Abstract
The recombinant φ11 endolysin hydrolyzed heat-killed staphylococci as well as staphylococcal biofilms. Cell wall targeting appeared to be a prerequisite for lysis of whole cells, and the combined action of the endopeptidase and amidase domains was necessary for maximum activity. In contrast, the φ12 endolysin was inactive and caused aggregation of the cells.
Infections caused by Staphylococcus aureus and Staphylococcus epidermidis still play a major role in human and animal disease. In particular staphylococcal biofilms on indwelling devices are difficult to treat due to their inherent antibiotic resistance (5, 7, 26). In this context, it is important to develop alternative treatment strategies to combat staphylococcal infections, particularly in view of the ability of staphylococci to acquire resistance to commonly used antibiotics (10, 23, 29). Phage lysins, or endolysins, have received considerable attention as possible antimicrobial agents against gram-positive bacteria and have been applied to a variety of pathogens, such as Bacillus anthracis (21), Streptococcus pneumoniae (9), and S. aureus (25). Endolysins are active against dead cells and even living, planktonic cells (9, 25), but their ability to lyse the complex structure of staphylococcal biofilms has not yet been investigated. In this approach, we cloned and heterologously overexpressed the lysis genes of the bacteriophages φ11 and φ12 of S. aureus NCTC8325 in Escherichia coli for subsequent analysis of the lytic activity of the enzymes and their single subdomains on cell walls, whole cells, and biofilms. Knowledge of the lytic activity of both endolysins is limited. Their nucleotide sequences have been published (16), and the φ11 endolysin has been shown to possess a d-alanyl-glycyl endopeptidase and an N-acetylmuramyl-l-alanine amidase activity on crude cell walls of S. aureus OS2 (24).
Sequence comparison, cloning, and overexpression of φ11 and φ12 endolysins.
The φ11 and φ12 endolysin sequences were BLAST searched against the NCBI protein database. Both endolysins are modular enzymes which consist of three distinct domains coding for an N-terminal CHAP (cysteine, histidine-dependent amidohydrolases/peptidases) domain with hydrolytic function, a central amidase domain (N-acetylmuramyl-l-alanine amidase), and a C-terminal SH3b domain, which is involved in cell wall recognition (1). In spite of their similar domain architecture, these endolysins show low sequence identity (26.9%).
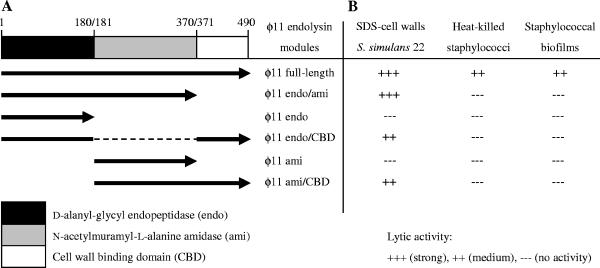
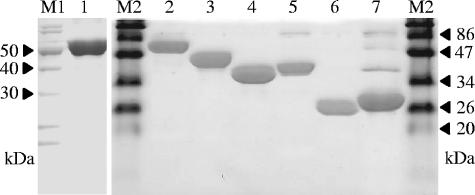
The endolysin genes of the bacteriophages φ11 (open reading frame [ORF] 53; 1,473 bp; accession number NC_004615) and φ12 (ORF 49; 1,455 bp; accession number NC_004616) were amplified by PCR from genomic DNA of S. aureus NCTC8325, using the primers listed in Table 1. In order to test the activity of the φ11 endolysin subunits, the endopeptidase unit (φ11endo, amino acids [aa] 1 to 180) and the amidase unit (φ11ami, aa 180 to 371) as well as each unit plus the cell wall binding domain (φ11endo/CBD, aa 1 to 180/371 to 490, and φ11ami/CBD, aa 180 to 490) were constructed separately (Fig. 1). In addition, the cell wall binding module was deleted from the φ11 endolysin (φ11endo/ami, aa 1 to 371). The amplification products were cloned into the multiple cloning site of the expression vector pET22b (Novagen) without the pelB leader tag to inhibit protein transport to the periplasm of the expression host. The resulting plasmids, pETerΔ11, pETerΔ12, pETendo11, pETendoCBD11, pETami11, pETamiCBD11, and pETendo/ami11, were used to overexpress each endolysin as a C-terminal six-His-tagged fusion protein. After subcloning of the plasmids in E. coli JM109, E. coli BL21(DE3) was used as a host for expression of each six-His-tagged endolysin. Expression cultures were grown in Luria-Bertani (LB) broth containing ampicillin (40 μg/ml) to an optical density at 600 nm (OD600) of 0.6. Then protein expression was induced by addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. Expression cultures were harvested after 4 h followed by protein purification steps under native conditions via nickel-nitrilotriacetic acid affinity chromatography (Fig. 2). Protein purification was also performed with cells harboring the empty vector, and the eluate served as a control in the activity tests.
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Relevant characteristic(s) or sequencea | Reference(s) or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| K-12 JM109 | Subcloning host for pET22b derivatives | 34 |
| BL21(DE3) | λDE3 lysogen, T7 RNA polymerase gene; expression host | 30 |
| S. aureus | ||
| NCTC8325 | Biofilm-positive, laboratory strain, lysogenic for bacteriophages φ11 and φ12 | 16 |
| Cowan 1 | MSSA, catheter infections by binding of fibrinogen and fibronectin | 6, 18 |
| Newman | MSSA | 18, 32 |
| Wood 46 | Protein A-deficient, alpha-toxin-producing strain | 6 |
| S. simulans 22 | Penicillin resistant, lysostaphin susceptible | 19 |
| S. epidermidis O-47 | Biofilm-positive, clinical isolate causing CVC-associated infections | 13, 28 |
| Plasmids | ||
| pET22b | C-terminal six-His-tagged expression vector, T7 lac promoter, β-lactamase gene (Ampr); in this study, without pelB leader tag | 30 |
| pETerΔ11 | pET22b + ORF 53 of φ11 (aa 1 to 490, 56.5 kDa) (I/II) | This study |
| pETendo/ami11 | pET22b + truncated ORF 53 of φ11 (aa 1 to 371, 43.2 kDa) (I/IX) | This study |
| pETendo11 | pET22b + truncated ORF 53 of φ11 (aa 1 to 180, 22.1 kDa) (I/V) | This study |
| pETendoCBD11 | pET22b + truncated ORF 53 of φ11 (Δaa 181 to 370, 35.2 kDa) (I/V; VI/VII) | This study |
| pETami11 | pET22b + truncated ORF 53 of φ11 (aa 180 to 371, 22.8 kDa) (VIII/IX) | This study |
| pETamiCBD | pET22b + truncated ORF 53 of φ11 (aa 180 to 490, 36.1 kDa) (VIII/II) | This study |
| pETerΔ12 | pET22b + ORF 49 of φ12 (aa 1 to 484, 55.3 kDa) (III/IV) | This study |
| Primer no. and name | ||
| I; phi11F | 5′ TTTGGATCCAATGAGTATCATCATGGAGGTG 3′ (BamHI) | |
| II; phi11R | 5′ GTCAAGCTTACTGATTTCTCCCCATAAGTCA 3′ (HindIII) | |
| III; phi12F | 5′ GTGGGATCCAATGTTGATAACAAAAAACCAA 3′ (BamHI) | |
| IV; phi12R | 5′ TAAAAGCTTAATCGTGCTAAACTTACCAAAAC 3′ (HindIII) | |
| V; endo11R | 5′ TTGAAGCTTAGCTGTTTCTTTTTTAGGT 3′ (HindIII) | |
| VI; CBD11F | 5′ ATGAAGCTTAAAATACCGGTTGCCACTGT 3′ (HindIII) | |
| VII; CBD11R | 5′ GTCCTCGAGACTGATTTCTCCCCATAAGTCA 3′ (XhoI) | |
| VIII; ami11F | 5′ GAACATATGAAGCCACAACCTAAAGCAGTAG 3′ (NdeI) | |
| IX; ami11R | 5′ CGGAAGCTTACCATCCATGTACGCCCTAA 3′ (HindIII) |
Roman numerals in parentheses are primer pairs used for amplification of ORFs (forward/reverse). Restriction site sequences are underlined, and the sites are given in parentheses. MSSA, methicillin-susceptible S. aureus; CVC, central venous catheter.
FIG. 1.
Recombinant murein hydrolases of the staphylococcal bacteriophage φ11. A. Schematic overview of the φ11 endolysin modules illustrating the constructs tested in this study. The φ11 endolysin features a modular design which consists of an N-terminal endopeptidase domain (endo), a central amidase domain (ami), and a C-terminal cell wall binding domain (CBD). The full-length and deletion constructs of the φ11 endolysin were overexpressed as six-His fusion proteins. B. Lytic activity of the staphylococcal bacteriophage φ11 endolysin domains.
FIG. 2.
SDS-polyacrylamide gel electrophoresis analysis of φ12 and φ11 endolysins and derived proteins purified by nickel-nitrilotriacetic acid affinity chromatography. The purified proteins were analyzed by 15% SDS-polyacrylamide gel electrophoresis and stained with PageBlue protein staining solution (Fermentas) according to the manufacturer's instructions. Lane M1, Fermentas Page Ruler unstained protein ladder; lanes M2, Fermentas prestained protein molecular weight marker; lane 1, φ12 endolysin (full length); lane 2, φ11 endolysin (full length); lane 3, φ11endo/ami; lane 4, φ11endo/CBD; lane 5, φ11ami/CBD; lane 6, φ11endo; lane 7, φ11ami.
Lytic activity of φ11 endolysin modules.
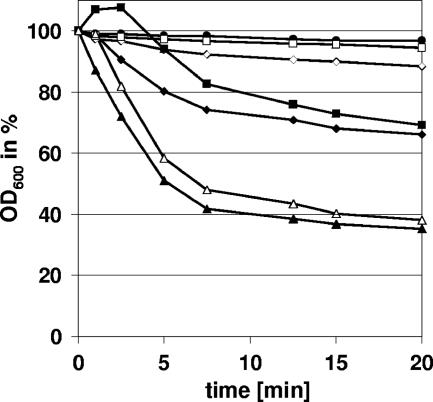
The lytic activities of the full-length φ11 endolysin and its deletion variants (each applied in a concentration of 20 μg/ml) were examined photometrically at 600 nm employing Staphylococcus simulans 22 cell walls (purified with sodium dodecyl sulfate [SDS]) (4) resuspended in incubation buffer (50 mM Tris-HCl, 100 mM NaCl; pH 7.5) to an OD600 of ∼0.3. All lysis experiments were performed in triplicate. The full-length enzyme showed efficient lysis of the peptidoglycan compared to the control (empty-vector eluate), and both the endopeptidase and the amidase module plus cell wall binding domain (φ11endo/CBD and φ11ami/CBD) were active and able to lyse cell walls. However, the full-length enzyme was more active than the isolated subdomains, and only the combination of φ11endo/CBD and φ11ami/CBD (20 μg/ml each) restored full lytic activity (Fig. 3). The effect of the truncation was even more pronounced with whole cells. To this end, cells of S. aureus NCTC8325 were grown overnight, diluted in incubation buffer to an OD600 of approximately 0.3, and pasteurized for 10 min at 80°C. After addition of equivalent amounts of endolysin (20 μg/ml), the lytic activity was determined as before. In contrast to the full-length protein (Fig. 4), the isolated amidase or endopeptidase domains were hardly active against heat-killed cells (data not shown). In conclusion, the two catalytic domains of the enzyme have to be combined with each other to cleave the cell wall of intact cells. Thus, the φ11 endolysin seems to belong to a group of endolysins which act as multifunctional hydrolases (2). Recently, the lytic enzyme of phage φWMY of Staphylococcus warneri M (LysWMY) was reported to show strong similarities in its domain architecture to the φ11 endolysin (35). In contrast to the φ11 endolysin, LysWMY retained its full activity even when both the amidase and cell wall binding domains had been deleted. This result indicates that combined action of different domains does not seem to be an obligatory characteristic even among closely related murein hydrolases.
FIG. 3.
Activity of the domains of the multifunctional φ11 endolysin. The lytic activities of purified enzyme samples of the full-length and truncated φ11 endolysin (20 μg/ml each) were examined using purified cell walls of S. simulans 22. The full-length enzyme (▴) was able to lyse the substrate efficiently compared to the protein eluate of the empty-vector control (•). The truncated enzymes φ11endo (□) and φ11ami (⋄) did not show mentionable lytic activity. The truncated enzymes containing the cell wall binding domain, φ11endo/CBD (▪) and φ11ami/CBD (⧫), showed enhanced lytic activity but still worked less effectively than the full-length enzyme. The lytic activity could be restored by the parallel use of φ11endo/CBD and φ11ami/CBD (▵).
FIG. 4.
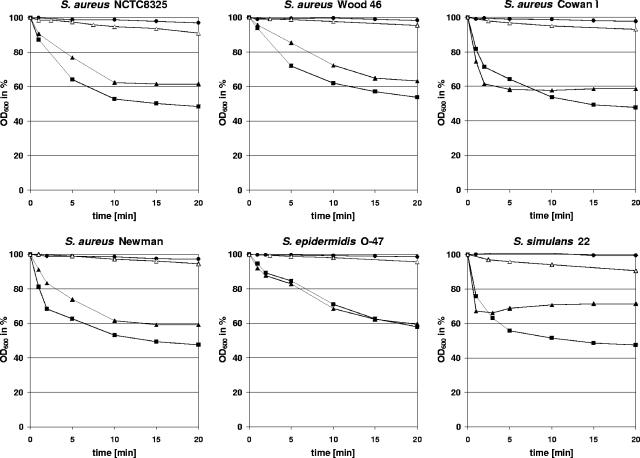
The φ11 endolysin lyses whole cells of several staphylococci. Light scattering data showed that the φ11 endolysin (▴; 20 μg/ml) rapidly decreased the optical density of heat-inactivated S. aureus NCTC8325, S. aureus Wood 46, S. aureus Cowan 1, S. aureus Newman, S. epidermidis O-47, and S. simulans 22 cultures compared to the protein eluate of the empty-vector control (•). Its lytic activity was comparable to the effect observed after addition of lysostaphin (▪; 5 μg/ml), which is a highly efficient glycyl-glycine endopeptidase. After deletion of the cell wall targeting domain, the φ11 enzyme (φ11endo/ami, ▵; 20 μg/ml) showed a significantly reduced activity, which indicates a fundamental role of the cell wall binding domain in efficient cell lysis.
Purified cell walls were a much more sensitive substrate for the enzymes than heat-killed cells were, since they showed the residual activities of the single subdomains much more clearly. In spite of this, the φ12 endolysin did not show any activity with S. simulans 22 cell walls (data not shown).
Substrate recognition is essential for full lytic activity.
Substrate recognition mediated by the cell wall binding domain, which is homologous to the C-terminal domain of lysostaphin (20), appeared to be necessary for high catalytic efficiency. Lytic activity of the φ11 endolysin on heat-killed staphylococcal cells was abolished after deletion of the cell wall binding domain (φ11endo/ami) (Fig. 4), while it retained nearly all of its lytic activity on SDS cell walls (data not shown). The single domains, φ11endo and φ11ami, showed significantly reduced lytic activity on SDS cell walls in the absence of the cell wall binding domain (Fig. 3). These findings are similar to the results obtained with lysostaphin and ALE-1, a glycyl-glycine endopeptidase homologous to lysostaphin, which showed a significant reduction in lytic activity on autoclaved staphylococci or viable cells, respectively, after deletion of their C-terminal cell wall targeting domains (1, 22), which bind to cross-linked peptidoglycan and recognize the Gly5 interpeptide cross bridge (12, 22). Our results suggest that a similar targeting mechanism exists for the φ11 endolysin and that, in this respect, the phage enzyme closely resembles its staphylococcal counterparts.
Activities of the endolysins with different staphylococcal strains.
We next analyzed the lytic activities of the φ11 and φ12 endolysins on whole cells of several staphylococcal strains. In addition to S. aureus NCTC8325, the full-length φ11 endolysin rapidly lysed heat-killed cells of S. aureus Wood 46, S. aureus Cowan 1, S. aureus Newman, S. epidermidis O-47, and S. simulans 22 (Fig. 4). Its lytic activity was equivalent to that of highly purified lysostaphin (5 μg/ml) (Dr. Petry Genmedics GmbH, Reutlingen, Germany).
Unlike φ11, the φ12 endolysin was not able to hydrolyze heat-killed cells of S. aureus Wood 46, S. aureus Cowan 1, or S. aureus Newman. However, after addition of the φ12 endolysin an increase in optical density of the cultures was observed (Fig. 5). This was accompanied and most probably caused by an aggregation of the cells that was macroscopically visible. This phenomenon was earlier described by Takano et al. (31), who investigated the influence of synthetic peptides derived from the S. aureus major autolysin Atl on autolysis. Sequence comparison with the homologous amidase-3 domains of the S. aureus bacteriophages φSA 2MW, L54a, φSLT, PVL, 96, 3a, 53, 77, ROSA, and φETA and amidases of Staphylococcus haemolyticus JCSC1435 and Staphylococcus epidermidis RP62a identified an amino acid exchange at position 260 in the amidase-3 domain of the φ12 endolysin. This exchange introduces a histidine and therefore an additional positive charge in a position that is occupied by glutamine, glutamate, or asparagine in the other staphylococcal enzymes. Whether this amino acid exchange or an inadequate folding of the His-tagged protein is involved in the loss of hydrolytic activity is part of current investigations. Most probably, the φ12 endolysin in our test system is unable to exert efficient hydrolytic activity on staphylococcal murein, while retaining its ability to bind the cell wall. The substrate binding ability of the enzyme may then lead to the enhanced adhesion between the cells.
FIG. 5.
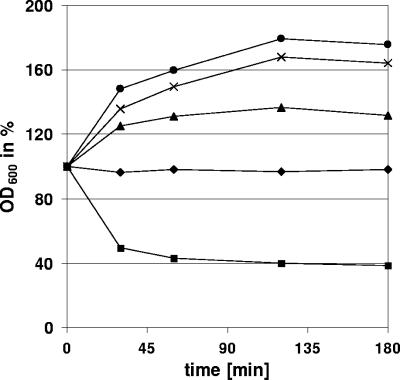
Increase of optical density after addition of φ12 endolysin. Unlike the φ11 enzyme, the φ12 endolysin was not able to lyse heat-killed cells of any tested staphylococcal strain. Upon addition of φ12 endolysin (15 μg/ml) we detected an obvious increase of optical density of S. aureus Wood 46 (•), S. aureus Cowan 1 (×), and S. aureus Newman (▴). The protein eluate of empty-vector control (⧫) showed no effect, whereas the control with lysostaphin (▪; 5 μg/ml) lysed all staphylococcal strains.
φ11 endolysin eliminates S. aureus NCTC8325 biofilm on artificial surfaces.
Finally, we determined the influence of the φ11 and φ12 endolysins on staphylococcal biofilms by a modified biofilm plate assay (33). To this end, an overnight culture was diluted 1:200 in tryptic soy broth medium supplemented with 0.25% d-(+)-glucose to a final volume of 200 μl in each well of a 96-well polystyrene microtiter plate (Nunc, Wiesbaden, Germany). The plates were incubated for 24 h or 48 h at 37°C under aerobic and O2-limited conditions, respectively. Afterwards, the wells were washed twice with incubation buffer. The biofilm-containing wells were then filled with incubation buffer plus endolysin. Fresh medium and incubation buffer alone or incubation buffer plus lysostaphin or lysozyme was employed as a control. Lysostaphin was used as a positive control, since it is able to lyse S. aureus and S. epidermidis biofilms (33). Following incubation for 2 hours at 37°C, the wells were washed again and stained with 0.1% safranin.
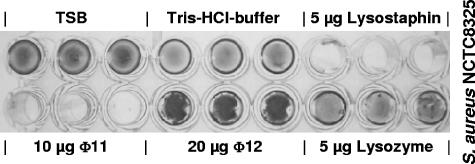
We found that the φ12 endolysin showed no hydrolytic activity on biofilms of S. aureus NCTC8325 and S. epidermidis O-47. In contrast, after addition of the φ12 endolysin we noticed an enhanced staining of the biofilms (Fig. 6). This effect is consistent with the results of the experiments performed with heat-killed cells and may be explained by a murein hydrolase-mediated adhesion of staphylococci (14, 15). In contrast, the purified φ11 endolysin was able to eliminate biofilms of S. aureus NCTC8325, with an efficiency that was comparable to or slightly lower than that of lysostaphin (Fig. 6). However, there was no effect on biofilms of S. epidermidis O-47. To our knowledge, no phage lysin has been reported so far to disrupt staphylococcal biofilms.
FIG. 6.
Biofilm plate assay of S. aureus NCTC8325. The φ11 endolysin lysed biofilms of S. aureus NCTC8325 with an efficiency comparable to that of lysostaphin. In contrast, addition of the φ12 endolysin resulted in enhanced staining of the cells. TSB, tryptic soy broth.
Bacterial biofilm formation is part of a survival strategy to resist suboptimal environmental conditions such as limited nutrient availability or lethal concentrations of antibiotics. Antimicrobial agents often show significantly reduced effects on biofilms, which is thought to be due to several biofilm-inherent properties. For example, a low growth rate impedes the action of antibiotics (8, 29), and evidence is emerging that the sessile cells in biofilms live in an altered metabolic state (3, 27). Furthermore, the diffusion velocity of antibiotics is limited within biofilms. A reduced rate of antibiotic penetration leads to a gradually increasing concentration of the antibiotic in the deeper layers of a biofilm, which permits adaptation of the biofilm cells to the antibiotic by stress-induced metabolic and transcriptional changes (17). The φ11 endolysin most probably destabilizes the biofilm structure by fast lysis of sessile cells, which are embedded in the extracellular matrix or at the interface of the matrix and surface, whereupon the biofilm is dissolved. Another possibility could be an influence of the intrinsic nature of the biofilm. Neither sodium metaperiodate nor proteinase K treatment could completely eradicate biofilms of S. aureus NCTC8325 grown in tryptic soy broth (data not shown), which suggests that an elevated portion of proteinogenous biofilm is present along with a biofilm mediated by the characteristic polysaccharide (PIA). PIA consists of β-1,6-linked N-acetylglucosamine residues (11) and is not the target of the φ11 murein hydrolase. In contrast to S. aureus NCTC8325, S. epidermidis O-47 biofilms are exclusively polysaccharide mediated. Therefore, different natures of the biofilm matrices tested here could be an explanation for the varying efficacy of the φ11 endolysin.
Considering the huge clinical relevance of staphylococcal biofilms in terms of human diseases, the φ11 endolysin may constitute a novel strategy to combat S. aureus nosocomial infections that are mediated by biofilm formation on medical devices.
Acknowledgments
This work was supported by a grant of the Competence Network PathoGenoMik (101.099-1/04-I.1.03) funded by the Bundesministerium für Bildung und Forschung of Germany.
Footnotes
Published ahead of print on 3 November 2006.
REFERENCES
- 1.Baba, T., and O. Schneewind. 1996. Target cell specificity of a bacteriocin molecule: a C-terminal signal directs lysostaphin to the cell wall of Staphylococcus aureus. EMBO J. 15:4789-4797. [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman, A., and N. D. Rawlings. 2003. The CHAP domain: a large family of amidases including GSP amidases and peptidoglycan hydrolases. Trends Biochem. Sci. 28:234-237. [DOI] [PubMed] [Google Scholar]
- 3.Beenken, K. E., P. M. Dunman, F. McAleese, D. Macapagal, E. Murphy, S. J. Projan, J. V. Blevins, and M. S. Smeltzer. 2004. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 186:4665-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bierbaum, G., and H. G. Sahl. 1987. Autolytic system of Staphylococcus simulans 22: influence of cationic peptides on activity of N-acetylmuramoyl-l-alanine amidase. J. Bacteriol. 169:5452-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton, J. W., K. J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 6.Delmi, M., P. Vaudaux, D. P. Lew, and H. Vasey. 1994. Role of fibronectin in staphylococcal adhesion to metallic surfaces used as models of orthopaedic devices. J. Orthop. Res. 12:432-438. [DOI] [PubMed] [Google Scholar]
- 7.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duguid, I. G., E. Evans, M. R. Brown, and P. Gilbert. 1992. Growth-rate-independent killing by ciprofloxacin of biofilm-derived Staphylococcus epidermidis; evidence for cell-cycle dependency. J. Antimicrob. Chemother. 30:791-802. [DOI] [PubMed] [Google Scholar]
- 9.Entenza, J. M., J. M. Loeffler, D. Grandgirard, V. A. Fischetti, and P. Moreillon. 2005. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob. Agents Chemother. 49:4789-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geisel, R., F. J. Schmitz, A. C. Fluit, and H. Labischinski. 2001. Emergence, mechanism, and clinical implications of reduced glycopeptide susceptibility in Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 20:685-697. [DOI] [PubMed] [Google Scholar]
- 11.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 12.Gründling, A., and O. Schneewind. 2006. Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus. J. Bacteriol. 188:2463-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heilmann, C., C. Gerke, F. Perdreau-Remington, and F. Götz. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect. Immun. 64:227-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heilmann, C., G. Thumm, G. S. Chhatwal, J. Hartleib, A. Uekotter, and G. Peters. 2003. Identification and characterization of a novel autolysin (Aae) with adhesive properties from Staphylococcus epidermidis. Microbiology 149:2769-2778. [DOI] [PubMed] [Google Scholar]
- 15.Heilmann, C., M. Hussain, G. Peters, and F. Götz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 16.Iandolo, J. J., V. Worrell, K. H. Groicher, Y. Qian, R. Tian, S. Kenton, A. Dorman, H. Ji, S. Lin, P. Loh, S. Qi, H. Zhu, and B. A. Roe. 2002. Comparative analysis of the genomes of the temperate bacteriophages phi 11, phi 12 and phi 13 of Staphylococcus aureus 8325. Gene 289:109-118. [DOI] [PubMed] [Google Scholar]
- 17.Jefferson, K. K., D. A. Goldmann, and G. B. Pier. 2005. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 49:2467-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juuti, K. M., B. Sinha, C. Werbick, G. Peters, and P. I. Kuusela. 2004. Reduced adherence and host cell invasion by methicillin-resistant Staphylococcus aureus expressing the surface protein Pls. J. Infect. Dis. 189:1574-1584. [DOI] [PubMed] [Google Scholar]
- 19.Kloos, W. E., and K. H. Schleifer. 1975. Isolation and characterization of staphylococci from human skin. II. Descriptions of four new species: Staphylococcus warneri, Staphylococcus capitis, Staphylococcus hominis, and Staphylococcus simulans. Int. J. Syst. Bacteriol. 25:62-79. [Google Scholar]
- 20.Loessner, M. J., S. Gaeng, G. Wendlinger, S. K. Maier, and S. Scherer. 1998. The two-component lysis system of Staphylococcus aureus bacteriophage Twort: a large TTG-start holin and an associated amidase endolysin. FEMS Microbiol. Lett. 162:265-274. [DOI] [PubMed] [Google Scholar]
- 21.Low, L. Y., C. Yang, M. Perego, A. Osterman, and R. C. Liddington. 2005. Structure and lytic activity of a Bacillus anthracis prophage endolysin. J. Biol. Chem. 280:35433-35439. [DOI] [PubMed] [Google Scholar]
- 22.Lu, J. Z., T. Fujiwara, H. Komatsuzawa, M. Sugai, and J. Sakon. 2006. Cell wall-targeting domain of glycylglycine endopeptidase distinguishes among peptidoglycan cross-bridges. J. Biol. Chem. 281:549-558. [DOI] [PubMed] [Google Scholar]
- 23.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 24.Navarre, W. W., H. Ton-That, K. F. Faull, and O. Schneewind. 1999. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage phi11. Identification of a d-alanyl-glycine endopeptidase activity. J. Biol. Chem. 274:15847-15856. [DOI] [PubMed] [Google Scholar]
- 25.O'Flaherty, S., A. Coffey, W. Meaney, G. F. Fitzgerald, and R. P. Ross. 2005. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J. Bacteriol. 187:7161-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Grady, N. P., M. Alexander, E. P. Dellinger, J. L. Gerberding, S. O. Heard, D. G. Maki, H. Masur, R. D. McCormick, L. A. Mermel, M. L. Pearson, I. I. Raad, A. Randolph, R. A. Weinstein, et al. 2002. Guidelines for the prevention of intravascular catheter-related infections. Pediatrics 110:e51. [DOI] [PubMed] [Google Scholar]
- 27.Resch, A., R. Rosenstein, C. Nerz, and F. Götz. 2005. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl. Environ. Microbiol. 71:2663-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rupp, M. E., P. D. Fey, C. Heilmann, and F. Götz. 2001. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J. Infect. Dis. 183:1038-1042. [DOI] [PubMed] [Google Scholar]
- 29.Stewart, P. S. 2002. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292:107-113. [DOI] [PubMed] [Google Scholar]
- 30.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 31.Takano, M., T. Oshida, A. Yasojima, M. Yamada, C. Okagaki, M. Sugai, H. Suginaka, and T. Matsushita. 2000. Modification of autolysis by synthetic peptides derived from the presumptive binding domain of Staphylococcus aureus autolysin. Microbiol. Immunol. 44:463-472. [DOI] [PubMed] [Google Scholar]
- 32.Wolz, C., D. McDevitt, T. J. Foster, and A. L. Cheung. 1996. Influence of agr on fibrinogen binding in Staphylococcus aureus Newman. Infect. Immun. 64:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu, J. A., C. Kusuma, J. J. Mond, and J. F. Kokai-Kun. 2003. Lysostaphin disrupts Staphylococcus aureus and Staphylococcus epidermidis biofilms on artificial surfaces. Antimicrob. Agents Chemother. 47:3407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 35.Yokoi, K. J., N. Kawahigashi, M. Uchida, K. Sugahara, M. Shinohara, K. Kawasaki, S. Nakamura, A. Taketo, and K. Kodaira. 2005. The two-component cell lysis genes holWMY and lysWMY of the Staphylococcus warneri M phage φWMY: cloning, sequencing, expression, and mutational analysis in Escherichia coli. Gene 351:97-108. [DOI] [PubMed] [Google Scholar]