Abstract
Laboratory-reared and field-collected Amblyomma americanum ticks were hosts of a Coxiella sp. and a Rickettsia sp. While the Coxiella sp. was detected in 50 of 50 field-collected ticks, the Rickettsia sp. was absent from 32% of ticks. The Coxiella sp. showed evidence of a reduced genome and may be an obligate endosymbiont.
The lone star tick Amblyomma americanum is a common pest of humans and domestic animals in the southern United States, and its range now extends into the Northeast. In parts of New Jersey A. americanum ticks are more commonly encountered than the deer tick Ixodes scapularis (13). While originally considered a nuisance species, A. americanum is now recognized as a vector of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis, and E. ewingii, a cause of granulocytic ehrlichiosis in humans and dogs (16). A. americanum is also the vector of Borrelia lonestari (1), an organism that has been implicated but not proven (5, 17) as a cause of a tick-associated rash illness in the southern United States (2, 9). According to Childs and Paddock, the “public health relevance of lone star ticks is no longer in question” (3). Yet comparatively little is known about the biology of this or other Amblyomma spp.
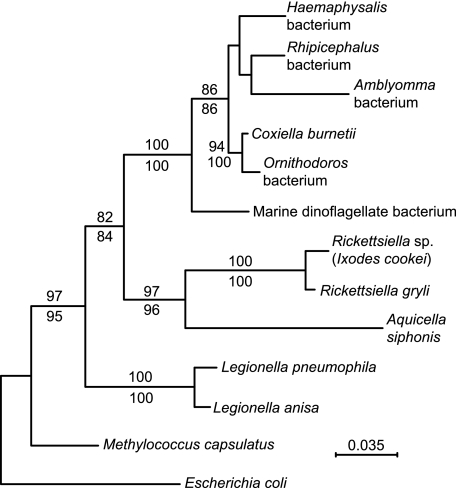
As part of a genetics study of A. americanum, we produced a cDNA library from RNA extracted from the midguts of laboratory-reared female A. americanum ticks. Upon sequencing cDNA clones, we found that 4% of ∼500 nonredundant cDNA sequences had closest matches to sequences of the Q-fever agent Coxiella burnetii (unpublished findings). These included coding sequences for DnaK (DQ912980); FusA, elongation factor G (DQ908900); RpsF, ribosomal protein S6 (DQ908901); RpsG, ribosomal protein S7 (DQ908902), and 16S rRNA gene (AY939824). Coxiella-like bacteria have been reported to have been present in the hard ticks Haemaphysalis longicornis and Rhipicephalus sanguineus and the soft tick Ornithodoros moubata (12), but these had not been further characterized. Figure 1 is a phylogram of 16S rRNA sequences of the Amblyomma bacterium, C. burnetii and related bacteria (12), other members of Legionellales, and two other γ-proteobacteria, Escherichia coli and the methanotroph Methylococcus capsulatus. The analysis indicates that C. burnetii, the bacteria of Haemaphysalis, Rhipicephalus, and Ornithodoros ticks, and the Amblyomma bacterium are monophyletic and that they, along with a symbiont of the marine dinoflagellate Heterocapsa circularisquama, constitute a clade separate from Legionella spp. and from a clade that comprises entomopathogenic Rickettsiella spp. and the protozoan-associated bacterium Aquicella siphonis. Similar phylogenetic analyses of the alignments of the housekeeping genes fusA, rpsF, and rpsG of the Amblyomma bacterium and corresponding genes of E. coli K-12 (NC_000913), M. capsulatus (NC_002977), L. pneumophila (NC_002942), and C. burnetii (NC_002971) confirmed that the A. americanum bacterium is a sister taxon to C. burnetii (data not shown). Nucleotide sequence identities between the Amblyomma bacterium and C. burnetii for fusA, rpsF, and rpsG were 72%, 64%, and 69%, respectively.
FIG. 1.
Phylogram of partial 16S rRNA genes of an Amblyomma americanum bacterium (accession number AY939824) and selected other γ-proteobacteria: Coxiella burnetii bacterium (NC_002971), Haemaphysalis longicornis bacterium (AY342035), Rhipicephalus sanguineus bacterium (D84559), Ornithodoros moubata bacterium (AB001521), a marine dinoflagellate bacterium (AB058918), Rickettsiella grylli (U97547), a Rickettsiella sp. of Ixodes woodi (AF383621), the protozoan-associated bacterium Aquicella siphonis (AY359283), Legionella pneumophila (NC_006369), Legionella anisa (AY744776), Methylococcus capsulatus (NC_002977), and Escherichia coli K-12 (NC_000913). Nucleotide positions with gaps in the aligned sequences were excluded. Bootstrap values of nodes with >80% values by maximum likelihood (500 replicates; shown above the line) or neighbor-joining (1,000 replicates; shown below the line) distance criteria are indicated and were estimated using PHYLO_WIN phylogenetic analysis software (http://pbil.univ-lyon1.fr/software/phylowin.html). Bar, nucleotide distance.
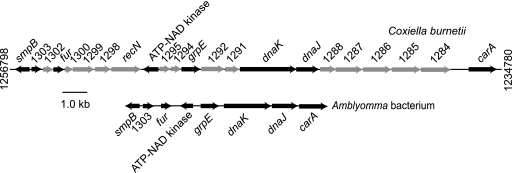
We next screened a lambda bacteriophage library of A. americanum genomic DNA, which we had previously produced from eggs (7), with the cloned dnaK cDNA of the symbiont as a probe, as described previously (7). A hybridizing clone was isolated, and the insert was sequenced over both strands on a CEQ 8000 automated sequencer (Beckman Coulter, Fullerton, CA) with custom primers. Figure 2 shows physical maps of 8,332 nucleotides of the Amblyomma bacterium (DQ912980) and 22,018 nucleotides of a homologous region of C. burnetii (NC_002971) (14). These two genome fragments had the same gene order for genes in common: smpB, fur, grpE, dnaK, dnaJ, and carA. But the Amblyomma bacterium's fragment lacked nearly all the hypothetical proteins, which are designated by number alone in the figure, of C. burnetii in this region. The Amblyomma bacterium may also lack the DNA repair function provided by recN. These findings provided further evidence that the tick bacterium is in the same lineage as C. burnetii but also that it has a reduced genome, a common feature of obligate endosymbionts of invertebrates (10).
FIG. 2.
Physical maps of 22 kb of the chromosome of Coxiella burnetii (accession number NC_002971) and a corresponding 9-kb region of an Amblyomma americanum bacterium (DQ912980). The arrows correspond to the lengths of the open reading frames (ORFs) and the presumed direction of transcription. Black arrows indicate ORFs found in both sequences. The numbers at the ends of the C. burnetii sequence are the genome nucleotide positions. ORFs with matches to known genes, e.g., dnaK, or with a protein family of presumed function are indicated. ORFs designated by numbers alone indicate hypothetical proteins of C. burnetii (14). Bar, sequence length.
Using quantitative PCR with specific primers and dye-labeled probes, we then characterized the distribution, prevalence, and copy numbers of the Coxiella sp. in A. americanum. We compared these results with those obtained for a Rickettsia bellii-like bacterium that had been identified in A. americanum (U11012) and Amblyomma spp. in Brazil (8). The targets for the Coxiella sp. and the Rickettsia sp. were the fusA gene and the citrate synthase gene (gltA; accession no. AY388956), respectively. The internal control for both PCR assays was an exon of the A. americanum gene for macrophage inhibitory factor (MIF), which we had previously demonstrated to be present in this species (7). The ratios of copies of fusA or gltA to the MIF gene provided estimates of the burden of the Coxiella sp. and Rickettsia sp. in the ticks. Total DNA was obtained by freezing and pulverizing adult ticks in liquid nitrogen and then extracting DNA as described previously (15). The 6-carboxyfluorescein-labeled probe, forward primer, and reverse primer specific for fusA were 5′ATTTACCTGCACCTACTGATATACCTGAT3′, 5′AGCCTTATTAGATGCTGTGGTTGA3′, and 5′CGTCTGCTTCTTCACCTCGAA3′, respectively. The probe, forward primer, and reverse primer for the MIF gene were 5′CACTGATGACCCATGCGCTATTGCAAAT3′, 5′CCACATCAACGCCGATCAG3′, and 5′TTTGTTCTCCTTTGGACTCAGACA3′, and the probe, forward primer, and reverse primer for gltA were 5′ATGCTTCTACTTCAACAGTCCGAATTGCCG3′, 5′TCCTACATGCCGACCATGAG3′, and 5′AAAGGGTTAGCTCCGGATGAG3′, respectively. PCR was carried out using a Rotor-Gene RG-3000 apparatus (Corbett Research, San Francisco, CA). The reactions were carried out with 1.5 mM MgCl2, 0.2 mM (each) deoxynucleoside triphosphates, 3 U Taq polymerase (Roche Diagnostics, Mannheim, Germany), and 0.1 μM of primers, and conditions were 1 cycle at 95°C for 4 min, 40 cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 1 min, and finally 72°C for 7 min. For standard curves, the PCR targets were cloned into the plasmid pCR2.1-TOPO (Invitrogen, San Diego, CA).
We found no evidence of this Coxiella sp. in five each of field-collected adult Ixodes scapularis and Dermacentor variabilis ticks by the fusA assay, and the MIF gene assay was specific for A. americanum (data not shown). Of 10 laboratory-reared A. americanum females at 3 months postmolt, which were obtained from Oklahoma State University, all were positive for the Coxiella sp. by PCR; the mean (95% confidence interval) fusA/MIF gene copy number ratio was 85 (range, 75 to 95). We then dissected four adult females as described previously (6) and extracted DNA. We found that the mean fusA/MIF ratios were 381 (range, 252 to 512) in midguts, 79 (range, 39 to 298) in ovaries, and 12 (range, 11 to 14) in salivary glands.
We examined the distribution of the Coxiella sp. and Rickettsia sp. in natural populations of A. americanum by randomly sampling 10 female ticks from collections of ticks from five different state parks in the United States in Maryland, Georgia, Kentucky, and Oklahoma. The ticks were collected in 2004 by drag sampling (4) and stored in 70% ethanol until DNA extraction and quantitative PCR were performed as described above. The results are shown in Table 1. The Coxiella sp. was found in all ticks at each location. In contrast, 16 (32%) of the 50 ticks did not have a detectable Rickettsia sp. at a ≥0.01 gltA/MIF copy ratio (2-tailed chi-square test; P < 0.0001). A mean fusA/MIF ratio of 129 (range, 81 to 177) for the Coxiella sp. approximated what was observed in laboratory ticks. Among field-collected ticks with the Rickettsia sp., the mean gltA/MIF copy ratio was 4.3 (range, 2.7 to 5.9), 30-fold lower than observed for the relative copy number of the Coxiella sp.
TABLE 1.
Prevalences and relative numbers of Coxiella sp. and Rickettsia sp. bacteria in female A. americanum ticks collected in five state parks in the United Statesa
| State | State park | No. of ticks with Coxiella sp. detected by PCR/ total no. of ticks | Mean fusA/MIF ratio (95% CI)b | No. of ticks with Rickettsia sp. detected by PCR/ total no. of ticks | Mean gltA/MIF ratio (95% CI)b |
|---|---|---|---|---|---|
| Maryland | Elk Neck | 10/10c | 52 (7-97) | 5/10c | 2.8 (0.8-4.9) |
| Georgia | Fort McAllister | 10/10 | 106 (8-205) | 7/10 | 4.1 (0.7-7.4) |
| Georgia | Hard Labor Creek | 10/10 | 79 (9-150) | 6/10 | 2.0 (0.1-4.0) |
| Kentucky | Green River Lake | 10/10 | 47 (4-91) | 9/10 | 2.2 (0.4-4.0) |
| Oklahoma | Osage Hill | 10/10 | 75 (15-135) | 7/10 | 2.3 (0.7-3.9) |
| Total | 50/50 | 72 (43-101) | 34/50 | 2.7 (1.8-3.6) |
fusA, Coxiella sp. elongation factor G gene; MIF, A. americanum macrophage inhibitory factor gene; gltA, Rickettsia sp. citrate synthase gene.
Values represent mean ratios of fusA or gltA to MIF gene copies per positive tick result (95% confidence interval).
The value for the Cochran-Mantel-Haenszel statistic of prevalence of Coxiella sp. versus Rickettsia sp. among sampled ticks, stratified by state park, was 18.7 with 1 degree of freedom (two-tailed test; P < 10−6) (StatXact v. 6, Cytel Software Corp.).
In summary, we identified a hitherto unknown Coxiella sp. in all laboratory-reared and field-collected A. americanum females. The bacterium was more prevalent and in higher densities than an R. bellii-like species in lone star ticks. The ubiquity of the Coxiella sp. and its presence in a genomic library derived from eggs indicate that it is an endosymbiont (11). Evidence of a reduced genome for the bacterium supports this proposal. The consequences of this bacterium for the fitness of its tick host, including nutrient provisioning, remain to be determined.
Acknowledgments
This work was supported by an Ellison Medical Foundation Senior Scholar in Global Infectious Diseases grant, National Institutes of Health grant AI065359, and Centers for Disease Control and Prevention cooperative agreement CI00017-03.
We thank Anne Gatewood and Durland Fish of Yale University for the field-collected ticks and Thomas McDonald and Hany Mattaous for technical assistance.
Footnotes
Published ahead of print on 3 November 2006.
REFERENCES
- 1.Barbour, A. G., G. O. Maupin, G. J. Teltow, C. J. Carter, and J. Piesman. 1996. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect. Dis. 173:403-409. [DOI] [PubMed] [Google Scholar]
- 2.Campbell, G. L., W. S. Paul, M. E. Schriefer, R. B. Craven, K. E. Robbins, and D. T. Dennis. 1995. Epidemiologic and diagnostic studies of patients with suspected early Lyme disease, Missouri, 1990-1993. J. Infect. Dis. 172:470-480. [DOI] [PubMed] [Google Scholar]
- 3.Childs, J. E., and C. D. Paddock. 2003. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu. Rev. Entomol. 48:307-337. [DOI] [PubMed] [Google Scholar]
- 4.Falco, R. C., and D. Fish. 1992. A comparison of methods for sampling the deer tick, Ixodes dammini, in a Lyme disease endemic area. Exp. Appl. Acarol. 14:165-173. [DOI] [PubMed] [Google Scholar]
- 5.James, A. M., D. Liveris, G. P. Wormser, I. Schwartz, M. A. Montecalvo, and B. J. Johnson. 2001. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J. Infect. Dis. 183:1810-1814. [DOI] [PubMed] [Google Scholar]
- 6.Jasinskas, A., and A. G. Barbour. 2005. The Fc fragment mediates the uptake of immunoglobulin C from the midgut to hemolymph in the ixodid tick Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 42:359-366. [DOI] [PubMed] [Google Scholar]
- 7.Jaworski, D. C., A. Jasinskas, C. N. Metz, R. Bucala, and A. G. Barbour. 2001. Identification and characterization of a homologue of the pro-inflammatory cytokine Macrophage Migration Inhibitory Factor in the tick, Amblyomma americanum. Insect Mol. Biol. 10:323-331. [DOI] [PubMed] [Google Scholar]
- 8.Labruna, M. B., T. Whitworth, D. H. Bouyer, J. McBride, L. M. Camargo, E. P. Camargo, V. Popov, and D. H. Walker. 2004. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the State of Rondonia, Western Amazon, Brazil. J. Med. Entomol. 41:1073-1081. [DOI] [PubMed] [Google Scholar]
- 9.Masters, E., S. Granter, P. Duray, and P. Cordes. 1998. Physician-diagnosed erythema migrans and erythema migrans-like rashes following lone star tick bites. Arch. Dermatol. 134:955-960. [DOI] [PubMed] [Google Scholar]
- 10.Moran, N. A., and J. J. Wernegreen. 2000. Lifestyle evolution in symbiotic bacteria: insights from genomics. Trends Ecol. Evol. 15:321-326. [DOI] [PubMed] [Google Scholar]
- 11.Munderloh, U. G., S. D. Jauron, and T. J. Kurtti. 2005. The tick: a different kind of host for human pathogens, p. 37-64. In J. L. Goodman, D. T. Dennis, and D. E. Sonenshine (ed.), Tick-borne diseases of humans. ASM Press, Washington, D.C.
- 12.Noda, H., U. G. Munderloh, and T. J. Kurtti. 1997. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ Microbiol. 63:3926-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulze, T. L., R. A. Jordan, C. J. Schulze, T. Mixson, and M. Papero. 2005. Relative encounter frequencies and prevalence of selected Borrelia, Ehrlichia, and Anaplasma infections in Amblyomma americanum and Ixodes scapularis (Acari: Ixodidae) ticks from central New Jersey. J. Med. Entomol. 42:450-456. [DOI] [PubMed] [Google Scholar]
- 14.Seshadri, R., I. T. Paulsen, J. A. Eisen, T. D. Read, K. E. Nelson, W. C. Nelson, N. L. Ward, H. Tettelin, T. M. Davidsen, M. J. Beanan, R. T. Deboy, S. C. Daugherty, L. M. Brinkac, R. Madupu, R. J. Dodson, H. M. Khouri, K. H. Lee, H. A. Carty, D. Scanlan, R. A. Heinzen, H. A. Thompson, J. E. Samuel, C. M. Fraser, and J. F. Heidelberg. 2003. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc. Natl. Acad. Sci. USA 100:5455-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsao, J. I., J. T. Wootton, J. Bunikis, M. G. Luna, D. Fish, and A. G. Barbour. 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl. Acad. Sci. USA 101:18159-18164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varela, A. S., V. A. Moore, and S. E. Little. 2004. Disease agents in Amblyomma americanum from northeastern Georgia. J. Med. Entomol. 41:753-759. [DOI] [PubMed] [Google Scholar]
- 17.Wormser, G. P., E. Masters, D. Liveris, J. Nowakowski, R. B. Nadelman, D. Holmgren, S. Bittker, D. Cooper, G. Wang, and I. Schwartz. 2005. Microbiologic evaluation of patients from Missouri with erythema migrans. Clin. Infect. Dis. 40:423-428. [DOI] [PMC free article] [PubMed] [Google Scholar]