Abstract
Osmotic stress activates mitogen-activated protein kinases (MAPKs) including c-Jun N-terminal kinase (JNK) and p38, which play important roles in cellular stress responses. TGF-β activated kinase 1 (TAK1) is a member of the MAPK kinase kinase (MAPKKK) family and can activate JNK and p38. TAK1 can also activate Iκ B kinase (IKK) that leads to degradation of IκB and subsequent NF-κB activation. We found that TAK1 is essential for osmotic stress-induced activation of JNK, but is not an exclusive mediator of p38 activation. Furthermore, we found that although TAK1 was highly activated upon osmotic stress, it could not induce degradation of Iκ B or activation of NF-κB. These results suggest that TAK1 activity is somehow modulated to function specifically in osmotic stress signaling, leading to the activation of JNK but not of IKK. To elucidate the mechanism underlying this modulation, we screened for potential TAK1 binding proteins. We found that Thousand-and-One Amino Acid Kinase 2 (TAO2) associates with TAK1, and can inhibit TAK1-mediated activation of NF-κ B but not of JNK. We observe that TAO2 can interfere with the interaction between TAK1 and IKK, and thus may regulate TAK1 function. TAK1 is activated by many distinct stimuli including cytokines and stresses, and regulation by TAO2 may be important to activate specific intracellular signaling pathways that are unique to osmotic stress.
MAPK cascades play important roles in many extracellular stimuli-activated intracellular signaling pathways (1-3). These stimuli include not only growth factors and cytokines but also chemical and physical stresses such as ultraviolet light irradiation and osmotic stress. Among the three well-characterized MAPK subfamilies; extracellular signal-regulated kinase (ERK), JNK and p38 MAPK; JNK and p38 are the major regulators of stress-induced cellular responses. In mammalian cell cultures, high osmolarity generated by adding salt or sugar into the culture medium activates JNK and p38, which in turn up-regulates a number of genes involved in cellular stress responses, such as production of osmolytes in renal cells (4). In addition, the JNK and p38 pathways promote cell death, which is important for the elimination of dysfunctional cells (5-7). JNK and p38 are activated by the MAPK kinases (MAPKKs) MKK4/MKK7 and MKK3/MKK6, respectively. The MAPKKs are normally activated by members of the MAPKK kinase (MAPKKK) family that includes MEKKs, mixed lineage kinases (MLKs), apoptosis-stimulating kinase 1 (ASK1) and others in response to engagement of cell surface receptors or stresses. Although the MAPKKKs are believed to confer signal- and stimuli-specificity, the particular MAPKKKs that are essential to each chemical and physical stress have not yet been well defined.
Transforming growth factor activated kinase 1 (TAK1) is a member of the MAPKKK family, and plays an essential role in tumor necrosis factor (TNF), interleukin 1 (IL-1), and Toll-like receptor(TLR) signaling pathways (8-13). TAK1 functions as a MAPKKK in these pathways, and is indispensable for the activation of JNK and p38. Furthermore, TAK1 is also essential for TNF-, IL-1- and TLR ligand-induced activation of IκB kinase (IKK), which leads to the subsequent degradation of IκB and activation of transcription factor NF-κB. TAK1-/- or TAK1Δ/Δ (expressing a kinase-dead TAK1) (Fig. 2A) cells do not activate JNK, p38 or NF-κB upon treatment by TNF, IL-1 or TLR ligands (9,10). Several earlier studies have been shown that TAK1 can be activated by chemical and physical stresses, including osmotic stress (14-16). However, the role of TAK1 in stress signaling has not yet been defined.
Fig. 2.
TAK1 is essential for osmotic stress-induced JNK but not p38.
(A) Schematic representation of TAK1 deletion.
(B) MEF cells and TAK1Δ/Δ were subjected to osmotic stress (0.5 M NaCl) for 10 or 30 min. Cell extracts were subjected to immunoblotting with anti-phospho JNK (top panel) and anti-phospho p38 (third panel). The amounts of endogenous JNK, p38, and TAK1 are shown in the second, fourth, and bottom panels, respectively. IB, immunoblotting.
(C) Mouse keratinocytes and TAK1Δ/Δ keratinocytes were subjected to osmotic stress (0.5 M NaCl) for 10 or 30 min. Cell extracts were subjected to immunoblotting with anti-phospho JNK (top panel) and anti-phospho p38 (third panel). The amounts of endogenous JNK, p38, and TAK1 are shown in the second, fourth, and bottom panels, respectively. IB, immunoblotting.
In this study, we demonstrate the essential role of TAK1 in osmotic stress signaling using TAK1Δ/Δ cells. We found that TAK1 is essential for osmotic stress-induced activation of JNK but not of p38. We also found that although TAK1 is highly activated upon osmotic stress treatment, this did not result in IκB degradation or subsequent activation of NF-κB. These results suggest that TAK1 is regulated to activate only the MAPK cascades and not the NF-κB pathway in osmotic stress signaling. We attempted to elucidate the mechanism underlying of this regulation of TAK1. We demonstrate here that a TAK1 binding kinase, Thousand-and-One Amino Acid Kinase 2 (TAO2) blocks the TAK1-mediated NF-κB pathway by preventing the interaction of TAK1 with IKK, yet did not inhibit TAK1-induced activation of JNK. These results suggest that binding by TAO2 may modulate the specific cellular responses of TAK1.
EXPERIMENTAL PROCEDURES
Cell culture and reagents
Human embryonic kidney 293 cells, 293 IL-1RI cells that stably expresses IL-1 receptor I (17), and TAK1Δ/Δ mouse embryonic fibroblasts (MEFs) (9) were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% Bovine Growth Serum (Hyclone) at 37°C in 5% CO2. TAK1 wild type and Δ/Δ keratinocytes(13) were cultured in Ca2+-free Eagle’s minimal essential medium (EMEM; BioWhittaker) supplemented with 4% Chelex-treated bovine growth serum, 10 ng/mL human epidermal growth factor (Invitrogen) and 0.05 mM calcium chloride at 33°C in 8% CO2. For transfection studies, 293 cells (1 x 106 or 1.6 x 105) were seeded onto 10 cm or 6-well (3.5 cm) dishes, respectively, and transfected with various expression vectors by the standard calcium phosphate precipitation method 24 hr after seeding. 36 hr after transfection, cells were harvested in 0.5% Triton X-100 lysis buffer containing 20 mM HEPES (pH 7.4), 150 mM NaCl, 12.5 mM β -glycerophosphate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM DTT, 1 mM sodium orthovanadate, 1 mM PMSF, and 20 μM aprotinin). To induce osmotic stress, cells were treated with NaCl (0.5 M or 0.7 M). For cytokine stimulation, 10 ng/ml IL-1-β or 20 ng/ml TNF-α (Roche) was used. Stress agents were added 3 to 30 min prior to harvest.
Expression plasmids
Expression vectors for HA-tagged and Flag-tagged TAO1 and TAO2 were generated from the plasmids pCMV5-HA-TAO1(1-1001), pCMV5-Myc-TAO2(1-993), and pCMV5-Myc-TAO2(1-993)D169A (provided by Dr. Melanie Cobb, University of Texas, Southwestern Medical Center, Dallas) (18-21). To generate the pCMV-HA-TAO1 vector, an SacI/NheI-digested fragment from pCMV5-HA-TAO1(1-1001) and a synthesized 50 bp deoxyoligonuleotide consisting of a 5’ EcoRI site, an ATG codon, and TAO1 sequences to the first SacI restriction site were subcloned into the pCMV-HA mammalian expression vector. The gene insert was verified by sequencing. To generate the pCMVHA-and pCMV-Flag-TAO2(1-993) and pCMV-HA-and pCMV-Flag-TAO2(1-993)D169A vectors, EcoRI/BamHI-digested fragments from pCMV5-Myc-TAO2(1-993) and pCMV5-Myc-TAO2(1-993)D169A were subcloned into the pCMV-HA and pCMV-Flag mammalian expression vectors. TAO2(1-993) was used instead of full-length TAO2 due to poor expression of the full-length gene (21). Mammalian expression vectors encoding T7-tagged TAK1 (T7-TAK1), T7-tagged TAB1 (T7-TAB1), and HA-tagged IKKα (HA-IKKα) were described previously (22,23).
Antibodies
Polyclonal rabbit antibodies to TAK1 (anti-TAK1) and TAB1 (anti-TAB1) were described previously (8). Polyclonal rabbit antibody against TAO2 (anti-TAO2) was produced against peptides corresponding to amino acids 21-33 (Operon Biotechnologies). Anti-phospho-SAPK/JNK (Thr-183/Tyr-185), anti-phospho-p38 (Thr180/Tyr182), anti-phospho-IκBα (Ser32) and anti-phospho-TAK1 (Thr187) rabbit polyclonal antibodies (Cell Signaling) were used to detect the phosphorylated forms of JNK, p38, IκBα and TAK1. Anti-JNK1 (FL), anti-p38 (N-20), anti-IκBα (C-21), anti-IKKα (H-744), and anti-NF-κB p65 (C-20) polyclonal antibodies (Santa Cruz), anti-HA (HA.11) monoclonal antibody (Covance), anti-HA (Y-11) polyclonal antibody (Santa Cruz), anti-T7 monoclonal antibody (Novagen), anti-Flag M2 monoclonal antibody (Sigma), were used for immunoprecipitation and immunoblotting.
Immunoprecipitation-immunoblotting
Cells were lysed in the 0.5% Triton X-100 lysis buffer described above. Cellular debris was removed by centrifugation at 10,000 g for 5 min at 4°C, and the supernatant was collected. Proteins from the lysates were immunoprecipitated with various antibodies. Immunoprecipitates and whole cell extracts were resolved by SDS-PAGE, and transferred to Hybond-P membranes (Amersham Biosciences). The membranes were immunoblotted with various antibodies, and the bound antibodies were visualized with horseradish peroxidase-conjugated antibodies against rabbit or mouse immunoglobulin G (IgG) using the ECL Western Blotting System (GE Healthcare).
Electrophoretic mobility shift assay (EMSA)
The binding reactions contained radio-labeled [32P]-NF-κB oligonucleotide probe (Promega), 40 μg of cell extracts, 4% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl, 10 mM Tris-HCl [pH 7.5], 500 ng poly (dI-dC) (GE Healthcare) and 10 μg BSA to a final volume of 15 μl. The reaction mixtures were incubated at 25°C for 30 min and separated by 5% (w/v) polyacrylamide gel and visualized by autoradiography.
In Vitro Activation
In vitro activation was performed as described previously (24,25). Briefly, 293 cells were washed twice with 1 x PBS and resuspended in hypotonic buffer (20 mM HEPES-KOH, 10 mM KCl, 1 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT [pH 7.5]) supplemented with protease inhibitors (10 mM NaF, 1 mM PMSF, and 20 μM aprotinin). Resuspended cells were lysed by passing through a 22G needle 10 times. Cellular debris was removed by centrifugation at 10,000 g for 20 min at 4°C followed by filtration at 0.45 μm. Clear cell lysates were collected and incubated at 37°C for the indicated times or left untreated on ice. After incubation, an equivalent volume of hypotonic buffer containing 0.1% NP-40 and 300 mM NaCl was added.
NF-κB Reporter Assay
293 cells (1.6 x 105 cells/well) were seeded onto 6-well (35 mm) dishes 24 hr before transfection. Cells in each well were transfected with 0.1 μ g of an Ig-κ B - luciferase reporter plasmid, 0.1 μg of a control reporter plasmid pAct-β-Gal, (β-galactosidase under the control of the β-actin promoter) and various expression constructs by the calcium phosphate precipitation method. The total amounts of DNA in each well were kept constant by supplementing with empty vector. After 36 hr, cells were treated with or without TNF (20 ng/ml) for 12 hr prior to harvesting using reporter lysis buffer (Promega), and luciferase activity was assessed via luminometry. Transfection efficiencies were normalized by β-galactosidase activities from pAct-β-Gal. The NF-κB luciferase activity was expressed as the mean of the normalized luciferase activity from duplicated experiments.
In Vitro Kinase Assays
TAK1 was immunoprecipitated and the immunoprecipitates were incubated with 5 μCi of [γ-32P]-ATP (3,000 Ci/mmol) and 1 μg of bacterially expressed His-MKK6 in 10 μl of kinase buffer containing 10 mM HEPES (pH 7.4), 1 mM DTT, 5 mM MgCl2 at 25°C for 2 min. IKK was immunoprecipitated, and the immunoprecipitates were incubated with 5 μCi of [γ-32P]-ATP (3,000 Ci/mmol) and 1 μg of bacterially expressed GST-IκB in 10 ml of kinase buffer containing 10 mM HEPES (pH 7.4), 1 mM DTT, 5 mM MgCl2 and 100 μM ATP at 30°C for 30 min. Samples were then separated by 10% SDS-PAGE and visualized by autoradiography.
siRNAs
siRNAs were purchased from Ambion. siRNA used as control was Silencer® Negative Control #1 siRNA. Human TAO2 siRNAs used were pre-designed siRNAs #118287 (TAO2 siRNA#1), and #118285 (TAO2 siRNA#2) (Ambion). 293 cells were plated onto 6-well plates and transfected with siRNAs (30-50 nmol) using siPORT Amine Transfection Agent (Ambion). Cells were incubated for 48 hr post transfection and were stimulated with 0.5 M NaCl. The reduction of TAO2 expression was monitored by real-time PCR using human TAO2 specific primer (Qiagen).
RESULTS
We have previously demonstrated that TAK1 activates JNK, p38 and NF-κB in the TNF and IL-1 signaling pathways (8,9,26). Ablation of TAK1 markedly impairs TNF- and IL-1-induced activation of JNK, p38 as well as NF-κB. TAK1 is activated not only by proinflammatory cytokines but also in response to physical and chemical stresses, including osmotic stress. We found that, among several physical and chemical stresses including oxidative stress, arsenic and ultraviolet light irradiation, osmotic stress is the most potent activator of TAK1 in human embryonic kidney 293 and mouse embryonic fibroblasts (MEFs) (data not shown). To begin to define the role of TAK1 in osmotic stress signaling, we examined activation of TAK1, JNK, p38 and NF-κB following treatment by IL-1 or osmotic stress. 293 IL-1RI cells that stably express the IL-1 receptor were treated with IL-1 or high osmolarity. The activation of TAK1 was monitored by in vitro kinase assay using MKK6 as an exogenous substrate (Fig. 1A). TAK1 was activated by osmotic stress at higher levels relative to activation by IL-1. Activation of TAK1 can be also assessed by the appearance of phosphorylated forms of TAK1 and its binding partner TAK1 binding protein 1 (TAB1), resulting in slower migration on SDS-PAGE (27) (Fig. 1A, second panel). Phosphorylation of TAK1 and TAB1 was also observed upon osmotic stress. JNK and p38 were activated following TAK1 activation at higher levels in cells subjected to osmotic stress compared IL-1 treated cells (Fig. 1A, third and forth panels). Activation of NF-κB was monitored by phosphorylation and degradation of IκBα (Fig. 1A, fifth and bottom panels). In contrast to JNK and p38, only minor phosphorylation and degradation of IκBα were observed in osmotic stress-treated cells. To further confirm this lack of NF-κB activation by osmotic stress, we examined DNA binding of NF-κB by electrophoretic mobility shift assay (EMSA) in 293 IL-1RI and parental 293 cells (Fig. 1B). We could not detect any activation of NF-κ B in cell subjected to osmotic stress (top panel). Under these conditions, IκBα phosphorylation was minor and no IκBα degradation was observed (middle and bottom panels). These results demonstrate that osmotic stress activates JNK and p38 but not NF-κB, even though TAK1 is activated.
Fig. 1.
Osmotic stress activates TAK1 and JNK but not NF-κB.
(A) 293 cells were subjected to 0.7 M sodium chloride or 5 ng/ml IL-1 for 3 or 15 min. Catalytic activity of endogenous TAK1 was measured in vitro kinase assay using MKK6 as an exogenous substrate (top panel). Activation-coupled migration shift of TAK1 and TAB1 is also shown (second panel). Activation of JNK and p38 were monitored by phospho-specific antibodies (third and forth panel). Activation of the NF-κB pathway is monitored by phosphorylation (fifth panel) and degradation (bottom panel) of IκBα. IP, immunoprecipitation; IB, immunoblotting.
(B) 293 IL-1RI and 293 cells were treated with 10 ng/ml IL-1 or 0.5 M sodium chloride for 3 to 30 min. NF-κB activation was monitored by EMSA (top panel). Activation of the NF-κB pathway was also monitored by transient phosphorylation (second panel) and degradation (bottom panel) of IκBα. Asterisk, non-specific binding; IB, immunoblotting.
To define the essential role of TAK1 in osmotic stress signaling, we utilized TAK1Δ/Δ MEFs that express a truncated form of TAK1 lacking kinase activity (Fig. 2A). This cell line was established by infecting a Cre-expressing retroviral vector into MEFs in which both TAK1 alleles are flanked by loxP (9). We examined activation of JNK and p38 following osmotic stress (Fig. 2B). We found that activation of JNK was greatly impaired in these TAK1-deleted cells, while p38 was activated at similar or slightly lower levels. These results suggest that TAK1 plays a major role in the activation of JNK in osmotic stress signaling. To determine whether this role of TAK1 is specific to fibroblasts or if TAK1 functions generally as a mediator of JNK activation in osmotic stress, we used TAK1 Δ/Δ keratinocytes, which were isolated from mice having a skin epidermis-specific deletion of TAK1 (13). Similar to our results with MEFs, we found that osmotic stress could not activate JNK in TAK1-deficient keratinocytes, while p38 was activated at slightly lower levels compared to wild type keratinocytes (Fig. 2C). These results demonstrate that TAK1 is an indispensable intermediate in osmotic stress-induced JNK activation. TAK1 and other signaling intermediates likely function redundantly to activate p38 in osmotic stress signaling.
TAK1 can activate JNK, p38 and NF-κB, when activated by IL-1 and TNF treatment or when overexpressed. However, our results show that activation of TAK1 by osmotic stress does not lead to the activation of NF-κB. This prompted us to speculate that under some conditions TAK1 activity may be modulated so that it does not activate NF-κB. One possibility is that TAK1 forms a complex with a molecule that interferes with the interaction between TAK1 and the IKK-NF-κB pathway. To identify such a putative molecule, we performed a yeast two-hybrid screen with TAK1 as bait as described previously (11,28). Along with TAB1 and TAB2, which are well-characterized TAK1 binding partners, we isolated Thousand-and-One Amino Acid Kinase 1 (TAO1) as a binding partner of TAK1. TAO1 shares substantial homology with a closely related kinase TAO2 (also called prostate-derived STE20-like kinase, PSK1) (18-20). TAO1 and TAO2 are of particular interest because they have been reported to be involved in osmotic stress signaling via activation of the MAPK pathway (20). We conducted a co-immunoprecipitation assay to assess the interaction of TAK1 with TAO1 and TAO2 (Fig. 3A and 3B). Both TAO1 and TAO2 could associate with TAK1. Interaction of TAO2 with TAK1 was independent of its catalytic activity, since a kinase-dead version of TAO2 (TAO2D169A) could also bind to TAK1. Because TAO2 showed a relatively higher affinity to TAK1 compared to TAO1 in several independent experiments, we conducted subsequent experiments using TAO2. We next investigated whether TAO2 and TAK1 could form a complex under more physiological conditions. We raised antibody against TAO2 and attempted to examine the interaction of endogenous TAK1 with TAO2. Although our TAO2 antibody could weakly detect endogenous TAO2 (see Figure 6A), the efficiency was not sufficient to examine the co-precipitation assay. We therefore ectopically expressed TAO2 and examined its interaction with endogenous TAK1. We could not detect any co-precipitation of TAO2 with TAK1 in the presence or absence of osmotic stress when we used a lysis buffer containing 0.5% Triton X, which we usually use for immunoprecipitation and immunoblotting. We anticipated that the TAK1-TAO2 complex is unstable under this lysis condition. It has been reported that some endogenous protein complexes can assemble spontaneously in cell lysates after incubation at 37°C, such as in the case of the apoptosome and caspase 2-containing complex (24,25,29). We performed co-precipitation assay with endogenous TAK1 following a similar incubation step at 37°C. TAO2 was found in the TAK1 complex after incubation for 3 hour (Fig. 3C). The interaction of TAO2 with TAK1 was specific, as no TAO2 was found in the control precipitates. These results suggest that TAO2 interacts with TAK1 in vivo.
Fig. 3.
TAOs interact with TAK1.
(A) 293 cells were transfected with the expression vectors for T7-TAK1 together with HA-TAO1 as indicated. Left panels: TAO1 was immunoprecipitated with anti-HA and co-precipitated TAK1 was detected (top panel). The amount of T7-TAK1 in the whole cell extracts (WCE) is shown in the bottom panel. Right panels: TAK1 was immunoprecipitated with anti-T7 and co-precipitated TAO1 was detected (top panel). The amount of HA-TAO1 in the whole cell extracts (WCE) is shown in the bottom panel. IP, immunoprecipitation; IB, immunoblotting.
(B) 293 cells were transfected with the expression vectors for T7-TAK1 together with either HA-TAO2 or a HA-tagged kinase dead version of TAO2 (HA-TAO2-D169A). Left panels: TAO2 was immunoprecipitated with anti-HA and co-precipitated TAK1 was detected (top panel). The amount of T7-TAK1 in the whole cell extracts (WCE) is shown in the bottom panel. Right panels: TAK1 was immunoprecipitated with anti-T7 and co-precipitated TAO2 was detected (top panel). The amount of HA-TAO2 in the whole cell extracts (WCE) is shown in the bottom panel. IP, immunoprecipitation; IB, immunoblotting.
(C) 293 cells transiently expressing Flag-TAO2 were lysed and incubated for the indicated times at 37°C to allow spontaneous formation of the TAK1-TAO2 complex. Anti-HA was used for a control precipitation. Anti-TAK1 and anti-HA immunoprecipitates were analyzed for the presence of TAO2 by immunoblotting. IP, immunoprecipitation; IB, immunoblotting; WCE, whole cell extracts.
Fig. 6.

Effect of TAO2 siRNA
(A) 293 cells were transfected with control siRNA and TAO2 siRNAs (TAO2 siRNA#1 and #2) using increasing amounts of transfection reagent. TAO2 expression was determined by real-time PCR using human TAO2 primers. Amount of TAO2 mRNA relative to that from control siRNA transfected cells is shown. The TAO2 protein was detected with anti-TAO2. β-catenin was used as a loading control. IB, immunoblotting.
(B) 293 cells were transfected with control siRNA and TAO2 siRNAs (#1, left panels, #2, right panels) using 10 μl of transfection reagent. Cells were subjected to osmotic stress (0.5 M NaCl) and cell extracts were immunoblotted with anti-phospho JNK (top panel) and anti-phospho TAK1 (third panel). The amounts of endogenous JNK and TAK1 are shown in the second and bottom panels, respectively. Signal intensity of phospho-JNK was quantitated and normalized to the levels of total JNK amounts. Relative activation levels of JNK are shown (fold). Representative experiments of four are shown. IB, immunoblotting.
(4) 293 cells were transfected with control siRNA and TAO2 siRNAs. Cells were subjected to osmotic stress (0.5 M NaCl) and cell extracts were subjected to monitor NF-κB activation by EMSA. The amount of p65 NF-κB subunit is shown as a loading control. A representative result of three is shown.
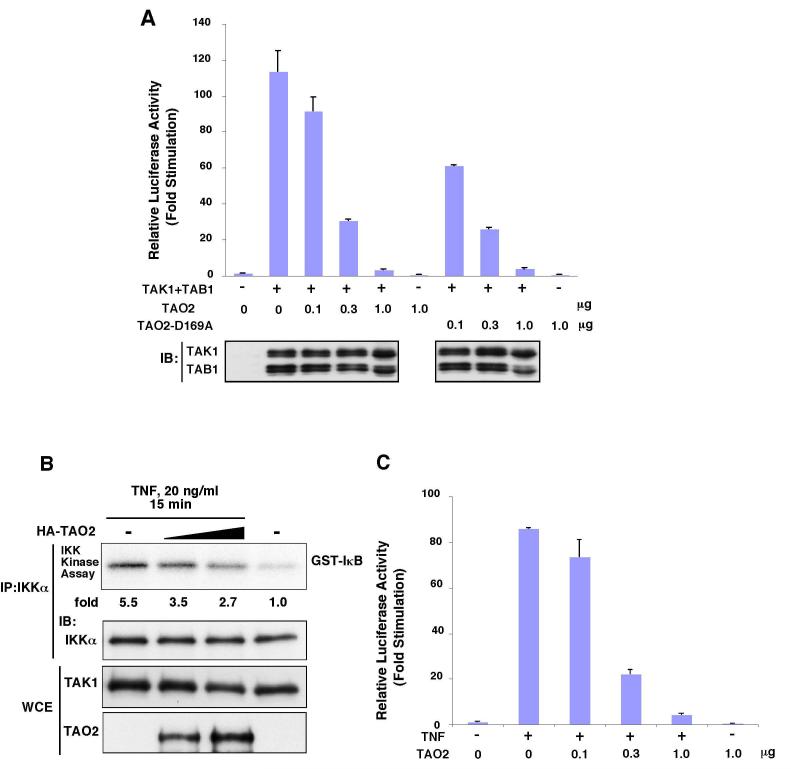
It has been demonstrated that TAO2 can activate JNK and p38 (19,20,30). However, the role of TAO2 in the NF-κB pathway has not been examined. We next tested whether TAO2 influences TAK1-mediated NF-κB activation. TAK1 is highly active when ectopically expressed in culture cells together with its activator subunit TAB1, and can activate JNK, p38 and NF-κB (8,27,28,31). We therefore examined NF-κB-dependent reporter activity in 293 cells coexpressing TAO2 with TAK1 and TAB1. TAO2 alone was unable to activate NF-κB. Furthermore, activation of NF-κB by ectopic expression of activated TAK1 was markedly reduced by coexpression of TAO2 (Fig. 4A). To assess whether TAO2 kinase activity is required for its effect on TAK1, we used a kinase-dead mutant of TAO2. We found that TAO2 could inhibit TAK1-mediated activation of NF-κB independent of its kinase activity. We next tested whether TAO2 could inhibit the TNF-induced NF-κB pathway by examining TNF activation of IKK and NF-κB-dependent transcription (Fig. 4B and C). We found that ectopic expression of TAO2 inhibited activation of IKK and subsequent transcription. These results suggest that TAO2 associates with TAK1 and prevents activation of the TAK1-mediated NF-κB pathway. To determine whether the action of TAO2 on TAK1 is specific to the TAK1-mediated NF-κ B pathway, we next examined JNK activation. We coexpressed TAO2 with an activated form of TAK1 (TAK1+TAB1) and monitored activation of JNK (Fig. 4D). Consistent with the earlier study, we found that TAO2 alone could activate JNK (30). Furthermore, TAO2 enhanced TAK1-mediated activation of JNK. These results suggest that the TAK1-TAO2 complex can activate JNK, but that binding by TAO2 blocks the TAK1-mediated NF-κ B pathway.
Fig. 4.

TAO2 inhibits TAK1-mediated activation of NF-κB pathway.
(A) 293 cells were transfected with NF-κB-dependent luciferase reporter together with expression vector for HA-TAO2 or kinase dead version of TAO2 (HA-TAO2-D169A) and TAK1+TAB1 (active TAK1). An internal control reporter (pAct-β-Gal) was used to normalize the transfection efficiency. Expression of TAK1 and TAB1 was monitored by immunoblotting (bottom panel). IB, immunoblotting.
(B) 293 cells were transfected with increasing amounts of HA-TAO2 or with 5 μg empty vector. Cells were either left untreated or treated with 20 ng/ml TNF for 15 min. IKKα was immunoprecipitated from the lysates with anti-IKKα and the immunoprecipitates were subjected to in vitro kinase assay using GST-IκBα as substrate (top panel). The amount of IKKα in the immunoprecipitates is shown in the middle panel. The amount of TAK1 and TAO2 in the whole cell extracts (WCE) is shown in the bottom panel. IKK activity was quantitated and normalized to the levels of IKKα amounts. Relative activation levels of IKK are shown (fold). IP, immunoprecipitation; IB, immunoblotting.
(C) 293 cells were transfected with a NF-κB-dependent luciferase reporter together with an expression vector for HA-TAO2. Cells were either left untreated or treated with 20 ng/ml TNF. An internal control reporter (pAct-β-Gal) was used to normalize the transfection efficiency. IB, immunoblotting.
(D) 293 cells were transfected with expression vectors for HA-TAO2 and T7-TAK1+ T7-TAB1 (active TAK1). Activated JNK was detected with anti-phospho-specific JNK (P-JNK) (top panel). The amounts of JNK, T7-TAK1+T7-TAB1, and HA-TAO2 in the whole cell extracts are shown in the second, third, and fourth panels, respectively. Signal intensity of phospho-JNK was quantitated and normalized to the levels of total JNK amounts. Relative activation levels of JNK are shown (fold). IB, immunoblotting.
To determine the mechanism by which TAO2 interferes with the TAK1-NF-κB pathway, we asked whether TAK1 activity is altered when TAO2 is coexpressed (Fig. 5A). We found that TAK1 activity was not changed by coexpression of TAO2, suggesting that association of TAO2 does not directly alter the activity of TAK1. This is consistent with results showing that TAO2 did not inhibit the TAK1-mediated JNK activation described above (Fig. 4D). We have previously demonstrated that TAK1 forms a complex with the IKKs (22), and that this is important for TAK1-mediated activation of the IKK-NF-κB pathway. We speculated therefore that TAO2 might affect the interaction of TAK1 with IKK. We transfected expression vectors for TAK1, IKK and increasing amounts of TAO2 and conducted coprecipitation assays in 293 cells to examine the relative amounts of TAK1 present in complexes with IKK (Fig. 5B). We found that increasing TAO2 blocked the formation of the TAK1-IKK complex. To further confirm the effect of TAO2 under more physiological condition, we examined endogenous interaction of TAK1 with IKK in the presence and absence of ectopic expression of TAO2 (Fig. 5C). Because interaction of endogenous TAK1 with IKK is unstable, we conducted co-precipitation assay using the 37°C incubation step as described above. We found that TAO2 blocked interaction of IKK with TAK1. These results collectively suggest that TAK1, when associated with TAO2, is sequestered away from the IKK complex, thereby activating the JNK, but not the NF-κB pathway.
Fig. 5.
TAO2 does not inhibit the catalytic activity of TAK1 but blocks the interaction of TAK1 with IKK.
(A) 293 cells were transiently transfected with fixed amounts of expression vectors for T7-TAK1 (1 μg) and T7-TAB1 (1 μg) and increasing amounts of HA-TAO2 as indicated. TAK1 was immunoprecipitated from the lysates with anti-TAK1 and the immunoprecipitates were subjected to in vitro kinase assay using His-MKK6 as substrate (top panel). The amount of T7-TAK1 in the immunoprecipitates is shown in the middle panel. The amount of HA-TAO2 in the whole cell extracts (WCE) is shown in the bottom panel. TAK1 activity was quantitated and normalized to the levels of TAK1 amounts. Relative activation levels of TAK1 are shown (fold). IP, immunoprecipitation; IB, immunoblotting.
(B) 293 cells were transiently transfected with fixed amounts of expression vectors encoding T7-TAK1 (0.5 μ g) and HA-IKKα (0.1 μ g) and increasing amounts of Flag-TAO2. HA-IKKα was immunoprecipitated from the lysates with anti-HA (second panel), and coprecipitated T7-TAK1 was detected with anti-T7 (top panel). The amounts of T7-TAK1 and Flag-TAO2 in the whole cell extracts (WCE) are shown in the third and fourth panels, respectively. IP, immunoprecipitation; IB, immunoblotting.
(C) 293 cells were transfected with an empty vector or an expression vector for HA-TAO2. Cells were lysed and incubated for the indicated times at 37°C to allow spontaneous formation of the TAK1-IKK or TAK1-HA-TAO2 complexes. Endogenous TAK1 was immunoprecipitated, and the precipitates were analyzed for the presence of endogenous IKKα or HA-TAO2 by immunoblotting. IP, immunoprecipitation; IB, immunoblotting; WCE, whole cell extracts.
We finally assessed whether TAO2 plays essential role in osmotic stress signaling by using TAO2 siRNAs. Two siRNAs showed ability to reduce expression of TAO2 about 60% (Fig. 6A). We examined the JNK and NF-κB activation upon osmotic stress in the TAO2 knockdown cells using these siRNAs (Fig. 6B and C). Two independent TAO2 siRNAs reduced osmotic stress-induced JNK activation, which suggest that TAO2 plays an at least partially essential role in osmotic stress-JNK pathway. Activation of TAK1 was determined by detecting the essential phosphorylation of its activity (16). We found that osmotic stress-induced activation of TAK1 was not altered by TAO2 knockdown (Fig. 6B, left side bottom panel). These results suggest that TAO2 is not an upstream kinase of TAK1 in osmotic stress signaling pathway but rather functions in parallel with TAK1 to lead JNK activation. NF-κB was slightly activated upon osmotic stress in TAO2 knockdown cells (Fig. 6C). These results further support our conclusion that TAO2 facilitates TAK1-JNK pathway but blocks TAK1-NF-κB pathway. However, the induction of NF-κB was marginal in TAO2 knockdown cells. This suggests that NF-κB activation might be blocked by not only TAO2 but also through other mechanisms.
DISCUSSION
TAK1 can be activated by diverse stimuli including the proinflammatory cytokines TNF, IL-1, Toll-like receptor ligands, and physical and chemical stresses. The role of TAK1 in each of these signaling pathways has been investigated using cells from genetically engineered TAK1-deficient mice, as well as with siRNA gene knockdown methods. TAK1 has been shown to be an indispensable intermediate of TNF, IL-1 and Toll-like receptor signaling in mammalian cells (9,10,13,26). In Drosophila, TAK1 is essential for activation of MAPK in response to innate immune stimuli, and plays a redundant role together with MEKK and MLK in stress signaling pathways (32). We have demonstrated here that TAK1 is an essential intermediate in osmotic stress induction of JNK in mammalian cells. We also showed that TAK1 is not an exclusive mediator of osmotic stress-induced p38 activation. TAK1 and other MAPKKK family members such as MEKK1 and MLK are likely to function redundantly in the p38pathway in response to osmotic stress in mammalian cells.
TAK1 is activated in response to many extracellular stimuli, yet TAK1 can initiate cellular responses that are unique to each stimulus. This suggests that TAK1 may be modified by some mechanism to selectively activate different downstream pathways. We have demonstrated here that TAO2 can interact with TAK1 and is responsible for the selective activation of JNK versus the NF-κB pathway. TAO2 is activated by osmotic stresses (19,20), therefore the TAK1-TAO2 complex may function to mediate osmotic stress signaling. Previously, Mochida et al. reported that the MAPKKK apoptosis stimulating kinase 1 (ASK1) interacts with and inhibits TAK1 by blocking the interaction of TAK1 with tumor necrosis factor receptor-associated factor 6 in the IL-1 signaling pathway (33). ASK1 is activated by oxidative stress, and participates in stress-induced apoptosis (34,35). Therefore, it may be possible that the TAK1-ASK1 complex mediates oxidative stress signaling that leads to apoptosis by activating JNK while inhibiting NF-κB.
In this study, we showed TAO2 knockdown only marginally induce NF-κB activation upon osmotic stress. This suggests that TAO2 is not solely responsible for blocking the NF-κB pathway under osmotic stress condition. TAO2 related kinase TAO1 may play redundantly to sequester TAK1 from IKK complex. In addition, to securely block NF-κB activation upon osmotic stress, it is likely that the NF-κB pathway is negatively regulated through several other mechanisms.
NF-κB is a major activator of cell survival signaling (36,37). In contrast, activation of JNK and p38 has been generally correlated with apoptotic as well as necrotic cell death (5-7,38,39). Proinflammatory cytokines such as TNF activate both NF-κB and JNK pathways through TAK1. Activation of both pathways is important for gene expression involving inflammatory responses. In inflammatory signaling, cell death is not a desired outcome. Therefore, it is believed that the proinflammatory cytokine-induced NF-κB pathway functions not only to activate inflammatory genes but also aid to cell survival by masking the pro-apoptotic actions of JNK. In contrast, cell death may be a desired outcome in response to excessive physical and chemical stress, and in these cases NF-κB is not activated and JNK is able to induce cell death. Such cell death is important to eliminate damaged and dysfunctioning cells from the body. Thus, TAK1 regulation in osmotic stress signaling, which activates only JNK while blocking the NF-κB pathway, is important for osmotic stress-induced cell death.
Glossary
The abbreviations used are
- IL-1
interleukin 1
- TNF
tumor necrosis factor
- TLR
Toll-like receptor
- TAK1
TGF-β activated kinase 1
- TAB1
TAK1-binding protein 1
- JNK
c-Jun N-terminal kinase
- NF-κB
nuclear factor-κB
- IκB
inhibitor of κB
- IKK
IκB kinase
- TAO1
thousand and one-amino acid protein kinase 1
- TAO2
thousand and one-amino acid protein kinase 2
- ASK1
apoptosis signal-regulating kinase 1
- MLK
mixed lineage kinase
- MEF
mouse embryonic fibroblast
- EMSA
electrophoretic mobility shift assay.
Footnotes
We thank Dr. Melanie Cobb for materials. This work was supported by special grants for SORST and Advanced Research on Cancer from the Ministry of Education, Culture and Science of Japan (KM), and by NIH grants GM068812 and AR050972 (JNT).
REFERENCES
- 1.Johnson GL, Lapadat R. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 2.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 3.Davis RJ. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 4.Sheikh-Hamad D, Gustin MC. Am J Physiol Renal Physiol. 2004;287:F1102–1110. doi: 10.1152/ajprenal.00225.2004. [DOI] [PubMed] [Google Scholar]
- 5.Varfolomeev EE, Ashkenazi A. Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- 6.Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K. Cell Death Differ. 2005 doi: 10.1038/sj.cdd.4401830. [DOI] [PubMed] [Google Scholar]
- 7.Wada T, Penninger JM. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 8.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 9.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Nat. Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 10.Shim J-H, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee K-Y, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, Matsumoto K. Mol. Cell. 2000;5:649–658. doi: 10.1016/s1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
- 12.Takaesu G, Ninomiya-Tsuji J, Kishida S, Li X, Stark GR, Matsumoto K. Mol. Cell. Biol. 2001;21:2475–2484. doi: 10.1128/MCB.21.7.2475-2484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC, Ninomiya-Tsuji J. J. Biol. Chem. 2006;281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirakabe K, Yamaguchi K, Shibuya H, Irie K, Matsuda S, Moriguchi T, Gotoh Y, Matsumoto K, Nishida E. J. Biol. Chem. 1997;272:8141–8144. doi: 10.1074/jbc.272.13.8141. [DOI] [PubMed] [Google Scholar]
- 15.Cheung PC, Campbell DG, Nebreda AR, Cohen P. EMBO J. 2003;22:5793–5805. doi: 10.1093/emboj/cdg552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H. J. Biol. Chem. 2005;280:7359–7368. doi: 10.1074/jbc.M407537200. [DOI] [PubMed] [Google Scholar]
- 17.Cao Z, Henzel WJ, Gao X. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 18.Hutchison M, Berman KS, Cobb MH. J. Biol. Chem. 1998;273:28625–28632. doi: 10.1074/jbc.273.44.28625. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Hutchison M, Cobb MH. J. Biol. Chem. 1999;274:28803–28807. doi: 10.1074/jbc.274.40.28803. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Cobb MH. J. Biol. Chem. 2001;276:16070–16075. doi: 10.1074/jbc.M100681200. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Raman M, Chen L, Lee SF, Gilman AG, Cobb MH. J. Biol. Chem. 2003;278:22278–22283. doi: 10.1074/jbc.M301173200. [DOI] [PubMed] [Google Scholar]
- 22.Kishida S, Sanjo H, Akira S, Matsumoto K, Ninomiya-Tsuji J. Genes Cells. 2005;10:447–454. doi: 10.1111/j.1365-2443.2005.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uemura N, Kajino T, Sanjo H, Sato S, Akira S, Matsumoto K, Ninomiya-Tsuji J. J. Biol. Chem. 2006 doi: 10.1074/jbc.M509834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tinel A, Tschopp J. Science. 2004;304:843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- 25.Janssens S, Tinel A, Lippens S, Tschopp J. Cell. 2005;123:1079–1092. doi: 10.1016/j.cell.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 26.Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB. J. Mol. Biol. 2003;326:105–115. doi: 10.1016/s0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- 27.Kishimoto K, Matsumoto K, Ninomiya-Tsuji J. J. Biol. Chem. 2000;275:7359–7364. doi: 10.1074/jbc.275.10.7359. [DOI] [PubMed] [Google Scholar]
- 28.Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- 29.Read SH, Baliga BC, Ekert PG, Vaux DL, Kumar S. J. Cell Biol. 2002;159:739–745. doi: 10.1083/jcb.200209004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore TM, Garg R, Johnson C, Coptcoat MJ, Ridley AJ, Morris JDH. J. Biol. Chem. 2000;275:4311–4322. doi: 10.1074/jbc.275.6.4311. [DOI] [PubMed] [Google Scholar]
- 31.Ono K, Ohtomo T, Sato S, Sugamata Y, Suzuki M, Hisamoto N, Ninomiya-Tsuji J, Tsuchiya M, Matsumoto K. J. Biol. Chem. 2001;276:24396–24400. doi: 10.1074/jbc.M102631200. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, White MA, Cobb MH. J. Biol. Chem. 2002;277:49105–49110. doi: 10.1074/jbc.M204934200. [DOI] [PubMed] [Google Scholar]
- 33.Mochida Y, Takeda K, Saitoh M, Nishitoh H, Amagasa T, Ninomiya-Tsuji J, Matsumoto K, Ichijo H. J. Biol. Chem. 2000;275:32747–32752. doi: 10.1074/jbc.M003042200. [DOI] [PubMed] [Google Scholar]
- 34.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 35.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karin M, Lin A. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 37.Hayden MS, Ghosh S. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 38.Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr., Davis RJ. Genes Dev. 2004;18:2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]