Abstract
Acetate is the most abundant intermediate of organic matter degradation in anoxic rice field soil and is converted to CH4 and/or CO2. Aceticlastic methanogens are the primary microorganisms dissimilating acetate in the absence of sulfate and reducible ferric iron. In contrast, very little is known about bacteria capable of assimilating acetate under methanogenic conditions. Here, we identified active acetate-assimilating microorganisms by using a combined approach of frequent label application at a low concentration and comparative RNA-stable isotope probing with 13C-labeled and unlabeled acetate. Rice field soil was incubated anaerobically at 25°C for 12 days, during which 13C-labeled acetate was added at a concentration of 500 μM every 3 days. 13C-labeled CH4 and CO2 were produced from the beginning of the incubation and accounted for about 60% of the supplied acetate 13C. RNA was extracted from the cells in each sample taken and separated by isopycnic centrifugation according to molecular weight. Bacterial and archaeal populations in each density fraction were screened by reverse transcription-PCR-mediated terminal restriction fragment polymorphism analysis. No differences in the bacterial populations were observed throughout the density fractions of the unlabeled treatment. However, in the heavy fractions of the 13C treatment, terminal restriction fragments (T-RFs) of 161 bp and 129 bp in length predominated. These T-RFs were identified by cloning and sequencing of 16S rRNA as from a Geobacter sp. and an Anaeromyxobacter sp., respectively. Apparently these bacteria, which are known as dissimilatory iron reducers, were able to assimilate acetate under methanogenic conditions, i.e., when CO2 was the predominant electron acceptor. We hypothesize that ferric iron minerals with low bioavailability might have served as electron acceptors for Geobacter spp. and Anaeromyxobacter spp. under these conditions.
In anoxic environments, a variety of microorganisms are involved in the degradation of organic matter by interacting with each other. The functions of the microorganisms consist of hydrolysis, fermentation, syntrophic oxidation, homoacetogenesis, methanogenesis, and oxidation coupled with the reduction of inorganic electron acceptors. Acetate is the most important intermediate under anaerobic conditions and is converted to CH4 and/or CO2 (21, 35, 48, 51). The degradation of acetate is strongly influenced by the availability of exogenous electron acceptors, such as sulfate or ferric iron. Thermodynamic theory predicts that in the presence of these electron acceptors (i.e., reduction phase), acetate is oxidized to CO2 associated with the reduction, while it is converted to CH4 and CO2 in the absence of these electron acceptors (i.e., methanogenic phase).
The reduction phase and the methanogenic phase proceed sequentially in flooded rice field soils (1, 40). The pathway of acetate degradation during these phases was demonstrated by turnover experiments using [2-14C]acetate. However, the carbon recovery of substrate and gas products was unexpectedly low in the methanogenic phase (4). This suggests that part of the acetate must have been assimilated by as-yet-unknown microorganisms. Whereas it is well known that aceticlastic methanogenic archaea (i.e., Methanosarcina spp. and Methanosaeta spp.) primarily degrade and assimilate acetate during the methanogenic phase (7, 17, 28, 29, 59), very little is known about the members of the domain Bacteria that are active under these conditions, probably because of the high diversity of the community composition and function.
Stable isotope probing (SIP) allows study of the ecophysiology of the microbial populations on the basis of the incorporation of a 13C-labeled substrate into biomass under quasinatural conditions (9, 24, 34, 43). SIP involves the following experimental steps: (i) microbial assimilation of a defined 13C-labeled substrate and 13C labeling of the nucleic acids, (ii) extraction of the total nucleic acids, (iii) separation of the labeled nucleic acids by isopycnic centrifugation, (iv) genetic profiling, and (v) cloning, sequencing, and phylogenetic analysis. High substrate concentrations and long incubation times should be avoided because they may cause a discrepancy between the experimental and the actual environmental conditions, for which the carbon flux may be high although the substrate pool is low (11). In this study, we added several small dosages of substrate at a low concentration (500 μM) to prevent enrichment conditions. Comparative SIP with 13C-labeled and unlabeled substrates was used to improve the detection of the 13C labeling of rRNA (31). Even if the substrate concentration and incubation time are insufficient for complete 13C labeling of nucleic acids, incorporation of substrate 13C into nucleic acids can be detected by comparison with a control using unlabeled substrate.
Rice fields are a significant source of atmospheric methane and have attracted much attention because of the strong impact on global warming (2, 15, 42). In-depth knowledge of the microbial interspecies relationships in anoxic rice fields might be of particular importance for developing options for mitigation of methane emission. However, which bacteria compete for substrate with methanogens is still mostly unclear. The objective of this study was to identify the acetate-assimilating microorganisms during the methanogenic phase in rice field soil by using a combination of frequent label application and comparative RNA-SIP.
MATERIALS AND METHODS
Anaerobic incubation of rice field slurry.
Rice field soil was collected from the rice fields at the Italian Rice Research Institute in Vercelli, Italy. Details of soil characteristics and the sampling site have been described in previous reports (52, 53). The soil was air dried, stored, and sieved as described previously (6). Prior to experimental use, a soil slurry was prepared by mixing the dry soil with distilled water at a ratio of 1:1. Aliquots (5 ml) of the homogenized soil slurry were placed in 25-ml serum vials, which were then sealed with butyl rubber septa. The soil slurry was preincubated anaerobically in the dark at 25°C for 30 days in order to allow for the activation of the soil slurry microorganisms and the reduction of available sulfate and ferric iron (28, 29, 30). After preincubation, the headspace of soil slurries was flushed with N2. Three treatments were prepared: (i) 13C treatment with [U-13C]acetate (99 atom%; Sigma, Taufkirchen, Germany) as the substrate, (ii) unlabeled treatment with unlabeled acetate as the substrate, and (iii) control treatment with distilled water instead of substrate. Each soil slurry treatment was run in triplicate with static incubation for 12 days at 25°C. The appropriate substrate was added to each vial at a final concentration of 500 μM on days 0, 3, 6, and 9. Samples of headspace gas, slurry water, and soil were removed every third day from each vial of each set of different slurry treatments done in triplicate. Total CH4 and CO2 in the headspace samples were analyzed using a standard gas chromatography method (49). The 13C atoms percent of CH4 and CO2 was analyzed by a gas chromatography-isotope ratio mass spectrometry method as described previously (8). The concentrations of volatile fatty acids from the slurry water sample were measured using a high-pressure liquid chromatography method (18). The soil samples were stored at −80°C for subsequent molecular analyses.
RNA extraction and density gradient centrifugation.
RNA was extracted from 0.5 ml of each soil slurry sample from one set of the 13C treatment and the unlabeled treatment after 12 days of incubation by a direct lysis protocol involving bead beating as described previously (39). Total RNA was quantified using the Ribogreen RNA quantification kit (Invitrogen, Karlsruhe, Germany) according to the manufacturer's instructions. RNA extracts (500 ng of RNA) of the 13C treatment and unlabeled treatment were mixed with cesium trifluoroacetate (CsTFA) (Amersham Biosciences, Freiburg, Germany) solution. The mixture was subjected to equilibrium density gradient centrifugation under the conditions reported previously (31). The centrifugation enabled the separation of RNA on the basis of density. Gradients of the density-separated RNA were fractionated, and the CsTFA buoyant density (BD) of each fraction was determined (31).
RT-PCR amplification and terminal restriction fragment length polymorphism analysis.
Each density fraction of RNA from the 13C treatment and unlabeled treatment was subjected to reverse transcription-PCR (RT-PCR) using a one-step RT-PCR system (Access Quick; Promega, Mannheim, Germany) for terminal restriction fragment length polymorphism (T-RFLP) fingerprinting. The PCR primer sets Ba27f-6-carboxyfluorescein (FAM)/Ba907r and Ar109f/Ar912rt-FAM were used to amplify the transcripts of bacterial and archaeal 16S rRNAs, respectively (29, 32). Reverse transcription was carried out at 48°C for 45 min, and PCR was started with an initial denaturation step at 94°C for 3 min. The thermal profile of PCR amplification consisted of 20 cycles under stringent conditions, each including 30 s at 94°C, 45 s at 52°C, and 90 s at 72°C, which was followed by a final extension step of 5 min at 72°C. The products were checked by electrophoresis on 1% agarose gels. RT-PCR amplicons were digested using MspI and TaqI for Bacteria and Archaea, respectively. Digested amplicons were desalted using Auto Seq G-50 columns (Amersham Biosciences). Prior to electrophoresis, 1 μl of the digest was suspended in 12 μl of Hi-Di formamide (Applied Biosystems, Weiterstadt, Germany) and 0.25 μl of carboxy-X-rhodamine (ROX)-labeled MapMarker 1000 ladder (Bio-Ventures, Murfreesboro, TN). The mixture was denatured at 95°C for 3 min and cooled immediately on ice. Size separation of terminal restriction fragments (T-RFs) was performed using an ABI 310 genetic analyzer (Applied Biosystems). The conditions of electrophoresis were the same as described previously (30).
Sequencing and phylogenetic analyses.
Selected density fractions of bacterial RNA were amplified for cloning using the primer set Ba27f/Ba907r under the thermal conditions mentioned above. The RT-PCR products were ligated into the plasmid vector pGEM-T Easy (Promega), and the ligation mixture was used to transform Escherichia coli JM109 supercompetent cells (Promega) according to the manufacturer's instructions. We randomly selected 71 clones from the 13C treatment and 67 clones from the unlabeled treatment. The 16S rRNA segments were sequenced at the automatic DNA isolation and sequencing facility of the Max-Planck-Institute for Plant Breeding Research, Cologne, Germany, using BigDye terminator cycle sequencing chemistry (Applied Biosystems) (25, 32). Chimeric structures were detected by separately analyzing the phylogenies of terminal stretches at the 5′ and 3′ ends (“fractional treeing”) (26, 27). Phylogenetic analyses were conducted using the ARB software package (http://www.arb-home.de) (26) as described previously (33). Phylogenetic trees with reference 16S rRNA sequences (>1,400 nucleotides) were calculated using neighbor-joining, maximum-parsimony, and maximum-likelihood methods. Bootstrap values were obtained from 1,000 replications. Partial 16S rRNA sequences (i.e., approximately 850 bp) obtained in this study were added to the trees by using the ARB parsimony tool. The similar topologies of the trees constructed with different algorithms were confirmed, and the trees best fitting the overall consensus were chosen for presentation.
Nucleotide sequence accession numbers.
The nucleotide sequence data obtained in this study have been deposited in the DDBJ nucleotide sequence database under accession numbers AB265824 to AB265961.
RESULTS
Biogeochemical activities in rice field slurry incubation with 13C-labeled and unlabeled acetate.
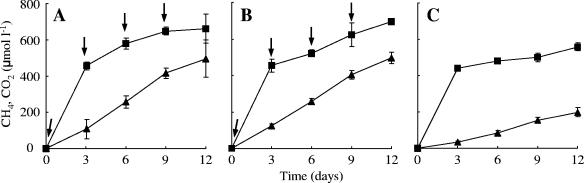
Anoxic preincubated rice field soil was repeatedly supplemented with 13C-labeled and unlabeled acetate. Before each new addition of substrate, the acetate concentration was monitored, and it was found to have decreased to less than 15 μM in all the treatments (i.e., 13C treatment, unlabeled treatment, and control treatment). Apparently, the size of the acetate pool was low in the rice field slurry and the added acetate was degraded within 3 days. Other volatile fatty acids (e.g., propionate or butyrate) were not detectable during the incubation. In total, 10 μmol of acetate was added to the 13C treatment and the unlabeled treatment. CH4 and CO2 were produced immediately after the beginning of the incubation in all three treatments (Fig. 1). The production of CH4 and CO2 in the control treatment (no acetate addition) was less than those in the 13C treatment and unlabeled treatment (Fig. 1A, B, and C). Addition of acetate increased the production of CH4 and CO2. There was little difference in CH4 and CO2 production between the 13C treatment and unlabeled treatment (Fig. 1A and B). CH4 increased in both treatments linearly to a concentration of approximately 500 μM during 12 days of the incubation. On the other hand, the CO2 concentration increased rapidly during the first 3 days of the incubation and thereafter increased gradually to 660 to 700 μmol liter−1 at day 12. The high CO2 concentration in the initial stage of the incubation was probably caused by degassing and establishment of the CO2-bicarbonate equilibrium. The similarity of biogeochemical activities in both the 13C treatment and the unlabeled treatment indicates similar physiological actions of the indigenous microbial community.
FIG. 1.
Change in concentrations of CH4 (▴) and CO2 (▪) during anaerobic incubation of a rice field slurry. (A) 13C treatment; (B) unlabeled treatment; (C) control treatment. Arrows indicate when acetate was added to each vial at a final concentration of 500 μM. The error bars represent the standard deviations of three replications.
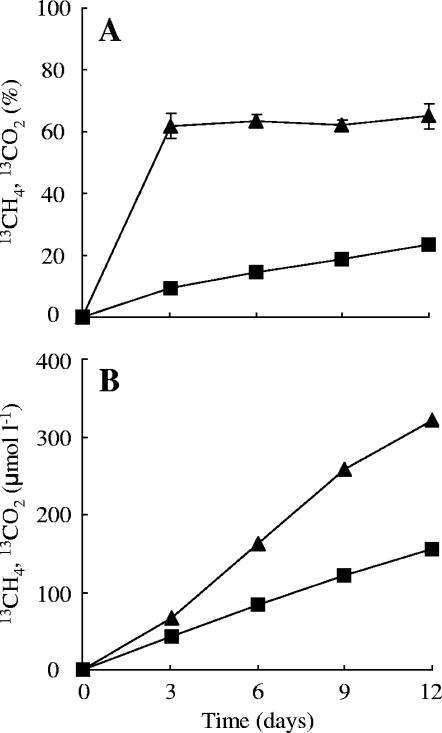
To resolve the fate of the substrate 13C, the 13C atoms percent of the products CH4 and CO2 were followed over time (Fig. 2). The 13C atoms percent of CH4 increased rapidly to more than 60% within the first 3 days of the incubation and afterwards remained at this level, while the 13C atoms percent of CO2 increased slowly to 23.4% at day 12 (Fig. 2A). The total amount of 13C-labeled CH4 and CO2 was calculated using the concentrations in the gas and liquid phases (Fig. 1A) and the 13C atoms percent (Fig. 2B). 13CH4 and 13CO2 were produced immediately upon the start of incubation and increased linearly to concentrations of 320 μmol and 160 μmol per liter of headspace at day 12, respectively. The total amount of 13C carbon from acetate added during the incubation was 20 μmol (2 × 10 μmol acetate). The amounts of 13CH4 and 13CO2 produced until day 12 were 6.4 and 3.1 μmol, respectively. Dissolved 13CO2 components were estimated to be 2.6 μmol by using the gaseous 13CO2 concentration and the pH of the rice field slurry (pH 7.7).
FIG. 2.
Conversion of 13C-labeled acetate to 13CH4 and 13CO2 during incubation of the 13C treatment. (A) Change in the 13C atoms percent of CH4 (▴) and CO2 (▪) in the headspace. Error bars indicates standard deviations of three replications. (B) Change in the concentrations of 13CH4 (▴) and 13CO2 (▪).
Comparative T-RFLP fingerprinting of bacterial and archaeal 16S rRNAs separated by density gradient centrifugation.
Total RNA was extracted from the soil samples of the 13C treatment and unlabeled treatment after 12 days of incubation, during which the added acetate (10 μmol) was mostly turned over. RNA was separated by isopycnic centrifugation according to molecular weight, and subsequently the gradients of the RNA were fractionated. Bacterial and archaeal rRNAs in each density fraction were used for RT-PCR-mediated T-RFLP analysis (Fig. 3). Incorporation of substrate 13C into the microbial population was detected by comparing the T-RFLP fingerprints of rRNA derived from the 13C treatment with those from the unlabeled treatment.
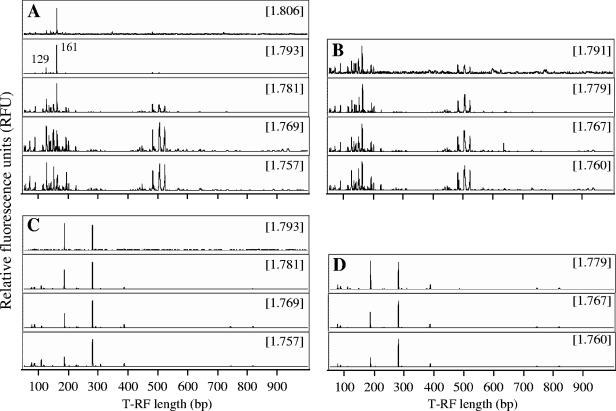
FIG. 3.
T-RFLP fingerprints of the bacterial (A and B) and archaeal (C and D) 16S rRNAs separated by isopycnic centrifugation from the 13C treatment (A and C) and the unlabeled treatment (B and D). The CsTFA BDs (g ml−1) of the fractions are shown in brackets.
Bacterial rRNA from the 13C treatment was successfully amplified in the fractions with BDs of 1.757 to 1.806 g ml−1, while that from unlabeled treatment was amplified in the fractions with BDs of 1.760 to 1.791 g ml−1. RNA obtained from the 13C treatment became slightly heavier than that from the unlabeled treatment. The T-RFLP fingerprint patterns of the 13C treatment and unlabeled treatment were similar in the low-density fractions with BDs of 1.757 to 1.769 g ml−1 (Fig. 3A and B). The fingerprints of the density fractions clearly showed a strong shift with increasing BD in the 13C treatment, but there was little change in the unlabeled treatment. In particular, a T-RF of 161 bp increased in relative frequency in the fractions with BDs of ≥1.781 g ml−1 and predominated in the fractions with BDs of ≥1.793 g ml−1. A T-RF of 129 bp could be detected as a second dominant peak in the fractions with BDs of ≥1.793 g ml−1.
Archaeal rRNA from the 13C treatment could be amplified only up to fractions with a BD of 1.757 to 1.793 g ml−1, which is less heavy than that of the bacterial RNA. This may be attributed to the low abundance of the archaeal RNA in the template, since the same cycle number for RT-PCR and the same amount of template were applied to the bacterial and archaeal fingerprintings. The archaeal population size was reported to be much lower than that of the bacterial population in rice field soil (30). A PCR product was obtained from a heavier fraction in the 13C treatment (BD of 1.793 g ml−1) compared to the unlabeled treatment (BD of 1.779 g ml−1); however, the fingerprints were undistinguishable. Two predominated archaeal T-RFs of 188 bp and 286 bp were detected in the fractions with BDs of ≥1.779 g ml−1 in both the 13C treatment and unlabeled treatment.
Comparative sequence analyses of bacterial 16S rRNAs from heavy gradient fractions.
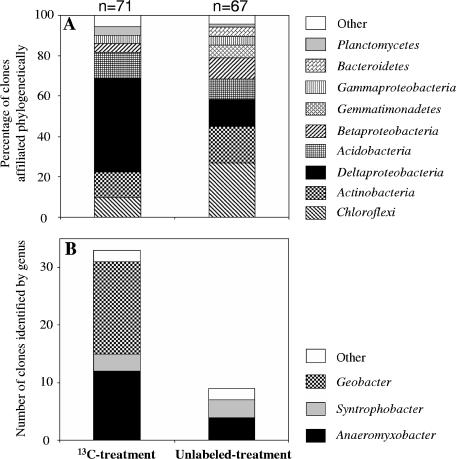
Two bacterial 16S rRNA clone libraries were constructed from the high (“H”)-density fractions (Fig. 4): (i) the LH library (n = 71 clones) represented the 13C treatment (BD of 1.793 g ml−1), and (ii) the UH library (n = 67 clones) represented the unlabeled treatment (BD of 1.791 g ml−1). The compositions of the LH and UH libraries were used to phylogenetically characterize the acetate-assimilating bacteria corresponding to the T-RFs of 161 bp and 129 bp found in the T-RFLP fingerprint analysis.
FIG. 4.
Comparison of bacterial 16S rRNA gene sequences obtained from selected density fractions from the 13C treatment and unlabeled treatment. (A) Relative proportions of 16S rRNA gene sequences affiliated phylogenetically. “Other” represents Alphaproteobacteria, Cyanobacteria, Deinococcus-Thermus, Firmicutes, or candidate division OP10 combined. n indicates the total numbers of clones obtained in this study. (B) Clone numbers of 16S rRNA gene sequences identified by genus within the Deltaproteobacteria. The phylogenetic relationships of the deltaproteobacterial clones are shown in Fig. 5.
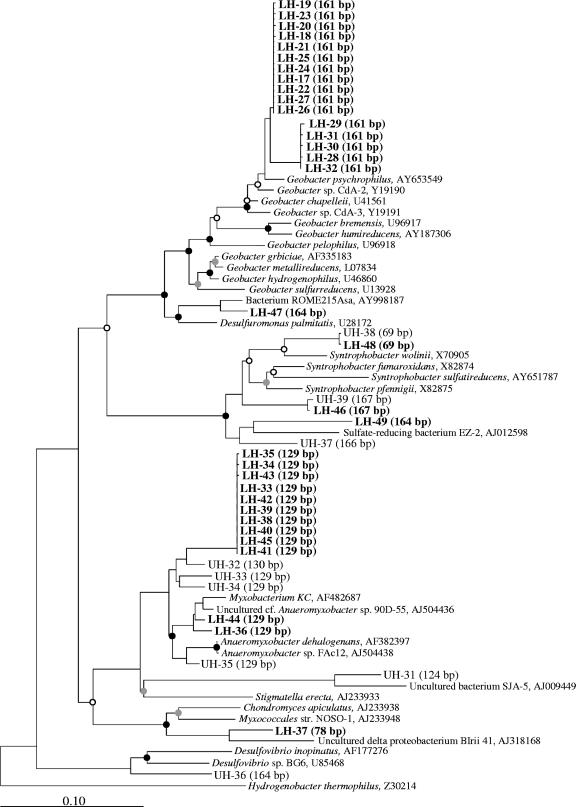
The composition of the LH library was less diverse than that of the UH library, as expected from the corresponding T-RFLP fingerprint patterns (Fig. 3A and B and 4A). The most remarkable difference between the LH and UH libraries concerned sequences within the class Deltaproteobacteria. In the LH library, the Deltaproteobacteria sequences were the primary component, accounting for 46% of the total. In contrast, in the UH library, Deltaproteobacteria sequences were less than 13%. The Deltaproteobacteria sequences in the LH and UH libraries were identified at the genus level (Fig. 4B). Sequences related to the genus Geobacter were detected only in the LH library and represented the largest part of the clones (i.e., 48% of total Deltaproteobacteria sequences). These clones were affiliated with two novel clusters in the phylogenetic tree, which were closely related to Geobacter psychrophilus (accession no. AY653549; 98% and 96% sequence identity) (Fig. 5) (38). The expected T-RF size was 161 bp, which corresponded to the dominant peak in the T-RFLP fingerprint (Fig. 3A). The number of sequences for the second identified genus, Anaeromyxobacter, was much larger in the LH library (n = 12) than in the UH library (n = 4). These clones in the LH library formed a novel cluster within the genus Anaeromyxobacter (Fig. 5). The expected T-RF size was 129 bp, which corresponded to the second dominant peak in the T-RFLP fingerprint (Fig. 3A).
FIG. 5.
Phylogenetic tree showing the relationships of 16S rRNA phylotypes affiliated with the Deltaproteobacteria. The basic tree of reference sequences was reconstructed using the maximum-parsimony method based on a comparison of more than 1,400 nucleotides. Bootstrap values were obtained from 1,000 replications, and >90%, 70 to 89%, and <69% are shown with black, gray, and open circles, respectively. Clones were designated LH (heavy fraction of the labeled 13C treatment; in boldface) and UH (heavy fraction of the unlabeled treatment). Numbers in parentheses represent the expected T-RF lengths of the clones. The scale bar represents 10% sequence divergence. GenBank accession numbers of reference sequences are given.
In addition to the class Deltaproteobacteria, the relative abundances of clones identified as Acidobacteria and Planctomycetes were higher in the LH library than in the UH library (Fig. 4A). However, these clones did not cluster into a specific branch of the phylogenetic trees (data not shown), and the expected T-RF sizes did not match with one of the dominant peaks in the T-RFLP fingerprint.
DISCUSSION
We investigated the acetate-assimilating microorganisms in a methanogenic rice field soil, in which acetate was immediately converted to CH4 and CO2. Frequent substrate supplementation at low concentration enabled the indigenous microorganisms to incorporate 13C substrate under conditions that are relatively close to those found in the natural environment (61). Comparative SIP with labeled and unlabeled substrates allowed the detection of RNA labeled with 13C from the assimilated acetate. The combination of repeated label addition at low dosage and comparative RNA-SIP thus provided insight into the microbial assimilation of acetate under methanogenic conditions in a rice field soil.
The CH4 produced in the control treatment was derived from endogenous organic matter present in the rice field soil (Fig. 1C). This organic matter was probably also degraded via acetate, as shown in numerous previous studies (21, 35, 48, 51) and supported by the observation of a low acetate concentration under steady-state conditions. The addition of acetate resulted in a 2.5-fold increase of the CH4 production activity (Fig. 1A and B). The carbon balance of the substrate and gas products showed that 20 μmol of acetate 13C was converted to at least 12.1 μmol of 13C-labeled gaseous products (CH4 plus CO2). The carbon recovery in the 13C treatment was approximately 60%, which is nearly consistent with the data of the previous turnover experiment using [2-14C]acetate (4). The carbon not recovered was probably assimilated into biomass including rRNA, which became labeled with 13C.
The CsTFA BD of the heaviest RNA obtained in this study was 1.806 g ml−1, which is not as high as that of the RNA fully labeled with 13C (BD of 1.815 g ml−1), as reported previously (31). Thus, the active microbial populations were not completely labeled but were heavily enriched with substrate 13C. Incorporation of the substrate 13C into RNA was clearly demonstrated by comparative T-RFLP analyses of the 13C treatment and unlabeled treatment (Fig. 3). The 13C treatment showed that the bacterial population detected clearly changed, because increasing BD of the density fraction and two T-RFs of 161 bp and 129 bp length predominated in the fingerprints of the high-density fraction (Fig. 3A and B). Clone library analyses revealed that the sequences of Geobacter spp. and Anaeromyxobacter spp. were detected preferentially in the LH library (Fig. 4B and Fig. 5). The expected sizes of T-RFs for Geobacter spp. and Anaeromyxobacter spp. were in accordance with those of the dominant peaks in the T-RFLP fingerprint (Fig. 3A and 5). These results demonstrate that Geobacter spp. and Anaeromyxobacter spp. played a role in acetate assimilation in methanogenic rice field soil. We believe that labeling of these bacteria with 13C was realistic for in situ conditions and was not caused by artificial enrichment due to the increased acetate concentrations, since the respective T-RFs were increased only upon addition of 13C-labeled acetate and not upon addition of unlabeled acetate.
Geobacter and Anaeromyxobacter spp., both of which are known as dissimilatory iron reducers (13, 22), have been found in a wide variety of anoxic environments (41, 54, 55), possibly because of their ability to oxidize acetate, which is a central intermediate in the anaerobic degradation of organic matter. It has been reported that on rice roots, where oxygen released from the aerenchyma is probably available for iron(II) oxidation, the reduction of ferric iron is an important biogeochemical process (10). Furthermore, rice roots were found to be colonized by uncultured Anaeromyxobacter species (see, e.g., environmental clone 90D-55 in Fig. 5) (57). In flooded rice field soils, fine-scale profiling of iron(II) and iron(III) oxide distributions has revealed a spatial and temporal activity of iron(III) reduction (19). Although iron reducers have not been detected as a dominant population by using culture-independent approaches (14, 19, 39), Anaeromyxobacter sp. strain FAc12 (shown in Fig. 5) was isolated from the bulk soil of rice microcosms by using acetate and ferrihydrite as substrates (57).
Presently, it is not known how Geobacter spp. and Anaeromyxobacter spp. can be metabolically active during the methanogenic phase in rice field soil. Acetate most likely was the energy substrate used for dissimilation. However, it is unclear how it might have been used other than by acetoclastic methanogenesis, since our experimental setup excluded electron acceptors such as O2, nitrate, or sulfate. Indeed, CH4 was the predominant product of 13C-labeled acetate, but 13CO2 was also formed, and we cannot exclude the possibility that some of the CO2 was formed from the methyl group of acetate, i.e., by acetate oxidation rather than methanogenic acetate cleavage. A fermentative type of metabolism has never been demonstrated so far for either Geobacter spp. (22) or Anaeromyxobacter spp. (13, 50, 57); also, it is unlikely that acetate alone could serve as a major carbon source for growth and energy metabolism, as this would require syntrophic acetate oxidation, i.e., that involving interspecies electron transfer (62), a process that has not been detected in rice field soil as yet (3). Oxidative acetate dissimilation may be achieved by using humic acids as primary electron acceptors (23). However, it is unclear whether humic acids in rice field soil have a large capacity for serving as ultimate oxidants for acetate. In lake sediments, for example, the electron uptake capacity of humic acids was found to be low in deeper layers, and humic acids were regarded as unlikely to be a significant oxidant (16). Rather than a final oxidant, humic acids seem to serve as an electron shuttle to ferric iron serving as terminal electron acceptor (16). In rice field soil, Geobacter spp. and Anaeromyxobacter spp. may oxidize acetate as an energy source by using such a shuttle mechanism. Alternatively, the Geobacter spp. and Anaeromyxobacter spp. identified might directly utilize iron(III) oxides that are only poorly available for dissimilatory iron reduction, e.g., crystalline rather than amorphous iron oxide phases (45, 46). In this study, the rice field slurry was preincubated for a long time, and bioavailable iron(III) oxides were therefore considered to be largely depleted. In another study (60), the production of Fe(II) was hardly detectable in rice field soil. However, Fe(II) production never completely balances the amount of ferric iron minerals originally present in rice field soil. Therefore, it is assumed that much of the Fe(III) minerals are left unreduced in the methanogenic rice field soil because they are hardly available to microbial metabolism. We therefore hypothesize that Geobacter spp. and Anaeromyxobacter spp. can utilize some of the barely accessible iron(III) oxides as electron acceptors under methanogenic condition in rice field soil, albeit at a low rate. Various strategies that may be used by microorganisms to access insoluble Fe(III) oxides have been suggested to date [e.g., production of electron-shuttling components and/or Fe(III) chelators, chemotaxis to contact with Fe(III) oxides, and electron transfer via microbial nanowires] (5, 36, 37, 44).
Environments in which iron(III) reduction occurs are geochemically characterized by low acetate and H2 concentrations (12, 56). Acetate-oxidizing iron reducers were reported to outcompete aceticlastic methanogens by maintaining the substrate concentration at levels too low for methanogenesis (20, 47). On the other hand, the reduction of Fe(III) is greatly influenced by the availability of ferric iron minerals. In this study, the steady-state acetate concentration of below 15 μM was lower than the acetate threshold concentration for aceticlastic methanogenesis, which ranges from 69 to 1,180 μM (58), indicating that a biogeochemical process that is more thermodynamically favorable than methanogenesis operated in the methanogenic rice field soil. Comparative SIP of RNA showed that while potential dissimilatory iron reducers of the genera Geobacter and Anaeromyxobacter were able to produce RNA from 13C-labeled acetate, the archaeal population assimilated only a small amount of the acetate 13C into their RNAs (Fig. 3). Energy obtained from iron(III) reduction is much greater than that from methanogenesis (1, 40). It is thought, therefore, that the iron(III) reducers can use acetate as a carbon source more readily than methanogens. The aceticlastic methanogens may have utilized acetate mainly for energy metabolism, with only a small amount being assimilated. Biomass production by methanogenic archaea probably was slight.
In summary, the results of this study showed that Geobacter spp. and Anaeromyxobacter spp. assimilated acetate during the methanogenic phase of a rice field soil. We assume that these genera were dissimilating acetate by reduction of crystalline ferric iron minerals. These bacteria would therefore compete for acetate with aceticlastic methanogens in situ but apparently did not outcompete them, since CH4 nevertheless was the primary metabolic product of acetate degradation. It has been reported that Geobacter and Anaeromyxobacter coexist in acidic contaminated sediments, depending on the environmental pH (41). The ability of Geobacter spp. and Anaeromyxobacter spp. to access different forms of ferric iron minerals should be investigated to learn more about the ecophysiological niches of these iron reducers in anoxic rice field soil.
Acknowledgments
We are grateful to Melanie Klose, Katja Meuser, Sonja Fleissner, and Peter Claus for their significant technical assistance. We also thank Shin Haruta and Masaharu Ishii (University of Tokyo) for their continuous encouragement.
This study was financially supported by the DFG within the special research program SFB395 and the Fonds der Chemischen Industrie. T. Hori received a scholarship from the University of Tokyo International Academic Exchange Activities Program and the Max Planck Society.
Footnotes
Published ahead of print on 27 October 2006.
REFERENCES
- 1.Achtnich, C., F. Bak, and R. Conrad. 1995. Competition for electron donors among nitrate reducers, ferric iron reducers, sulfate reducers, and methanogens in anoxic paddy soil. Biol. Fertil. Soils 19:65-72. [Google Scholar]
- 2.Bouwman, A. F. 1990. Exchange of greenhouse gases between terrestrial ecosystems and the atmosphere, p. 78-97. In A. F. Bouwman (ed.) Soils and the greenhouse effect. John Wiley, New York, NY.
- 3.Chidthaisong, A., and R. Conrad. 2000. Turnover of glucose and acetate coupled to reduction of nitrate, ferric iron and sulfate and to methanogenesis in anoxic rice field soil. FEMS Microbiol. Ecol. 31:73-86. [DOI] [PubMed] [Google Scholar]
- 4.Chidthaisong, A., B. Rosenstock, and R. Conrad. 1999. Measurement of monosaccharides and conversion of glucose to acetate in anoxic rice field soil. Appl. Environ. Microbiol. 65:2350-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Childers, S. E., S. Ciufo, and D. R. Lovley. 2002. Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis. Nature 416:767-769. [DOI] [PubMed] [Google Scholar]
- 6.Chin, K. J., and R. Conrad. 1995. Intermediary metabolism in methanogenic paddy soil and the influence of temperature. FEMS Microbiol. Ecol. 18:85-102. [Google Scholar]
- 7.Conrad, R., C. Erkel, and W. Liesack. 2006. Rice cluster I methanogens, an important group of Archaea producing greenhouse gas in soil. Curr. Opin. Biotechnol. 17:262-267. [DOI] [PubMed] [Google Scholar]
- 8.Conrad, R., M. Klose, and P. Claus. 2000. Phosphate inhibits acetotrophic methanogenesis on rice roots. Appl. Environ. Microbiol. 66:828-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumont, M. G., and J. C. Murrell. 2005. Stable isotope probing—linking microbial identity to function. Nat. Rev. Microbiol. 3:499-504. [DOI] [PubMed] [Google Scholar]
- 10.Frenzel, P., U. Bosse, and P. H. Janssen. 1999. Rice roots and methanogenesis in a paddy soil: ferric iron as an alternative electron acceptor in the rooted soil. Soil Biol. Biochem. 31:421-430. [Google Scholar]
- 11.Friedrich, M. W. 2006. Stable-isotope probing of DNA: insights into the function of uncultivated microorganisms from isotopically labeled metagenomes. Curr. Opin. Biotechnol. 17:59-66. [DOI] [PubMed] [Google Scholar]
- 12.He, Q., and R. A. Sanford. 2004. Acetate threshold concentrations suggest varying energy requirements during anaerobic respiration by Anaeromyxobacter dehalogenans. Appl. Environ. Microbiol. 70:6940-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, Q., and R. A. Sanford. 2003. Characterization of Fe(III) reduction by chlororespiring Anaeromyxobacter dehalogenans. Appl. Environ. Microbiol. 69:2712-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hengstmann, U., K. J. Chin, P. H. Janssen, and W. Liesack. 1999. Comparative phylogenetic assignment of environmental sequences of genes encoding 16S rRNA and numerically abundant culturable bacteria from an anoxic rice paddy soil. Appl. Environ. Microbiol. 65:5050-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Intergovernmental Panel on Climate Change. 2001. Climate change 2001: the scientific basis. In J. T. Houghton, Y. Ding, D. J. Griggs, M. Noguer, P. J. van der Linden, and D. Xiaosu (ed.), Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, England.
- 16.Kappler, A., M. Benz, B. Schink, and A. Brune. 2004. Electron shuttling via humic acids in microbial iron(III) reduction in a freshwater sediment. FEMS Microbiol. Ecol. 47:85-92. [DOI] [PubMed] [Google Scholar]
- 17.Krüger, M., P. Frenzel, D. Kemnitz, and R. Conrad. 2005. Activity, structure and dynamics of the methanogenic archaeal community in a flooded Italian rice field. FEMS Microbiol. Ecol. 51:323-331. [DOI] [PubMed] [Google Scholar]
- 18.Krumböck, M., and R. Conrad. 1991. Metabolism of position-labeled glucose in anoxic methanogenic paddy soil and lake sediment. FEMS Microbiol. Ecol. 85:247-256. [Google Scholar]
- 19.Liesack, W., S. Schnell, and N. P. Revsbech. 2000. Microbiology of flooded rice paddies. FEMS Microbiol. Rev. 24:625-645. [DOI] [PubMed] [Google Scholar]
- 20.Lovley, D. R., and E. J. Phillips. 1987. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl. Environ. Microbiol. 53:2636-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovley, D. R., and M. J. Klug. 1982. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl. Environ. Microbiol. 43:552-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovley, D. R., D. E. Holmes, and K. P. Nevin. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49:219-286. [DOI] [PubMed] [Google Scholar]
- 23.Lovley, D. R., J. D. Coates, E. L. Blunt-Harris, E. J. P. Phillips, and J. C. Woodward. 1996. Humic substances as electron acceptors for microbial respiration. Nature 382:445-448. [Google Scholar]
- 24.Lu, Y., and R. Conrad. 2005. In situ stable isotope probing of methanogenic archaea in the rice rhizosphere. Science 309:1088-1090. [DOI] [PubMed] [Google Scholar]
- 25.Lu, Y., D. Rosencrantz, W. Liesack, and R. Conrad. 2006. Structure and activity of bacterial community inhabiting rice roots and the rhizosphere. Environ. Microbiol. 8:1351-1360. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig, W., O. Strunk, S. Klugbauer, N. Klugbauer, M. Weizenegger, J. Neumaier, M. Bachleitner, and K. H. Schleifer. 1998. Bacterial phylogeny based on comparative sequence analysis. Electrophoresis 19:554-568. [DOI] [PubMed] [Google Scholar]
- 28.Lueders, T., and M. Friedrich. 2000. Archaeal population dynamics during sequential reduction processes in rice field soil. Appl. Environ. Microbiol. 66:2732-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lueders, T., and M. W. Friedrich. 2002. Effects of amendment with ferrihydrite and gypsum on the structure and activity of methanogenic populations in rice field soil. Appl. Environ. Microbiol. 68:2484-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lueders, T., B. Pommerenke, and M. W. Friedrich. 2004. Stable-isotope probing of microorganisms thriving at thermodynamic limits: syntrophic propionate oxidation in flooded soil. Appl. Environ. Microbiol. 70:5778-5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lueders, T., M. Manefield, and M. W. Friedrich. 2004. Enhanced sensitivity of DNA- and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environ. Microbiol. 6:73-78. [DOI] [PubMed] [Google Scholar]
- 32.Lueders, T., B. Wagner, P. Claus, and M. W. Friedrich. 2004. Stable isotope probing of rRNA and DNA reveals a dynamic methylotroph community and trophic interactions with fungi and protozoa in oxic rice field soil. Environ. Microbiol. 6:60-72. [DOI] [PubMed] [Google Scholar]
- 33.Lueders, T., K. J. Chin, R. Conrad, and M. Friedrich. 2001. Molecular analyses of methyl-coenzyme M reductase alpha-subunit (mcrA) genes in rice field soil and enrichment cultures reveal the methanogenic phenotype of a novel archaeal lineage. Environ. Microbiol. 3:194-204. [DOI] [PubMed] [Google Scholar]
- 34.Manefield, M., A. S. Whiteley, R. I. Griffiths, and M. J. Bailey. 2002. RNA stable isotope probing, a novel means of linking microbial community function to phylogeny. Appl. Environ. Microbiol. 68:5367-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mountfort, D. O., and R. A. Asher. 1978. Changes in proportions of acetate and carbon dioxide used as methane precursors during the anaerobic digestion of bovine waste. Appl. Environ. Microbiol. 35:648-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nevin, K. P., and D. R. Lovley. 2002. Mechanisms for Fe(III) oxide reduction in sedimentary environments. Geomicrobiol. J. 14:141-159. [Google Scholar]
- 37.Nevin, K. P., and D. R. Lovley. 2002. Mechanisms for accessing insoluble Fe(III) oxide during dissimilatory Fe(III) reduction by Geothrix fermentans. Appl. Environ. Microbiol. 68:2294-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nevin, K. P., D. E. Holmes, T. L. Woodard, E. S. Hinlein, D. W. Ostendorf, and D. R. Lovley. 2005. Geobacter bemidjiensis sp. nov. and Geobacter psychrophilus sp. nov., two novel Fe(III)-reducing subsurface isolates. Int. J. Syst. Evol. Microbiol. 55:1667-1674. [DOI] [PubMed] [Google Scholar]
- 39.Noll, M., D. Matthies, P. Frenzel, M. Derakshani, and W. Liesack. 2005. Succession of bacterial community structure and diversity in a paddy soil oxygen gradient. Environ. Microbiol. 7:382-395. [DOI] [PubMed] [Google Scholar]
- 40.Peters, V., and R. Conrad. 1996. Sequential reduction processes and initiation of CH4 production upon flooding of oxic upland soils. Soil Biol. Biochem. 28:371-382. [Google Scholar]
- 41.Petrie, L., N. N. North, S. L. Dollhopf, D. L. Balkwill, and J. E. Kostka. 2003. Enumeration and characterization of iron(III)-reducing microbial communities from acidic subsurface sediments contaminated with uranium(VI). Appl. Environ. Microbiol. 69:7467-7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prinn, R. G. 1994. Global atmospheric-biospheric chemistry, p. 1-18. In R. G. Prinn (ed.), Global atmospheric-biospheric chemistry. Plenum, New York, NY.
- 43.Radajewski, S., P. Ineson, N. R. Parekh, and J. C. Murrell. 2000. Stable-isotope probing as a tool in microbial ecology. Nature 403:646-649. [DOI] [PubMed] [Google Scholar]
- 44.Reguera, G., K. D. McCarthy, T. Mehta, J. S. Nicoll, M. T. Tuominen, and D. R. Lovley. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098-1101. [DOI] [PubMed] [Google Scholar]
- 45.Roden, E. E. 2006. Geochemical and microbiological controls on dissimilatory iron reduction. C. R. Geosci. 338:456-467. [Google Scholar]
- 46.Roden, E. E. 2003. Diversion of electron flow from methanogenesis to crystalline Fe(III) oxide reduction in carbon-limited cultures of wetland sediment microorganisms. Appl. Environ. Microbiol. 69:5702-5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roden, E. E., and R. G. Wetzel. 2003. Competition between Fe(III)-reducing and methanogenic bacteria for acetate in iron-rich freshwater sediments. Microb. Ecol. 45:252-258. [DOI] [PubMed] [Google Scholar]
- 48.Rothfuss, F., and R. Conrad. 1993. Thermodynamics of methanogenic intermediary metabolism in littoral sediment of Lake Constance. FEMS Microbiol. Ecol. 12:265-276. [Google Scholar]
- 49.Roy, R., H. D. Klüber, and R. Conrad. 1997. Early initiation of methane production in anoxic rice soil despite the presence of oxidants. FEMS Microbiol. Ecol. 24:311-320. [Google Scholar]
- 50.Sanford, R. A., J. R. Cole, and J. M. Tiedje. 2002. Characterization and description of Anaeromyxobacter dehalogenans gen. nov., sp. nov., an aryl-halorespiring facultative anaerobic myxobacterium. Appl. Environ. Microbiol. 68:893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sansone, F. J., and C. S. Martens. 1981. Methane production from acetate and associated methane fluxes from anoxic coastal sediments. Science 211:707-709. [DOI] [PubMed] [Google Scholar]
- 52.Schütz, H., W. Seiler, and R. Conrad. 1989. Processes involved in formation and emission of methane in rice paddies. Biogeochemistry 7:33-53. [Google Scholar]
- 53.Schütz, H., A. Holzapfel-Pschorn, R. Conrad, H. Rennenberg, and W. Seiler. 1989. A 3-year continuous record on the influence of daytime, season, and fertilizer treatment on methane emission rates from an Italian rice paddies. J. Geophys. Res. 94:16405-16416. [Google Scholar]
- 54.Snoeyenbos-West, O. L., K. P. Nevin, R. T. Anderson, and D. R. Lovley. 2000. Enrichment of Geobacter species in response to stimulation of Fe(III) reduction in sandy aquifer sediments. Microb. Ecol. 39:153-167. [DOI] [PubMed] [Google Scholar]
- 55.Stein, L. Y., M. T. La Duc, T. J. Grundl, and K. H. Nealson. 2001. Bacterial and archaeal populations associated with freshwater ferromanganous micronodules and sediments. Environ. Microbiol. 3:10-18. [DOI] [PubMed] [Google Scholar]
- 56.Sung, Y., K. E. Fletcher, K. M. Ritalahti, R. P. Apkarian, N. Ramos- Hernandez, R. A. Sanford, N. M. Mesbah, and F. E. Loffler. 2006. Geobacter lovleyi sp. nov. strain SZ, a novel metal-reducing and tetrachloroethene-dechlorinating bacterium. Appl. Environ. Microbiol. 72:2775-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Treude, N., D. Rosencrantz, W. Liesack, and S. Schnell. 2003. Strain FAc12, a dissimilatory iron-reducing member of the Anaeromyxobacter subgroup of Myxococcales. FEMS Microbiol. Ecol. 44:261-269. [DOI] [PubMed] [Google Scholar]
- 58.Westermann, P., B. K. Ahring, and R. A. Mah. 1989. Threshold acetate concentrations for acetate catabolism by aceticlastic methanogenic bacteria. Appl. Environ. Microbiol. 55:514-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu, X. L., M. W. Friedrich, and R. Conrad. 2006. Diversity and ubiquity of thermophilic methanogenic archaea in temperate anoxic soils. Environ. Microbiol. 8:394-404. [DOI] [PubMed] [Google Scholar]
- 60.Yao, H., and R. Conrad. 2000. Effect of temperature on reduction of iron and production of carbon dioxide and methane in anoxic wetland rice soils. Biol. Fertil. Soils 32:135-141. [Google Scholar]
- 61.Yao, H., R. Conrad, R. Wassmann, and H. U. Neue. 1999. Effect of soil characteristics on sequential reduction and methane production in sixteen rice paddy soils from China, the Philippines, and Italy. Biogeochemistry 47:269-295. [Google Scholar]
- 62.Zinder, S. H. 1994. Syntrophic acetate oxidation and “reversible acetogenesis,” p. 387-415. In H. L. Drake (ed.), Acetogenesis. Chapman and Hall, New York, NY.