Abstract
Peroxisome proliferator-activated receptor binding protein (PBP), a nuclear receptor coactivator, interacts with estrogen receptor α (ERα) in the absence of estrogen. This interaction was enhanced in the presence of estrogen but was reduced in the presence of antiestrogen, tamoxifen. Transfection of PBP in CV-1 cells resulted in enhancement of estrogen-dependent transcription, indicating that PBP serves as a coactivator in ER signaling. To examine whether overexpression of PBP plays a role in breast cancer because of its coactivator function in ER signaling, we determined the levels of PBP expression in breast tumors. High levels of PBP expression were detected in ≈50% of primary breast cancers and breast cancer cell lines by ribonuclease protection analysis, in situ hybridization, and immunoperoxidase staining. Fluorescence in situ hybridization of human chromosomes revealed that the PBP gene is located on chromosome 17q12, a region that is amplified in some breast cancers. We found PBP gene amplification in ≈24% (6/25) of breast tumors and ≈30% (2/6) of breast cancer cell lines, implying that PBP gene overexpression can occur independent of gene amplification. This gene comprises 17 exons that, together, span >37 kilobases. The 5′-flanking region of 2.5 kilobase pairs inserted into a luciferase reporter vector revealed that the promoter activity in CV-1 cells increased by deletion of nucleotides from −2,500 to −273. The −273 to +1 region, which exhibited high promoter activity, contains a typical CCAT box and multiple cis-elements such as C/EBPβ, YY1, c-Ets-1, AP1, AP2, and NFκB binding sites. These observations, in particular PBP gene amplification, suggest that PBP, by its ability to function as ERα coactivator, might play a role in mammary epithelial differentiation and in breast carcinogenesis.
Steroids, retinoids, peroxisome proliferators, and other ligands, capable of interacting with specific members of the nuclear receptor superfamily, activate transcription of genes that regulate biological processes such as cell growth, differentiation, and neoplastic conversion (1). Despite the specificity of ligand–receptor interactions, activation of cell specific target gene transcription in eukaryotes is a complex process that involves the participation of other proteins, such as coactivators that link the receptors with the basal transcription apparatus (2). To date, several coactivators that interact with nuclear receptors in a ligand-dependent fashion have been cloned, and these include steroid receptor coactivator 1 [SRC-1 (3)], SRC-2 [TIF-2/GRIP-1 (4, 5)], SRC-3 [ACTR (6), AIB1 (7), p/CIP (8), RAC3 (9)], p300/cAMP response element-binding protein-binding protein [CBP (10, 11)], peroxisome proliferator-activated receptor (PPAR) binding protein [PBP/PPARBP (12), TRAP220 (13), RB18A (14), DRIP230 (15), CRSP p200 (16)], PPARγ coactivator 1 [PGC-1(17)], and ARA70 (18), among others (3, 19). Most of our current knowledge regarding these proteins is based on their ability to bind liganded receptors and enhance transcriptional activation in transfection experiments. Although some of these coactivators, for example CBP/p300 (20), SRC-1 (21), and ACTR (6), possess intrinsic histone acetyltransferase activities capable of modifying the chromatin organization of the target gene promoter regions, very little is known as to which of these coactivators, if any, are necessary and sufficient for the transcriptional activation of specific nuclear receptors under in vivo conditions.
In an effort to determine whether the expression of certain coactivators parallels the expression of a specific nuclear receptor in certain cell types in vivo, we determined the expression of PPARγ and its two coactivators, SRC-1 (22) and PBP (12), and provided evidence for differential expression of these three genes in breast epithelium and in colonic and urinary bladder mucosa by in situ hybridization (23). Most importantly, expression of SRC-1 and PBP appeared highly restricted and confined to cells in the crypts whereas PPARγ expression was seen in the entire villus (23). Recently, the expression of estrogen receptors (ERα) and SRC-1 has been demonstrated elegantly to be segregated in distinct subsets of cells within the epithelium of estrogen responsive rat mammary gland by immunohistochemical methods (24). These observations, taken together with the results of recent studies in SRC-1 null mice (25, 26), raise the possibility of redundancy of coactivators in receptor-mediated transcriptional activity or suggest that they may have other functions. The amplification and overexpression of coactivator AIB1, in some breast and ovarian cancers, suggest the possibility that dysregulation of coactivators may play a role in neoplasia (7). In this study, we show that PBP functions as a coactivator for ER and present data on the overexpression and amplification of this coactivator in a proportion of primary breast cancers and breast cancer cell lines. The human PBP gene is localized to chromosome 17q12, a region known to contain the oncogene erbB-2/HER-2, which is amplified in ≈20–30% of breast cancers (27).
MATERIALS AND METHODS
Plasmids and in Vitro Binding Assay.
Plasmid pCMX-PBP was described previously (12). pCMV-ERα and ERE-TK-LUC were provided by L. Madison (Northwestern University). Glutathione S-transferase (GST)–ERα was constructed by inserting the entire coding sequence of ERα into the EcoRI/SalI site of pGEX-4T-1. GST and GST-ER were produced in Escherichia coli and were bound to GST-Sepharose beads (Amersham Pharmacia). A 10-μg aliquot of GST-ER fusion protein loaded on GST-Sepharose beads was incubated with 6 μl of [35S]methionine-labeled full-length PBP protein for 2 hr in 600 μl of NETN (20 mM Tris⋅HCl, pH 7.5/100 mM KCl/0.7 mM EDTA/0.5% Nonidet P-40/1 mM phenylmethylsulfonylfluoride), and beads were washed four times with NETN. β-estrogen and tamoxifen were used as ER ligands at the final concentration of 1 and 2 μM, respectively. The protein bound to the beads was eluted and analyzed by SDS/PAGE (12).
Cell Culture and Transfection.
CV-1 cells (1 × 105) were plated in DMEM containing 10% fetal calf serum in six-well plates and were cultured for 24 hr before transfection. Transfections were performed as described (12), using β-galactosidase expression vector pCMVβ as a cotransfectant to serve as the control for transfection efficiency. Cell extracts were prepared 36 hr after transfection and were assayed for luciferase and β-galactosidase activities.
Tissue and Cell Samples.
Established breast cancer cell lines MCF-7, MDA-MB-134-VI, MDA-MB-361, MDA-MB-157, MDA-MB-468, and MDA-MB-453 were obtained from the American Type Culture Collection. Surgically removed breast cancer specimens were from the tumor bank maintained by the Lurie Cancer Center, Northwestern University Medical School (Chicago).
Northern Blot and Ribonuclease Protection Assays.
Random primed full-length PBP cDNA was used as the probe to hybridize multisample breast tumor blot (Invitrogen), containing total RNA (20 μg each sample) isolated from four different individuals. Ribonuclease protection assays were performed essentially as described (28). Total RNA (10 μg) extracted from frozen breast tumor samples and breast cancer cell lines by Trizol reagent (GIBCO/BRL) was used for hybridization. Human PBP cDNA fragment corresponding to 2,950–3,095 nucleotides that protected a 165-bp region in PBP mRNA was amplified and subcloned into pGEM-T vector (Promega). Radiolabeled antisense probes were generated by transcription with T7 RNA polymerase. Nuclease resistant fragments were resolved on 6% polyacrylamide-urea gels. As control for RNA amount, a human glyceraldehyde-3-phosphate dehydrogenase probe protecting a 316 fragment derived from exons 5–8 was added into each hybridization reaction.
Immunohistochemistry and in Situ Hybridization.
Antibodies against a GST-PBP polypeptide (mouse PBP amino acids 488–739) (12) were raised in rabbits, and specificity has been established by immunoprecipitation and Western blotting (data not shown). For immunohistochemical and in situ hybridization studies, breast tumor tissue was fixed in 4% formaldehyde and sections (4 μm thick) were processed as described (12).
Southern and Slot Blot Analysis.
For Southern blot analysis, genomic DNA (5 μg) digested with PstI was probed with 32P-labeled 1.4-kilobase (kb) human PBP cDNA fragment (nucleotides 1,607–3,116) or by β-actin cDNA. For slot blot analysis, DNA (2 μg) was loaded into each slot and was denatured and processed as described (28). The probe used for PBP slot blot was the same as that used for Southern blotting.
Fluorescence in Situ Chromosomal Hybridization.
Human metaphase cells were prepared from phytohemagglutinin-stimulated peripheral blood lymphocytes. A 3.4-kb human PBP cDNA fragment containing nucleotides 1,478–4,878 was labeled by nick translation using Bio-16-dUTP (Enzo Diagnostics). Fluorescence in situ hybridization was performed as described (29). Hybridization was detected with fluorescein-conjugated avidin (Vector Laboratories), and chromosomes were identified by staining with 4′, 6-diamidino-2-phenylindole-dihydrochloride.
Isolation of Human Genomic Clone and Localization of Intron/Exon Junctions.
Two P1 genomic clones (19107 and 19108) containing the PBP gene were obtained by screening a human foreskin fibroblast P1 bacteriophage library (Genome Systems, St. Louis) using cDNA probe covering nucleotides 35–919. The P1 clone was digested with appropriate restriction enzymes and was subcloned as necessary. The intron sizes and positions were determined by Southern blotting, restriction mapping, PCR analysis, and sequencing, and by comparing homologous sequences from the cDNA and genomic DNA.
Primer Extension Analysis.
The sequence of the oligonucleotide primer used for primer extension was 5′-GCCGCCGGGGACTTCAGATTGATCCTTCCCGG-3′ from the first exon. The oligonucleotide was end-labeled by using [γ-32P]dATP (Amersham Pharmacia) and T4 polynucleotide kinase at 37°C for 45 min. Labeled oligonucleotide was annealed with 2 μg of human mammary gland poly (A+) RNA (CLONTECH) at 42°C and was extended by using avian myeloblastosis virus reverse transcriptase (Promega). Extension products were resolved on 6% polyacrylamide-urea gels.
Cloning of the Human PBP Promoter and Construction of Reporter Plasmids.
The 2.5-kb 5′-flanking genomic fragment was amplified from PBP human genomic P1 clone by PCR using the forward primer 5′-GCCTGGGAGCTCTAGTGAAACTCTGTCTCT-3′ and the reverse primer (+125) 5′-CGGGATGCTAGCACGCAGGGCACCAGCAGT-3′ and was inserted into the NheI and SacI sites of pGL3-Basic vector (Promega). Additional 5′-end deletion constructs were generated by PCR using the +125 reverse primer and 5′-GCTAGTGAGCTCGTACAGGGATCTGGAGAT-3′, or 5′-CACTGGGAGCTCACGGCGAAACCC-3′. They were digested with NheI and SacI and were inserted into pGL3-Basic NheI/SacI sites.
RESULTS
PBP Is a Coactivator for ER.
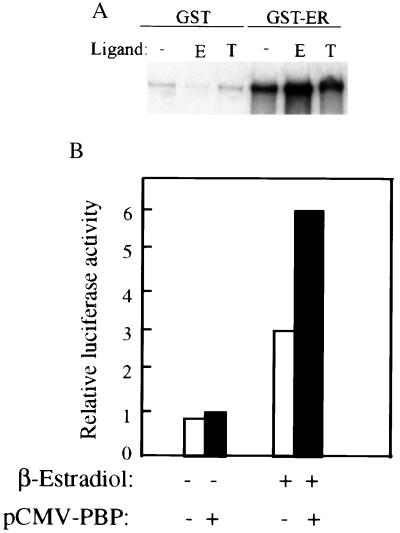
Given the role of PBP as a coactivator for PPAR (12) and thyroid hormone receptor (12, 13), we analyzed the direct interaction between PBP and ER. The in vitro binding was assayed with bacterially generated GST-ER fusion protein and in vitro translated full-length PBP. A matrix-bound GST-ER, but not GST alone, retained the radiolabeled PBP both in the presence or absence of β-estrogen, but the presence of β-estrogen increased the physical interaction (Fig. 1A). Inclusion of tamoxifen, a potent ER antagonist in the binding assay, reduced the direct interaction between ER and PBP. To determine the functional relevance of the binding of PBP with ER, we then tested the effect of expression of PBP on the transcription of ER-dependent reporter in CV-1 cells. Coexpression of PBP resulted in a 2-fold increase in transcription of luciferase gene in the presence of estrogen (Fig. 1B). This increase was not detected when estrogen was replaced by tamoxifen (data not shown).
Figure 1.
Binding of PBP to ER and potentiation of estrogen dependent transcription from ER. (A) Binding of PBP to ER. [35S] methionine-labeled PBP generated by in vitro translation was incubated with glutathione-Sepharose beads containing GST, or GST-ER, in the presence of estrogen (E) or tamoxifen (T) or absence of the ligand (-). The bound protein was eluted and analyzed by SDS/PAGE and was autoradiographed. PBP binds to ER in the absence of estrogen and in the presence of tamoxifen, but estrogen increases the interaction of ER with PBP. No binding was seen for GST alone, indicating that the interaction is specific for ER. (B) PBP potentiates the transcriptional activity of ER. CV-1 cell were transiently cotransfected with 1.5 μg of reporter construct ERE-TK-luc, 0.25 μg of pCMV-ER, 0.5 μg of pCMX-PBP or pCMX, and 0.5 μg of pCMVβ in the presence of β-estradiol or absence of ligand. PBP increased the transcription of luciferase gene ≈2- fold in the presence of β-estradiol. Results are the mean of four independent transfections and are normalized to the internal control β-galactosidase expression.
PBP Overexpression in Breast Tumors.
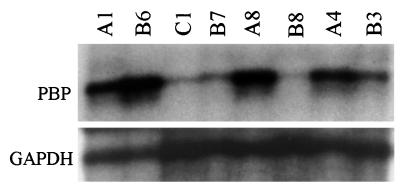
Because estrogen and ER play a prominent role in human breast tumorigenesis, we decided to ascertain the levels of expression of PBP. Human breast tumor multisample blot (Invitrogen) was initially used to ascertain possible changes in the expression level of PBP. In this blot, each slot contained total RNA isolated from four different breast tumor samples. RNA from the adjacent normal breast tissue was used as the control. Random-primed full-length human PBP cDNA was used as the probe. The same blot was stripped and reprobed with an 18 S rRNA probe to confirm the equal loading of RNA in all lanes. PBP mRNA concentration was high in one of these four tumors as compared with the levels in the other three tumors and adjacent normal breast (not illustrated). These results prompted us to investigate changes in PBP expression levels in 15 primary breast cancer samples and 6 established breast cancer cell lines by using ribonuclease protection analysis. An ≈6- to 15-fold increase in the amount of PBP mRNA was observed in 8 of 15 primary breast cancers (see Fig. 2, lanes A1, B6, A8, and B3 for representative examples); and 5 of 8 tumors with PBP overexpression also exhibited PBP gene amplification (see below). Of the six breast cancer cell lines examined (MCF-7, MDA-MB-134-VI, MDA-MB-361, MDA-MB-157, MDA-MB-468, and MDA-MB-453), three cell lines, namely MDA-MB-361 (Fig. 2, lane A4), MDA-MB-453, and MDA-MB-468 revealed overexpression of PBP mRNA.
Figure 2.
Ribonuclease protection assay of PBP transcript levels. Total RNA (10 μg) was hybridized with radiolabeled antisense PBP probe generated by in vitro transcription. Glyceraldehyde-3-phosphate dehydrogenase probe was included in each hybridization reaction as control. Nuclease resistant fragments were resolved on 6% polyacrylamide-urea gels. High levels of expression of PBP mRNA are seen in breast tumor samples A1, A8, B3, and B6 and in MDA-MD-361 breast cancer cell line (A4). Samples B7 and B8 are breast tumors with no appreciable increase in PBP transcript, and sample C1 is normal human mammary gland RNA.
In Situ Hybridization and Immunohistochemical Analysis.
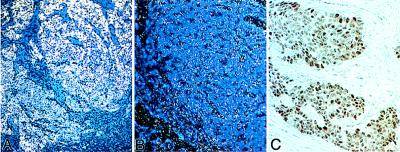
To evaluate expression of PBP in primary breast cancers more fully, in situ hybridization was performed by using a riboprobe (nucleotides 2,960–3,116 of human PBP) prepared from the human PBP and sections of breast cancers. Strong labeling for PBP was noted in the breast carcinomas showing PBP overexpression by ribonuclease protection assays. Examples of high and low levels of expression are shown in Fig. 3A and B, respectively. We further evaluated PBP protein expression in 10 breast carcinomas by immunoperoxidase staining and found variable degree of nuclear staining in different tumors ranging from weak to intensely strong reaction product (Fig. 3C). Although the numbers of samples analyzed are small, the results suggest that PBP expression is up-regulated in some breast cancers.
Figure 3.
In situ analysis of PBP mRNA expression and immunoperoxidase localization of PBP in breast carcinoma. Shown are examples of high (A) and low (B) levels of PBP mRNA expression in breast carcinoma. The connective tissue stroma surrounding the tumor tissue exhibits low signal (A). Immunohistochemical analysis showed the nuclear staining of PBP (C).
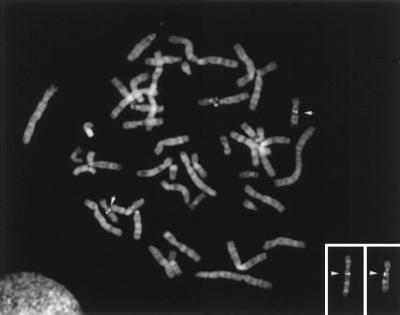
Amplification of PBP Gene in Breast Cancers. To localize the human PBP gene, we performed fluorescence in situ hybridization of a biotin-labeled human PBP probe to normal human metaphase chromosome. The PBP probe was a cDNA fragment containing nucleotides 1,478–4,878. Hybridization of the hPBP cDNA probe resulted in specific labeling of chromosome 17 (Fig. 4). Specific labeling of 17q12–21.2 was observed on four (5 cells), three (12 cells), two (5 cells), or one (3 cells) chromatid(s) of the chromosome 17 homologues in 25 cells examined. Of 71 signals observed, 69 (97%) were located at 17q12–21.2. Of these, 54 (78%) signals were located at 17q12; of the remaining 15 signals, 9 (13%) were at 17q21.1, and 6 (9%) at 17q21.2. Two background signals were observed at another chromosomal site (2q12). We observed specific signal at 17q12 in an additional hybridization experiment using the same probe. These results suggest that the PBP gene is localized to chromosome 17, band q12 (Fig. 4).
Figure 4.
Chromosomal assignment of the human PBP gene. Shown is in situ hybridization of a biotin-labeled 3.4-kb human PBP cDNA probe to human metaphase cells from phytohemagglutinin-stimulated peripheral blood lymphocytes. The chromosome 17 homologues are identified with arrows; specific labeling was observed at 17q12 (arrowheads).
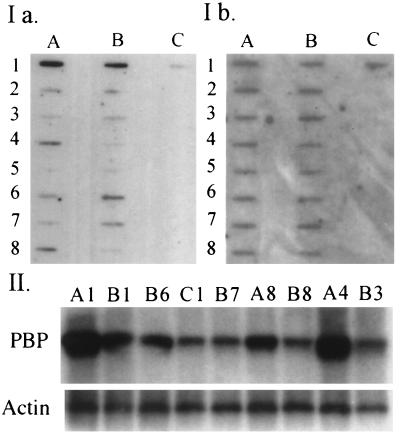
As the region in the chromosome 17q12 contains erbB-2/HER-2 that is amplified in ≈20–30% of breast tumors (27), we investigated the possibility of PBP gene amplification that may be responsible for its overexpression. PBP gene amplification was first ascertained by using slot blot procedure in the genomic DNA obtained from 25 primary breast cancers and 6 breast cancer cell lines. Genomic DNA from normal mammary gland was used as the control. When density ratios between the PBP gene and the reference gene were calculated, 6 of 25 primary breast cancers and 2 of 6 breast cancer cell lines (MDA-M13–361 and MDA-MB-453) showed ≈3- to 10-fold increase in PBP gene copy number (see Fig. 5I for representative examples). These results were confirmed by Southern blot analysis (Fig. 5II). All five tumors with amplified PBP gene (the quality of RNA was not good in one tumor) revealed increased RNA levels, suggesting a correlation between gene amplification and overexpression. Two tumors (samples B6 and A8) that had relatively low PBP gene copy number showed higher levels of PBP mRNA than that observed in a tumor with high PBP gene copy number (See A1, B6, and A8 in Figs. 2 and 5). In addition, three breast tumors and one cell line (MDA-MB-468) with no PBP gene amplification showed increased levels of PBP mRNA (tumor B3 in Figs. 2 and 5; data not shown). Furthermore, four tumors (samples A1, A4, A8, and B6) and one cell line (MDA-MB-361) that had been shown to contain amplified PBP gene also revealed erbB-2/HER-2 gene amplification (data not presented).
Figure 5.
Analysis of DNA from breast tumors and breast cancer cell lines for PBP gene amplification. I represents semiquantitative slot blots of DNA. DNA (2 μg) was loaded into each slot and was denatured and hybridized with [32P]-labeled cDNA probes. Ia represents the blot probed with PBP. This blot then was stripped and reprobed with Cyp4A1cDNA to assess DNA loading (Ib). A4 (MDA-MB-361) and B4 (MDA-MB-134VI) represented breast cancer cell lines. The rest of samples were primary breast tumors. Samples A1, A4, A8, B1, and B6 suggest amplification of PBP gene. II represents the Southern blot of representative samples. Genomic DNA (10 μg) digested with PstI was probed with full-length PBP cDNA (upper panel marked PBP) and was reprobed with β-actin cDNA to serve as a control. PBP gene amplification is clearly evident in samples A1, A4, A8, B1, and B6.
Genomic Organization and the Characterization of the 5′-Flanking Region of the Human PBP Gene. Because PBP gene amplification only partially accounts for PBP overexpression, other mechanisms, such as transcriptional regulation, can influence the overall functional activity. As a first step to elucidate the mechanism, we isolated the PBP gene and characterized its promoter region. To isolate the PBP gene, a human P1 bacteriophage library was screened, and two P1 clones (19107 and 19108) were obtained. These two P1 clones exhibited similar restriction fragment patterns; hence, we characterized only clone 19107, which contained the entire PBP gene. The introns of the PBP gene were amplified by XL-PCR, and the intron/exon boundaries were determined by nucleotide sequencing. The PBP gene spans ≈37 kb and contains 17 exons ranging in size from 29 bp (exon 6) to 4,148 bp (exon 17). The size of introns varies between 150 bp (intron 6) and 5 kb (intron 5) (data not shown). The sequences at all of the exon/intron junctions conformed to the GT/AG rule (30). The transcription start site was determined by primer extension analysis using poly (A+) RNA from normal human breast. We used a 32-mer oligonucleotide labeled with 32P at the end to perform primer extension and obtained a major band corresponding to the position G 280 nucleotides upstream of the AUG translation initiator codon (data not presented), and this nucleotide G has been designated as +1 (Fig. 6). The 5′-flanking region contained a typical CCAAT box (between nucleotide −74 and −70), but a typical TATA box was not discerned (Fig. 6). The 5′-flanking region contains a number of putative response elements; these include sequence matches for C/EBPβ (−268 to −262), HFH (−233 to −221), YY1 (−207 to −198), c-Ets-1 (−156 to −150), C-Ets-2 (−118 to −113), AP2 (−147 to −138), AP1 (−80 to −70), and NF-E (−49 to −46). Overlapping the transcription initiation site is the NF-κB binding site (+1 to +10).
Figure 6.
The nucleotide sequence of the 5′-flanking region of the human PBP gene. Nucleotide positions −308 to +58 relative to the transcription initiation site are shown. A major transcription start site is indicated by an asterisk and is designated as +1, and the ATG methionine is shown (boldface type and underlined). The position of intron 1 is shown. Underlined sequences indicate the putative binding sites for transcription factors.
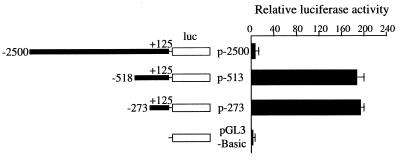
To determine the important regulatory regions for the expression of the PBP gene, the region between −2,500 and +125 as well as two deletion constructs of this promoter were subcloned into the pGL3-Basic. The promoter activity from these three constructs is shown in Fig. 7. In CV-1 cells, the plasmid p-273 showed the highest promoter activity (190 ± 5) as compared with the baseline activity of pGL3-Basic vector (Fig. 7). Although the p-513 showed a similar promoter activity as p-273, the luciferase activity of −2,500 PBP was lower, suggesting that the region between −518 and −2,500 strongly represses the transcriptional activity of PBP promoter (Fig. 7).
Figure 7.
The human PBP promoter activity. The schematic diagram on the left represents each deletion construct of the PBP gene (dark box) fused into the upstream region of the luciferase gene (luc, white box), with variable 5′-ends to the +125 nucleotide relative to the transcription start site. Each construct was transiently transfected into CV-1 cells. The promoter activity is expressed as fold induction relative to the activity of promoterless pGL3-Basic. The data are the mean ± standard error of triplicate determinations. A repressor region is located between bp −2,500 and −518, 5′-of the PBP transcription start site.
DISCUSSION
Given the complexity of eukaryotic transcriptional machinery, and the unexpected multiplicity and apparent promiscuity of coactivators toward nuclear receptors, it will be important to characterize various components of this complex system to understand the molecular governance of ligand- induced transcriptional activation of specific genes in specific cell types (32). During the past 5 years, many nuclear receptor coactivators have been identified, and of these, the PBP coactivator cloned (12) appears to be distinct from the SRC-1 family of coactivators, despite the fact that all of these coactivators contain LXXLL nuclear receptor interacting motif (s) (2, 6, 7, 12, 15, 17). First, unlike SRC-1 family of coactivators (6, 21), PBP does not have the basic helix-loop-helix structure, and it lacks histone acetyltransferase activity (ref. 12; Y.Z. and J.K.R., unpublished observations). Second, in addition to its ability to interact with several nuclear receptors (12, 13), the human and mouse PBP bind to p53 (ref. 14; Y.Z. and J.K.R., unpublished data). More recently, PBP was found to be a component of multiple protein complexes involved in eukaryotic transcription, namely TRAP (thyroid hormone receptor associated proteins) (13), CRSP (cofactor required for SP1 activation) (16), and DRIP (vitamin D-receptor interacting protein)/ARC (activator-recruited cofactor) (15, 33). In view of the potential importance of PBP in eukaryotic transcription, and high levels of expression of this gene in a proliferating subset of colonic mucosal cells and in the mammary epithelium of pregnant mouse breast (23) and in the seminiferous epithelium (12), functional and structural studies of PBP become necessary.
In this study, we first established that PBP binds to ER and increases ER ligand-dependent transactivation in CV-1 cells. It has been shown that PBP interacts with several nuclear receptors, such as PPARα, PPARγ, RXRα, RARα, TRβ1, and TRα (12, 13), and, in this regard, the coactivator role of PBP in ER transcription is not unexpected. Considering the importance of estrogen in the breast tumorigenesis, we further investigated the expression of the coactivator PBP in breast cancer and found PBP overexpression in ≈50% of the breast tumors examined. In situ hybridization revealed low levels of PBP mRNA in breast epithelium and high levels of PBP expression in some breast cancers. It would be of interest to ascertain whether the PBP and ER are expressed within the same cell or in different subsets of breast epithelium, such as that noted for SRC-1 (24).
The human PBP gene, which comprises 17 exons that together span ≈37 kb, was localized to chromosome 17q12. This 17q12 region is also the location of the protooncogene erbB-2/HER-2 that is commonly amplified in ≈20–30% of breast cancers (27, 34). We show that the PBP gene is amplified in ≈25% (6 of 25) of primary breast tumors analyzed in this study. Of the six tumors with PBP gene amplification, five were analyzed for PBP mRNA, and in each case amplification was associated with PBP overexpression. Of the 25 tumors used in this study for PBP gene amplification analysis, seven revealed erbB-2/HER-2 gene overexpression (data not included), and, of these seven tumors, six had PBP gene amplification. The absence of PBP gene amplification in one tumor with erbB-2/HER-2 overexpression suggests that these two events do not always occur in conjunction. In addition, two of the six breast cancer cell lines revealed PBP gene amplification. Of special interest is that three of the cell lines used in this study (MDA-MB-468, MDA-MB-453, and MDA-MB-361) were ER negative and showed no amplification or overexpression of AIB1 (SRC-3), a steroid receptor coactivator (7). Nevertheless, these three cell lines revealed PBP overexpression, and two also displayed PBP gene amplification, suggesting that PBP gene amplification and overexpression are not uncommon in ER negative cell lines and, in this regard, the events leading to PBP overexpression may differ from those leading to AIB1 overexpression and that PBP might play a role in the estrogen-independent growth of tumors. We found 8 of 15 primary breast tumors with high levels of PBP mRNA. In five of eight tumors, there was a concordance with PBP gene amplification, and, in three tumors, PBP overexpression was independent of gene amplification, indicating that overexpression can occur by mechanisms other than gene amplification. Although a large number of breast cancers need to be further analyzed, the present study suggests that overexpression of PBP gene can manifest independently of alterations in AIB1 and erbB-2/HER-2 genes in breast cancers and may have implications in breast carcinogenesis. Gene amplification is an important process in human cancers and has prognostic significance (34–36), and, because many genes amplified in breast cancers appear to function as oncogenes (27, 35), it is conceivable that PBP may be involved globally in growth regulation and differentiation. Our finding that PBP is overexpressed and amplified in breast cancers is of importance, because of the recent demonstration by Lin et al. (35), that the fraction of transcripts exhibiting significant differences between normal and cancer cells was relatively small, representing 1.5% of the 300,000 transcripts derived from at least 45,000 different genes.
The promoter activity of the PBP 5′-flanking region was assessed after insertion upstream of the luciferase coding sequence in the pGL3-basic vector. The high activity of PBP promoter (−518 to +125 and −273 to +125) was detected in CV-1 cells whereas the longer construct, −2,500 to +1, exerted lower promoter activity. The region highly active as a promoter (−273 to +125) reveals several putative transcription factor binding sites. The 5′ segment of PBP gene surrounding the transcriptional initiation site is GC-rich and lacks a typical TATA box, a feature that is consistently observed in many housekeeping and transcription factor genes. This −273 to +1 region contains putative binding sites for several transcription factors, such as C/EBPβ, HFH, NF-κB, c-Ets-1, c-Ets-2, NF-E, AP1, and AP2 (31). The AP-2 gene family of transcription factors appears to regulate ER and erbB-2/HER-2 expression during progression from normal breast epithelium to breast cancer (37). Further study is required to determine which transcription factors play a central role in the PBP gene transcription. These putative landmarks in 5′-flanking region should facilitate further study of the mechanisms involved in the transcriptional regulation of PBP gene expression and its role in ligand-dependent transcription of nuclear receptors.
This work was supported by Public Health Service/National Institutes of Health Grants GM23750 (to J.K.R.), CA40046 (to M.M.L.), and DK43806 (to M.A.L.), Veterans Affairs Merit Review grants (to A.V.Y. and M.S.R.), and the Joseph L. Mayberry, Sr. Endowment Fund.
ABBREVIATIONS
- PPAR
peroxisome proliferator activated receptor
- PBP or PPARBP
PPAR binding protein
- ER
estrogen receptor
- GST
glutathione S-transferase
- SRC-1
steroid receptor coactivator 1
- kb
kilobase
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF157513).
References
- 1.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schultz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass C K, Rose D W, Rosenfeld M G. Curr Opin Cell Biol. 1997;9:222–232. doi: 10.1016/s0955-0674(97)80066-x. [DOI] [PubMed] [Google Scholar]
- 3.Onate S A, Tsai S Y, Tsai M-J, O’Malley B W. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 4.Voegel J J, Heine M J S, Zechel C, Chambon P, Gronemeyer H. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 5.Hong H, Kohli K, Trivedi A, Johnson D L, Stallcup M R. Proc Natl Acad Sci USA. 1996;93:4948–4952. doi: 10.1073/pnas.93.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, H., Lin, R. J., Schiltz, R. L., Chakravarti, D., Nash, A., Nagy, L., Privalsky, M. L., Nakatani, Y. & Evans, R. M. Cell90, 569–580. [DOI] [PubMed]
- 7.Anzick S L, Kononen J, Walker R L, Azorsa D O, Tanner M M, Guan X Y, Sauter G, Kallioniemi O P, Trent J M, Meltzer P S. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 8.Torchia J, Rose D W, Inostroza J, Kamei Y, Westin S, Glass C K, Rosenfeld M G. Nature (London) 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Gomes P J, Chen J D. Proc Natl Acad Sci USA. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S-C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 11.Yao T-P, Ku G, Zhou N, Scully R, Livingston D M. Proc Natl Acad Sci USA. 1996;93:10626–10631. doi: 10.1073/pnas.93.20.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y, Qi C, Jain S, Rao M S, Reddy J K. J Biol Chem. 1997;272:25500–25506. doi: 10.1074/jbc.272.41.25500. [DOI] [PubMed] [Google Scholar]
- 13.Yuan C-X, Ito M, Fondell J D, Fu Z-Y, Roeder R G. Proc Natl Acad Sci USA. 1998;95:7939–7944. doi: 10.1073/pnas.95.14.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drane P, Barel M, Balbao M, Frade R. Oncogene. 1997;15:3013–3024. doi: 10.1038/sj.onc.1201492. [DOI] [PubMed] [Google Scholar]
- 15.Rachez C, Lemon B D, Suldan Z, Bromleigh V, Gamble M, Naar A M, Erdjument-Bromage H, Tempst P, Freedman L P. Nature (London) 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 16.Ryu S, Zhou S, Ladurer A G, Tjian R. Nature (London) 1999;397:446–450. doi: 10.1038/17141. [DOI] [PubMed] [Google Scholar]
- 17.Puigserver P, Wu Z, Park C W, Graves R, Wright M, Spiegelman B M. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 18.Yeh S, Chang C. Proc Natl Acad Sci USA. 1997;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voegel J J, Heine M J S, Tinin M, Vivat V, Chambon P, Gronemeyer H. EMBO J. 1998;17:507–519. doi: 10.1093/emboj/17.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogryzko V, Schiltz R A, Russanova V, Howard B H, Nakatani Y. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 21.Spencer T E, Jenster G, Burcin M M, Allis C D, Zhou J, Mizzen C A, McKenna N J, Onate S A, Tsai S Y, Tsai M-J, O’Malley B W. Nature (London) 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Qi C, Calandra C, Rao M S, Reddy J K. Gene Expression. 1996;6:185–195. [PMC free article] [PubMed] [Google Scholar]
- 23.Jain S, Pulikuri S, Zhu Y, Qi C, Kanwar Y S, Yeldandi A Y, Rao M S, Reddy J K. Am J Pathol. 1998;153:349–354. doi: 10.1016/s0002-9440(10)65577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shim W-S, DiRenzo J, DeCaprio J A, Santen R J, Brown M, Jeng M-H. Proc Natl Acad Sci USA. 1999;96:208–213. doi: 10.1073/pnas.96.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Qiu Y, DeMayo F J, Tsai S Y, Tsai M J, O’Malley B W. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 26.Qi C, Zhu Y, Pan J, Yeldandi A V, Rao M S, Maeda N, Subbarao V, Pulikuri S, Hashimoto T, Reddy J K. Proc Natl Acad Sci USA. 1999;96:1585–1590. doi: 10.1073/pnas.96.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slamon D J, Godolphin W, Jones L A, Holt J A, Wong S C, Keith D E, Levin W J, Stuart S G, Udove J, Ullrich A, Press M F. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 28.Ausubel F M, Brent R, Kingston R E, M ore D D, Seidman J G, Smith J A, Struhl K, editors. Current Protocols in Molecular Biology. New York: Wiley; 1993. [Google Scholar]
- 29.Rowley J D, Diaz M O, Espinosa R, Patel Y D, van Melle E, Ziemin S, Taillon-Miller P, Lichter P, Evans G A, Kersey J D, et al. Proc Natl Acad Sci USA. 1990;87:9358–9362. doi: 10.1073/pnas.87.23.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breathnach R, Bednoist C, O’Hare K, Gannon F, Chambon P. Proc Natl Acad Sci USA. 1978;82:8567–8571. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locker J, editor. Transcription Factors. Oxford: Wiley; 1996. [Google Scholar]
- 32.Tjian R, Maniatis T. Cell. 1994;77:5–8. doi: 10.1016/0092-8674(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 33.Naar A M, Beaurang P A, Zhou S, Abraham S, Solomon W, Tijan R. Nature (London) 1999;398:828–832. doi: 10.1038/19789. [DOI] [PubMed] [Google Scholar]
- 34.Lengauer C, Kinzler K W, Vogelstein B. Nature (London) 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 35.Lin Z, Wei Z, Velculescu V E, Kern S E, Hruban R H, Hamilton S R, Vogelstein B, Kinzler K W. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 36.Devilee P, Schuuring E, van de Vijver M J, Cornelisse C J. Crit Rev Oncog. 1994;5:247–270. doi: 10.1615/critrevoncog.v5.i2-3.70. [DOI] [PubMed] [Google Scholar]
- 37.Bosher J M, Totty N F, Hsuan J J, Williams T, Hurst H C. Oncogene. 1996;13:1701–1707. [PubMed] [Google Scholar]