Abstract
The accumulation of mutant genotypes within a biofilm evokes the controversy over whether the biofilm environment induces adaptive mutation or whether the accumulation can be explained by natural selection. A comparison of the virulence of two strains of the dental pathogen Streptococcus mutans showed that rats infected with one of the strains accumulated a high proportion (average, 22%) of organisms that had undergone a deletion between two contiguous and highly homologous genes. To determine if the accumulation of deletion mutants was due to selection or to an increased mutation rate, accumulations of deletion mutants within in vitro planktonic and biofilm cultures and within rats inoculated with various proportions of deletion organisms were quantified. We report here that natural selection was the primary force behind the accumulation of the deletion mutants.
There is a 150-year-old controversy over whether selective stress can influence mutation rate (13). It has led to the concept of adaptive mutation, a process whereby environmental stress can induce the cellular mechanisms that lead to mutations. Experiments by Cairns et al. (2, 3) figured prominently in fueling the controversy as they demonstrated the reversion of a Lac− phenotype in resting Escherichia coli plated on lactose-containing medium. However, the controversy lingers, primarily because it is difficult to isolate the effects of mutations on growth rates or the growth of subpopulations under conditions that would ordinarily suppress growth (13).
One source of stress that has been proposed to increase the mutation rate is the environment within a biofilm (8), though a biofilm is in reality a collection of environments. In a trial to compare the virulence of the wild-type dental pathogen Streptococcus mutans and that of a genetically engineered mutant that no longer expressed a glucan-binding protein (Gbp) designated GbpA, it was noted that rats infected with the gbpA mutant accumulated high proportions (average, 22%) of organisms that had undergone a deletion (7). Recovery of deletion mutants from wild-type-infected rats was rare. The basis for the deletion was a recombination between two contiguous and highly homologous glucosyltransferase (Gtf) genes (Fig. 1). This system offered the opportunity to investigate the basis for the accumulation of the deletion mutants. We do not propose to end the controversy over adaptive mutation. However, we present evidence that natural selection was the primary basis for the accumulation of deletion mutants in this system.
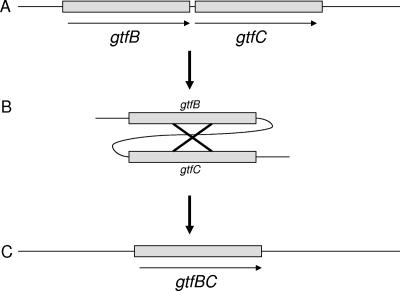
FIG. 1.
(A) The S. mutans chromosomal gtfB (4.4-kb) and gtfC (4.2-kb) genes share 98% identity over a 2,000-bp stretch (15) between the regions encoding the signal peptide and the glucan-binding domain. (B) Homologous recombination occurs at random sites within the highly homologous regions in the two genes. (C) Due to the high level of homology, the precise sites of the deletion are difficult to determine. However, the single hybrid gene that results is thought to encode the gtfB active site and the gtfC glucan-binding domain. The resulting hybrid gtfBC gene encodes a Gtf that retains enzymatic activity, though the absolute amount of activity of each clone may vary. The glucan product is more similar to that catalyzed by the gtfC product than the gtfB product.
The choice of S. mutans was based on ongoing studies of the virulence mechanisms of this species. Already, it was known that the most prominent virulence attributes of S. mutans were its ability to produce copious amounts of acid from the fermentation of dietary carbohydrates, its acid tolerance, and its ability to utilize sucrose to synthesize polymers of glucose, called glucans, that promote adhesion and accumulation on tooth surfaces (1). Due to the central role of glucans, S. mutans proteins possessing an affinity for glucan have been proposed to contribute to the establishment of the dental plaque biofilm and have been designated Gbps.
The Gbps of Streptococcus mutans include the Gtfs, responsible for catalyzing the synthesis of extracellular glucan, and non-Gtf Gbps that piggyback onto glucan while making a variety of contributions to the biology of the organism. In particular, GbpA has been reported to affect the depth and distribution of microcolonies (aggregates of organisms that extend outward from the substratum surface) within in vitro biofilms formed by S. mutans (6). It is likely that the change in biofilm architecture extends to the in vivo environment, as rats monoinfected with a gbpA strain of S. mutans displayed a higher caries rate than the wild type and accumulated deletion mutants that had undergone a recombination between two gtf genes (7). The accumulation of deletion mutants, designated gtfBC mutants, averaged 22% and mitigated the increase in virulence and resulted in a partial restoration of the biofilm phenotype associated with the loss of GbpA.
In vitro, the RecA-dependent recombination of the nearly identical gtfB and gtfC genes occurs at a frequency of 10−3 and results in the formation of a single Gtf that catalyzes the synthesis of mainly water-soluble glucans (14). It is speculated that gtfBC mutants may be more likely to dissociate from a biofilm, allowing them to colonize other sites. In rats colonized with gbpA mutants, the accumulation of gtfBC mutants may be a response to increased stress within the altered biofilm. The accumulation of gtfBC mutants may be explained by natural selection, an increased frequency of recombination, or a combination of both factors. The importance of distinguishing between these possibilities is fundamental to understanding the mechanism behind the contributions of GbpA and to understanding the evolutionary forces that have an impact on biofilm ecology. An increase in recombination frequency would indicate a direct or indirect influence on gene expression or enzyme activity related to basic cellular functions, like recombination. Confirmation of natural selection would suggest that a major contribution of GbpA is to the development of an optimal plaque biofilm that minimizes the stress to the bacterial population as a whole. Accordingly, experiments were undertaken to distinguish between these possibilities.
In an animal study designed to investigate the virulence of a gbpA strain of S. mutans, the accumulation of gtfBC mutants in gbpA mutant-colonized rats averaged 22% when the starting inoculum harbored approximately 0.001% gtfBC mutants (7). This accumulation of deletion mutants occurred over a thirty-day period of colonization. To begin to understand the forces behind the ascendancy of gtfBC mutants, another rat infection study was carried out; in this study, the inocula were varied with respect to the ratio of gtfBC deletion to nondeletion gbpA strains of S. mutans. The infection protocol was as described before (7) and involved inoculating germfree rats by swabbing their teeth on two consecutive days, allowing the rats to feed on a 5% sucrose diet ad libitum for thirty days, and then collecting plaque samples to enumerate the proportion of deletion mutants. The gbpA strain was engineered to harbor a kanamycin resistance cassette so that new deletion mutants could be distinguished from gtfBC mutants that were part of the original inoculum. Deletion mutants were recognized by their smooth-colony morphology on mitis salivarius agar plates containing sucrose (7). As shown in Table 1 , when the gtfBC mutants were in the majority in the inoculum, their percentages decreased by the end of thirty days, whereas if the starting percentage was only 25%, their proportion increased. Reversion of the gtfBC deletion to the wild type was not possible, but there was a finite possibility that intact gtfB and gtfC genes could be reintroduced via natural transformation. When the inoculum was 100% gtfBC deletion mutants, no rough colonies (indicative of wild-type gtf genes) were detected after thirty days. New gtfBC mutants were minimal, indicating that changes in the proportions of gtfBC mutants were strongly influenced by natural selection. The observation that proportions of gtfBC mutants moved higher or lower depending on their starting percentage indicates that neither strain enjoyed a clear growth advantage in vivo and that natural selection could work to reduce the population of deletion mutants as well as increase their representation.
TABLE 1.
Changes in the percentages of gtfBC deletion mutants over time in rats
| % gtfBC mutants
|
Frequency of new gtfBC mutantsa | |
|---|---|---|
| Initial inoculum | After 30 days | |
| 25 | 34 | Not detected |
| 64 | 48 | 0.004 |
| 81 | 51 | Not detected |
| 96 | 69 | 0.020 |
| 100 | 100 | |
New gtfBC mutants are those arising from organisms in the initial inoculum, which started with intact gtfB and gtfC genes. The new gtfBC mutants could be distinguished from the initial gtfBC mutant population by selection on medium containing kanamycin.
Next, the in vitro accumulation of gtfBC mutants was examined. It was reasoned that, if the loss of GbpA affected recombination frequency, the change in frequency might be detected in both planktonic and biofilm cultures. Alternatively, if natural selection was the primary force behind the accumulation of gtfBC mutants, then greater recovery of deletion mutants should take place only from biofilm cultures. For these experiments, two-day biofilm or planktonic cultures were grown with a change of medium after 24 h. (For planktonic cultures, the bacteria were pelleted by centrifugation prior to the addition of new prewarmed medium). The cultures were then sonicated to break up bacterial aggregates, diluted, and plated to obtain isolated colonies (30 to 300 per plate) on brain heart infusion medium with 1% sucrose. Smooth and rough colonies were then enumerated and are presented in Table 2. The highest frequency for recovering gtfBC mutants was from biofilm cultures of the gbpA strain. This frequency was significantly higher than that from planktonic cultures of the gbpA strain as determined by a chi-square analysis. Conversely, there was no statistical difference between the frequencies of recovering gtfBC mutants from planktonic and biofilm cultures of wild-type S. mutans. No deletion mutants were recovered from planktonic or biofilm cultures of a recA strain.
TABLE 2.
Frequency of recovery of gtfBC deletion mutants in vitro
| Strain and growth condition (culture) | No. of gtfBC mutants | Total no. of colonies | Frequency of recovery of gtfBC mutants |
|---|---|---|---|
| Wild type, planktonic | 43 | 6,630 | 0.006 |
| Wild type, biofilm | 48 | 5,232 | 0.009 |
| gbpA mutant, planktonic | 37 | 6,151 | 0.006 |
| gbpA mutant, biofilm | 66 | 5,266 | 0.013 |
One possible basis for the observed natural selection was that pH differences existed between cultures of the two strains. To be sure that the loss of GbpA did not result in a greater reduction in pH, the terminal pH was measured in the planktonic phase above biofilms formed by the gbpA and wild-type strains of S. mutans. No difference was detected (4.3 for the wild-type versus 4.4 for the gbpA strain; n = 4). The rate of pH decline also did not differ between the two strains. If pH is a factor in the selection for gtfBC mutants, it must be so within localized areas of the biofilm.
Localized differences in pH are a possibility, since the loss of GbpA altered biofilm architecture (6), and rats colonized with the gbpA strain of S. mutans experienced a higher caries rate than rats colonized with the wild type (7). The accumulation of gtfBC deletion mutants (averaging 22%) mitigated both of these phenotypes (6, 7). It could be speculated that the accumulation of deletion mutants was due to different selection forces that accompany the change in biofilm architecture or a difference in the acid response of the gbpA strain.
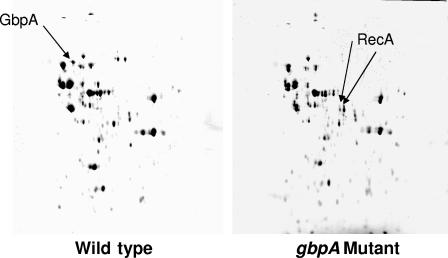
The latter possibility was investigated by utilizing two-dimensional gel electrophoresis to compare proteins recovered from biofilms formed by the gbpA and wild-type strains. Several spots differed between the strains (Fig. 2). An exhaustive proteomic comparison was not an objective; however, several spots corresponding to the greatest differences in magnitude between strains were sent out for identification (Steven H. Seeholzer, Fox Chase Cancer Institute, Philadelphia, PA). Interestingly, unique or more intense spots from the gbpA mutant biofilms included two isoforms of RecA. These results provided the first evidence that the frequency of recombination, in addition to natural selection, could be a factor contributing to the accumulation of gtfBC mutants.
FIG. 2.
Two-dimensional gel electrophoresis of proteins recovered from 48-h biofilms formed by the wild-type (left) and gbpA mutant (right) strains of Streptococcus mutans. The first-dimension isoelectric focusing employed a pH range of 4 to 8. The second-dimension sodium dodecyl sulfate-polyacrylamide gel electrophoresis was on an 8% polyacrylamide gel that was run until the dye front migrated off the gel. The gels were stained with Coomassie brilliant blue. The GbpA protein in the gel of wild-type proteins and the unique RecA isoforms in the gel of mutant proteins are indicated by arrows. Protein spots of interest were directly excised from the gel and sent to the Fox Chase Institute (Philadelphia, PA) for identification using mass spectrometry.
To determine if the protein spots coincided with an increased transcription of the recA gene, the relative expression of recA within gbpA or wild-type biofilms was measured via real-time reverse transcription-PCR. Conditions for biofilm growth matched those employed for the two-dimensional gel analysis. Instead of an increase, a slight decrease in transcription of recA was detected when normalized to the expression of the gyrA gene (Table 3). This decrease was not statistically significant. Several groups have catalogued changes in gene or protein expression in response to pH in S. mutans (4, 9, 16, 17) or in other species (5, 11). One of these (9) reports an increase in RecA in response to acidic pH. This may have occurred in our system, but we compared expression levels only between strains grown under identical conditions, not between pH levels. We cannot rule out the possibility that the unique RecA isoforms in the gbpA mutant biofilms catalyzed recombination more efficiently or that there was a transient increase in recA expression in the gbpA biofilm at an earlier time point.
TABLE 3.
Gene expression ratios for wild-type and gbpA strains of S. mutansa
RNA isolation, purification, and real-time PCR were performed as previously described (10). The expression ratios were calculated using the Pfaffl method (12). For this experiment, the ratios of gene expression were calculated from the critical-threshold values for the 400-pg cDNA dilutions.
Primers (113-bp product) for recA were 5′-TGATGTCCGTGGCAATACT-3′ and 5′-CCTTAAATGGTGGAGCAACTT-3′.
Primers (123-bp product) for gyrA were 5′-AACGCGTGACCTAATGGAAGT-3′ and 5′-CGAGCACGCAAAACAATAGA-3′.
Cumulatively, the results support the conclusion that natural selection is the predominant force behind the accumulation of gtfBC mutants in biofilms formed by a gbpA strain of S. mutans. The in vivo fluctuations in gtfBC mutant proportions were almost exclusively independent of new recombination events. In vitro, gtfBC mutants were recovered at the highest frequency from biofilms formed by the gbpA strain, and this frequency was statistically higher than the recovery from gbpA planktonic cultures. We believe that the change in biofilm architecture that accompanies the loss of GbpA yields the conditions that drive the natural selection of gtfBC mutants. A model is proposed in Fig. 3. A consequence of the change in biofilm structure may be local pH drops that overwhelm the ability of the organism to adapt to an acidic environment. Although unique RecA isoforms were identified in gbpA biofilms, the preponderance of data make it doubtful that recombination frequency plays more than an ancillary role in the accumulation of gtfBC mutants. The fact that the forces of natural selection change within the context of gbpA biofilms highlights the contribution GbpA provides to the formation of an optimal biofilm environment. The presence of the contiguous, highly homologous gtfB and gtfC genes allows S. mutans to respond to biofilm stress without a need for adaptive mutation.
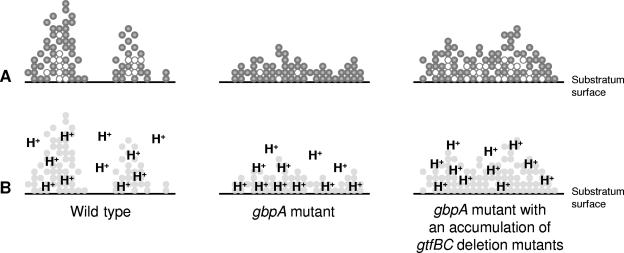
FIG. 3.
A model of biofilm architecture (A) and acid distribution (B) to explain the natural selection of gtfBC deletion mutants. In part A, the filled circles represent metabolically active bacteria and the unfilled circles represent metabolically less active organisms. In biofilms formed by the wild-type strain, microcolonies build outward from the substratum surface (left panel). Metabolically less active organisms are confined to the inner portions of microcolonies. There are portions of the substratum that are only sparsely coated with bacteria. When GbpA is missing, S. mutans loses the ability to build tall microcolonies and the substratum is more evenly coated (middle panel). Consequently, there are higher numbers of metabolically active bacteria in contact with the substratum surface and a greater acid burden that could explain the higher caries rates of the gbpA mutant in rats. But this biofilm is more stressful for the bacteria, and so there is a selection for gtfBC mutants that can partially restore the biofilm architecture (right panel). The restoration of architecture adds depth to the biofilm that, in turn, sequesters some bacteria in contact with the substratum, rendering them less metabolically active and leading to a more benign distribution of acid molecules.
Acknowledgments
We acknowledge the support of grants DE10058 (J.A.B.) and DE09081 (S.M.M.) from the National Institute of Dental and Craniofacial Research.
Footnotes
Published ahead of print on 3 November 2006.
REFERENCES
- 1.Banas, J. A. 2004. Virulence properties of Streptococcus mutans. Front. Biosci. 9:1267-1277. [DOI] [PubMed] [Google Scholar]
- 2.Cairns, J., J. Overbaugh, and S. Miller. 1988. The origin of mutants. Nature 335:142-145. [DOI] [PubMed] [Google Scholar]
- 3.Cairns, J., and P. L. Foster. 1991. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chia, J.-S., Y.-Y. Lee, P.-T. Huang, and J.-Y. Chen. 2001. Identification of stress-responsive genes in Streptococcus mutans by differential display reverse transcription-PCR. Infect. Immun. 69:2493-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotter, P. D., and C. Hill. 2003. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67:429-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazlett, K. R. O., J. E. Mazurkiewicz, and J. A. Banas. 1999. Inactivation of the gbpA gene of Streptococcus mutans alters structural and functional aspects of plaque biofilm which are compensated by recombination of the gtfB and gtfC genes. Infect. Immun. 67:3909-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hazlett, K. R. O., S. M. Michalek, and J. A. Banas. 1998. Inactivation of the gbpA gene of Streptococcus mutans increases virulence and promotes in vivo accumulation of recombinations between the glucosyltransferase B and C genes. Infect. Immun. 66:2180-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krašovec, R., and I. Jerman. 2003. Bacterial multicellularity as a possible source of antibiotic resistance. Med. Hypoth. 60:484-488. [DOI] [PubMed] [Google Scholar]
- 9.Len, A. C. L., D. W. S. Harty, and N. A. Jacques. 2004. Stress-responsive proteins are upregulated in Streptococcus mutans during acid tolerance. Microbiology 150:1339-1351. [DOI] [PubMed] [Google Scholar]
- 10.Mattos-Graner, R. O., K. A. Porter, D. J. Smith, Y. Hosogi, and M. J. Duncan. 2006. Functional analysis of glucan binding protein B from Streptococcus mutans. J. Bacteriol. 188:3813-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson, E. R. 1993. Influence of pH on bacterial gene expression. Mol. Microbiol. 8:5-14. [DOI] [PubMed] [Google Scholar]
- 12.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res. 29:2002-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth, J. R., E. Kugelberg, A. B. Reams, E. Kofoid, and D. I. Andersson. 2006. Origin of mutations under selection: the adaptive mutation controversy. Annu. Rev. Microbiol. 60:477-501. [DOI] [PubMed] [Google Scholar]
- 14.Ueda, S., and H. K. Kuramitsu. 1988. Molecular basis for the spontaneous generation of colonization-defective mutants of Streptococcus mutans. Mol. Microbiol. 2:135-140. [DOI] [PubMed] [Google Scholar]
- 15.Ueda, S., T. Shiroza, and H. K. Kuramitsu. 1988. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene 69:101-109. [DOI] [PubMed] [Google Scholar]
- 16.Welin, J., J. C. Wilkins, D. Beighton, K. Wrzesinski, S. J. Fey, P. Mose-Larsen, I. R. Hamilton, and G. Svensäter. 2003. Effect of acid shock on protein expression by biofilm cells of Streptococcus mutans. FEMS Microbiol. Lett. 227:287-293. [DOI] [PubMed] [Google Scholar]
- 17.Wilkins, J. C., K. A. Homer, and D. Beighton. 2002. Analysis of Streptococcus mutans proteins modulated by culture under acidic conditions. Appl. Environ. Microbiol. 68:2382-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]