Abstract
Time-delay circuitries in which a transcription factor processes independent input parameters can modulate NF-κB activation, manage quorum-sensing cross-talk, and control the circadian clock. We have constructed a synthetic mammalian gene network that processes four different input signals to control either immediate or time-delayed transcription of specific target genes. BirA-mediated ligation of biotin to a biotinylation signal-containing VP16 transactivation domain triggers heterodimerization of chimeric VP16 to a streptavidin-linked tetracycline repressor (TetR). At increasing biotin concentrations up to 20 nM, TetR-specific promoters are gradually activated (off to on, input signal 1), are maximally induced at concentrations between 20 nM and 10 μM, and are adjustably shut off at biotin levels exceeding 10 μM (on to off, input signal 2). These specific expression characteristics with a discrete biotin concentration window emulate a biotin-triggered bandpass filter. Removal of biotin from the culture environment (input signal 3) results in time-delayed transgene expression until the intracellular biotinylated VP16 pool is degraded. Because the TetR component of the chimeric transactivator retains its tetracycline responsiveness, addition of this antibiotic (input signal 4) overrides biotin control and immediately shuts off target gene expression. Biotin-responsive immediate, bandpass filter, and time-delay transcription characteristics were predicted by a computational model and have been validated in standard cultivation settings or biopharmaceutical manufacturing scenarios using trangenic CHO-K1 cell derivatives and have been confirmed in mice. Synthetic gene circuitries provide insight into structure–function correlations of native signaling networks and foster advances in gene therapy and biopharmaceutical manufacturing.
Keywords: biological circuit, biopharmaceutical manufacturing, gene switch, synthetic biology, biotin
Synthetic biologists have begun designing artificial gene networks from modular well characterized and compatible genetic components (1–5). Such networks have not only enabled a better understanding of cellular signal processing circuitries (6–12), but have provided solutions for economic biopharmaceutical manufacturing of protein therapeutics and small-molecule drugs (13, 14), precise and timely molecular interventions in prototype gene therapy scenarios (15), and rational reprogramming of therapeutic cell phenotypes in tissue engineering (16). Pioneering synthetic networks using repressors to create transcription feedback loops in Escherichia coli have provided insight into noise suppression (7), bistability (10, 17), and oscillations (8). More recently, newly developed transcription regulators (18, 19), interoperating as genetic transistor replicas with adjustable signal processing, have been assembled to create semisynthetic transcription cascades, which interface with host regulatory networks (20), epigenetic expression imprinting (21), and hysteretic expression memories (22) in mammalian cells and mice. However, compared with electronic circuitries that combine diodes, capacitors, resistors, and transistors to achieve complex in silico information processing, the assembly of synthetic mammalian gene networks remains limited to the functional interconnection of transistor-like switches emulated by trigger-inducible promoters (23, 24). By rational assembly of protein modification and transcription-control components we designed a synthetic mammalian time-delay circuit that emulates an electronic switchboard with sequential wiring of diodes, capacitors, resistors, and transistors. Time-delay circuitries are a recurrent control motif in nature, which evolved, for example, to (i) create stationary patterns from a diffusive gradient that grows with time during interpopulation quorum-sensing cross-talk (25, 26), (ii) modulate parallel Toll-like receptor 4 signaling pathways activating NF-κB from isolated damped oscillatory to stable behavior (27), and (iii) adjust the circadian clock by delayed phosphorylation-dependent degradation of FRQ (28) or differential ubiquitinylation of CRY2 and CRY1 (29).
Results
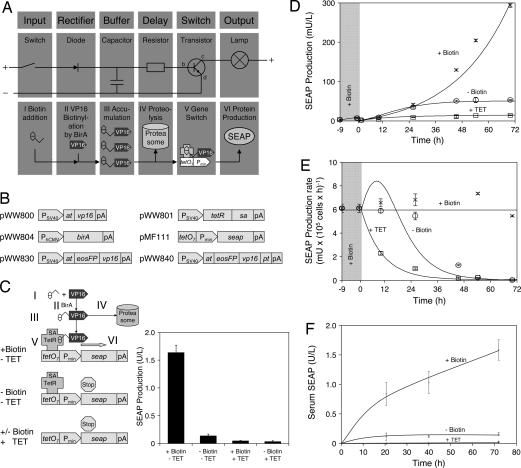
Information processing in the synthetic mammalian time-delay circuit consists of (i) administration of the bioavailable and nontoxic input molecule biotin (vitamin H) (switch; Fig. 1AI), (ii) irreversible ligation of biotin to the synthetic AVITAG peptide (30) fused to the Herpes simplex-derived VP16 transactivation domain (31) by ectopic expression of heterologous E. coli BirA biotin ligase (32) (diode; Fig. 1AII), (iii) an AVITAG-VP16-based buffer, which retains biotin and sustains signal processing even after biotin withdrawal from the culture medium (capacitor; Fig. 1AIII), (iv) proteasome-dependent degradation of biotin-AVITAG-VP16 domains, which controls capacitor discharge and delays signal referral (resistor; Fig. 1AIV), and (v) binding of biotin-AVITAG-VP16 to streptavidin fused to the E. coli Tn10-derived tetracycline repressor (TetR) (33), resulting in a chimeric transactivator (TetR-streptavidin-biotin-AVITAG-VP16), which binds and activates TetR-specific target promoters (PhCMV∗-1) that contain heptameric operator sites (tetO7; PhCMV∗-1, tetO7-PhCMVmin) (transistor; Fig. 1AV). This promoter drives transcription of the human glycoprotein SEAP (human placental secreted alkaline phosphatase) in a tetracycline-adjustable manner until the biotin-AVITAG-VP16 buffer is degraded (output; Fig. 1AVI). Functionality of individual circuitry components was validated by cotransfection of Chinese hamster ovary (CHO-K1) cells with expression vectors encoding AVITAG-VP16 (pWW800), TetR-streptavidin (pWW801), BirA (pWW804), and a TetR-responsive SEAP expression vector (pMF111) (Fig. 1B). This vector set enabled biotin-triggered SEAP production (heterodimerization and PhCMV∗-1 binding of TetR-streptavidin-biotin-AVITAG-VP16), which could be repressed by vitamin H deficiency (no heterodimerization) or addition of tetracycline (heterodimerization, no PhCMV∗-1 binding) (Fig. 1C).
Fig. 1.
Design and functionality of the electronic and biologic time-delay circuits. (A) Electronic time-delay circuitry. Activating the switch triggers a current through the diode into the capacitor, which becomes charged and remains charged even when the switch is interrupted again. The capacitor's charge is slowly dissipated to the base of the resistor (b) where it triggers a current from collector (c) to emitter (e), resulting in activation of the output lamp. After complete capacitor draining the transistor switches back to off. In the synthetic biologic counterpart, the biotin input (I) results in VP16 biotinylation (II) and accumulation of biotinylated VP16 (III) in the cell buffer from which it is slowly degraded (IV). Biotinylated VP16 attaches to streptavidin fused with TetR, which binds specific tetO7 operator sites upstream of a minimal human cytomegalovirus promoter (Pmin), resulting in promoter activation (V) and production (VI) of the reporter SEAP. (B) Expression vectors used for construction of the biologic time-delay circuitry. at, AVITAG biotinylation signal; birA, E. coli biotin ligase; eosFP, photoswitchable fluorescent protein; pA, polyadenylation site; PCMV, human cytomegalovirus immediate early promoter; Pmin, minimal human cytomegalovirus promoter; PSV40, simian virus 40 promoter; pt, ubiquitinylation signal (PEST-sequence); sa, streptavidin; seap, human placental secreted alkaline phosphatase; tetO7, heptameric TetR-specific operator site; tetR, tetracycline-responsive repressor; vp16, activation domain of Herpes simplex viral protein 16. (C) Functional schematic of regulatory modules. In the presence of biotin (+Biotin; I) VP16 is biotinylated by BirA (II), accumulates in the cell (III), and activates (V) the minimal promoter (Pmin) by interaction with TetR-fused streptavidin (SA-TetR) bound to the tetO7 operator site in the absence of tetracycline, resulting in SEAP production (VI). Gene expression is reversed when biotinylated VP16 is degraded (IV). In the absence of biotin (−Biotin), VP16 is not biotinylated and the promoter remains silent. Upon addition of tetracycline (+TET) TetR-SA dissociates from tetO7 and the minimal promoter is not activated, irrespective of whether biotin is present or not. (D) In vitro and in silico validation of the time-delay circuitry. CHOTIME cells (50,000 cells per ml) were cultivated in standard biotin-containing (20 nM) medium for 9 h (gray shaded area) before switching (at t = 0 h) to biotin-free (−Biotin, circles), biotin-containing (+Biotin, 20 nM, crosses) or tetracycline-containing (+TET, 2 μg/ml, squares) medium. Time courses of SEAP production under these conditions include experimental data (symbols) and in silico predictions (lines). Details on the derivation of mathematical model, parameter values, and initial conditions are provided in supporting information (SI) Text and SI Tables 1 and 2. (E) Specific SEAP production rates calculated from finite differences of experimental data shown in D (symbols) and corresponding computational predictions (lines). (F) In vivo validation of the time-delay circuitry. Biotin-adapted (20 nM) CHOTIME cells were microencapsulated in alginate-poly-l-lysine-alginate microcapsules and i.p.-injected into mice followed by administration of biotin (100 μg/kg), tetracycline (100 mg/kg), or 0.9% NaCl as control (−Biotin). Serum samples were taken after 20, 40, and 72 h for quantification of SEAP production.
Because biotin is covalently linked to AVITAG-VP16 and thus remains sequestered in the cell, it continues to promote heterodimerization of the chimeric transactivator even after biotin withdrawal from the culture medium. SEAP production continues in a time-delayed manner until it plateaus as the biotin-AVITAG-VP16 capacitor pool is degraded. SEAP levels then remain at a constant level caused by the stability of this protein in culture [t1/2 (SEAP) = 502 h (34)]. Time-delay profiles of biotin-triggered protein production were characterized in CHOTIME, a stable CHO-K1 derivative tetra-transgenic for pWW800, pWW801, pWW804, and pMF111 (Fig. 1B) and cultivated in the presence of 20 nM biotin for 9 h before switching to biotin-free, biotin-containing, or tetracycline-supplemented medium and subsequent SEAP profiling (Fig. 1D). Whereas biotin-supplemented populations exhibited steady increases in SEAP production, the SEAP levels of biotin-deprived cultures reached 50 milliunits/liter during the first 50 h (time delay) and stagnated thereafter (Fig. 1D). Addition of tetracycline overrode biotin-triggered and time-delayed SEAP production (Fig. 1D). The corresponding time delay became obvious when we calculated the specific SEAP production rates: the decay of SEAP production rates in tetracycline-containing medium, which represents shut-off of a simple transcription-control system, occurred ≈30 h before a corresponding decrease in the time-delayed circuit (Fig. 1E). Time-delayed SEAP production was also observed in the serum of biotin-deficient mice implanted with CHOTIME, whereas control animal populations, kept on a normal diet, showed steady increases in their SEAP serum levels (Fig. 1F). In both situations, tetracycline administration repressed transgenic SEAP production (Fig. 1F). This successful in vivo validation suggests that the biotin-triggered, time-delay circuitry may provide a temporally defined epigenetic expression window, independent of inducer fluctuations, and so enable stable therapeutic interventions in future gene therapy scenarios.
To characterize the signal processing capacity of the time-delay circuitry in greater detail we used a differential algebraic equation model, coordinated to molecular and biochemical parameters, to simulate SEAP production profiles (Eqs. 1–6):
with
 |
Changes in the concentration of the biotinylated AVITAG-VP16 [VB] depend on the extracellular biotin concentration [B] and the concentration of free AVITAG-VP16 [V]. Transport and conversion by BirA are represented by the first term of Eq. 1 (SI Text for all details of model derivation), while linear degradation (parameter d1) and dilution caused by cell growth (growth rate μ) diminish the pool of biotinylated AVITAG-VP16. Similarly, the dynamics of SEAP mRNA [M] is described by a Michaelis-Menten-like term for production that considers the gene dose [G], the concentration of TetR-streptavidin-biotin-AVITAG-VP16 [VBT], and other factors (see SI Text and SI Figs. 4 and 5 for details). By using steady-state assumptions, [VBT] can be derived from the total concentrations of TetR-streptavidin (T0), AVITAG-VP16 (V0), and tetracycline ([TC]) according to Eq. 6. The dynamic Eq. 3 describes the production of extracellular SEAP protein from SEAP mRNA, which is proportional to the cell count [X]. Again, linear degradation of SEAP protein is included in the model. Finally, we use two state variables for the cell count [X] (Eq. 4; linear growth rate μ) and the gene dose [G] (Eq. 5). Eq. 5 is relevant only for transiently transfected cells because plasmids are not replicated; in this case, plasmid dilution is represented by setting d4 = μ. Parameter values and initial conditions for all simulations are provided in SI Text and SI Tables 1 and 2. Simulation results in Fig. 1 D and E show that the model, which was adapted to the corresponding experimental data, was able to describe the system's dynamics quantitatively, using realistic parameter values. The transient increase of specific SEAP production after biotin removal suggested by the model predictions (Fig. 1E) results from the abolished competition of biotin-AVITAG-VP16 with free biotin for TetR-streptavidin.
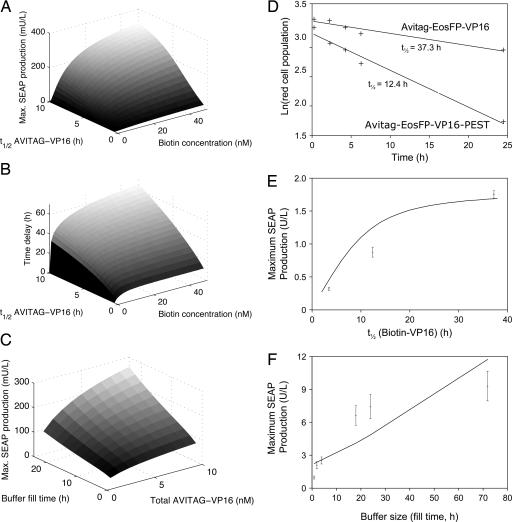
We next characterized different input parameters to assess whether their impact on the time-delay profile of the biotin-triggered genetic circuit is similar to electronic circuitries, in which capacitor discharge resulting in time-delayed signal processing is a function of capacity (i.e., biotin-AVITAG-VP16 concentration) and outflow resistance (i.e., biotin-AVITAG-VP16 degradation rate). In silico simulations indicated that increased stability of biotin-AVITAG-VP16 results in longer time delays and higher maximum SEAP production levels, whereas short-lived biotin-AVITAG-VP16 results in almost immediate signal processing (Fig. 2 A and B), a situation reminiscent of classical transcription control modalities where transgene transcription shut-off occurs immediately after changes in the inducer concentration (19, 35) (e.g., addition of tetracycline in Fig. 1 D–F). Accordingly, a greater biotin buffer or increased buffer fill time produce higher SEAP levels (Fig. 2C), and maximum SEAP production was found to increase monotonically with the half-life of biotin-AVITAG-VP16 (Fig. 2A).
Fig. 2.
In silico and in vitro functional characterization of the biologic time-delay circuit and associated molecular building blocks. (A) Computational prediction of maximum SEAP production in a 72-h time interval after switching from biotin-containing medium (12-h cultivation) to biotin-free medium as a function of biotin concentration and AVITAG-VP16 half-life (t1/2 AVITAG-VP16). (B) Predicted time delays defined as the differences between the points in time of reaching 50% SEAP production rate after transcriptional shut-off caused by switching to tetracycline-containing and biotin-free medium, respectively. (C) Corresponding in silico-determined switching characteristics of the biologic time-delay circuitry as a function of the buffer size of total VP16 molecules and the buffer fill time (time of cultivation in 20 nM biotin medium). (D) Quantification of half-lives of different chimeric transactivators. CHO-K1 were transfected with plasmids pWW830 (PSV40-AVITAG-EosFP-VP16-pA) or pWW840 (PSV40-AVITAG-EosFP-VP16-PEST-pA) and cultivated for 48 h before switching the EosFP-fusion proteins from green to red by a 20-s UV (390 nm) light pulse. FACS analysis was used at the indicated time points to score the decrease in red fluorescence. (E) In vitro investigation of the impact of VP16 degradation kinetics on switching characteristics as represented by maximum SEAP production. CHO-K1 was transfected with pWW801, pWW804, pMF111, and either pWW800, pWW830, or pWW840 and cultivated for 2 h in the presence of biotin before switching to biotin-free conditions for 72 h and profiling of maximum SEAP production. Maximum SEAP production was correlated with the half-lives of AVITAG-VP16 variants (pWW800, 3.5 h; pWW840, 12.4 h; pWW830, 37.3 h) as predicted by the mathematical model (line). (F) In vitro investigation of the impact of the buffer size on switching characteristics represented by maximum SEAP production. CHO-K1 were transfected with plasmids pWW801, pWW804, pWW830, and pMF111 and cultivated in biotin-containing (20 nM) medium for the indicated periods before switching to biotin free conditions for another 48 h. Maximum SEAP production was determined and correlated with the time required to replenish the biotinylated VP16 buffer (line indicates model predictions).
For in vitro confirmation of these in silico predictions, AVITAG-VP16 was fused (i) to EosFP (36), a fluorescent protein variant exhibiting a green-to-red fluorescence switch after illumination at 390 nm (pWW830, PSV40-AVITAG-EosFP-VP16-pA; Fig. 1B) and (ii) to the ornithine decarboxylase-derived proteasome-specific degradation domain [residues 422–461, PEST (37)] (pWW840, PSV40-AVITAG-EosFP-VP16-PEST-pA; Fig. 1B). FACS-based dynamic analysis of 390-nm pulsed CHO-K1 populations, transgenic for either pWW830 or pWW840, revealed half-lives of 37.3 and 12.4 h for PEST-free and PEST-containing circuit components, respectively (Fig. 2D). Proteasome-based degradation of AVITAG-EosFP-VP16-PEST was confirmed by addition of the proteasome inhibitor MG132 (38) (half-lifes: 22.4 h, +MG132; 12.4 h, −MG132). The impact of VP16 stability was evaluated by cotransfection of CHO-K1 cells with expression vectors encoding BirA (pWW804), TetR-streptavidin (pWW801), a TetR-responsive SEAP expression vector (pMF111), and AVITAG-VP16 variant (pWW800, pWW830 or pWW840) and subsequent cultivation for 2 h in the presence of biotin to replenish the inducer buffer and then for 72 h in biotin-free medium before profiling SEAP production. Maximum SEAP production showed a monotonic relation between the half-life of the VP16 fusion protein and the maximum SEAP production levels, as predicted by theoretical considerations (Fig. 2E). Note that these quantitatively accurate theoretical predictions were truly independent (data not used for model adjustment, adaptation only of experiment-specific parameters such as the specific growth rate). The impact of the buffer size on time-delay dynamics was analyzed by cultivating transfected (pWW801, pWW830, pWW804, and pMF111) CHO-K1-derived cell populations for different intervals in biotin-containing medium to charge the vitamin H buffer to various extents and profiling maximum SEAP production after a subsequent 48-h cultivation period in biotin-free medium (Fig. 2F). Maximum SEAP production profiles linearly correlated with the biotin buffer capacity of the time-delay circuitry, which confirmed the in silico data and suggests that the transgene expression delay is primarily a function of transactivator stability and inducer availability (Fig. 2 E and F).
Besides providing insight into natural time-delay signal processing (27, 28), biotin-triggered transcription control could impact biopharmaceutical manufacturing and gene therapy applications because of the bioavailability of the nontoxic physiologic inducer biotin (with no known side effects), its excellent regulation performance, and its inducible dimerization-based control characteristics. Furthermore, dose–response profiling of biotin-controlled SEAP expression revealed three independent biotin-response characteristics: (i) adjustable dose-dependent induction of SEAP expression up to 20 nM biotin, (ii) maximum SEAP production plateau within a specific biotin concentration window (20 nM to 10 μM), and (iii) adaptable transgene repression beyond 100 μM (Fig. 3A). As indicated by the mathematical model, transcription shut-off at high biotin concentrations is probably caused by free biotin-based saturation of the TetR-streptavidin and a corresponding reduction of transcription-competent complexes with biotin-AVITAG-VP16. Adjustable induction and repression signaling by addition of a single inducer molecule is unique and exhibits band-pass filter-like characteristics, which have thus far been achieved only by combining different transistor-type transcription control modalities (39) or by assembly of complex networks mimicking developmental pattern formation (25). With its off-to-on and on-to-off responsiveness to different biotin concentrations (Fig. 3A), the time-delayed expression characteristics after biotin removal, and the sensitivity to tetracycline (Fig. 1C), this system combines four signal processing capacities into a single synthetic transcription factor.
Fig. 3.
Validation of biotin-dependent expression signaling for bioprocess applications and in vivo gene therapy studies. (A) Biotin dose–response study. CHO-K1 were transfected with plasmids pWW800, pWW801, pWW804, and pMF111 and cultivated in the presence of different biotin concentrations for 48 h before measuring SEAP production. The experimental data were used to estimate model parameters; simulation results are shown by the line. (B) Bioreactor compatibility of biotin-regulated gene expression. CHO-K1 cells, stably transfected with plasmids pWW800, pWW801, pWW804, and pMF111, were grown in a 1L BioWave reactor, and SEAP expression was induced at 48 h by addition of 100 nM biotin (gray area) and subsequently reversed at 125 h by the addition of 200 μM biotin. Lines indicate model predictions. (C) The same as B except that expression was reversed at 125 h by the addition of 2 μg/ml tetracycline. (D) Production of the toxic death domain of the human receptor-interacting protein (RipDD). CHO-K1 cells were transfected with plasmids pWW800, pWW801, pWW804, and pWW810 (PhCMV∗-1-RipDD-HIS6-pA) and cultivated in 20 nM biotin for the indicated time periods before Western blot-based RipDD quantification (IOD, integrated optical density) and assessment cell death (annexin and propidium iodide stainings) 48 h after transfection. (E) Validation of biotin-dependent signaling for in vivo gene expression control. CHO-K1 cells, stably transfected with plasmids pWW800, pWW801, pWW804, and pMF111, were microencapsulated and implanted into mice fed with a standard biotin-containing diet. Mice were injected with increasing biotin doses, and SEAP serum levels were assessed after 48 h.
Reversible off-to-on and on-to-off switches, through administration of a single biocompatible signal molecule, are particularly attractive for precise transgene expression control in complex systems including bioreactors, from which inducer molecules must be eliminated by cost-intensive medium exchange or filtration, and organisms, which depend on the pharmacokinetics of the inducer molecule for reversal of the transgene expression status when using classical transistor-type control modalities (35). Transgene control modalities are particularly valuable for the manufacture of difficult-to-produce (e.g., cytotoxic) protein therapeutics. In this system, the administration of biotin, to induce the desired levels of protein production, will result in an identical media composition to that currently found in standard licensed processes, thereby alleviating the need for expensive downstream processing procedures to remove the inducer molecule. To validate the potential of biotin-triggered transgene control for biopharmaceutical manufacturing, we profiled heterologous protein production of CHOTIME, transgenic for biotin-inducible SEAP expression in 2-liter bioreactors. After a 48-h cultivation period in biotin-free media, which repressed SEAP to the detection limit, the production culture was exposed to 100 nM biotin, which triggered sustained SEAP production until the production status was reversed by the addition of 200 μM biotin (Fig. 3B) or 2 μg/ml tetracycline (Fig. 3C). Again, the corresponding time courses could be predicted quantitatively with the mathematical model. Besides providing precise fine-tuning of product levels, the biotin-triggered time-delay circuitry enables genetic programming of bioprocess dynamics. After initial setting of the buffer size and resistor value, the time-delay circuitry automates production kinetics and product levels. To demonstrate the usefulness of the time-delay circuitry for production of toxic proteins in bioreactors, we expressed the apoptosis-inducing death domain of the human receptor-interacting protein (RipDD) (40–42). Because the buffer size defined the maximum RipDD production it could be used to program the optimal balance of RipDD expression and apoptosis to maximize product yield (Fig. 3D). In contrast, constitutive expression resulted in decreased RipDD titer (90.3 ± 0.8 integrated optical density) caused by extensive cell death (68% viability). To validate biotin-induced transgene expression in a prototype gene therapy scenario including a standard biotin-containing diet we implanted microencapsulated CHOTIME i.p. into mice sustained on a standard biotin-containing diet. Administration of increasing vitamin H doses triggered decreasing serum SEAP levels, which confirmed adjustable gene switch operation in wild-type mice exposed to a standard diet with normal supplies of biotin (Fig. 3E).
Discussion
Recent advances in understanding NF-κB signaling have revealed that mammalian cells can convert transactivator functions, which exhibit oscillatory readouts, into stable expression patterns through overlay of a time-delay signal processing network (25, 27). We have used experimental and theoretical approaches to design a synthetic time-delay circuit mimicking NF-κB processing functionality and characterized key parameters required for efficient operation in mammalian cells, mice, and bioreactors. Biotin-triggered transgene expression emulated the signal processing characteristics found in the digital electronics environment of diodes, capacitors, resistors, and transistors. For the basic research community, synthetic transcription factor can process five input signals. In addition, it represents a transgene control system that can be induced and repressed by the same nontoxic molecule vitamin H. Biotin-triggered signal processing may foster advances in understanding cellular control circuitries, therapeutic reprogramming of system deficiencies, and biopharmaceutical manufacturing of difficult-to-produce protein therapeutics.
Methods
Plasmid Constructions.
pWW800 (PSV40-AVITAG-VP16-pA) was constructed by PCR-mediated amplification of VP16 encoded on pSAM200 (18) with oligonucleotides OWW800 [5′-gatcgaattcccaccatgggtctgaacgacatcttcgaggctcagaaaatcgaatggcacgaaTCCGCG-TACAGCCGCGCG-3′, AVITAG (30) in italics, annealing sequence uppercase, EcoRI site underlined] and OWW801 (5-′gatcggatccCTACCCACCGTACTCGTC-3′, annealing sequence uppercase, BamHI site underlined) and cloning of the resulting AVITAG-VP16 fragment (EcoRI/BamHI) into pSAM200. To construct pWW801 (PSV40-TetR-strepdavidin-pA), streptavidin was PCR-amplified from pUC8-SZ (43) using OWW802 (5′-gatcgcgcgcctatggctagcatgactggtggacagcaaatgggtcgcgaccagGAGG-CCGGCATCACCGGCACCTGG-3′, annealing sequence uppercase, BssHII site underlined) and OWW803 (5′-gatcggatccctacagggacccCTGCTGAACGGCGTCGAGCG-GGTT-3′, annealing sequence uppercase, BamHI site underlined) and ligated (BssHII/BamHI) into pSAM200. The E. coli biotin ligase BirA (32) was excised (EcoRI/SpeI) from pGEM-SD2 (J. Strouboulis, personal communication) and ligated (EcoRI/XbaI) into pMF150, which resulted in pWW804 (PhCMV-BirA-pA). pWW830 (PSV40-AVITAG-eosFP-VP16-pA) was constructed by inserting the BssHII-generated eosFP (36)-encoding fragment of pd2EosFP (Genscript, Piscataway, NJ) in frame and sense orientation into the BssHII site of pWW800. The PEST domain was PCR-amplified from pd2EYFP (Clontech, Mountain View, CA) and fused in-frame 3′ to the VP16 domain of pWW830 in a multistep cloning procedure (available on request), resulting in pWW840 (PSV40-AVITAG-eosFP-VP16-PEST-pA). pMF111 (PhCMV∗-1-SEAP-pA; PhCMV∗-1, tetO7-PhCMVmin) has been described (18). For construction of pWW810 (PhCMV∗-1-RipDD-HIS6-pA) PhCMV∗-1 was excised from pCF201 (unpublished work) by SspI/AscI and cloned into the SspI/BssHII sites of pWW326 (41).
Cell Culture and Cell Line Design.
Chinese hamster ovary cells (CHO-K1, CCL-61; ATCC, Manassas, VA), and the CHO-K1-derived transgenic cell line CHOTIME were cultivated in biotin-free DMEM (Invitrogen, Carlsbad, CA) or biotin-free ChoMaster HTS medium (Cell Culture Technology, Gravesano, Switzerland) supplemented with either 10% FCS (PAN Biotech, Aidenbach, Germany) or 10% biotin-free knockout serum replacement (KOSR; Invitrogen) and selective antibiotics G418 (600 μg/ml) and puromycin (1 μg/ml) (CHOTIME only). Transfections were performed according to an optimized calcium phosphate protocol (19). CHOTIME was constructed by sequential cotransfection and single-cell cloning of CHO-K1 with (i) pWW800, pWW801, and pSV2neo (Clontech) (selection in G418-containing medium) and (ii) pWW804, pMF111, and pPUR (Clontech) (selection in puromycin-containing medium).
Bioreactor Operation.
Cells were cultivated in a BioWave 20SPS-F bioreactor (Wave Biotech, Tagelswangen, Switzerland) equipped with 2-liter Wave Bags and inlet gas humidification (HumiCare 200; Gruendler Medical, Freudenstadt, Germany) and the following parameter setting: temperature, 37°C; aeration, 100 ml/min humidified air (>95% relative humidity) containing 5% CO2; rocking rate, 16 min−1; rocking angle, 5°.
Analytics.
SEAP production was quantified as detailed by Schlatter et al. (34). EosFP-based red fluorescence dynamics was analyzed by using either a DM RB fluorescence microscope (Leica, Vienna) equipped with 520-nm (excitation) and 570-nm (emission) filters or a Cytomics FC500 flow cytometer (Beckman Coulter, Fullerton, CA) with CFP analysis software (Beckman Coulter) set for 488-nm excitation and recording at 620 nm. Cell viability and cell death was assessed by FACS using an annexin V/propidium iodide-based apoptosis assay (Invitrogen). Affinity-purified RipDD (Qiagen, Valencia, CA) was quantified by Western blot using anti-hexahistine antibodies (Novagen, San Diego, CA) and ECL-Plus detection reagents (Amersham, Piscataway, NJ) and Chemilux hardware (Intas, Göttingen, Germany).
Inducer Molecules.
Biotin (Acros Organics, Geel, Belgium) was dissolved in water and used at a final concentration of 20 nM unless stated otherwise. Tetracycline (Sigma, St. Louis, MO) was prepared as a stock solution of 1 mg/ml in water and used at a final concentration of 2 μg/ml.
Animal Studies.
CHOTIME was encapsulated in 400 μm alginate-PLL-alginate beads by using the Encapsulator Research IE-50R (Inotech Biotechnologies, Basel, Switzerland) according to the manufacturer's protocol and the following specific settings: 0.2-mm nozzle, 20-ml syringe at a flow rate of 325 units, nozzle vibration frequency 1,088 s−1, voltage for bead dispersion 1,300 V. Female OF1 mice were obtained from Iffa-Credo (Les Oncins, France) and kept on a biotin-free diet (Société SAFE, Augy, France) for 2 weeks, whereas the control mice were fed the same diet supplemented with 4 mg/kg biotin. Biotin deficiency was monitored by using the forced swim test (44), resulting in swim times of 58 ± 28 and 132 ± 30 s for biotin-deficient and control mice, respectively. Seven hundred microliters of FMX-8 medium containing 50% capsules (2 × 106 cells, 200 cells per capsule) was injected i.p. into the mice. When appropriate, additional biotin was dissolved in 0.9% (wt/vol) NaCl and administered by i.p. injection 1 h after capsule implantation. Blood samples were collected retroorbitally, and serum was produced by using microtainer SST tubes (Becton Dickinson, Franklin Lakes, NJ). All experiments involving animals were approved by the French Ministry of Agriculture and Fishery and performed by M.D.-E.B.
Supplementary Material
Acknowledgments
We thank David Greber, Beat Kramer, Thomas Ulrich, and Marcia Schoenberg for critical comments on the manuscript. This work was supported by Swiss National Science Foundation Grant 631-065946, the Swiss State Secretariat for Education and Research within European Community Framework 6, and Cistronics Cell Technology (Zurich, Switzerland).
Abbreviations
- TetR
tetracycline repressor
- SEAP
human placental secreted alkaline phosphatase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0606398104/DC1.
References
- 1.Endy D. Nature. 2005;438:449–453. doi: 10.1038/nature04342. [DOI] [PubMed] [Google Scholar]
- 2.Gibbs WW. Sci Am. 2004;290:74–81. [PubMed] [Google Scholar]
- 3.Hasty J, McMillen D, Collins JJ. Nature. 2002;420:224–230. doi: 10.1038/nature01257. [DOI] [PubMed] [Google Scholar]
- 4.Sprinzak D, Elowitz MB. Nature. 2005;438:443–448. doi: 10.1038/nature04335. [DOI] [PubMed] [Google Scholar]
- 5.Guido NJ, Wang X, Adalsteinsson D, McMillen D, Hasty J, Cantor CR, Elston TC, Collins JJ. Nature. 2006;439:856–860. doi: 10.1038/nature04473. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson MR, Savageau MA, Myers JT, Ninfa AJ. Cell. 2003;113:597–607. doi: 10.1016/s0092-8674(03)00346-5. [DOI] [PubMed] [Google Scholar]
- 7.Becskei A, Serrano L. Nature. 2000;405:590–593. doi: 10.1038/35014651. [DOI] [PubMed] [Google Scholar]
- 8.Elowitz MB, Leibler S. Nature. 2000;403:335–338. doi: 10.1038/35002125. [DOI] [PubMed] [Google Scholar]
- 9.Elowitz MB, Levine AJ, Siggia ED, Swain PS. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- 10.Gardner TS, Cantor CR, Collins JJ. Nature. 2000;403:339–342. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- 11.Pedraza JM, van Oudenaarden A. Science. 2005;307:1965–1969. doi: 10.1126/science.1109090. [DOI] [PubMed] [Google Scholar]
- 12.Fussenegger M, Bailey JE, Varner J. Nat Biotechnol. 2000;18:768–774. doi: 10.1038/77589. [DOI] [PubMed] [Google Scholar]
- 13.Fussenegger M, Schlatter S, Datwyler D, Mazur X, Bailey JE. Nat Biotechnol. 1998;16:468–472. doi: 10.1038/nbt0598-468. [DOI] [PubMed] [Google Scholar]
- 14.Martin VJ, Pitera DJ, Withers ST, Newman JD, Keasling JD. Nat Biotechnol. 2003;21:796–802. doi: 10.1038/nbt833. [DOI] [PubMed] [Google Scholar]
- 15.Rivera VM, Wang X, Wardwell S, Courage NL, Volchuk A, Keenan T, Holt DA, Gilman M, Orci L, Cerasoli F, Jr, et al. Science. 2000;287:826–830. doi: 10.1126/science.287.5454.826. [DOI] [PubMed] [Google Scholar]
- 16.Niwa H, Miyazaki J, Smith AG. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi H, Kaern M, Araki M, Chung K, Gardner TS, Cantor CR, Collins JJ. Proc Natl Acad Sci USA. 2004;101:8414–8419. doi: 10.1073/pnas.0402940101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fussenegger M, Morris RP, Fux C, Rimann M, von Stockar B, Thompson CJ, Bailey JE. Nat Biotechnol. 2000;18:1203–1208. doi: 10.1038/81208. [DOI] [PubMed] [Google Scholar]
- 19.Weber W, Fux C, Daoud-el Baba M, Keller B, Weber CC, Kramer BP, Heinzen C, Aubel D, Bailey JE, Fussenegger M. Nat Biotechnol. 2002;20:901–907. doi: 10.1038/nbt731. [DOI] [PubMed] [Google Scholar]
- 20.Kramer BP, Fischer M, Fussenegger M. Metab Eng. 2005;7:241–250. doi: 10.1016/j.ymben.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Kramer BP, Viretta AU, Daoud-El-Baba M, Aubel D, Weber W, Fussenegger M. Nat Biotechnol. 2004;22:867–870. doi: 10.1038/nbt980. [DOI] [PubMed] [Google Scholar]
- 22.Kramer BP, Fussenegger M. Proc Natl Acad Sci USA. 2005;102:9517–9522. doi: 10.1073/pnas.0500345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer BP, Fischer C, Fussenegger M. Biotechnol Bioeng. 2004;87:478–484. doi: 10.1002/bit.20142. [DOI] [PubMed] [Google Scholar]
- 24.Kramer BP, Weber W, Fussenegger M. Biotechnol Bioeng. 2003;83:810–820. doi: 10.1002/bit.10731. [DOI] [PubMed] [Google Scholar]
- 25.Basu S, Gerchman Y, Collins CH, Arnold FH, Weiss R. Nature. 2005;434:1130–1134. doi: 10.1038/nature03461. [DOI] [PubMed] [Google Scholar]
- 26.Viretta AU, Fussenegger M. Biotechnol Prog. 2004;20:670–678. doi: 10.1021/bp034323l. [DOI] [PubMed] [Google Scholar]
- 27.Covert MW, Leung TH, Gaston JE, Baltimore D. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 28.Bratsun D, Volfson D, Tsimring LS, Hasty J. Proc Natl Acad Sci USA. 2005;102:14593–14598. doi: 10.1073/pnas.0503858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forger DB, Peskin CS. Proc Natl Acad Sci USA. 2003;100:14806–14811. doi: 10.1073/pnas.2036281100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckett D, Kovaleva E, Schatz PJ. Protein Sci. 1999;8:921–929. doi: 10.1110/ps.8.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triezenberg SJ, Kingsbury RC, McKnight SL. Genes Dev. 1988;2:718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- 32.Chapman-Smith A, Mulhern TD, Whelan F, Cronan JE, Jr, Wallace JC. Protein Sci. 2001;10:2608–2617. doi: 10.1110/ps.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillen W, Berens C. Annu Rev Microbiol. 1994;48:345–369. doi: 10.1146/annurev.mi.48.100194.002021. [DOI] [PubMed] [Google Scholar]
- 34.Schlatter S, Rimann M, Kelm J, Fussenegger M. Gene. 2002;282:19–31. doi: 10.1016/s0378-1119(01)00824-1. [DOI] [PubMed] [Google Scholar]
- 35.Weber W, Rimann M, Spielmann M, Keller B, Daoud-El Baba M, Aubel D, Weber CC, Fussenegger M. Nat Biotechnol. 2004;22:1440–1444. doi: 10.1038/nbt1021. [DOI] [PubMed] [Google Scholar]
- 36.Wiedenmann J, Ivanchenko S, Oswald F, Schmitt F, Rocker C, Salih A, Spindler KD, Nienhaus GU. Proc Natl Acad Sci USA. 2004;101:15905–15910. doi: 10.1073/pnas.0403668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corish P, Tyler-Smith C. Protein Eng. 1999;12:1035–1040. doi: 10.1093/protein/12.12.1035. [DOI] [PubMed] [Google Scholar]
- 38.Gruendler C, Lin Y, Farley J, Wang T. J Biol Chem. 2001;276:46533–46543. doi: 10.1074/jbc.M105500200. [DOI] [PubMed] [Google Scholar]
- 39.Freundlieb S, Schirra-Muller C, Bujard H. J Gene Med. 1999;1:4–12. doi: 10.1002/(SICI)1521-2254(199901/02)1:1<4::AID-JGM4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 40.Boorsma M, Nieba L, Koller D, Bachmann MF, Bailey JE, Renner WA. Nat Biotechnol. 2000;18:429–432. doi: 10.1038/74493. [DOI] [PubMed] [Google Scholar]
- 41.Link N, Aubel C, Kelm JM, Marty RR, Greber D, Djonov V, Bourhis J, Weber W, Fussenegger M. Nucleic Acids Res. 2006;34:e16. doi: 10.1093/nar/gnj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 43.Argarana CE, Kuntz ID, Birken S, Axel R, Cantor CR. Nucleic Acids Res. 1986;14:1871–1882. doi: 10.1093/nar/14.4.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osada K, Komai M, Sugiyama K, Urayama N, Furukawa Y. Int J Vitam Nutr Res. 2004;74:334–340. doi: 10.1024/0300-9831.74.5.334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.