Abstract
HIV infection is accompanied by an early immune dysfunction limiting host control of virus and likely contributing to difficulties in achieving a successful vaccine against HIV. We report here that the HIV Tat protein is strongly immunosuppressive, both immediately after immunization of mice with soluble protein (sTat), and in seroconverting humans, and propose that Tat-induced suppression cripples immune surveillance to HIV infection. We show that macrophages are sensitive to sTat stimulation at concentrations 1,000-fold lower (500 pM) than T cells, and this stimulation is accompanied by the immunosuppressive induction of Fas ligand on the macrophage. T cell proliferative defects induced by sTat in vitro can be completely (at lower concentrations of sTat) or partially (at higher concentrations) reversed by antagonists to Fas/Fas ligand interaction. We further report a method to preserve immunogenicity while inactivating Tat immunosuppression through oxidation, which advances the use of oxidized Tat as a component of an anti-HIV vaccine. These observations define additional methods to study the immunosuppressive functions of sTat that now may be rapidly applied to primary isolates from individuals with differing clinical courses. Our findings have immediate relevance for vaccine development, by describing and supporting a strategy that includes inactivated sTat in a multicomponent, anti-HIV vaccine.
During the initial phase of HIV infection, immune dysfunction exceeds CD4+ T cell infection and loss (1). This early immune impairment is observed in vitro as diminished T cell responses to antigen-specific stimulation (2), and in the infected individual as persistent replication (3). Although the lag time between infection and immunodeficiency is not fully defined, in many cases HIV replication is never completely controlled by an immune response, suggesting that immunosuppression subsequent to HIV infection may be practically immediate (4). Accumulation of soluble immune suppressants of host or HIV origin might explain abnormalities in uninfected cells. Accelerated apoptosis in several types of immune responder cells has been shown during HIV infection and has been proposed to contribute to the general immune dysfunction observed in HIV disease (5, 6).
The HIV-1 Tat protein functions as a soluble effector (sTat; ref. 7) that, in addition to transactivating the HIV ltr and other genes (7), potently stimulates T cells to undergo apoptosis in vitro (8–10). The translation of this observation to HIV disease is under vigorous investigation. The concentration of Tat protein required to activate (11) or to directly induce apoptosis of CD4+ T cells varies from 50 nM to 2 mM in different experimental systems (8–11). Tat has been shown to induce abnormalities in other types of immune cells, including macrophage (MΦ) antigen-presenting cells (APCs) (12). Recently, infected as well as bystander MΦs, but not dendritic cells, from HIV-infected individuals were shown to aberrantly overexpress Fas ligand (FasL) (13). This overexpression would kill antigen-responding T cells activated to express Fas (CD95) (14) through apoptosis mediated by Fas/FasL interaction (10, 15–17). In contrast, activated MΦs may acquire a resistance to suicide by uncoupling Fas-mediated signaling, as demonstrated in vitro after tumor necrosis factor (TNF) α or lipopolysaccharide (LPS) stimulation (18).
No vaccine capable of eliciting protective immunity to HIV infection has been formulated. HIV presents a formidable challenge to immune surveillance based on many factors, including hypervariability of its principal neutralizing domain (V3) (19), concealment of critical, functional domains in the external envelope glycoprotein (gp120) behind inessential structures (20), and infection of APCs resulting in their dysfunction (21). Substantial progress has been made recently in defining neutralizing domains within the HIV envelope, and in augmenting the immune response to HIV proteins (22). Despite these important advances, an effective HIV vaccine remains elusive, we propose, because the immediate immunodeficiency accompanying HIV infection creates another obstacle to a successful vaccine (23). Here we investigate the validity of this hypothesis, the mechanism by which HIV induces immediate immunosuppression, and a strategy by which this immunosuppression might be overcome.
MATERIALS AND METHODS
Murine Immunizations and Immune Responses.
At week 0, mice were bled for preimmune sera, and then immunized with 5 μg of recombinant Tat protein, or, in the case of the mixing experiment, with 5 μg recombinant Tat and/or recombinant p24 (Chiron) in 100 μl of complete Freund’s adjuvant administered s.c. in the flanks. Subsequently, sera were collected every other week for antibody response (up to 10 weeks), or lymph nodes were harvested at 6 weeks for T cell proliferation assays.
ELISAs.
Briefly, protein at 1 μg/ml was applied to plastic 96-microwell plates in carbonate/bicarbonate coating buffer, pH 9.6 overnight at 4°C, and blocked overnight at 4°C in PBS, pH 7.4, with 0.05% Tween-20, 2.5% BSA (Sigma), and 5% FCS (GIBCO) (blocking buffer). Sera, diluted 1:100, 1:1,000, and 1:10,000 into assay buffer (PBS + 0.05% Tween 9:1 blocking buffer), were incubated on the coated plates for 1 hr at 37°C. Reactions were developed with affinity-purified, horseradish peroxidase-conjugated anti-human IgG or IgM, or anti-mouse IgG (Kirkegaard & Perry Laboratories) for 30 min at 37°C, followed by tetra methyl benzidine substrate, and stopped in 4 N H2SO4. Anti-p24 antibodies were measured by commercial ELISA. Plates were read (V Max Pro, Molecular Devices) for the difference in OD between 450 nM (signal) and 575 nM (background).
Isolation of Murine MΦs.
Eight- to 12-week-old BALB/c mice were injected i.p. with 3 ml of 2.9% thioglycolate (TG) (National Institutes of Health media unit). At 4 days, mice were sacrificed, and peritoneal exudate cells highly enriched for MΦs were harvested by i.p. lavage with ice-cold DMEM containing 5% FCS.
Classification of Human Groups.
The criteria for long-term nonprogressors (LTNPs) were: >12 years with HIV infection and >500 CD4+ cells/μl; for immediate seroconverters (ISs): symptomatic individuals, with seroconversion and HIV isolation, during the period of initial presentation; for progressors (Ps): individuals with recurrent high viral loads (>5,000 copies/ml) and declining CD4+ concentrations (range 134–348 cells/μl) observed over 3 years; for random HIV-infected individuals (HIV positive, HP): individuals without symptoms or AIDS at least 3 years after HIV infection; for the uninfected controls: individuals shown to be HIV-negative by prior commercial ELISA. All sera first were prescreened for (predominantly IgM) “natural antibodies” crossreactive to Tat (24); 2/25 (one control and one P) sera fulfilling this criterion were excluded from further study. A new control was substituted but another P serum was not.
Tat Protein.
Recombinant Tat was prepared, as described (8), under mildly denaturing conditions, and was renatured in the presence of 0.1 mM DTT. Three preparations of sTat, assayed to be endotoxin-free (<25 pg/ml), were used in these studies. The generation and maintenance of long-term, peripheral blood cell-derived CD4+ T cell lines overexpressing Tat of primary HIV isolates (Tat Tcls) will be described elsewhere.
Flow Cytometry.
Flow cytometry was performed as described (9) by staining MΦs with the anti-FasL mAb, Nok 1 (PharMingen) and analyzing stained cells by FACScan (Becton Dickinson).
T Cell Proliferation Assays.
Human peripheral blood mononuclear cells (PBMCs) were purified as described (9) and plated at 105 cells/100 μl in 96-well plates in RPMI medium supplemented with 5 × 10−5 β-mercaptoethanol and 5% human AB serum. [3H]thymidine was added over the last 18 hr of culture.
Protein Immunoblots.
Protein lysates were resolved by 12% SDS/PAGE, transferred to nitrocellulose (Hybond, Amersham Pharmacia), probed with human antibodies from a LTNP (SF) or an IS (RR), and blots were developed by enhanced chemical luminescence with a secondary goat anti-human antibody conjugated with horseradish peroxidase (Kirkegaard & Perry Laboratories).
RESULTS
Oxidation of Tat Protein Inactivates Immunosuppression and Preserves Immunogenicity.
While making antibodies to Tat in mice, we found stronger immune responses to inactivated, oxidized protein than to native protein (Table 1, Exp. 1), which was surprising because antibodies frequently are directed against native conformations (25). To explore the possibility that this resulted because native sTat was directly immunosuppressive in mice, we analyzed four groups immunized either with p24 (HIV gag) (I), sTat + p24 (II), oxidized Tat (oxTat) + p24 (III), or ox p24 (IV), reasoning that sTat immunosuppression could extend to a coadministered antigen by affecting APC function (13). sTat was a 10-fold poorer immunogen than inactivated Tat (Table 1, Exp. 2, αTAT group II vs. III, P < 0.0004, t test). Coimmunization of sTAT with p24 resulted in a 7-fold suppression of the anti-p24 response (P < 0.0047, ANOVA) compared with the anti-p24 responses to p24 alone (I), ox p24 (IV), or p24 coadministered with inactivated Tat (III), which were all comparable immunogens. This immunosuppression derived at least in part from a sustained impairment in T cell function, because recall proliferative responses to a wide range of p24 concentrations (0.02, 0.2, and 2 μg/ml) were strongly suppressed (100%, 73%, and 56%, respectively) in lymph node T cells harvested from mice 6 weeks post-sTat+p24 (II) challenge, when compared with group III (oxTat+p24) mice.
Table 1.
Native sTat, but not oxTat, suppresses antibody response to a coadministered antigen in mice
| Group | Immunogen | Exp. 1
|
|||
|---|---|---|---|---|---|
| αTat titer
|
Exp. 2
|
||||
| Wk 2 | Wk 6 | αTat titer | αp24 titer | ||
| sTat | <100 | 922 (822) | |||
| oxTat | 12,750 (670) | 17,675 (4,925) | |||
| I | p24 | 0 | 9,109 (1,337) | ||
| II | sTat + p24 | 983 (317) | 1,100 (384) | ||
| III | oxTat + p24 | 9,033 (657) | 8,013 (1,410) | ||
| IV | oxp24 | 0 | 7,944 (1,245) | ||
Analysis by ELISA of antibody responses to recombinant native Tat, or to recombinant p24 protein (SF2, Chiron). Titers were determined by linear regression analysis of serial 1:100, 1:1,000, and 1:10,000 dilutions. Results are the mean (SD). Statistical analysis (P) was performed by two-tailed Student’s t test (two-way), or by ANOVA (four-way). In Exp. 1, P < 0.07; in Exp. 2, P < 0.0004 (II vs. III, αTat titer) and P < 0.0047 (α p24 titer).
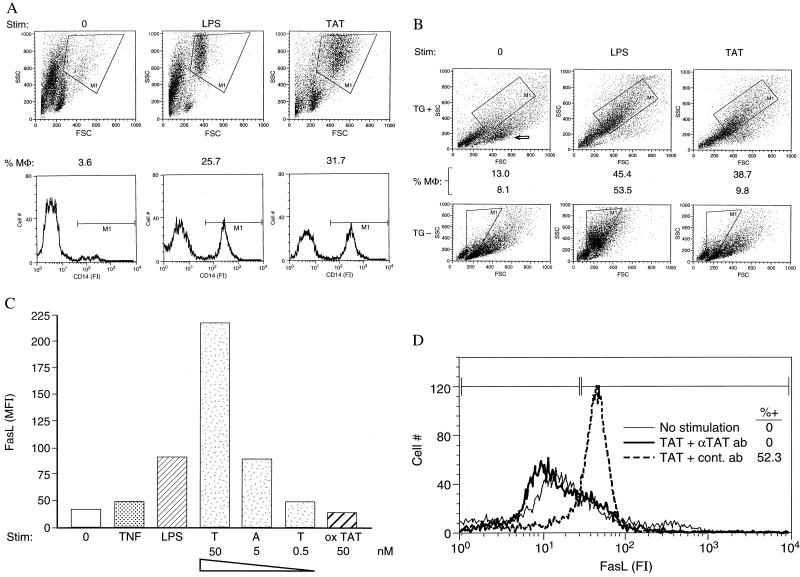
Picomolar Concentrations of sTat Stimulate FasL on Purified Populations of MΦ. The immune cell most responsive in vitro to sTat-mediated immunosuppression has not been determined, and one obvious target, the CD4+ T lymphocyte, is relatively insensitive to its activity. Our immunizations (Table 1) suggested that sTat induced an APC defect. To determine whether MΦ APCs, recently correlated with apoptosis in HIV-infected individuals (13), were sensitive to sTat, we first studied elutriated human peripheral blood monocytes (>90% monocytes at culture initiation), which might be more receptive to Tat activity than murine MΦs in the context of a (partial) species specificity of Tat (26, 27). Human monocytes were not viable over time in the absence of stimulation (11), resulting in the outgrowth of smaller, CD14− PBMCs (Fig. 1A). By comparison, LPS- (Fig. 1A) or TNF-α (not shown)-treated monocytes remained viable. Interestingly, sTat significantly promoted the survival of CD14+ cells (Fig. 1A), which diffusely enlarged (Fig. 1A, Upper, M1 gate) and became adherent, suggesting their differentiation into MΦs. sTat stimulation of monocytes began at 500 pM, 100-fold below its threshold for activating human T cells (11), and 1,000-fold below the concentration directly triggering apoptosis of CD4+ T cells (10).
Figure 1.
Tat promotes viability and stimulates human and murine MΦs in culture. (A) Human MΦs. Fluorescence-activated cell sorter (FACscan, Becton Dickinson) analysis of monocytes, enriched from peripheral blood by centrifugal elutriation, cultured for 6 days with either no stimulus (0), 100 ng/ml of LPS, or 50 nM Tat. (Upper) Scatter plot, the large, CD14+ MΦs (M1, 100% CD14+) are gated. FSC, forward scatter. (Lower) Immunofluorescence of all cells in culture after staining with a rhodamine-labeled anti-CD14 antibody (PharMingen). The CD14+ cells (M1, MΦs), as a % of all cells in culture at time of assay, are indicated above, and agree closely with the % MΦs determined by scatter plot. FI, fluorescence intensity. (B) Tat promotes viability of murine MΦs that have been previously activated in vivo. Mouse peritoneal washout cells, a MΦ-rich population, were isolated either 3 days after i.p. TG injection (TG+), or without this in vivo stimulation (TG−). Murine MΦs were cultured for 5 days either in the absence of additional stimulation (0), with LPS (100 ng/ml), or with sTat (100 nM), and then analyzed by flow cytometry scatter plot for a large population of cells (gate, M1, %MΦ) staining for murine MΦ markers (not shown). Arrow indicates population of apoptotic, annexin-reactive cells accumulating in unstimulated cultures. FSC, forward scatter. (C) Median fluorescence (MFl) of monocytes, cultured for 6 days either with no stimulus (0), 50 ng/ml TNF-α, 100 ng/ml LPS, decreasing concentrations of sTat (T, A, T), or 50 nM oxTat (3% H2O2 for 1 hr at 25°C), and stained with an anti-FasL mAb (Nok 1, PharMingen), followed by a fluoresceinated goat anti-mouse polyclonal antibody (Amersham Pharmacia). (D) Neutralization of Tat-mediated induction of FasL on human MΦs by anti-Tat polyclonal antibodies. MΦs cultured for 2 days either with no stimulus, with 50 nM Tat pretreated for 1 hr with murine polyclonal antibodies (1:100 dilution) prepared to oxTat, or with preimmune serum (1:100) were analyzed by FACs after staining with an anti-FasL mAb (Nok 1, PharMingen). FI, fluorescence intensity.
Moreover, sTat did enhance the viability of cultured murine MΦs, as long as the MΦs first were activated in vivo [with TG (TG+) , compared with no prior activation (TG−) (Fig. 1B)]; and stimulated with relatively high concentrations of Tat (100 nM). By comparison, LPS promoted the viability of murine MΦs independently from in vivo stimulation, and at the same concentration effective for human MΦs (Fig. 1B). We interpreted the partial resistance to Tat activity to imply a barrier to Tat uptake in mice, which could be partially overcome when MΦ phagocytosis was stimulated.
An acquired resistance to Fas-initiated apoptosis that accompanies an induction of FasL has been proposed to explain the viability of TNF-α- or LPS-stimulated monocytes in culture (18). To learn whether sTat also induced FasL on human monocytes, we assayed 6-day cultures for surface FasL expression after either no stimulation, stimulation with increasing concentrations of sTat ranging from 500 to 50,000 pM, with TNF-α, and with LPS (Fig. 1C), all of which enhanced MΦ viability (Fig. 1A). Although TNF-α (weakly) and LPS (more strongly) induced FasL on MΦs, sTat was the most potent inducer of FasL (median fluorescence of 216.7 vs. 90.6 for LPS, Fig. 1C). The sTat induction of FasL on MΦs was dose and time dependent, was competed (100% over 48-hr culture) by murine (Fig. 1D) or human (not shown) polyclonal antibodies to Tat, and was not stimulated by sTat that had been oxidized (Fig. 1C), with 3% H202 for 1 hr at 25°C, before addition to the monocytes. Several other surface proteins capable of regulating T cell responses, including B7–1, B7–2, CCR5, and CXCR4, were not affected by sTat treatment of MΦs (not shown). The smaller, CD14− cells in these cultures (consisting of contaminating T and B lymphocytes) did not express detectable amounts of FasL (not shown). Purified dendritic cells treated with sTat also did not induce FasL (not shown).
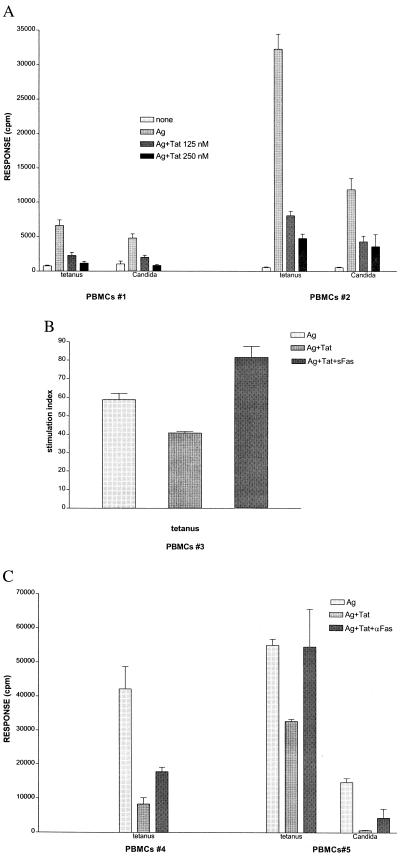
Tat Suppresses Helper Cell Activation in Vitro That at Lower Concentrations (50 nM) Is Fully Reversed by FasL Inhibitors.
To better define a pathway of Tat immunosuppression, through FasL induction (on the MΦ), resulting in loss of helper T cell recall responses, we performed T cell proliferation assays using recall antigens, sTat (2), and FasL antagonists. T cell proliferation to tetanus antigen (mean inhibition 70% at 125 nM and 84% at 250 nM, Fig. 2A) and to Candida antigen (mean inhibition 72% at 125 nM and 81% at 250 nM, Fig. 2A) was suppressed in a dose-dependent fashion by sTat, whereas phytohemagglutinin-induced proliferation was less sensitive to Tat suppression (29% at 250 nM, not shown). At lower concentrations of sTat (50 nM), Tat-induced immunosuppression (31%, Fig. 2B) was not only fully reversed by the addition of soluble Fas (sFas, 50 μg/ml), but under these conditions sTat actually became stimulatory (11) (141% relative to antigen treatment alone, Fig. 2B) in these short-term cultures. An antibody (ZB4) that inhibits FasL by antagonizing its receptor (Fas) on the T cell, partially (42%, Fig. 2C, PBMCs #4) or fully (96%, Fig. 2C, PBMCs #5) reversed sTat immunosuppression of tetanus responses, and enhanced a Candida response 6.3-fold relative to sTat treatment alone (Fig. 2C, PBMCs #5). The FasL antagonists, ZB4 and sFas, did not enhance T cell proliferation independently from sTat treatment (not shown). We interpreted these results to indicate that a portion of sTat-induced immunosuppression was contributed by induction of FasL, although other Tat-induced factors also could participate in suppressing T cell proliferative responses, particularly at higher concentrations of sTat (12).
Figure 2.
Participation of FasL in Tat-mediated suppression of lymphocyte proliferation. (A) Human PBMCs from two individuals (PBMCs #1 and #2) cultured in triplicate for 6 days in medium alone, tetanus (0.3 Lf/ml) antigen (Ag) or Candida (4 μg/ml) Ag, or Ags with additionally 125 or 250 nM recombinant Tat protein, were pulsed with [3H]thymidine over the last 18 hr before harvest. Results are representative of 10 experiments with different PBMCs. (B) Human PBMCs from one individual (PBMCs #3) cultured in triplicate for 5 days in either medium (not shown), tetanus antigen (Ag, 0.3 Lf/ml), antigen with the further addition of 50 nM Tat (Ag+Tat), or Ag with 50 nM Tat and recombinant sFas protein (25 μg/ml) to block surface FasL (Ag+Tat+sFas). [3H]thymidine was added over the last 18 hr, and results are graphed as stimulation index (mean cpm stimulated culture/mean cpm medium control). Results are representative of three experiments. (C) Proliferation of PBMCs cultured 6 days with either tetanus (PBMCs #4 and #5) or Candida (PBMCs #5) antigen (Ag) alone as in A, compared with cultures in which Tat (Ag+Tat, 125 nM), or Tat (125 nM) and the antagonistic anti-Fas antibody, ZB4 (250 μg/ml, Upstate Biotechnology) also were added (Ag+Tat+αFas). Results are representative of three experiments.
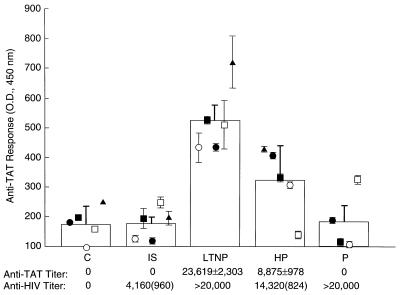
In HIV-Infected Individuals, the Antibody Response to Tat Is Delayed and Difficult to Maintain.
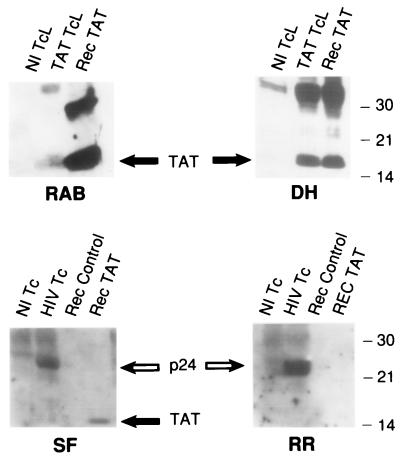
To analyze the effect(s) of extracellular Tat in HIV-infected individuals, we explored the relationship(s) between anti-Tat antibodies and disease course. We reasoned that antibodies to sTat would neutralize its proapoptotic effect and slow progression, whereas its apoptotic function would impair initiation and maintenance of any immune response directed to Tat. Sera from ISs, HP individuals, Ps during rapid disease progression, LTNPs, and uninfected controls were analyzed by ELISA for antibodies to recombinant sTat. Interestingly, neither the ISs or the Ps had any antibody directed against Tat (Fig. 3, graph), whereas they had antibodies to other HIV proteins (Fig. 3, Lower). The LTNPs had the highest anti-Tat antibody titer (23,619 ± 2,303), whereas the HP individuals had an intermediate response (Fig. 3). This relationship among the four groups of HIV-infected individuals was highly statistically significant (P < 0.0001, ANOVA). The higher response in the LTNP compared with the HP group was also significant (P < 0.02, two-tailed t test). Protein immunoblots confirmed that the five ISs made no anti-Tat antibodies, whereas they already made antibodies to other viral proteins, including p24 (Fig. 4, RR, open arrow). All the LTNP sera (Fig. 4, examples DH and SF) and a rabbit antiserum to recombinant Tat (Fig. 4, RAB) recognized both recombinant (Rec) and native Tat (Tat TcL) (Fig. 4, dark arrow).
Figure 3.
Absence of Tat-directed antibody response in ISs and Ps. Antibodies to Tat in sera from uninfected controls (C), ISs, LTNPs, random HP individuals, and Ps were measured by ELISA against recombinant Tat produced in bacteria, and the OD (multiplied by 1,000 to reflect the dilution), averaged from triplicate determinations, is reported as a point for each individual. The mean for each group is indicated by a bar, with SEM flags. (Lower) Mean antibody titer of each group to Tat and HIV proteins (Abbott Diagnostic Labs, Chicago, to gp41 envelope and p24 nucleocapsid) determined by linear regression analysis of ELISA readings at 1:10,000, 1:1,000, and 1:100 dilutions for Tat, and at 1:1,600 and 1:400 dilutions for HIV. The anti-HIV titers are a lower estimate as some samples exceeded the maximum range of this assay (>20,000). The difference in anti-Tat antibody response between the LTNP and either the IS or the P groups is highly statistically significant (P < 0.0003 vs. IS, P < 0.0024 vs. P, unpaired t test), whereas the difference in antibody between the LTNP and the HP groups is also statistically significant (P < 0.02, unpaired t test).
Figure 4.
Analysis of anti-Tat and anti-p24 antibody responses by protein immunoblot. (Upper) Protein lysate from a normal T cell line (Nl TcL), lysate from a PBMC-derived T cell line engineered to overexpress the Tat protein (Tat TcL), and recombinant Tat protein (Rec Tat) were resolved by 12% SDS/PAGE, transferred to nitrocellulose, and probed with a rabbit polyclonal antiserum (RAB) to recombinant Tat, or with human antibody from a LTNP (DH). Protein markers (kDa) and expected migration of the Tat protein (filled arrow) are indicated. (Lower) Protein lysate from normal phytohemagglutinin-stimulated CD4+ PBMCs (Nl Tc), lysate from identical CD4+ cells infected with a dualtropic HIV-1 isolate (HIV Tc), recombinant human retinoblastoma 25-kDa fragment (Rec Control), and recombinant Tat (Rec TAT) were resolved by 12% SDS/PAGE, transferred to nitrocellulose, and probed with human antibodies from a LTNP (SF) or an IS (RR). Expected migration of p24 (open arrow) and Tat (filled arrow) proteins, and relative position of protein markers (kDa) are indicated.
DISCUSSION
Our previous work (8, 11), and the well-conserved Tat sequence among various viral strains as reviewed by others (23), suggest the potential for Tat as a target for immunotherapy and AIDS vaccine development. We have shown here that sTat functions as an acute immunosuppressant in vivo, supporting the inclusion of inactivated Tat as a vaccine against AIDS.
Tat inhibited recall activation of antigen-specific T cells. Antibody to Tat is relatively difficult to produce both in humans and in mice, and the response in humans is delayed compared with other HIV antigens, including p24. The maintenance of anti-Tat antibodies in the infected individual is one factor associated with slower disease progression (28). Tat acutely suppressed the immune response to a coadministered antigen, p24, whereas p24 was not immunosuppressive (29). In our mixing experiment, p24 was selected as a second immunogen because antibodies to it, like Tat, are lost over time, possibly owing to its strong T helper cell dependence (30).
Our finding that sTat functions to up-regulate FasL on MΦ APCs provides a consistent explanation for our observations. Importantly, 1,000-fold lower concentrations of sTat (500 pM) trigger this effect on the MΦ, as compared with the concentration required to initiate direct apoptosis of CD4+ T cells (approximately 500 nM). MΦs stimulated by Tat, either by HIV infection or by sTat uptake, would induce FasL, which in turn would trigger the apoptosis of antigen-reacting, Fas-expressing helper T cells. When a Tat-activated MΦ presented more than one antigen, either because of HIV infection or phagocytosis of soluble antigens, immune responses directed toward the other antigens would be suppressed as well. This process would blunt T-cell dependent, cellular, and humoral immune responses. The properties of sTat in vivo inevitably will be complex and dose dependent, reflecting a mixture of stimulatory (ref. 11, Fig. 3B) and apoptotic (8–10) effects on the T cell and immunosuppressive effects from the MΦs.
Our work defines two useful assays for evaluating this acutely immunosuppressive function of sTat in the context of HIV isolates from infected individuals with varying disease progression. The simplest assay will be to compare different sTats for induction of FasL on the MΦ. The small animal model for evaluating immune disregulation in HIV disease has important similarities to transgenic mice in stressing the role of APCs in HIV immune dysfunction (31–33), but has critical differences by emphasizing the outcome of an acute challenge. This acute scenario seems more relevant to the development of an effective HIV vaccine.
The findings presented here strongly support the inclusion of inactivated Tat in immunization protocols designed primarily to elicit protective anti-HIV immunity to structural proteins. We have provided a safe and practical method for inactivating Tat that maintains the immunogenicity of the protein. Vaccination to oxTat would neutralize the immune paralysis otherwise mediated by native, active Tat during viral challenge, and thereby sustain protective responses. By these criteria, immunization with inactivated, oxTat warrants further immediate study in vaccine protocols in primates, and potentially in humans.
Acknowledgments
We thank William E. Paul for helpful discussions and review of the manuscript; Ligia Pinto, Jolynne Tschetter, Andrew Blauvelt, and Gene Shearer for assistance; the AIDS Research and Reference Repository and Marjorie-Robert Guroff for reagents; and C. Darden for assistance with manuscript preparation. We are grateful for funds provided by Queens College, City University of New York used in the completion of this work.
ABBREVIATIONS
- sTat
soluble Tat
- MΦ
macrophage
- APC
antigen-presenting cell
- FasL
Fas ligand
- TNF
tumor necrosis factor
- LPS
lipopolysaccharide
- LTNP
long-term nonprogressor
- IS
immediate seroconverter
- P
progressor
- HP
HIV positive
- PBMC
peripheral blood mononuclear cell
- oxTat
oxidized Tat
- TG
thioglycolate
- sFas
soluble Fas
References
- 1.Pantaleo G, Fauci A. Annu Rev Immunol. 1995;248:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 2.Viscidi R P, Mayur K, Lederman H M, Frankel A D. Science. 1989;246:1606–1608. doi: 10.1126/science.2556795. [DOI] [PubMed] [Google Scholar]
- 3.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. Nature (London) 1993;362:355–359. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 4.Piatak M, Saag M S, Yang L C, Clark S J, Kappes J C, Luk K C, Hahn B H, Shaw G M, Lifson J D. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- 5.Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen J C. J Exp Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banda N K, Bernier J, Kurahara D K, Kurrie R, Haigwood N, Sekaly R P, Finkel T H. J Exp Med. 1992;176:1099–1106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frankel A D, Pabo C O. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 8.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 9.Kolesnitchenko V, King L, Riva A, Tani Y, Korsmeyer S J, Cohen D I. J Virol. 1997;71:9753–9763. doi: 10.1128/jvi.71.12.9753-9763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K M, Krammer P H. Nature (London) 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 11.Li C J, Ueda Y, Shi B, Borodyansky L, Huang L, Li Y-Z, Pardee A B. Proc Natl Acad Sci USA. 1997;94:8116–8120. doi: 10.1073/pnas.94.15.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu M X, Schlossman S F. Proc Natl Acad Sci USA. 1997;94:13832–13837. doi: 10.1073/pnas.94.25.13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dockrell D H, Badley A D, Villacian J S, Heppelmann C J, Algeciras A, Ziesmer S, Yagita H, Lynch D H, Roche P C, Leibson P J, Paya C V. J Clin Invest. 1998;101:2394–2405. doi: 10.1172/JCI1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 15.Dhein J, Walczak H, Baumler C, Debatin K M, Krammer P. Nature (London) 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 16.Brunner T, Mogil R J, LaFace D, Yoo N J, Mahboubi A, Echeverri F, Martin S J, Force W R, Lynch D H, Ware C F. Nature (London) 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 17.Ju S T, Panka D J, Cui H, Ettinger R, el-Khatib M, Sherr D H, Stanger B Z, Marshak-Rothstein A. Nature (London) 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 18.Kiener P A, Davis P M, Starling G C, Mehlin C, Klebanoff S J, Ledbetter J A, Liles W C. J Exp Med. 1997;185:1511–1516. doi: 10.1084/jem.185.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emini E A, Putney S D. Biotechnology. 1992;20:309–326. doi: 10.1016/b978-0-7506-9265-6.50019-1. [DOI] [PubMed] [Google Scholar]
- 20.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Nature (London) 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuitemaker H, Meyaard L, Kootstra N A, Dubbes R, Otto S A, Tersmette M, Heeney J L, Miedema F. J Infect Dis. 1993;168:1140–1147. doi: 10.1093/infdis/168.5.1140. [DOI] [PubMed] [Google Scholar]
- 22.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. Nature (London) 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein G. Nat Med. 1996;2:960–964. doi: 10.1038/nm0996-960. [DOI] [PubMed] [Google Scholar]
- 24.Rodman T C, Pruslin F H, To S E, Winston R. J Exp Med. 1992;175:1247–1253. doi: 10.1084/jem.175.5.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berzofsky J A, Berkower I J. In: Fundamental Immunology. 2nd Ed. Paul W E, editor. New York: Raven; 1989. pp. 177–182. [Google Scholar]
- 26.Wei P, Garber M, Fang S M, Fischer W H, Jones K A. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 27.Gazzinelli R T, Sher A, Cheever A, Gerstberger S, Martin M A, Dickie P. J Exp Med. 1996;183:1645–1655. doi: 10.1084/jem.183.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Re M C, Furlini G, Vignoli M, Ramazzotti E, Zauli G, La Placa M. Clin Diagn Lab Immunol. 1996;3:230–232. doi: 10.1128/cdli.3.2.230-232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luban J, Bossolt K L, Franke E K, Kalpana G V, Goff S P. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 30.Binley J M, Klasse P J, Cao Y, Jones I, Markowitz M, Ho D D, Moore J P. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanna Z, Kay D G, Cool M, Jothy S, Rebai N, Jolicoeur P. J Virol. 1998;72:121–132. doi: 10.1128/jvi.72.1.121-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vellutini C, Horschowski N, Philippon V, Gambarelli D, Nave K A, Filippi P. Aids Res Hum Retroviruses. 1995;11:21–29. doi: 10.1089/aid.1995.11.21. [DOI] [PubMed] [Google Scholar]
- 33.Brady H J, Abraham D J, Pennington D J, Miles C G, Jenkins S, Dziezak E A. J Virol. 1995;69:7622–7629. doi: 10.1128/jvi.69.12.7622-7629.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]