Abstract
Glucocorticoid treatment can lead to the development of glaucomatous ocular hypertension and a secondary open-angle glaucoma due to increased aqueous humor outflow resistance that is associated with morphological and biochemical changes in the trabecular meshwork (TM). The cellular responses of glucocorticoids are achieved by binding to the glucocorticoid receptor α (GRα), a ligand-activated transcription factor. An alternatively spliced variant, glucocorticoid receptor β (GRβ), has dominant negative activity on GRα and has been implicated in a variety of steroid-resistant diseases. We previously showed that GRβ can block dexamethasone (DEX) responsiveness in TM cells. TM cells are actively phagocytic and function in the removal of debris, pigment and other materials from the aqueous outflow drainage pathway. A decrease in phagocytic activity has been proposed in the pathogenesis of glaucoma and glucocorticoid-induced glaucoma. In this study, we investigated the effect of DEX and GRβ on phagocytosis in normal and glaucomatous TM cells. Human transformed normal NTM-5 and primary normal NTM174-00 cells, which express relatively high amounts of GRβ, and transformed glaucomatous GTM-3 and primary glaucomatous GTM520-05 cells, which have lower GRβ expression, were treated with 100 nM DEX or vehicle control for 24 hours. NTM cells also were transfected with a control or GRβ expression plasmid to examine the effect of GRβ on phagocytic activity. The cells were incubated with Alexa 488 conjugated Straphylococcus aureus bioparticles opsonized with rabbit IgG for one hour, followed by fixation and incubation with Alexa 633 conjugated goat anti-rabbit IgG to distinguish ingested from extracellular bioparticles. DAPI nuclear staining was used to quantify cell numbers. Cells and bioparticles were visualized by confocal microscopy. We found that NTM-5 cells ingested more bioparticles than GTM-3 cells. DEX treatment significantly decreased the phagocytosis of bioparticles in NTM-5 and GTM-3 cells, while GTM-3 cells were more responsive to DEX, compared to NTM-5 cells. In primary cell culture, NTM174-00 also engulfed more bioparticles than GTM520-05 cells. DEX treatment significantly decreased the phagocytic activity in GTM 520-05, but not in NTM174-00 cells.Transient transfection of pCMX-hGRβ plasmid increased the expression of GRβ and consequently maintained the phagocytotic activity of NTM-5 cells in the presence of DEX. Our data demonstrated that the expression level of GRβ in TM cells can regulate DEX-induced suppression of phagocytotic activity. The lower expression of GRβ in glaucomatous TM cells may contribute to the altered phagocytic function of TM cells, and may lead to the increased aqueous humor outflow resistance mediated by glucocorticoids.
Keywords: Glucocorticoid Receptor β, Trabecular Meshwork, Dexamethasone, Phagocytosis, Glaucoma
Introduction
Glucocorticoid (GC) therapy can lead to the development of glaucomatous ocular hypertension and secondary open-angle glaucoma that is clinically similar to primary open-angle glaucoma (POAG) (Clark and Morrison, 2002). The elevated intraocular pressure (IOP) is due to increased aqueous humor outflow resistance and is associated with morphological and biochemic changes in the trabecular meshwork (TM) (Wordinger and Clark, 1999). These changes are associated with increased deposition of extracellular matrix material in the outflow pathway, which may be due, in part, to a decrease in TM phagocytosis. Most of the effects of GCs on TM cells and tissues are likely due to GC-mediated TM cell gene expression, including the induction of myocilin, fibronection and other genes (Steely et al., 1992, Nguyen et al., 1998, Ishibashi et al., 2002, Lo et al., 2003) (Rozsa et al., 2006). It is currently unclear which of these effects or combinations of effects accounts for GC-induced ocular hypertension.
There are differences in steroid sensitivity among the population. Topical ocular administration of GCs produces measurable increases in IOP in a greater percentage of patients with POAG and their descendants compared with normal individuals (Armaly, 1963, Becker and Hahn, 1964, Armaly and Becker, 1965). The molecular basis of the increase in IOP experienced by patients with glaucoma and subjects receiving GCs is not well understood. Cellular effects of GCs are achieved by binding to glucocorticoid receptor α (GRα), which functions as a ligand-activated transcription factor (Evans, 1988). Interestingly, the alternative splicing variant, human glucocorticoid receptor β (GRβ), has been shown to have a dominant negative activity on GRα (Bamberger et al., 1995, Oakley et al., 1996, Oakley et al., 1999, Hauk et al., 2002, Zhang et al., 2005). In addition, GRβ has been implicated in a variety of steroid-resistant diseases, including rheumatoid arthritis, asthma, and inflammatory bowel diseases (Sousa et al., 2000, Derijk et al., 2001, Orii et al., 2002, Goleva et al., 2005). Recently, we reported that GRβ regulates GC responsiveness in TM cells, with glaucomatous TM cells having lower levels of GRβ (Zhang et al., 2005).
TM cells have well-established phagocytic properties, which has been implicated as an essential function to eliminate debris and in maintaining the aqueous outflow pathway in both normal and disease states (Bill, 1975, Johnson et al., 1989, Buller et al., 1990, Murphy et al., 1992, Schlotzer-Schrehardt and Naumann, 1995). Clinically, TM cells phagocytose autogenous blood (Grierson and Lee, 1973), melanin granules (Richardson et al., 1977), and photocoagulation debris from the iris (Grierson and Chisholm, 1978). Under experimental conditions, TM cells are reported to avidly ingest a variety of substances, such as India ink, colloidal gold, mercuric sulfide (Rohen and van der Zypen, 1968), sickled red blood cells (Goldberg and Tso, 1978), pigment (Epstein et al., 1986), zymosan particles (Sherwood and Richardson, 1988), and latex spheres (Johnson et al., 1989). This property of phagocytosis can be demonstrated in cultured TM cells, trabeculectomy specimens, and organ culture systems. Failure to phagocytose could give rise to abnormalities in the clearance mechanism and at least in part, may contribute to a number of pathological conditions including POAG, pigmentary glaucoma, exfloliation glaucoma, and GC-induced glaucoma. TM cell phagocytosis is also involved in the turnover of extracellular matrix (Acott, 1994), and any alteration in the turnover of the extracellular matrix may be involved in the increased outflow resistance seen in POAG.
Corticosteroids inhibit phagocytosis in cultured TM cells (Li and Zhang, 2004) and in organ cultures (Matsumoto and Johnson, 1997a). Previously, we reported that glaucomatous TM cells had a relatively lower expression of GRβ and had increased responsiveness to DEX (Zhang et al., 2005). Increased expression of GRβ blocked the DEX induction of myocilin and fibronectin in cultured human TM cell lines. In this study, we further investigated the effect of GRβ on the regulation of phagocytosis and the action of DEX treatment in normal and glaucomatous TM cells.
Materials and methods
Cell Culture
Normal primary TM (NTM174-00) and glaucomatous primary GTM520-05 cells were generated and characterized as previously described (Steely et al., 1992; Clark et al., 1994). In addition to these cell lines, transformed normal NTM-5 and and glaucomatous GTM-3 cells (Pang et al., 1994) were cultured in 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, penicillin and streptomycin, and glutamate (Invitrogen-Gibco, Grand Island, NY, USA). Cells were cultured on cover-slides in 6 well plates in 5% FBS DMEM with vehicle (0.1% ethanol) or 100 nM DEX (Sigma Chemical, St Louis, MO, USA) for 24 hours prior to phagocytosis.
Immunolabeling of Alexa Flour 488 conjugated S. aureus Bioparticles
Alexa 488 conjugated Straphylococcus aureus bioparticles (heat- or chemically killed) (Molecular Probes, Eugene, OR, USA) were incubated with S. aureus bioparticle opsonizing reagent (purified rabbit polyclonal IgG antibodies) (Molecular Probes, Eugene, OR, USA) at 37°C for 1 hr, followed by a PBS wash to remove excess antibody from bioparticles according to the manufacturer's protocol. This opsonizing reagent is derived from purified rabbit polyclonal IgG antibodies that are specific for the S. aureus particles and is used for enhancing the uptake of these particles. After this incubation, the opsonized bioparticles (bioparticles coated with antibody) were immunoreacted with secondary antibody Alexa Flour 633 goat anti-rabbit IgG for 1hr at room temperature and placed on a glass slide under a cover slide, followed by confocal scanning laser microscopy (model LSM-410; Carl Zeiss Meditec, Inc., Thornwood, NY) using double fluorescence (excitation wavelengths 488 nm and 633 nM) to examine the color differences. For phagocytosis, opsonized bioparticles were incubated with TM cells cultured on coverslides for 1 hour, followed by fixation and incubation of Alexa Flour 633 goat anti-rabbit IgG. The purposes of this opsonization and secondary antibody incubation were to enhance the cellular uptake of bioparticles and to allow differentiation of intracellular bioparticles from bioadherent extracellular bioparticles that were not internalized. Ingested bioparticles were protected from secondary antibody and showed green fluorescence under double fluorescent microscopy. The secondary antibody binds to the extracellular bioparticles opsonized with rabbit IgG, and the bioparticles fluoresce both green and red, which in overlay becomes orange or yellow.
Phagocytosis Assay
Typically, human neutrophils incubated with fluorescein-labelled bacteria become saturated after a phagocytic challenge of 1 hour (Oben and Foreman, 1988). Many studies have shown that 30 minutes to 1hour incubation time is needed to obtain maximal uptake of bioparticles, including S. aureus, E. coli, or Zymosan by immune cells (Cantinieaux et al., 1989, Wan et al., 1993, Zhu et al., 1995, Mold et al., 2001). Individual TM cells can complete the entire sequence of phagocytic events by 1 hour (Sherwood and Richardson, 1988), and TM cells also actively metabolize foreign particles via lysosomes (Polansky et al., 1984, Yue et al., 1987). We therefore used a 1 hour incubation time for our phagocytic studies. TM cells were cultured on cover-slides in 6-well plates. After vehicle (ethanol) or DEX treatment for 24 hours, TM cells were shifted to serum-free medium and then incubated with rabbit IgG opsonized Alexa 488 conjugated S. aureus bioparticles at a ratio of 50 bioparticles per cell at 37°C for 1 hr according to the manufacturer's protocol. After incubation, cover-slides were washed with PBS three times to remove free bioparticles and cells were fixed with a 4% paraformaldehyde solution for 30 minutes. After blocking with 5% Albumin + 5% goat pre-immune serum for 30 minutes, cells were incubated with Alexa Flour 633 goat anti-rabbit IgG secondary antibody for 1 hour. The extra secondary antibody was removed by a PBS wash. To determine the number of cells used in the experiment, cells were incubated with DAPI for 10 minutes to stain nuclei. Cover-slides were mounted and cellular phagocytosis of bioparticles, DAPI nuclear staining and cellular morphology were visualized and imaged with a confocal scanning laser microscope system (model LSM-410; Carl Zeiss Meditec, Inc., Thornwood, NY) using double fluorescence (excitation wavelengths 488 nm and 633 nm), UV light (wavelength 343) and transmission light, respectively. The number of phagocytic bioparticles was quantified by counting individual TM cells (DAPI stained nuclei) and total bioparticles ingested by these TM cells (# bioparticles/100 cells).
Transfection of Human GRβ Vector
A pCMX-hGRβ expression vector was generated as described previously (Zhang et al., 2005). NTM-5 cells were seeded into 6-well cover-slides overnight, then transfected with the control pCMX or pCMX-hGRβ expression vectors using lipofectamine (BD Biosciences. San Jose, CA) in serum-free medium when cells were about 60% confluent. 1 μg of vector and 1.5 μl of lipofectamine per coverslide was used and cells were transfected for 9 hours, followed by a post-transfection incubation in serum medium for 24 hours. Cells were either fixed for GRβ immunofluorescence staining or switched to serum free medium and treated with vehicle or 100 nM DEX for another 24 hours. Phagocytosis assays were conducted as described above.
Immunocytochemistry Assay
TM cells were fixed in 4% paraformaldehyde for 30 min, permeabilized in 0.2% Triton X-100 for 15 min, incubated in 0.2M glycine for 30 min, and blocked with 5% bovine serum albumin + 5% normal goat serum for 30 min. The cells were incubated overnight at 4°C with anti- GRβ antibody (PA3–514, Affinity Bioreagents, Golden, CO, USA) and subsequently incubated with Alexa 633 goat anti-rabbit IgG (Molecular Probe, Eugene, Oregon, USA) for 1 hour. Coverslides were mounted and visualized with a confocal scanning laser microscope system.
Statistical Analysis
Phagocytic values were determined by counting total DAPI stained nuclei (assuming that one nucleus represents one cell) and ingested bioparticles by these cells. The phagocytic index was expressed as number of particles per 100 cells. All values are listed as means ± SEM. The data were analyzed with analysis of variance with One-way ANOVA and Student-Newman-Keuls multiple comparison test for comparing many groups or with t-test for comparing two groups. Significance was set at p < 0.05.
Results
Immunolabeling of Alexa Flour 488 conjugated S. aureus Bioparticles to distinguish intracellular vs extracellular bioparticles
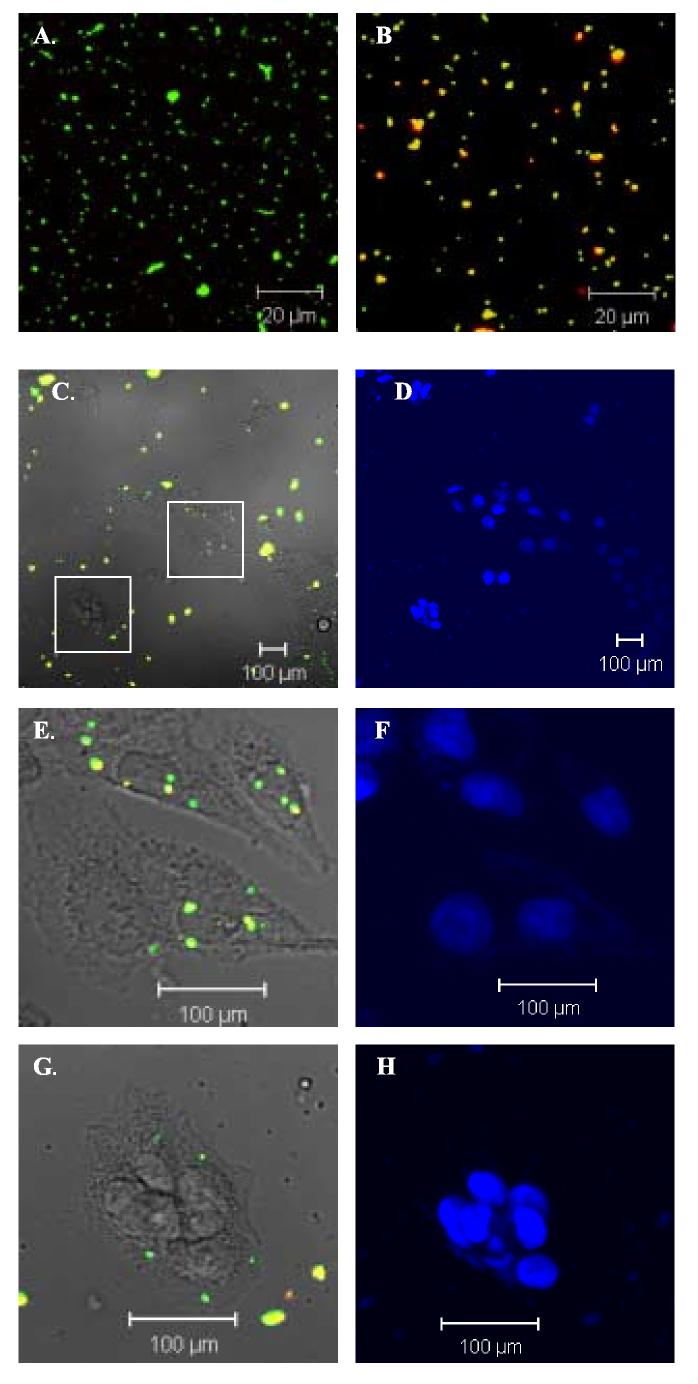
In order to differentiate ingested particles from extracellular ones, two immunofluorescence color plots, which have been utilized previously for trabecular meshwork cell phagocytosis assays (Barak et al., 1988, Matsumoto and Johnson, 1997a, Matsumoto and Johnson, 1997b), were used in this study. Initially, we verified the color difference of S. aureus bioparticles alone with or without incubation of opsonizing reagent (rabbit IgG), followed by incubation with secondary antibody Alexa Flour 633 conjugated goat anti-rabbit IgG. As shown in Fig. 1A and 1B, the S. aureus bioparticles without incubation with opsonizing reagent were green (Fig. 1A) as the Alexa Flour 488 is inherent in the S. aureus bioparticles at the time of manufacture. However, after incubation with opsonizing reagent and secondary antibody Alexa Flour 633 conjugated goat anti-rabbit IgG, these bioparticles appeared yellow (Fig. 1B), because of the overlay of green and red colors. In order to use this method to distinguish extracellular versus intracellular bioparticles, experiments were conducted using a sparse cultured NTM-5 cells at about 30% confluency challenged with the fluorescent bioparticles, followed by visualization with confocal double fluorescence microscopy. As illustrated in Fig. 1 C and high magnification images E and G, cultured NTM-5 cells actively ingested bioparticles, and the green intracellular and yellow or orange extracellular bioparticles were easily discriminated from each other. By counting DAPI stained nuclei (Fig. 1 D, F and H) and green bioparticles, we could determine the total ingested bioparticles by these cells, expressed as number of particles per 100 cells.
Fig.1.
Phagocytosis of bioparticles by NTM-5 cells. A-B: Images were taken under 488 nm and 633 nm excitation wavelengths using confocal double fluorescence microscopy. Fluorescence image of Alexa Flour 488 (green) conjugated S. aureus bioparticles without (A) and with (B) incubation with oposonizing reagent rabbit IgG and secondary antibody Alexa Flour 633 (red) conjugated goat anti-rabbit IgG. Green = ingested bioparticles. Yellow = extracellular adherent bioparticles (green + red = yellow). C: Light microscopy of NTM-5 cells and confocal double fluorescence image of bioparticles after phagocytosis. E and G: High magnification of two areas in image C (indicated as two squares). D, F and H: DAPI nuclear staining. Opsonized bioparticles were incubated with subconfluent NTM-5 cells at a ratio of 50:1 at 37°C for 1 hour. Cells were then fixed and incubated with Alexa Flour 633 conjugated secondary antibody. A representative experiment of three is shown.
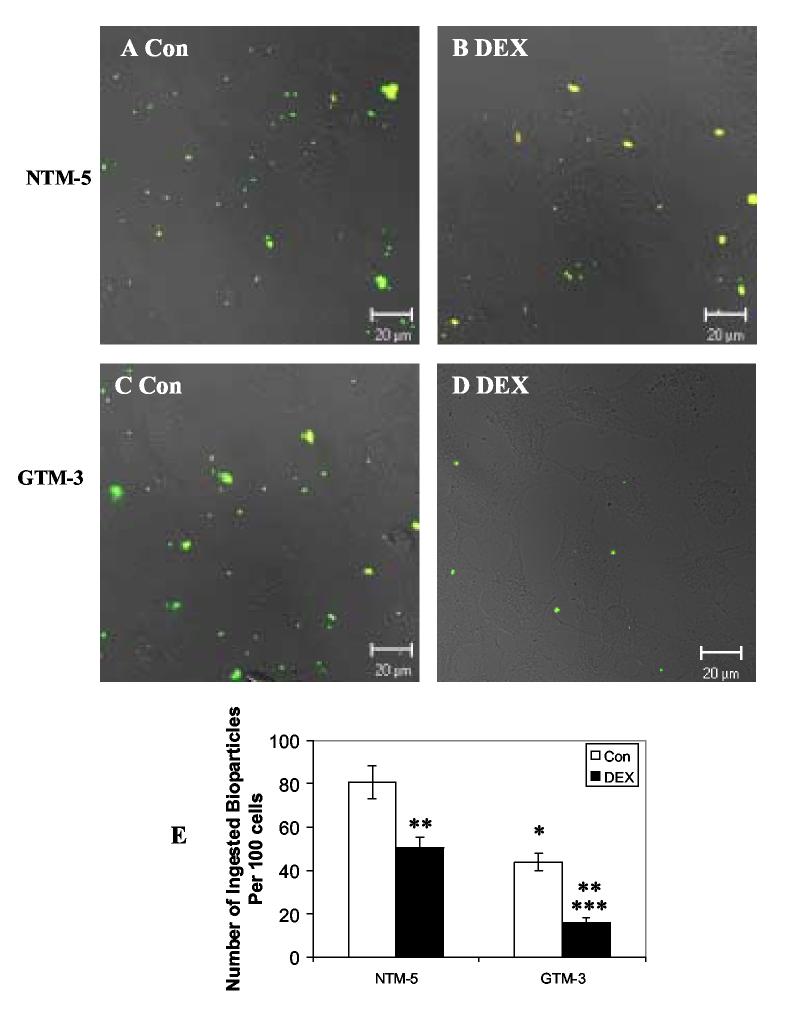
Effects of Dexamethasone on Phagocytotic Activity in Normal NTM-5 and Glaucomatous GTM-3 cells
Previously we reported that glaucomatous GTM-3 cells expressed lower levels of GRβ compared to normal NTM-5 cells, and the expression level of GRβ regulated the cellular responsiveness to glucocorticoids by decreasing targeted glucocorticoid gene expression (Zhang et al., 2005). DEX treatment has been shown to inhibit the phagocytotic capability in TM cells (Matsumoto and Johnson, 1997a, Li and Zhang, 2004). In our study, we determined whether DEX would also inhibit phagocytosis in GTM-3 and HTM-5 cells and further investigated whether altered expression of GRβ could differentially influence DEX-reduced phagocytosis of normal versus glaucomatous TM cells. In three independent experiments, we showed that both normal and glaucomatous TM cells were able to phagocytose these bioparticles with most of the bioparticles inside the cell (green) and only a few bioparticles outside the cells (yellow) (Fig. 2. A-D). DEX treatment decreased phagocytotic activity in both NTM-5 and GTM-3 cells (Fig. 2 B, D, and E). DEX-treated NTM-5 cells ingested significantly fewer bioparticles than controls (51 ± 5 bioparticles/ 100 cells following DEX treatment versus 81 ± 8 bioparticles/ 100 cells in controls; mean ± SEM, p< 0.05 by One-way ANOVA) resulting in a 37% reduction in ingested bioparticles. Compared to NTM-5 cells, GTM-3 cells ingested significantly less bioparticles under control conditions (p< 0.05 by One-way ANOVA), and DEX treatment further decreased this phagocytosis (16 ± 2 bioparticles/ 100 cells in DEX treated cells versus 44 ± 4 bioparticles/ 100 cells in controls; mean ± SEM, p< 0.05 by One-way ANOVA). DEX treatment resulted in a 64% reduction in ingested bioparticles.
Fig. 2.
Effects of Dexamethsone on Phagocytosis in Normal NTM-5 and Glaucomatous GTM-3 cells. NTM-5 and GTM-3 cells were treated with 100 nM DEX for 24 hours. Opsonized bioparticles were then incubated with TM cells at a ratio of 50:1 at 37°C for 1 hour. After phagocytosis, cells were fixed and incubated with Alexa Flour 633 conjugated secondary antibody. A-D: Light microscopy of TM cells and confocal double fluorescence microscopy of bioparticles after phagocytosis. E: Phagocytosis was scored as the number of phagocytic bioparticles per 100 cells. Results are expressed as the mean value of three independent experiments. At least 1000 cells were examined in each experiment. * denotes statistical significance of mean phagocytic index of GTM-3 versus that of NTM-5, ** denotes statistical significance of mean phagocytic index of NTM-5 and GTM-3 from DEX treatment versus that of control, and *** denotes statistical significance of mean phagocytic index of GTM-3 from DEX treatment versus that of NTM-5 from DEX treatment as determined by One-way ANOVA and Student-Newman-Keuls multiple comparison test at p<0.05.
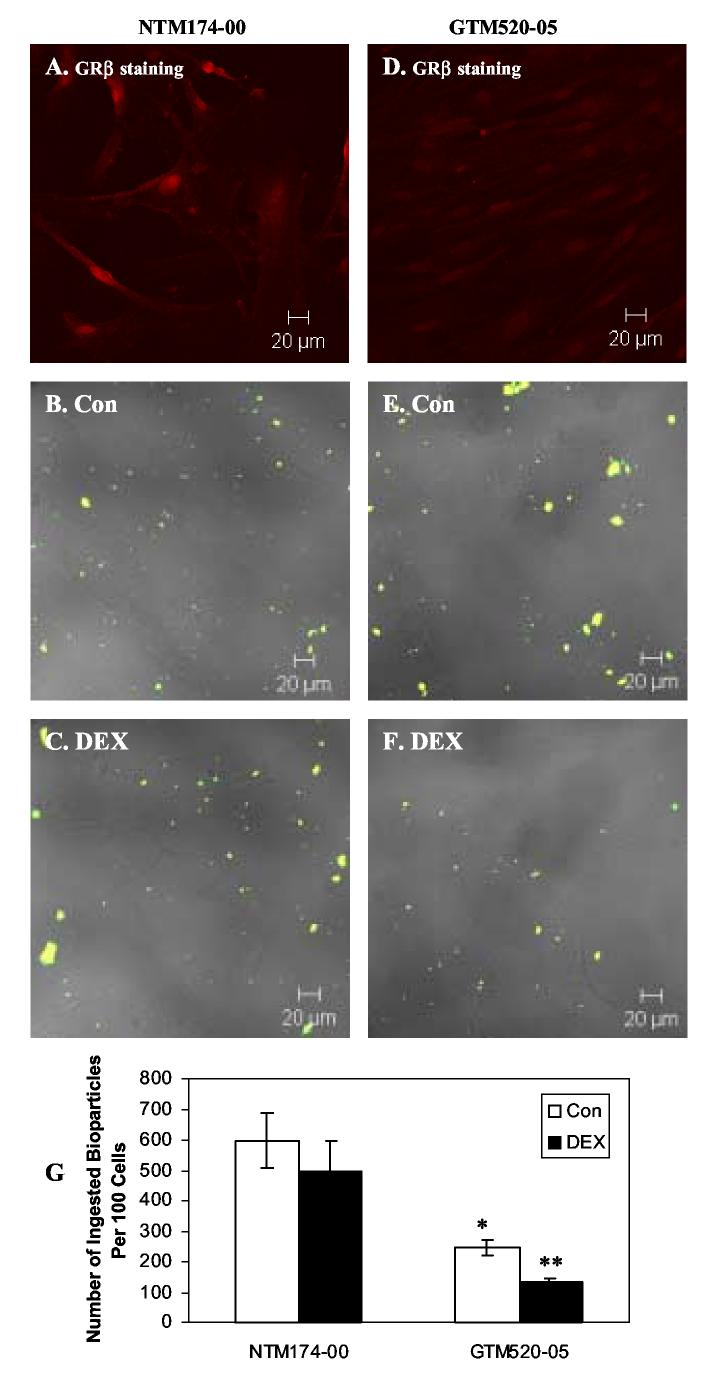
Effects of Dexamethasone on Phagocytotic Activity in Primary Normal NTM174-00 and Primary Glaucomatous GTM520-05 cells
In primary cultured TM cell lines, we showed that primary NTM174-00 expressed relatively high amount of GRβ in their nucleus, compared to GTM520-05 (Fig. 3. A and D). This is consistent with the previous report that normal TM cells had a higher GRβ expression compared to glaucomatous TM cells did (Zhang et al., 2005). In general, primary cultured TM cells appeared to phagocytose more bioparticles than transformed TM cells. The larger cell size, flat shape, and larger surface area of primary TM cells may contribute to this difference. In NTM174-00 cells, there was a non-significant decrease of phagocytic bioparticles between control and DEX treatment (Fig. 3. B-C and G: 499 ± 97 bioparticles/ 100 cells following DEX treatment versus 597 ± 90 bioparticles/ 100 cells in controls; mean ± SEM, p>0.05 by One-way ANOVA and t-test). GTM520-05 cells ingested significantly less bioparticles than NTM174-00 under control conditions (Fig. 3. B, E and G: p<0.05, by One-way ANOVA and t-test) and DEX treatment further decreased this phagocytosis in GTM520-05 cells (Fig. 3. E-G: 132 ± 11 bioparticles/ 100 cells in DEX treated cells versus 245 ± 26 bioparticles/ 100 cells in controls; mean ± SEM, p< 0.05 by t-test), with DEX treatment resulting in a 46% reduction in ingested bioparticles.
Fig. 3.
Effects of Dexamethasone on Phagocytosis in Primary Normal NTM174-00 and Primary Glaucomatous GTM520-05 Cells. A and D: GRβ staining in NTM174-00 and GTM520-05 cells, respectively. B-C and E-F: NTM174-00 and GTM520-05 cells were treated with 100 nM DEX for 24 hours. Opsonized bioparticles were then incubated with TM cells at a ratio of 50:1 at 37°C for 1 hour. After phagocytosis, cells were fixed and incubated with Alexa Flour 633 conjugated secondary antibody. B-C: Light microscopy of NTM174-00 cells and confocal double fluorescence microscopy of bioparticles after phagocytosis. E-F: Light microscopy of GTM520-05 cells and confocal double fluorescence microscopy of bioparticles after phagocytosis. G: Phagocytosis was scored as the number of phagocytic bioparticles per 100 cells. * denotes statistical significance of mean phagocytic index of GTM-520-05 versus that of NTM174-00 as determined by One-way ANOVA and Student-Newman-Keuls multiple comparison test at p<0.05, ** denotes statistical significance of mean phagocytic index of GTM520-05 DEX versus that of GTM520-05 Con as determined by t-test at p<0.05 (n = 3).
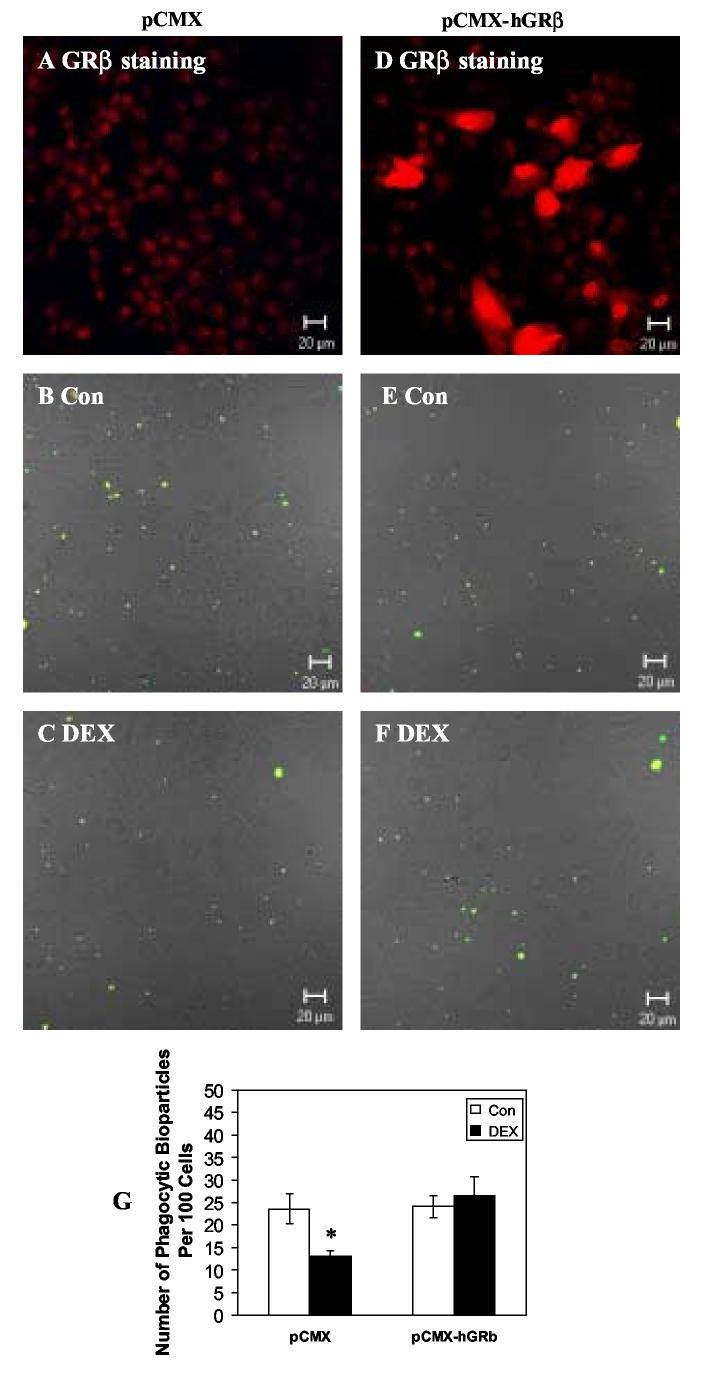
Effects of GRβ Overexpression on DEX-induced decrease in Phagocytosis of NTM-5 cells
In order to determine whether GRβ expression can alter DEX effects on TM cell phagocytosis, NTM-5 cells were transfected with control or GRβ expression vectors and then incubated with or without DEX prior to the phagocytosis assay. Immunohistochemical GRβ protein expression was increased in pCMX-hGRβ-transfected NTM-5 cells compared with NTM-5 cells transfected with a pCMX empty vector (Fig. 4A and 4D). In the empty vector pCMX-transfected NTM-5 cells, DEX treatment significantly decreased phagocytosis (13 ± 1 bioparticles/ 100 cells with DEX treatment versus 24 ± 3 bioparticles/ 100 cells in controls; (mean ± SEM, p< 0.05 by t-test) (Fig. 4B, C and G). In pCMX-hGRβ-transfected NTM-5 cells, there was no significant difference in ingested bioparticles between DEX and control treatments (27 ± 4 bioparticles/ 100 cells with DEX treatment versus 24 ± 2 bioparticles/ 100 cells in controls) (Fig. 4E, F, and G). Overexpression of GRβ blocked DEX mediated effects on TM cell phagocytotic activity.
Fig. 4.
Effects of Overexpression of GRβ on DEX-decreased Phagocytosis in NTM-5 cells. NTM-5 cells were transfected with control empty vector (A-C) or GRβ expression vector pCMX-hGRβ (D-F). A and D: After transfection, cells were fixed and stained for GRβ. B-C and E-F: After transfection, cells were treated with ethanol control or 100 nM DEX for 24 hours. Opsonized bioparticles were then incubated with cells at a ratio of 50:1 at 37°C for 1 hour. After phagocytosis, cells were fixed and incubated with Alexa Flour 633 conjugated secondary antibody. Light microscopy of TM cells and confocal double fluorescence microscopy of bioparticles were performed. G: Phagocytosis was scored as the number of phagocytic bioparticles per 100 cells. Results are expressed as the mean value of three independent experiments. At least 1000 cells were examined in each experiment. * denotes statistical significance of mean phagocytic index after DEX versus that after control treatment in NTM-5 transfected with empty vector pCMX as determined by t-test at p< 0.05.
Discussion
In this study, we found different phagocytic abilities between cultured normal NTM and glaucomatous GTM cells, with a reduced phagocytosis in GTM cells. Although only a few cell lines were used in this study, our results support the hypothesis of reduced phagocytic activity in glaucomatous TM cells (Bill, 1975). However, one study did not find any differences in TM phagocytosis between perfusion cultured human glaucomatous and normal anterior segments (Matsumoto & Johnson 1997b). Phagocytosis of TM cells is an important property that helps maintain normal aqueous humor outflow (Grierson and Lee, 1973, Fink et al., 1978, Polansky et al., 1984, Buller et al., 1990). Our data also supports the idea that glaucomatous TM cells have a decreased phagocytic capability, and this phagocytosis abnormality by glaucomatous TM cells might be related to the increased outflow resistance that occurs in POAG. We also demonstrated that DEX treatment significantly decreased the phagocytosis of bioparticles in NTM-5, GTM-3, and GTM520-05 cells. The glaucomatous cells (GTM-3 and GTM520-05) were more responsive to DEX compared to normal TM cells. The inhibitory effect of DEX on phagocytosis is consistent with results shown for TM cell monolayers in culture (Li and Zhang, 2004) and in TM from perfusion organ cultured anterior segments (Matsumoto and Johnson, 1997a). This increased responsiveness to DEX-induced phagocytosis inhibition in glaucomatous TM cells could exacerbate the increased aqueous humor outflow resistance present in glaucoma.
Previously, we reported that GTM-3 cells expressed relatively lower levels of GRβ and were more responsive to DEX in the induction of fibronectin, myocilin, and a GRE-luciferase reporter gene (Zhang et al., 2005). We showed that GRβ was a key regulator of this increased responsiveness, and suggested that GRβ may modulate DEX mediated suppression of TM cell phagocytosis. In order to further confirm the regulatory role of GRβ, we transienly transfected cells with a pCMX-hGRβ plasmid to increase the expression level of GRβ. We found that the empty vector treated NTM-5 cells responded to DEX with the typical decrease in phagocytosis, while overexpression of GRβ decreased the DEX response and consequently these cells retained their phagocytotic activity even following the DEX challenge. However, the overall phagocytic activity of TM cells was decreased after transfection. Although the reason for this decreased phagocytosis after transfection is not clear, it could involve lipofectamine induced changes in the TM cell membrane properties, which may interfere with the cell's ability to phagocytose. In addition, transfection itself requires the cell to ingest the lipid-plasmid complex, which may reduce the phagocytic ability of cells prior to the phagocytic challenge with S. aureus bioparticles. Overall, our data demonstrate that the expression level of GRβ in TM cells can regulate the cell's ability to respond to DEX, and thereby inhibit the DEX suppression of phagocytic activity.
How GRβ regulates DEX effects on phagocytosis is not known. The actin cytoskeleton is involved in phagocytosis, and disturbing or modifying the actin cytoskeletal organization can alter TM cell phagocytosis (Tamura and Iwamoto, 1989, Park and Latina, 1993). DEX treatment causes a progressive reorganization of microfilaments to form cross-linked actin networks in cultured TM cells and tissues, which are associated with decreased proliferation and migration and may be responsible for the DEX inhibition of phagocytosis (Clark et al., 1994, Clark et al., 2005). Thus, the inhibition of DEX-decreased phagocytosis by overexpression of GRβ results in the maintenance of the cytoskeletal integrity and prevents the actin cytoskeketal reorganization mediated by DEX.
In summary, we demonstrated that the expression level of GRβ can regulate the cellular responsiveness to DEX not only in gene expression, as reported previously, but also in regulating phagocytosis activity. The lower expression of GRβ in glaucomatous TM cells could contribute to increased aqueous humor outflow resistance by exacerbating a steroid-induced decrease in phagocytosis.
Acknowledgments
The authors wish to thank Ifan-Chang for her assistance with the confocal fluorescence microscopy and Raghu Krishnamoorthy, and Christina Jackson for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acott TS. Biochemistry of aqueous humor outflow. In: Kaufman PL, Mittag TQ, editors. Vol. 1994. Glaucoma. Lonson; Mosby: 1994. pp. 1.47–1.78. [Google Scholar]
- Armaly MF. Effect of Corticosteroids on Intraocular Pressure and Fluid Dynamics. I. The Effect of Dexamethasone in the Normal Eye. Arch Ophthalmol. 1963;70:482–491. doi: 10.1001/archopht.1963.00960050484010. [DOI] [PubMed] [Google Scholar]
- Armaly MF, Becker B. Intraocular pressure response to topical corticosteroids. Fed Proc. 1965;24:1274–1278. [PubMed] [Google Scholar]
- Bamberger CM, Bamberger AM, de Castro M, Chrousos GP. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest. 1995;95:2435–2441. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak MH, Weinreb RN, Ryder MI. Quantitative assessment of cynomolgus monkey trabecular cell phagocytosis and adsorption. Curr Eye Res. 1988;7:445–448. doi: 10.3109/02713688809031796. [DOI] [PubMed] [Google Scholar]
- Becker B, Hahn KA. Topical Corticosteroids and Heredity in Primary Open-Angle Glaucoma. Am J Ophthalmol. 1964;57:543–551. doi: 10.1016/0002-9394(64)92500-0. [DOI] [PubMed] [Google Scholar]
- Bill A. Editorial: The drainage of aqueous humor. Invest Ophthalmol. 1975;14:1–3. [PubMed] [Google Scholar]
- Buller C, Johnson DH, Tschumper RC. Human trabecular meshwork phagocytosis. Observations in an organ culture system. Invest Ophthalmol Vis Sci. 1990;31:2156–2163. [PubMed] [Google Scholar]
- Cantinieaux B, Hariga C, Courtoy P, Hupin J, Fondu P. Staphylococcus aureus phagocytosis. A new cytofluorometric method using FITC and paraformaldehyde. J Immunol Methods. 1989;121:203–208. doi: 10.1016/0022-1759(89)90161-0. [DOI] [PubMed] [Google Scholar]
- Clark A, Morrison JM. Corticosteriod glaucoma. In: Morrison JC, Pollack IP, editors. Glaucoma: Science and Practice. Thieme Medical Publishers, Inc.; New York: 2002. pp. 197–206. [Google Scholar]
- Clark AF, Brotchie D, Read AT, Hellberg P, English-Wright S, Pang IH, Ethier CR, Grierson I. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton. 2005;60:83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994;35:281–294. [PubMed] [Google Scholar]
- Derijk RH, Schaaf MJ, Turner G, Datson NA, Vreugdenhil E, Cidlowski J, de Kloet ER, Emery P, Sternberg EM, Detera-Wadleigh SD. A human glucocorticoid receptor gene variant that increases the stability of the glucocorticoid receptor beta-isoform mRNA is associated with rheumatoid arthritis. J Rheumatol. 2001;28:2383–2388. [PubMed] [Google Scholar]
- Epstein DL, Freddo TF, Anderson PJ, Patterson MM, Bassett-Chu S. Experimental obstruction to aqueous outflow by pigment particles in living monkeys. Invest Ophthalmol Vis Sci. 1986;27:387–395. [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink AI, Felix MD, Fletcher RC. The anatomic basis for glaucoma. Ann Ophthalmol. 1978;10:397–411. [PubMed] [Google Scholar]
- Goldberg MF, Tso MO. Rubeosis iridis and glaucoma associated with sickle cell retinopathy: a light and electron microscopic study. Ophthalmology. 1978;85:1028–1041. doi: 10.1016/s0161-6420(78)35587-1. [DOI] [PubMed] [Google Scholar]
- Goleva E, Li LB, Eves PT, Strand MJ, Martin RJ, Leung DY. Increased Glucocorticoid Receptor Beta Alters Steroid Response in Glucocorticoid Insensitive Asthma. Am J Respir Crit Care Med. 2005 doi: 10.1164/rccm.200507-1046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson I, Chisholm IA. Clearance of debris from the iris through the drainage angle of the rabbit's eye. Br J Ophthalmol. 1978;62:694–704. doi: 10.1136/bjo.62.10.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson I, Lee WR. Erythrocyte phagocytosis in the human trabecular meshwork. Br J Ophthalmol. 1973;57:400–415. doi: 10.1136/bjo.57.6.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk PJ, Goleva E, Strickland I, Vottero A, Chrousos GP, Kisich KO, Leung DY. Increased glucocorticoid receptor Beta expression converts mouse hybridoma cells to a corticosteroid-insensitive phenotype. Am J Respir Cell Mol Biol. 2002;27:361–367. doi: 10.1165/rcmb.4861. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Takagi Y, Mori K, Naruse S, Nishino H, Yue BY, Kinoshita S. cDNA microarray analysis of gene expression changes induced by dexamethasone in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002;43:3691–3697. [PubMed] [Google Scholar]
- Johnson DH, Richardson TM, Epstein DL. Trabecular meshwork recovery after phagocytic challenge. Curr Eye Res. 1989;8:1121–1130. doi: 10.3109/02713688909000037. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang H. [Effect of dexamethasone on adhesion and phagocytosis of bovine trebacular meshwork cells] Yan Ke Xue Bao. 2004;20:127–130. [PubMed] [Google Scholar]
- Lo WR, Rowlette LL, Caballero M, Yang P, Hernandez MR, Borras T. Tissue differential microarray analysis of dexamethasone induction reveals potential mechanisms of steroid glaucoma. Invest Ophthalmol Vis Sci. 2003;44:473–485. doi: 10.1167/iovs.02-0444. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Johnson DH. Dexamethasone decreases phagocytosis by human trabecular meshwork cells in situ. Invest Ophthalmol Vis Sci. 1997a;38:1902–1907. [PubMed] [Google Scholar]
- Matsumoto Y, Johnson DH. Trabecular meshwork phagocytosis in glaucomatous eyes. Ophthalmologica. 1997b;211:147–152. doi: 10.1159/000310782. [DOI] [PubMed] [Google Scholar]
- Mold C, Gresham HD, Du Clos TW. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine Fc gamma Rs. J Immunol. 2001;166:1200–1205. doi: 10.4049/jimmunol.166.2.1200. [DOI] [PubMed] [Google Scholar]
- Murphy CG, Johnson M, Alvarado JA. Juxtacanalicular tissue in pigmentary and primary open angle glaucoma. The hydrodynamic role of pigment and other constituents. Arch Ophthalmol. 1992;110:1779–1785. doi: 10.1001/archopht.1992.01080240119043. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Jewell CM, Yudt MR, Bofetiado DM, Cidlowski JA. The dominant negative activity of the human glucocorticoid receptor beta isoform. Specificity and mechanisms of action. J Biol Chem. 1999;274:27857–27866. doi: 10.1074/jbc.274.39.27857. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J Biol Chem. 1996;271:9550–9559. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- Oben JA, Foreman JC. A simple quantitative fluorimetric assay of in vitro phagocytosis in human neutrophils. J Immunol Methods. 1988;112:99–103. doi: 10.1016/0022-1759(88)90039-7. [DOI] [PubMed] [Google Scholar]
- Orii F, Ashida T, Nomura M, Maemoto A, Fujiki T, Ayabe T, Imai S, Saitoh Y, Kohgo Y. Quantitative analysis for human glucocorticoid receptor alpha/beta mRNA in IBD. Biochem Biophys Res Commun. 2002;296:1286–1294. doi: 10.1016/s0006-291x(02)02030-2. [DOI] [PubMed] [Google Scholar]
- Pang IH, Shade DL, Clark AF, Steely HT, DeSantis L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Curr Eye Res. 1994;13:51–63. doi: 10.3109/02713689409042398. [DOI] [PubMed] [Google Scholar]
- Park CH, Latina MA. Effects of gamma-interferon on human trabecular meshwork cell phagocytosis. Invest Ophthalmol Vis Sci. 1993;34:2228–2236. [PubMed] [Google Scholar]
- Polansky JR, Wood IS, Maglio MT, Alvarado JA. Trabecular meshwork cell culture in glaucoma research: evaluation of biological activity and structural properties of human trabecular cells in vitro. Ophthalmology. 1984;91:580–595. doi: 10.1016/s0161-6420(84)34241-5. [DOI] [PubMed] [Google Scholar]
- Richardson TM, Hutchinson BT, Grant WM. The outflow tract in pigmentary glaucoma: a light and electron microscopic study. Arch Ophthalmol. 1977;95:1015–1025. doi: 10.1001/archopht.1977.04450060101010. [DOI] [PubMed] [Google Scholar]
- Rohen JW, van der Zypen E. The phagocytic activity of the trabecularmeshwork endothelium. An electron-microscopic study of the vervet (Cercopithecus aethiops) Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1968;175:143–160. doi: 10.1007/BF02385060. [DOI] [PubMed] [Google Scholar]
- Rozsa FW, Reed DM, Scott KM, Pawar H, Moroi SE, Kijek TG, Krafchak CM, Othman MI, Vollrath D, Elner VM, Richards JE. Gene expression profile of human trabecular meshwork cells in response to long-term dexamethasone exposure. Mol Vis. 2006;12:125–141. [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Naumann GO. Trabecular meshwork in pseudoexfoliation syndrome with and without open-angle glaucoma. A morphometric, ultrastructural study. Invest Ophthalmol Vis Sci. 1995;36:1750–1764. [PubMed] [Google Scholar]
- Sherwood ME, Richardson TM. Phagocytosis by trabecular meshwork cells: sequence of events in cats and monkeys. Exp Eye Res. 1988;46:881–895. doi: 10.1016/s0014-4835(88)80040-x. [DOI] [PubMed] [Google Scholar]
- Sousa AR, Lane SJ, Cidlowski JA, Staynov DZ, Lee TH. Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. J Allergy Clin Immunol. 2000;105:943–950. doi: 10.1067/mai.2000.106486. [DOI] [PubMed] [Google Scholar]
- Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1992;33:2242–2250. [PubMed] [Google Scholar]
- Tamura M, Iwamoto Y. The effect of platelet-derived growth factor on phagocytosis of cultured human trabecular cells. Exp Eye Res. 1989;48:761–770. doi: 10.1016/0014-4835(89)90062-6. [DOI] [PubMed] [Google Scholar]
- Wan CP, Park CS, Lau BH. A rapid and simple microfluorometric phagocytosis assay. J Immunol Methods. 1993;162:1–7. doi: 10.1016/0022-1759(93)90400-2. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999;18:629–667. doi: 10.1016/s1350-9462(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Yue BY, Elner VM, Elner SG, Davis HR. Lysosomal enzyme activities in cultured trabecular-meshwork cells. Exp Eye Res. 1987;44:891–897. doi: 10.1016/s0014-4835(87)80051-9. [DOI] [PubMed] [Google Scholar]
- Zhang X, Clark AF, Yorio T. Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-beta. Invest Ophthalmol Vis Sci. 2005;46:4607–4616. doi: 10.1167/iovs.05-0571. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Bao Z, Li J. MacMARCKS mutation blocks macrophage phagocytosis of zymosan. J Biol Chem. 1995;270:17652–17655. doi: 10.1074/jbc.270.30.17652. [DOI] [PubMed] [Google Scholar]