the absence of CO2 in the inlet gas and the presumed insignificant quantity of CO2 generated by cells when they are not growing, succinate accumulated to 7 g/liter during the anaerobic phase of YYC202 fermentations (Table 1). This surprising result led us to speculate that succinate production during the anaerobic phase might best be prevented by the deletion of one or more of the genes in the succinate pathway (Fig. 1).
One strategy proposed to prevent succinate generation was to knock out ppc in YYC202 and thereby prevent the formation of oxaloacetate from PEP via PEP carboxylase (Fig. 1). Previous reports indicate that E. coli with a ppc knockout does not grow on glucose as the sole carbon source (1, 17, 22, 24, 33). In our present study, ALS961 (YYC202 ppc) similarly did not grow aerobically in shake flasks with GAM medium. Furthermore, ALS961 did not grow in GAM medium supplemented with citrate or fumarate, and growth was poor in GAM medium supplemented with succinate. However, ALS961 grew in GAM medium supplemented with 5 g/liter Casamino Acids or in GAM medium when glucose was replaced by succinate or fumarate. We also examined growth when GAM medium was supplemented with a single amino acid (each one individually except isoleucine, which was present in GAM medium). Of the 19 amino acids examined, growth was observed only in the presence of glutamate, aspartate, or glutamine, and no significant difference was observed when the concentration was 0.1 g/liter or 1.0 g/liter.
A two-phase fermentation of ALS961 was completed with GAM medium supplemented with 1 g/liter glutamate. The maximum specific growth rate was about 60% less than that observed for YYC202. Furthermore, growth stalled when the OD600 reached about 4.5, and at this time, the conditions were switched to those of the anaerobic phase. As expected, during the 8 h of anaerobic conditions, succinate did not accumulate (Table 1). Despite the low OD600 and thus the low-volumetric lactate formation rate, the specific lactate formation rate was 1.0 g/g · h, about the same as that observed in the YYC202 anaerobic phase.
Effect of an frdABCD knockout.
Although YYC202 containing the ppc mutation showed no growth in GAM medium, the strain successfully prevented the formation of the by-product succinate when grown in GAM medium supplemented with glutamate. Since the growth was still unacceptably slow and eventually stalled altogether, we next constructed and examined a YYC202 frdABCD mutant, ALS974. Unlike ALS961, ALS974 attained the same growth rate as YYC202 in GAM medium. Using Ca(OH)2 for pH control, 92 g/liter lactate was generated in 15 h of a production phase, or about 32 h total fermentation time, results very similar to those obtained using YYC202 (Table 1). The yield during the anaerobic production phase was over 0.99 g/g (0.81 g/g for both phases). The volumetric productivity decreased from about 9 g/liter · h to 6 g/liter · h during the anaerobic phase, a change attributable to volume increase due to glucose feeding and base control. The specific lactate productivity was 0.9 to 1.1 g/g · h for the duration of the production phase. Surprisingly, succinate was also formed in ALS974 during the anaerobic phase, although its final concentration was always less than 3 g/liter. Furthermore, the accumulation of succinate correlated with the consumption of acetate, some residual amount of which remained in the culture at the end of the growth phase. In those experiments in which the acetate concentration happened to be low at the end of the growth phase, that remaining acetate was slowly consumed during the anaerobic phase at about 0.03 to 0.05 g/g · h, and the accumulation of succinate ceased when acetate was depleted. In those experiments in which the residual acetate concentration was comparatively high, acetate was also slowly consumed during the anaerobic phase. However, succinate generation ceased at 3 g/liter. Then, while acetate consumption continued, ethanol accumulated instead of succinate. The consumption of acetate and formation of either succinate or ethanol did not appear to influence the production of lactate.
13C NMR analysis.
Since succinate accumulation in ALS974 correlated with acetate consumption during the anaerobic phase, we speculated that succinate was formed from acetate through the glyoxylate shunt. To test this hypothesis, we repeated the fed-batch fermentation with ALS974 but introduced uniformly 13C-labeled acetate into the bioreactor 1 hour after the beginning of the anaerobic phase. If the glyoxylate shunt was the route from uniformly labeled acetate to generate succinate, then doublets for the C-1 and C-2 carbons of succinate would be observed in the NMR spectrum as a result of spin-spin coupling.
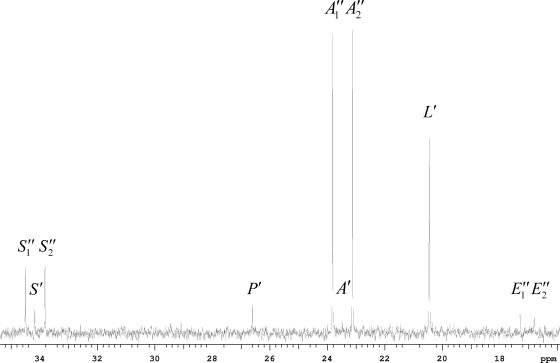
After the labeled acetate was added, the expected doublet peaks were observed at the positions of the acetate methyl (C-2) and carboxylic (C-1) carbons, and the integral areas of these peaks decreased as the anaerobic phase progressed. Moreover, doublet peaks appeared at the positions of the succinate methylene (C-2/C-3) and carboxylic (C-1/C-4) carbons, and these peak areas increased as the anaerobic phase progressed (Fig. 5). Doublets of the doublet peaks were not observed for methylene carbons, demonstrating the absence of significant succinate labeled at each of the four carbons. Doublet peaks also appeared at positions for ethanol methyl and hydroxyl carbons 2 hours after acetate addition, which increased as the anaerobic phase progressed. Doublet peaks were not observed for glucose, lactate, or pyruvate. No other unidentified peaks were observed in any of the NMR studies.
FIG. 5.
Portion of 13C NMR spectrum of fermentation medium 2 hours after the addition of uniformly labeled acetate into the anaerobic phase of ALS974 fermentation. S1′′ and S2′′ correspond to the doublet of the CH2 group of succinate; S′ corresponds to the singlet of the CH2 group of succinate; P′ corresponds to the singlet of the CH3 group of pyruvate; A1′′ and A2′′ correspond to the doublet of the CH3 group of acetate; A′ corresponds to the singlet of the CH3 group of acetate; L′ corresponds to the singlet of the CH3 group of lactate; E1′′ and E2′′ correspond to the doublet of the CH3 group of ethanol.
We next estimated the quantities of succinate and ethanol generated using the integral areas of the doublet peaks. At the beginning of the anaerobic phase (1 to 2 h after the start of the phase), about 50% of the labeled acetate was recovered in succinate, and the balance was unaccounted for. Later in the anaerobic phase (5 to 6 h), about 50% of the labeled acetate was converted to succinate and about 50% was converted to ethanol.
Prolonged fermentation.
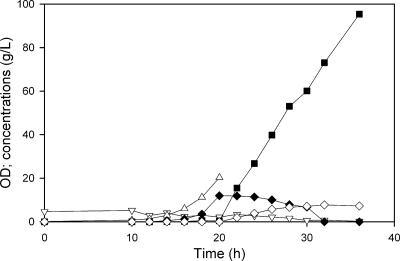
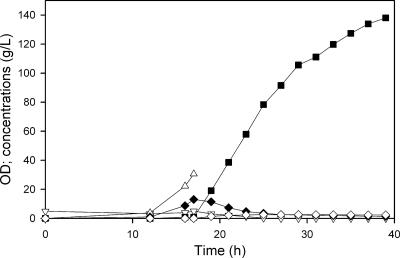
In the previous fermentations using ALS974, the volume changed from 1.5 liters to about 2.4 liters during the course of the fed-batch process due to glucose feeding and base control, both of which served to dilute the nongrowing cells. Since the fermentor capacity was about 2.4 liters, the experiments did not address the question of the maximum concentration of (calcium) lactate which could be produced. We therefore repeated the ALS974 fermentation with a 1.0-liter initial volume and using 30% Ca(OH)2 as the base. In this case, a final lactate concentration of 138 g/liter (1.55 M, 97% of the total carbon products) was achieved in 39 h (Fig. 6) in a final volume of 2.0 liters, while other products (3%) included pyruvate (1.5 g/liter), succinate (3 g/liter), and ethanol (0.3 g/liter). The average anaerobic phase productivity was 6.3 g/liter · h (Table 1).
FIG. 6.
Production of lactate during the two-phase fermentation of ALS974. Cells were grown aerobically to an OD600 of 30, with NH4OH for pH control, and then switched to anaerobic conditions (at approximately 14 h), with 30% Ca(OH)2 for pH control. After the initial 20 g/liter glucose was reduced to 10 g/liter, glucose was automatically fed to maintain a concentration above 10 g/liter. The figure shows OD600s (▵) and concentrations of lactate (▪), acetate (▿), pyruvate (♦), and succinate (⋄).
DISCUSSION
Lactate is produced by E. coli to achieve redox balance under anaerobic conditions and in the absence of external electron acceptors (7). Lactate is generated from pyruvate by LDH (EC 1.1.1.28) encoded by ldhA, which is coupled to the oxidation of NADH to NAD+. Due to the prospect for both a carbon and a redox balance in the conversion of glucose exclusively to lactate, homolactate fermentation can theoretically be achieved in E. coli with a lactate yield of 2 mol/mol glucose (1 g/g). Achieving high-yield homolactate production requires the absence of competing pathways which would serve to oxidize NADH and/or direct carbon to other products. Of course, oxygen and other electron acceptors must also be avoided to prevent oxidation of NADH by any means other than via lactate dehydrogenase.
E. coli YYC202, with mutations in the pflB, aceEF, poxB, and pps genes, was previously constructed and observed to accumulate nearly 70 g/liter pyruvate and 26 g/liter lactate aerobically (32). This strain has knockouts in genes which encode the pyruvate dehydrogenase complex and the enzymes PFL, pyruvate oxidase (POX), and PEP synthase and is therefore ideally suited for studying the potential for homolactate fermentation by E. coli. Consequences of these multiple mutations include an inability to grow anaerobically and a growth requirement for acetate. The absence of growth under anaerobic conditions necessitates a two-phase aerobic-anaerobic process, but it also provides a means for achieving high lactate yield by preventing glucose (or other carbon sources) from being diverted to biomass. Furthermore, pyruvate accumulation during growth may increase the flux toward lactate due to the allosteric activation of LDH (30).
Using YYC202, we observed lactate concentrations consistently over 70 g/liter, with overall productivities (i.e., including those for the aerobic growth phase) greater than 2.5 g/liter · h and yields greater than 0.7 g/g. Two important results were observed in these fermentations. First, Ca2+ is superior to Na+ as a base counterion. Although the specific cause for this difference remains unknown, the E. coli growth rate has been demonstrated to be much more sensitive to Na+ under anaerobic conditions than under aerobic conditions, apparently as a result of the cell's reduced extrusion activity for this specific ion (25). The effect of Na+ is particularly intriguing in this study because it occurs in cells which are not growing, demonstrating that elevated Na+ levels not only affect the growth rate (25) but also affect product formation independently. The results do not distinguish as to whether the observed Na+ “inhibition” is related to cell energetics or whether Na+ merely inhibits a key enzyme specifically. Additional studies with other cations, such as K+ (not examined in this study), would help clarify the inhibitory effects of Na+. The difference observed between Ca2+ and Na+ suggests that Ca(OH)2 may benefit other E. coli fermentation processes which require a high level of base addition. Second, despite the use of N2 at a low flow rate to maintain anaerobic conditions, as much as 7 g/liter succinate was observed at the end of the fermentations. Based on the assumption that a portion of this succinate was derived via PEP carboxylase (Fig. 1), two strategies were employed: knockouts of either ppc or frdABCD were introduced into YYC202.
In ALS961 (YYC202 containing a ppc knockout), we observed only limited growth when the medium was supplemented with glutamate. Although lactate accumulated only slowly at the low cell density achieved, the specific rate equaled the rate observed with YYC202. Moreover, accumulation of succinate was indeed prevented. Thus, homolactate fermentation by ALS961 might be quite successful if the growth characteristics of this strain were improved, for example, by allowing the cometabolism of a four-carbon substrate.
Our studies further clarify the growth requirements of E. coli lacking the enzyme PEP carboxylase. The tricarboxylic acid (TCA) cycle is an important source of intermediates necessary for biosynthesis (22, 24), and as they are withdrawn, these biochemicals must be replenished using anaplerotic pathways. PEP carboxylase is the primary anaplerotic pathway in E. coli, and as expected, a ppc mutation prevents growth on glucose minimal medium (1, 18, 22, 24, 33). Although PEP carboxykinase (encoded by the pckA gene) and malic enzymes (encoded by maeB and sfcA) also catalyze reversible C-3 carboxylation/C-4 decarboxylation reactions, these two pathways appear to be responsible for decarboxylation in E. coli rather than for carboxylation (22). Moreover, the pckA, maeB, and sfcA genes are subject to catabolite repression in the presence of glucose (19, 21, 22). Theoretically, the glyoxylate shunt could compensate for a ppc mutation by generating TCA cycle intermediates from acetate. Despite reports of the shunt also being subject to glucose catabolite repression (19, 21, 22), our NMR results demonstrate that this shunt is at least able to generate succinate from acetate. Why was this succinate unable to support growth in the absence of PEP carboxylase? The low rate of acetate consumption (only 0.03 g/g · h) may have been insufficient to support the anaplerotic requirement for growth. That ALS961 grew on medium supplemented with any one of the three amino acids which are metabolically linked to the TCA cycle demonstrates that these amino acids could, at least to some degree, fulfill the anaplerotic demand in the absence of PEP carboxylase.
Growth of ALS961 was not observed in medium supplemented with the TCA cycle intermediate citrate or fumarate, and growth was very poor with succinate. Most E. coli strains do not consume citrate aerobically due to the lack of a functional transport system (20). Also, the main aerobic C-4 dicarboxylate transport gene (dctA) is subject to cyclic AMP receptor protein-mediated catabolite repression by glucose (9, 15). The slight growth observed with succinate implicates a DctA-independent carrier that is able to transport succinate but not fumarate (14). Growth of ALS961 when glucose was replaced by fumarate or succinate (but not when glucose and either substrate were present) further supports the hypothesis that glucose repression prevented the growth of ALS961 in a medium supplemented with fumarate or succinate compared to what occurred in a medium supplemented with a key amino acid.
The second strategy examined for prevention of succinate accumulation was a knockout of frdABCD (3, 33). In E. coli, two distinct enzymes catalyze succinate oxidation and fumarate reduction (4): succinate-ubiquinone oxidoreductase functions aerobically as part of the TCA cycle, and menaquinol-fumarate oxidoreductase (QFR) is mainly used for anaerobic respiration and succinate production. Since E. coli accumulates succinate via QFR, a mutation in frdABCD encoding QFR should not affect aerobic growth but would prevent anaerobic succinate by this route. Using ALS974 reduced the final succinate concentration from 7 g/liter to about 2 g/liter, with residual succinate linked by NMR to acetate consumption. As noted above, the activity of the glyoxylate shunt was sufficient to convert the remaining acetate to succinate anaerobically but was insufficient to allow significant aerobic growth in the absence of PEP carboxylase. Precise control of the acetate concentration to ensure a near-zero concentration at the transition between aerobic growth and anaerobic production might achieve a further reduction in the formation of by-product succinate.
Our NMR results (Fig. 5) shed additional light on the metabolic pathways used by E. coli during the anaerobic nongrowth phase. Normally, 13C labeling experiments are conducted to provide information for stoichiometric metabolic flux analysis (23, 29). In a metabolic flux analysis experiment, a metabolic and isotopic steady state should be achieved using either a chemostat or a well-controlled fed batch (23). In the present study, a chemostat could not be applied since ALS974 does not grow under anaerobic conditions. Instead, we used uniformly labeled acetate to determine merely whether acetate was the source of succinate or other products during the anaerobic nongrowth phase. The 13C—13C structure of uniformly labeled acetate has doublet peaks for both carbons in the 13C NMR spectrum due to 13C—13C spin coupling (23). By virtue of the presence of doublets, we were able to determine products of acetate metabolism for pathways in which the 13C—13C bond of acetate was not broken. That no doublet was observed for glucose, lactate, or pyruvate indicates that acetate was not converted into these compounds, at least not without the breaking of the C—C acetate bond. Acetate was confirmed to be a source of both succinate and ethanol since doublet peaks corresponding to these compounds appeared soon after the addition of the labeled acetate. We conclude therefore that the production of succinate from acetate is the result of the enzymatic sequence converting acetate to acetyl-CoA, acetyl-CoA and oxaloacetate to citrate and then isocitrate, and isocitrate to succinate and glyoxylate via the enzyme isocitrate lyase (Fig. 7).
FIG. 7.
Proposed pathways used by E. coli ALS974 during anaerobic conversion of glucose and acetate to principally lactate and some ethanol and succinate. 13C NMR results demonstrate that malate is not significantly converted into oxaloacetate.
If succinate is derived from acetate via isocitrate lyase, then what happens to glyoxylate, the other product of isocitrate lyase? Glyoxylate resulting via isocitrate lyase would not obtain labeled carbons from uniformly labeled acetate. The action of the second enzyme of the glyoxylate shunt, malate synthase, would subsequently convert glyoxylate and additional acetyl-CoA into malate. Because a large portion of the acetyl-CoA was uniformly labeled in our NMR study, a significant amount of malate would be labeled simultaneously at C-3 and C-4. If it were converted into oxaloacetate, malate labeled at C-3 and C-4 would lead via the TCA cycle to a small but readily detectable concentration of uniformly labeled succinate. However, we observed only one doublet pattern at the methylene carbon of succinate, consistent with the absence of uniformly labeled succinate. This result indicates either that the glyoxylate shunt is incomplete during the anaerobic nongrowth phase or that the malate generated from the glyoxylate shunt is not converted to oxaloacetate. The first explanation is not supported: we did not observe the accumulation of glyoxylate or malate. However, we also did not observe the accumulation of any other labeled compound. We therefore speculate that malate generated from glyoxylate was converted into a compound that we could not measure, such as pyruvate via malic enzyme (Fig. 7). This pathway would have broken the 13C—13C bond introduced into malate from uniformly labeled acetate and generated 13CO2 and 13C-3-pyruvate. Interestingly, this proposed route would lead to a small quantity of lactate generated from acetate. The high concentration of naturally labeled lactate and pyruvate relative to the amount of acetate consumed prevented accurate confirmation of this pathway by 13C NMR analysis. Although some CO2 would be generated by the oxidation of malate to pyruvate, the source of the CO2 for the conversion of PEP to oxaloacetate during the N2-supplied nongrowth phase remains unexplained. Additional studies are needed to identify whether this small amount of CO2 comes from the pentose phosphate pathway, the remaining activities of TCA cycle enzymes, or another pathway.
During the first 3 h of the anaerobic phase, we observed 13C labeling patterns consistent with succinate generation occurring via the glyoxylate shunt and with glyoxylate being converted to pyruvate via malate. Not considering ATP, the metabolism of acetate early in the anaerobic phase thus may be summarized as follows (Fig. 7):
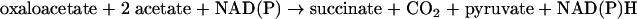 |
The fraction of ethanol generated slowly increased so that by 3 to 6 h after the beginning of the anaerobic phase, we observed approximately equimolar succinate and ethanol production from acetate, with the latter being presumably formed directly:
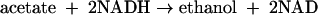 |
The first reaction generates reduced cofactors, while the second consumes reduced cofactors, although together they are not redox balanced. So why does the organism initially generate succinate and then increasingly ethanol from acetate? First, in comparison to the flux through glycolysis and toward lactate, the fluxes toward these two products are very small (less than 5%). Thus, the presence of these two pathways for acetate assimilation does not significantly affect the overall redox balance and may merely offer the cells a means to fine tune the redox environment as the culture enters and during anaerobic conditions. Second, previous research with wild-type E. coli indicates that the NADH/NAD ratio under aerobic conditions is less than half of the value under anaerobic conditions (17). The sudden change from aerobic growth to anaerobic conditions could therefore result in a temporary deficit in NADH until a new, higher steady-state NADH/NAD ratio is attained. During such transient conditions, the cell may actually prefer reactions which generate NADH compared to those which consume NADH. If this is the case, it is reasonable that initially more succinate was generated from acetate than from ethanol, as we observed. Accumulation of succinate in homolactate fermentation might be avoided by the careful control of acetate so that this substrate is not present at the onset of the anaerobic phase.
With ALS974, we report the highest levels of lactate achieved for E. coli in a defined medium (Table 1): a final lactate concentration of 138 g/liter (97% of the total amount of carbon products), an overall productivity of 3.5 g/liter · h (6.3 g/liter · h anaerobic productivity), and an overall yield of 0.86 g/g glucose (0.99 g/g anaerobic yield).
Acknowledgments
We thank the USDA-NRI program (2003-35504-13666) and the Georgia Experiment Station for financial support of this research.
We also thank John E. Cronan, Jr., for E. coli strain YYC202 and Jeffrey Urbauer, Sarah Lee, and Ronni Altman for technical assistance.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Ashworth, J. M., H. L. Kornberg, and R. L. Ward. 1965. Role of phosphoenolpyruvate carboxylase in Escherichia coli. Biochem. J. 94:28P. [Google Scholar]
- 2.Blomqvist, J. 2001. RIS Metropolis Monte Carlo studies of poly(L-lactic), poly(L,D-lactic) and polyglycolic acids. Polymer 42:3515-3521. [Google Scholar]
- 3.Causey, T. B., K. T. Shanmugam, L. P. Yomano, and L. O. Ingram. 2004. Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc. Natl. Acad. Sci. USA 101:2235-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecchini, G., I. Schroder, R. P. Gunsalus, and E. Maklashina. 2002. Succinate dehydrogenase and fumarate reductase from Escherichia coli. Biochim. Biophys. Acta 1553:140-157. [DOI] [PubMed] [Google Scholar]
- 5.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, D. E., H. C. Jung, J. S. Rhee, and J. G. Pan. 1999. Homofermentative production of d- or l-lactate in metabolically engineered Escherichia coli RR1. Appl. Environ. Microbiol. 65:1384-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark, D. P. 1989. The fermentation pathways of Escherichia coli. FEMS Microbiol. Rev. 63:223-234. [DOI] [PubMed] [Google Scholar]
- 8.Datta, R., and M. Henry. 2006. Lactic acid: recent advances in products, process and technologies—a review. J. Chem. Technol. Biotechnol. 81:1119-1129. [Google Scholar]
- 9.Davies, S. J., P. Golby, D. Omrani, S. A. Broad, V. L. Harrington, J. R. Guest, D. J. Kelly, and S. C. Andrews. 1999. Inactivation and regulation of the aerobic C-4-dicarboxylate transport (dctA) gene of Escherichia coli. J. Bacteriol. 181:5624-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong, S. J., B. van Eerdenbrugh, C. F. van Nostrum, J. J. Kettenes-van de Bosch, and W. E. Hennink. 2001. Physically crosslinked dextran hydrogels by stereocomplex formation of lactic acid oligomers: degradation and protein release behavior. J. Control Release 71:261-275. [DOI] [PubMed] [Google Scholar]
- 11.Dien, B. S., N. N. Nichols, and R. J. Bothast. 2001. Recombinant Escherichia coli engineered for production of L-lactic acid from hexose and pentose sugars. J. Ind. Microbiol. Biotechnol. 27:259-264. [DOI] [PubMed] [Google Scholar]
- 12.Eiteman, M. A., and M. J. Chastain. 1997. Optimization of the ion-exchange analysis of organic acids from fermentation. Anal. Chim. Acta 338:69-75. [Google Scholar]
- 13.Hofvendahl, K., and B. Hahn-Hägerdal. 2000. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb. Technol. 26:87-107. [DOI] [PubMed] [Google Scholar]
- 14.Janausch, I. G., O. B. Kim, and G. Unden. 2001. DctA- and Dcu-independent transport of succinate in Escherichia coli: contribution of diffusion and of alternative carriers. Arch. Microbiol. 176:224-230. [DOI] [PubMed] [Google Scholar]
- 15.Kay, W. W., and H. L. Kornberg. 1971. The uptake of C4-dicarboxylic acids by Escherichia coli. Eur. J. Biochem. 18:274-281. [DOI] [PubMed] [Google Scholar]
- 16.Kharas, G. B., F. Sanchez-Riera, and D. K. Severson. 1994. Polymers of lactic acid, p. 93-137. In D. P. Mobley (ed.), Plastics from microbes: microbial synthesis of polymers and polymer precursors. Hanser Publishers, Munich, Germany.
- 17.Leonardo, M. R., Y. Dailly, and D. P. Clark. 1996. Role of NAD in regulating the adhE gene of Escherichia coli. J. Bacteriol. 178:6013-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAlister, L. E., E. L. Evans, and T. E. Smith. 1981. Properties of a mutant Escherichia coli phosphoenolpyruvate carboxylase deficient in coregulation by intermediary metabolites. J. Bacteriol. 146:200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phue, J. N., S. B. Noronha, R. Hattacharyya, A. J. Wolfe, and J. Shiloach. 2005. Glucose metabolism at high density growth of E. coli B and E. coli K: differences in metabolic pathways are responsible for efficient glucose utilization in E. coli B as determined by microarrays and Northern blot analyses. Biotechnol. Bioeng. 90:805-820. [DOI] [PubMed] [Google Scholar]
- 20.Pos, K. M., P. Dimroth, and M. Bott. 1998. The Escherichia coli citrate carrier CitT: a member of a novel eubacterial transporter family related to the 2-oxoglutarate/malate translocator from spinach chloroplasts. J. Bacteriol. 180:4160-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saier, M. H., and T. M. Ramseier. 1996. The catabolite repressor/activator (Cra) protein of enteric bacteria. J. Bacteriol. 178:3411-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sauer, U., and B. J. Eikmanns. 2005. The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol. Rev. 29:765-794. [DOI] [PubMed] [Google Scholar]
- 23.Stephanopoulos, G. N., A. A. Aristidou, and J. Nielsen. 1998. Metabolic engineering—principles and methodologies. Academic Press, San Diego, CA.
- 24.Theodore, T. S., and E. Englesberg. 1964. Mutant of Salmonella typhimurium deficient in carbon dioxide-fixing enzyme phosphoenolpyruvic carboxylase. J. Bacteriol. 88:946-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trchounian, A., and H. Kobayashi. 1999. Fermenting Escherichia coli is able to grow in media of high osmolarity, but is sensitive to the presence of sodium ion. Curr. Microbiol. 39:109-114. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji, F. 2002. Autocatalytic hydrolysis of amorphous-made polylactides: effects of L-lactide content, tacticity, and enantiomeric polymer blending. Polymer 43:1789-1796. [Google Scholar]
- 27.Vickroy, T. B. 1985. Lactic acid, p. 761-776. In M. Moo-Young, H. W. Blanch, S. Drew, and D. I. C. Wang (ed), Comprehensive biotechnology, vol. 3. Pergamon Press, New York, NY. [Google Scholar]
- 28.Wehrenberg, R. H. 1981. Lactic-acid polymers—strong, degradable thermoplastics—research at Battelle proves the value of limited-lifetime plastics made from renewable resources. Mater. Eng. 94:63-66. [Google Scholar]
- 29.Wiechert, W. 2001. 13C metabolic flux analysis. Metab. Eng. 3:195-206. [DOI] [PubMed] [Google Scholar]
- 30.Yang, Y.-T., G. N. Bennett, and K.-Y. San. 2001. The effects of feed and intracellular pyruvate levels on the redistribution of metabolic fluxes in Escherichia coli. Metab. Eng. 3:115-123. [DOI] [PubMed] [Google Scholar]
- 31.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelic, B., T. Gerharz, M. Bott, D. Vasič-Racki, C. Wandrey, and R. Takors. 2003. Fed-batch process for pyruvate production by recombinant Escherichia coli YYC202 strain. Eng. Life Sci. 3:299-305. [Google Scholar]
- 33.Zhou, S. D., T. B. Causey, A. Hasona, K. T. Shanmugam, and L. O. Ingram. 2003. Production of optically pure d-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl. Environ. Microbiol. 69:399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou, S. D., K. T. Shanmugam, and L. O. Ingram. 2003. Functional replacement of the Escherichia coli d-(−)-lactate dehydrogenase gene (ldhA) with the l-(+)-lactate dehydrogenase gene (ldhL) from Pediococcus acidilactici. Appl. Environ. Microbiol. 69:2237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]