Abstract
TAK1 is a serine/threonine kinase that is a mitogen-activated protein kinase kinase kinase (MAPKKK), and an essential intracellular signaling component in inflammatory signaling pathways. Upon stimulation of cells with inflammatory cytokines, TAK1 binds proteins that stimulate autophosphorylation within its activation loop, and is thereby catalytically activated. This activation is transient; it peaks within a couple of minutes and is subsequently down-regulated rapidly to basal levels. The mechanism of down-regulation of TAK1 has not yet been elucidated. In this study, we found that toxin inhibition of type 2A protein phosphatases greatly enhances IL-1-dependent phosphorylation of Thr-187 in the TAK1 activation loop, as well as the catalytic activity of TAK1. From proteomic analysis of TAK1 binding proteins, we identified protein phosphatase 6 (PP6), a type-2A phosphatase, and demonstrated that PP6 associated with and inactivated TAK1 by dephosphorylation of Thr-187. Ectopic and endogenous PP6 co-precipitated with TAK1 and expression of PP6 reduced IL-1 activation of TAK1 but did not affect osmotic activation of MLK3, another MAPKKK. Reduction of PP6 expression by small interfering RNA enhances IL-1-induced phosphorylation of Thr-187 in TAK1. Enhancement occurred without change in levels of PP2A showing specificity for PP6. Our results demonstrate that PP6 specifically down-regulates TAK1 through dephosphorylation of Thr-187 in the activation loop, which is likely important for suppressing inflammatory responses via TAK1 signaling pathways.
TAK1 (transforming growth factor β (TGF-β) activated kinase 1) is a member of the mitogen-activated protein kinase kinase kinases (MAPKKK) family, and is activated not only by TGF-βbut also by proinflammatory cytokines including interleukin-1 (IL-1) and tumor necrosis factor (TNF) (1-3). Genetic studies using TAK1-deficient cells have demonstrated that TAK1 is an indispensable signaling intermediate in TNF and IL-1 signaling pathways (4-6). In the proinflammatory signaling pathways, TAK1 is activated through ligand-dependent assembly of a TAK1 signaling complex containing TNF receptor associated factor (TRAF), TAK1 binding partners, TAK1 binding protein 1, 2 and 3 (TAB1, TAB2 and TAB3) (2,4-13). TAK1, in turn, stimulates two downstream pathways; one is the MAPK cascades to activate c-Jun N-terminal kinase (JNK) and p38 MAPK; and the other is the IκB kinase (IKK) pathway ultimately leading to NF-κB activation(2,4,5,14).
Many kinases are phosphorylated in the kinase activation loop located between the conserved sequence DFG of kinase subdomain VII and APE of kinase subdomain VIII (15). The activation loop is important for substrate recognition, and phosphorylation in this segment is required to allow correct alignment of the substrates to the catalytic site. In many cases, phosphorylation within the activation loop is mediated by upstream kinases in the kinase cascades (16). However, in some kinases, autophosphorylation occurs in this segment following stimuli-dependent conformational change (17-20). The kinase activation loop of TAK1 contains phosphorylation sites at Thr-184, Thr-187 and Ser-192. Unphosphorylatable amino acid substitutions of any of these residues abolish the catalytic activity of TAK1 (8,21). Proinflammatory cytokines increase phosphorylation of TAK1 within the activation loop (8,21). Catalytic activity of TAK1 is required for this phosphorylation, suggesting that TAK1 autophosphorylates its activation loop. Upon cytokine stimulation, TAK1 autophosphorylation is induced presumably through the conformational change due to assembly of the signaling complex, which converts TAK1 into a catalytically active form. Among the phosphorylation sites in the TAK1 activation loop, it has so far been established that phosphorylation at Thr-187 correlates with activation of TAK1 (22).
TAK1 is activated in a transient manner (23). IL-1 activates TAK1 within 1-2 min, and the activation peaks at 3-5 min and declines to the basal levels within 15-30 min after stimulation. Although TAK1 activation has been determined to some extent as described above, the mechanism by which TAK1 is down-regulated remains largely unknown. In general, the level of protein phosphorylation is controlled by the balanced activities of protein kinases and protein phosphatases. Indeed, TAK1 activity is known to be regulated by protein phosphatase PP2C family members in the unstimulated state (24,25). In this study, we found that inhibition of type 2A protein phosphatases results in hyperphosphorylation and hyperactivation of TAK1 in response to IL-1 stimulation. Protein Ser/Thr phosphatase (PPP) family comprises the type 1 and type 2A phosphatases, and these are the major protein phosphatases that play an important role in the regulation of cell growth and a diverse set of cellular proteins, including metabolic enzymes, ion channels, hormone receptors, and kinase cascades (26). Protein phosphatase 4 (PP4) and protein phosphatase 6 (PP6) have been identified as novel phosphatases and have been classified as type 2A phosphatase family members based on their sequence homology (27-29). However, relative to PP2A, much less is known about the functions of PP4 and PP6. Recently, PP6 has been implicated in opposing NF-κB activation by control of IκBε degradation (30). We here found that TAK1 associates with PP6, and that PP6 dephosphorylates and inactivates TAK1. We also show that reduction of PP6 expression increases phosphorylation of IL-1-induced TAK1. Our results suggest that PP6 is a negative regulator of TAK1.
EXPERIMENTAL PROCEDURES
Chemicals, plasmids and antibodies
Tautomysin, Okadaic Acid, Cyclosporin A, and Calculin A were purchased from Calbiochem. Recombinant human IL-1β was purchased from Roche Applied Science. The mammalian expression vectors for HA-tagged TAK1 (HA-TAK1), FLAG-tagged TAK1 (FLAG-TAK1), and TAB1 have been described previously (2,10,23). A catalytically inactive version of PP6, PP6-D84N was prepared by Quick Change (Stratagene) according to manufacturer’s instruction. Anti-phospho-TAK1 (Thr-187) antibody (Cell Signaling), anti-TAK1 antibody (2), anti-HA monoclonal antibody 16B12 (Covance), anti-FLAG monoclonal antibody M2 (Sigma), anti-mixed-lineage protein kinase 3 (MLK3), anti-phospho-MLK3 (Thr-277/Ser-281) (Cell Signaling), anti-PP2A (Santa Cruz), anti-PP6 antibody (Sigma) and anti-β -catenin (BD Biosciences) were used.
Cell cultures
293 cells, 293-IL-1RI cells were cultured in DMEM plus 10% bovine growth serum (BGS, HyClone) or fetal bovine serum. Transfection of 293 cells was carried outaccording to the calcium phosphate precipitation method.
siRNAs
siRNAs targeted against human PP6 and purchased from Dharmacon Inc. Two siRNA against the sequence, PP6-1 (47GCA AGT ACC TGC CAG AGA A65) and PP6-2 (893GAA CGA CAA CGC CAT ATT T911) were used. A pool of three siRNAs for human PP2A was purchased from B-Bridge, (target sequences, 333CAC CAT TCT TCG AGG GAA T351, 663GCA AGA TAT TTC TGA GAC A681, and 3’ untranslated region GGA AAT GGG AAG AGC AAC A). Control siRNA against unrelated nucleotide sequence was purchased from Ambion (Silencer negative Control 1 siRNA). The siRNA duplexes were transfected into 293 IL-1R cells using oligofectamine reagent (Invitrogen). Cells were incubated in 30% fetal bovine serum for 48h post transfection and then stimulated with IL-1.
Immunoprecipitation and immunoblotting
Whole cell extracts were prepared in lysis buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, 1 mM PMSF, 20 μ M aprotinin, 0.5 % Triton X-100). Proteins from these cell lysates were immunoprecipitated with 1 μ g of various antibodies and 15 μl of protein G-Sepharose (GE Heathcare). The immune complexes were washed three times with wash buffer containing 20 mM HEPES (pH 7.4), 500 mM NaCl and 10 mM MgCl2 and once with rinse buffer containing 20 mM HEPES (pH 7.4), 150 mM NaCl and 10 mM MgCl2 and suspend in 30 μl of rinse buffer. For immunoblotting, the immunoprecipitates or cell lysates were resolved on SDS-PAGE and transferred to Hybond-P membranes (GE Heathcare). The membranes were immunoblotted with various antibodies, and bound antibodies were visualized with horseradish peroxidase-conjugated antibodies against rabbit, or mouse IgG using the ECL Western blotting system (GE Heathcare) or SuperSignal West Femto Sensitivity Substrate (PIERCE).
In vitro kinase assay
Immunoprecipitates were incubated with 1 μg of bacterially expressed MKK6 in 10 μl of kinase buffer containing 10 mM HEPES (pH 7.4), 1 mM dithiothreitol, 5 mM MgCl2, and 5 μCi of [γ-32P] ATP (3,000 Ci/mmol) at 25 oC for 2 min. Samples were fractionated by 10 % SDS-PAGE and visualized by autoradiography.
In vitro dephosphorylation assay
Purified PP2A was purchased from Millipore/Upstate. HA-TAK1 was activated by co-expression of TAB1 in 293 cells and was immunoprecipitaed with anti-HA, incubated with the purified PP2A for 30 min at 30 oC.
RESULTS
Inhibition of type 2A Phosphatase activity increases IL-1-induced phosphorylation of TAK1
TAK1 is activated through its autophosphorylation within the activation loop induced by binding of proteins such as TAB1, TAB2 and TRAFs, and is rapidly down-regulated (8,22,23). The mechanism by which TAK1 is down-regulated has not yet been elucidated, but it is likely to involve protein phosphatases. Indeed, both TAK1 phosphorylation and activation are regulated by PP2C family (a.k.a. MPP) phosphatases, which participate in silencing TAK1 basal activity in the unstimulated state (24,25). To further address which protein phosphatases reverse stimuli-induced TAK1 activation, we examined the effects of protein phosphatase inhibitors on IL-1-induced TAK1 phosphorylation. The 293 IL-1RI cell line, which stably expresses IL-1 receptor, was stimulated by IL-1 in the presence or absence of different phosphatase inhibitors: 1) tautomycin, an inhibitor of PP1; 2) okadaic acid (OA), an inhibitor of the PP2A family; 3) cyclosporin A, an inhibitor of PP2B; or 4) calyculin A, an inhibitor of both PP1 and PP2A families (Fig. 1). Although OA can inhibit both PP1 and PP2A at high concentrations, OA at the concentration of 100 nM was used for relatively selective inhibition of the PP2A family (31). Upon IL-1 treatment TAK1 was autophosphorylated and it migrated slower than unstimulated TAK1 on SDS-PAGE (Fig. 1, upper panel) as described previously (8). Phosphorylation of TAK1 in the activation loop was monitored by immunoblotting with a phosphospecific antibody that recognized phosphorylated TAK1 at Thr-187 (anti-P-Thr-187) (Fig. 1, lower panel). IL-1-induced phosphorylation at Thr-187 was barely detected without phosphatase inhibitor, but either OA or calyculin A greatly enhanced the IL-1-dependent phosphorylation at Thr-187 and showed a large mobility shift of TAK1 on SDS-PAGE. In contrast, neither tautomycin nor cyclosporine A enhanced the TAK1 phosphorylation at Thr-187 or altered mobility in SDS-PAGE. These results suggest that the inhibition of PP2A family enhances the phosphorylation of TAK1 at Thr-187. To assess the role of PP2A family members on TAK1, we used 100 nM OA for the subsequent experiments.
Fig. 1.
Effect of protein phosphatase inhibitors on TAK1 phosphorylation.
Cultures of 293 IL-1RI cells were treated with 1 μM tautomycin (Tm), 100 nM Okadaic acid (OA), or 1 μM cyclosporin A (CysA) for 6 h, or 20 nM calyculin A (CalA) for 40 min, or the vehicle, DMSO or ethanol (EtOH) for 6 h followed by treatment with IL-1 (5 ng/ml) for 5 min. Cell lysates were subjected to immunoblotting (IB) with anti-TAK1 and anti-P-Thr-187.
Inhibition of PP2A family increases IL-1-induced activation of TAK1
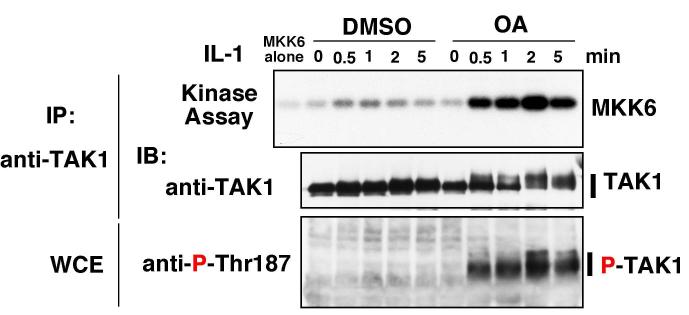
We examined the kinetics of TAK1 activation in the absence and presence of OA (Fig. 2). Catalytic activity of TAK1 was measured using MKK6 as a specific substrate. IL-1 addition to cells stimulated TAK1 activity at 0.5 and 1.0 min, and the activity declined rapidly thereafter returning to basal by 5 min. Addition of OA significantly enhanced IL-1-dependent activation of TAK1. The peak levels of TAK1 catalytic activity were greatly increased, and the activity peaked at 2 min and was sustained longer (> 5 min) compare to that in OA-untreated cells. The phosphorylation of TAK1 at Thr-187 enhanced by OA was correlated with the kinetics of OA-induced TAK1 activation. These data demonstrate that inhibition of PP2A family phosphatases by OA enhances IL-1-dependent phosphorylation and activation of TAK1.
Fig. 2.
Effect of OA on IL-1-induced activation of TAK1
293 IL-RI cells were pretreated with 100 nM Okadaic acid or vehicle (DMSO) for 5 h followed by addition of IL-1 (5 ng/ml). Proteins from the cell lysates were immunoprecipitated with anti-TAK1, and subjected to in vitro kinase assay using MKK6 as an exogenous substrate (top panel) and immunoblotting with anti-TAK1 (middle panel). The phosphorylated TAK1 was detected with anti-P-Thr-187 (bottom panel). IP, immunoprecipitation; IB, immunoblotting; WCE, whole cell extracts.
PP2A can dephosphorylate and inactivate TAK1 in vitro
Upon cell stimulation, TAK1 binds to the TRAF6-containing complex and autophosphorylates Thr-187 in the activation loop, thereby activating its catalytic activity (8). Therefore, we speculate that a PP2A family phosphatase may down-regulate TAK1 by dephosphorylating Thr-187 in the TAK1 activation loop. We examined whether PP2A could directly dephosphorylate Thr-187 and inactivate TAK1. We isolated an active form of TAK1 from cells co-expressing TAK1 together with its activator subunit TAB1. Ectopically expressed TAB1 induces autophosphorylation of TAK1 at the activation loop as does treatment of cells with IL-1 (8). The activated TAK1 was immunoprecipitated and incubated with purified PP2A (Fig. 3). PP2A dephosphorylated Thr-187 in the TAK1 activation loop and inactivated TAK1. Collectively, these results suggest that a PP2A family phosphatase negatively regulates TAK1 by dephosphorylating Thr-187 in the activation loop of TAK1.
Fig. 3.
PP2A dephosphorylates and inactivates TAK1.
HA-TAK1 and TAB1 were co-expressed in 293 cells and precipitated with anti-HA. The immunoprecipitate first was incubated with 0.02 or 0.2 U purified PP2A (dimer of AC subunits) and subjected to in vitro kinase assay using MKK6 as an exogenous substrate (top panel). Total TAK1 was detected with anti-TAK1 (middle panel) and phosphorylated TAK1 was detected with anti-P-Thr-187 (bottom panel). IB, immunoblotting.
PP6 interacts with TAK1
To identify the TAK1 endogenous phosphatase, we immunoprecipitated FLAG-tagged TAK1 from transiently transfected cells. The immunocomplex was digested and the peptides were analyzed by using a nano-scale liquid chromatography system with collision-induced dissociation tandem mass spectrometry (32). Several peptides were found to correspond to TAB1. One peptide yielded the sequence YGNANAWRYCTK and it corresponded to protein phosphatase 6 (PP6) catalytic subunit. To confirm the interaction of TAK1 with PP6, we conducted the immunoprecipitation assay (Fig. 4A). FLAG-TAK1 and HA-tagged PP6 catalytic subunit (HA-PP6) were co-expressed in 293 cells. FLAG-TAK1 coprecipitated with HA-PP6 and the interaction was confirmed by the reciprocal anti-HA immunoprecipitation. We asked whether stimulation of TAK1 and the catalytic activity of PP6 affect the interaction. We coexpressed FLAG-TAK1 with HA-PP6 wild type and a catalytically inactive form of PP6 (PP6-D84N) in 293 IL-1RI cells, and treated cells with IL-1 (Fig. 4B). TAK1 associated with PP6 independent of catalytic activity of PP6. The interaction was not altered by IL-1 stimulation. These results suggest that PP6 is a constitutive binding partner of TAK1 with or without IL-1 stimulation.
Fig. 4.
Association of TAK1 with PP6
(A) 293 cells were transfected with expression vectors for FLAG-TAK1 and HA-PP6. Proteins from the cell lysates were immunoprecipitated with anti-HA or with anti-FLAG, and immunoblotted with anti-FLAG and anti-HA. IP, immunoprecipitation; IB, immunoblotting; WCE, whole cell extracts.
(B) 293 IL-1RI cells were transfected with expression vectors for FLAG-TAK1, HA-PP6 wild type (WT) or a catalytically inactive version of HA-PP6 D84N (DN). At 36 h post transfection, cells were treated with 5 ng/ml IL-1. Proteins from the cell lysates were immunoprecipitated with anti-HA, and immunoblotted with anti-FLAG and anti-HA. IP, immunoprecipitation; IB, immunoblotting; WCE, whole cell extracts.
(C) 293 IL-1RI cells were treated with or without 5 ng/ml IL1 for 1 min. Endogenous proteins were immunoprecipitated with anti-TAK1 (TAK1) or with control IgG (C), and co-precipitated PP6 was detected with anti-PP6. IP, immunoprecipitation; IB, immunoblotting; WCE, whole cell extracts.
To verify the physiological TAK1-PP6 interaction, we examined whether endogenous TAK1 associated with PP6. Cell extracts from cells treated with or without IL-1 were prepared and endogenous TAK1 was immunoprecipitated (Fig. 4C). PP6 was co-precipitated with TAK1 with and without IL-1 stimulation of cells. This indicated that endogenous PP6 interacts with TAK1.
PP6 dephosphorylates and inactivates TAK1
We examined whether PP6 can dephosphorylate TAK1. PP6 wild type or catalytically inactive form of PP6 was overexpressed in 293 IL-1RI cells, and cells were stimulated with IL-1. The phosphorylation of TAK1 was monitored by anti-P-Thr-187 antibody (Fig. 5A). We found that increased expression of PP6 reduced IL-1-dependent phosphorylation at Thr-187 of TAK1. To determine whether PP6 action is specific to TAK1 among the family members of MAPKKKs, we examined the effect of PP6 overexpression on osmotic stress-induced MLK3 activation. MLK3 is phosphorylated and activated upon osmotic stress. 293 cells were treated with 0.5 M sorbitol and the phosphorylated form of MLK3 was detected with a phospho specific MLK3 antibody (Fig. 5B). Ectopic expression of PP6 did not alter the phosphorylation status of MLK3.
Fig. 5.
Phosphorylation of TAK1 depends on PP6
(A) 293 IL-1RI cells were transfected with an empty vector (-) or with expression vectors for HA-PP6 wild type (WT) and a catalytically inactive version of HA-PP6 D84N (DN). At 48 h post transfection, cells were treated with 5 ng/ml IL-1 for 1 min. Cell lysates were subjected to immunoblotting with anti-P-Thr-187, anti-TAK1, and anti-PP6. IB, immunoblotting.
(B) 293 cells were transfected with an empty vector (-) or with expression vector for HA-PP6 wild type (WT). At 48 h post transfection, cells were treated with 0.5 M sorbitol for 10 or 30 min. Cell lysates were subjected to immunoblotting with anti-phospho-MLK3, anti-MLK3, and anti-PP6. IB, immunoblotting.
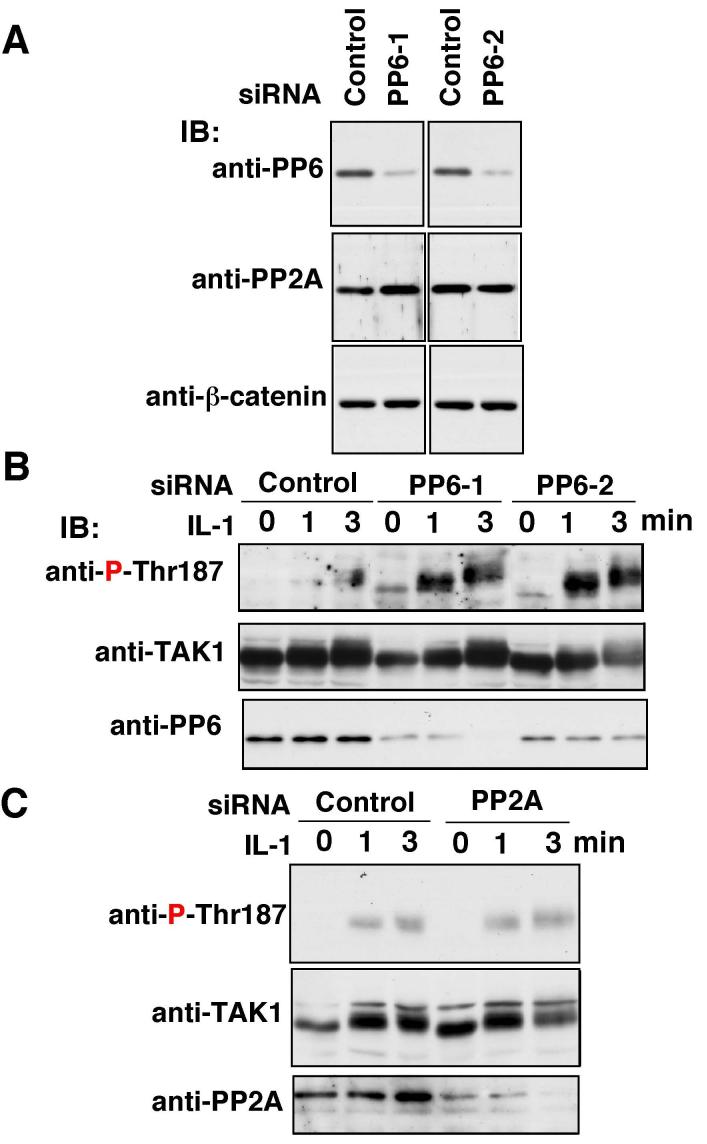
We examined whether PP6 levels affected regulation of TAK1. We tested down-regulation of PP6 by siRNA in IL-1-induced activation of TAK1. Different siRNAs (PP6-1 and PP6-2) targeted against two sequences in human PP6 were used. The synthetic siRNA duplexes were transfected into 293 IL-1RI cells and the reduction of PP6 expression were monitored by immunoblotting of PP6 (Fig. 6A). Both siRNAs resulted in a 60-80% decrease in the level of PP6 protein. Specificity of these siRNAs was confirmed by showing no change in the protein levels of PP2A and the unrelated protein β-catenin. Knockdown of PP6 led to an increased phosphorylation of Thr-187 TAK1 in IL-1 stimulated cells (Fig. 6B). In contrast, siRNAs targeted against PP2A did not alter the phosphorylation of Thr-187 of TAK1 (Fig. 6C). These results confirm that PP6 is the negative regulator of TAK1 activation by IL-1 stimulation.
Fig. 6.
Effect of PP6 knockdown
(A) Two independent siRNAs targeted against PP6 (PP6-1 and PP6-2) or control non-targeting siRNA (Control) were transfected into the 293 IL-1RI cells. The efficiency of PP6 knockdown was examined at 48 hr post transfection. PP6 was detected with anti-PP6, and anti-PP2A and β-catenin blottings were used to assess specificity of the siRNAs.
(B) Cells were transfected with PP6 siRNA or control siRNA, and at 48 hr post transfection cells were treated with 5 ng/ml IL-1 for 1-3 min. Cell lysates were subjected to immunoblotting with anti-P-Thr-187, and anti-TAK1. IB, immunoblotting.
(C) A pool of siRNA for PP2A or control siRNA were transfected into the 293 IL-1RI cells. At 48 hr post transfection cells were treated with 5 ng/ml IL-1 for 1-3 min. Cell lysates were subjected to immunoblotting with anti-P-Thr-187, and anti-TAK1. IB, immunoblotting.
DISCUSSION
TAK1 is an essential intermediate of IL-1 signaling pathway, and it is activated following association with the TRAF6-containing complex. This binding presumably alters TAK1 conformation and thereby induces autophosphorylation within the activation loop at Thr-187. TAK1 is in turn catalytically activated at 0.5-3 min after IL-1 stimulation. This activation is rapidly down-regulated. The mechanism of TAK1 down-regulation has not yet been defined. There are several possible mechanisms to reduce the TAK1 activity; 1) dissociation of TAK1 from the TRAF6-containing complex; 2) modification of TAK1 itself or its regulatory subunits; and 3) dephosphorylation of Thr-187 in the activation loop. We have previously reported that TAK1 is dissociated from the TRAF6-complex 10-30 min after IL-1 stimulation (23). This dissociation is likely to convert TAK1 conformation from active to inactive state and thereby blocks further autophosphorylation of TAK1. Cheung et al. has proposed that p38 MAPK is involved in negative regulation of TAK1 (14). p38 is activated through TAK1 pathway in response to IL-1 stimulation. They have shown that p38 phosphorylates the TAK1 activator subunit TAB1 resulting in inactivation of TAK1 in a negative feedback loop. This modification serves to inhibit catalytically active (autophosphorylated) forms of TAK1, which may enhance the down-regulation of TAK1. We show here that PP6 interacts with TAK1, dephosphorylates Thr-187 in the activation loop of TAK1 and inactivates TAK1. Furthermore, our knockdown of PP6 demonstrated that PP6 is an essential negative regulator of TAK1 activation. Therefore, TAK1 is down-regulated through several distinct mechanisms, and PP6-mediated direct dephosphorylation of Thr-187 is one of major mechanism to inactivate TAK1.
Both PP2C and PP6 can dephosphorylate and inactivate TAK1. The dephosphorylation site(s) by PP2C has not yet been defined. However, because TAK1 activity is dependent on phosphorylation at Thr-184, Thr-187 and Ser-192 within its activation loop, it is possible that both PP6 and PP2C dephosphorylate those sites. While PP6 constitutively associates with TAK1, PP2C is found to dissociate form TAK1 upon IL-1 stimulation (25), which suggests that PP2C functions to inhibit the TAK1 activity in unstimulated state and releasing PP2C from TAK1 complex may participate in TAK1 activation. In contrast, in this study, we found that knockdown of PP6 only affects IL-1-stimulted activation of TAK1, but it does not alter basal TAK1 activity seen without IL-1 stimulation. Therefore, PP6 is primarily involved in downregulation of activated forms of TAK1 generated upon stimulation. Collectively, both PP6 and PP2C regulate TAK1 activity by dephosphorylation, but they seems to function on different forms of TAK1.
Genetic studies using a mouse model have revealed that TAK1 plays a non-redundant role in innate immune responses as well as adaptive immunity in vivo (4-6). TAK1 is a master regulator of both NF-κB and JNK, which are major pathways to control inflammation. Because TAK1 plays such a key role in intracellular signaling to activate inflammation, the effective down-regulation of TAK1 following the stimuli-dependent rapid activation is important to prevent excessive immune responses. Dysregulation of inflammation is implicated in pathogenesis of many chronic diseases, such as psoriasis, rheumatoid arthritis, inflammatory bowel disease, and asthma. Furthermore, the chronic inflammation often associates with tumor development (33). Elucidation of the mechanism of TAK1 down-regulation is essential for better understanding of the regulatory mechanism of inflammation. In this study, we demonstrated that PP6 is a potent negative regulator of TAK1 in a physiological setting. PP6 is likely to play a role that regulates inflammatory signaling in vivo, and hence PP6 may be a potential novel target for pharmacological therapy of inflammatory diseases as well as inflammation-associated tumors.
Glossary
The abbreviations used are
- TAK1
transforming growth factor β activated kinase 1
- MAPKKK
mitogen-activated protein kinase kinase kinase
- IL-1
interleukin-1
- TNF
tumor necrosis factor
- TAB1
TAK1 binding protein 1
- JNK
c-Jun N-terminal kinase
- PP6
protein phosphatase 6
- PP2A
type 2A protein phosphatase
- OA
okadaic acid
- MLK
mixed-lineage protein kinase 3
- siRNA
small interfering RNA.
Footnotes
We thank S. Tamura for discussion. This work was supported by special grants for SORST and Advanced Research on Cancer from the Ministry of Education, Culture and Science of Japan (KM), and by NIH grants GM068812, AR050972 (JNT) and CA077584 (DLB).
REFERENCES
- 1.Akira S, Takeda K. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 4.Shim J-H, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee K-Y, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Nat. Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 6.Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC, Ninomiya-Tsuji J. J. Biol. Chem. 2006;281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishitani T, Takaesu G, Ninomiya-Tsuji J, Shibuya H, Gaynor RB, Matsumoto K. EMBO J. 2003;22:6277–6288. doi: 10.1093/emboj/cdg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishimoto K, Matsumoto K, Ninomiya-Tsuji J. J. Biol. Chem. 2000;275:7359–7364. doi: 10.1074/jbc.275.10.7359. [DOI] [PubMed] [Google Scholar]
- 9.Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- 10.Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, Matsumoto K. Mol. Cell. 2000;5:649–658. doi: 10.1016/s1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
- 11.Cheung PC, Nebreda AR, Cohen P. Biochem. J. 2004;378:27–34. doi: 10.1042/BJ20031794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ. Mol. Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Jin G, Klika A, Callahan M, Faga B, Danzig J, Jiang Z, Li X, Stark GR, Harrington J, Sherf B. Proc. Natl. Acad. Sci. U S A. 2004;101:2028–2033. doi: 10.1073/pnas.0307314101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung PC, Campbell DG, Nebreda AR, Cohen P. EMBO J. 2003;22:5793–5805. doi: 10.1093/emboj/cdg552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson LN, Noble ME, Owen DJ. Cell. 1996;85:149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- 16.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 17.Deak JC, Templeton DJ. Biochem. J. 1997;322(Pt 1):185–192. doi: 10.1042/bj3220185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung IW, Lassam N. J. Biol. Chem. 2001;276:1961–1967. doi: 10.1074/jbc.M004092200. [DOI] [PubMed] [Google Scholar]
- 19.Posas F, Saito H. EMBO J. 1998;17:1385–1394. doi: 10.1093/emboj/17.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siow YL, Kalmar GB, Sanghera JS, Tai G, Oh SS, Pelech SL. J. Biol. Chem. 1997;272:7586–7594. doi: 10.1074/jbc.272.12.7586. [DOI] [PubMed] [Google Scholar]
- 21.Sakurai H, Miyoshi H, Mizukami J, Sugita T. FEBS Lett. 2000;474:141–145. doi: 10.1016/s0014-5793(00)01588-x. [DOI] [PubMed] [Google Scholar]
- 22.Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H. J. Biol. Chem. 2005;280:7359–7368. doi: 10.1074/jbc.M407537200. [DOI] [PubMed] [Google Scholar]
- 23.Takaesu G, Ninomiya-Tsuji J, Kishida S, Li X, Stark GR, Matsumoto K. Mol. Cell. Biol. 2001;21:2475–2484. doi: 10.1128/MCB.21.7.2475-2484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanada M, Ninomiya-Tsuji J, Komaki K.-i., Ohnishi M, Katsura K, Kanamaru R, Matsumoto K, Tamura S. J. Biol. Chem. 2001;276:5753–5759. doi: 10.1074/jbc.M007773200. [DOI] [PubMed] [Google Scholar]
- 25.Li MG, Katsura K, Nomiyama H, Komaki K.-i., Ninomiya-Tsuji J, Matsumoto K, Kobayashi T, Tamura S. J. Biol. Chem. 2003;278:12013–12021. doi: 10.1074/jbc.M211474200. [DOI] [PubMed] [Google Scholar]
- 26.Janssens V, Goris J. Biochem. J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastians H, Ponstingl H. J. Cell Sci. 1996;109(Pt 12):2865–2874. doi: 10.1242/jcs.109.12.2865. [DOI] [PubMed] [Google Scholar]
- 28.Brewis ND, Street AJ, Prescott AR, Cohen PT. EMBO J. 1993;12:987–996. doi: 10.1002/j.1460-2075.1993.tb05739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen PT. Trends Biochem. Sci. 1997;22:245–251. doi: 10.1016/s0968-0004(97)01060-8. [DOI] [PubMed] [Google Scholar]
- 30.Stefansson B, Brautigan DL. J. Biol. Chem. 2006;281:22624–22634. doi: 10.1074/jbc.M601772200. [DOI] [PubMed] [Google Scholar]
- 31.Honkanen RE, Golden T. Curr. Med. Chem. 2002;9:2055–2075. doi: 10.2174/0929867023368836. [DOI] [PubMed] [Google Scholar]
- 32.Natsume T, Yamauchi Y, Nakayama H, Shinkawa T, Yanagida M, Takahashi N, Isobe T. Anal. Chem. 2002;74:4725–4733. doi: 10.1021/ac020018n. [DOI] [PubMed] [Google Scholar]
- 33.Coussens LM, Werb Z. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]