Abstract
Enterobacter sakazakii is associated with neonatal infections and is occasionally present at low levels (<1 CFU/g) in powdered infant formula milk (IFM). It has been previously reported that some E. sakazakii strains do not grow in standard media for Enterobacteriaceae and coliform bacteria; therefore, a reliable method is needed for recovery of the organism. Three E. sakazakii enrichment broths—Enterobacteriaceae enrichment broth (EE), E. sakazakii selective broth (ESSB), and modified lauryl sulfate broth (mLST)—were compared with a novel broth designed for maximum recovery of E. sakazakii, E. sakazakii enrichment broth (ESE). One hundred seventy-seven strains (100%) grew in ESE, whereas between 2 and 6% of strains did not grow in EE, mLST, or ESSB. E. sakazakii possesses α-glucosidase activity, and a number of selective, chromogenic agars for E. sakazakii isolation based on this enzyme have been developed. E. sakazakii isolation agar produced fewer false-positive colonies than did Druggan-Forsythe-Iversen agar. However, the latter supported the growth of more E. sakazakii strains. It was also determined that 2% of E. sakazakii strains did not produce yellow pigmentation on tryptone soya agar at 25°C, a characteristic frequently cited in the identification of E. sakazakii. The recovery of desiccated E. sakazakii (0.2 to 2000 CFU/25 g) from powdered IFM in the presence of a competing flora was determined with various enrichment broths and differential selective media. Current media designed for the isolation and presumptive identification of E. sakazakii do not support the growth of all currently known E. sakazakii phenotypes; therefore, improvements in the proposed methods are desirable.
Enterobacter sakazakii is an occasional contaminant of powdered infant formula milk (IFM) and is a rare cause of neonatal infections (7, 9, 20, 22). Although not all cases have been attributed to the ingestion of IFM, the microbiological safety and preparation of IFM are of concern (4, 5). The Codex Alimentarius Commission is currently reviewing the code of hygienic practices for foods for infants and children, and the European Union has introduced microbiological criteria (2).
Several methods have been proposed for the enrichment and isolation of E. sakazakii (6, 11, 21). An integral part of all methods is the α-glucosidase test (16). However, a number of other Enterobacteriaceae are α-glucosidase positive (14, 15), and coisolation of these organisms lowers the efficiency of chromogenic media for the isolation of E. sakazakii. Although E. sakazakii can be recovered from 3 to 14% of IFM samples, reported levels have never exceeded 1 CFU g−1 (10, 17, 18). Therefore, specific and sensitive enrichment is required for isolation of the organism.
Farmer et al. (3) reported reduced plating efficiency of E. sakazakii strains on media commonly used in enteric bacteriology. It has also been noted that some E. sakazakii strains are unable to grow in lauryl sulfate broth (LST) or brilliant green bile broth (12). As the latter strains also failed to grow in Enterobacteriaceae enrichment broth (EE), it was deemed necessary to design a modified enrichment medium to aid comparison of the selective media. This study reports a comparison between currently proposed enrichment and isolation media for the detection of E. sakazakii.
(A preliminary report of this work was presented at the 105th General Meeting of the American Society for Microbiology [13]).
MATERIALS AND METHODS
Microbiological strains.
Proposed media for the isolation of E. sakazakii were assessed with over 250 Enterobacteriaceae isolates from the culture collection of Nottingham Trent University, Nottingham, United Kingdom. The E. sakazakii isolates (n = 177) were from clinical, food, and environmental sources and have been described previously (10, 11). The competing α-glucosidase-positive Enterobacteriaceae were Buttiauxella noakiae and strains from two as-yet-unnamed species identified as distinct 16S cluster groups (14, 15). The remaining strains were Enterobacter pyrinus, Enterobacter cloacae, Citrobacter koseri, Citrobacter freundii, Citrobacter braakii, Enterobacter asburiae, Enterobacter aerogenes, Enterobacter amnigenus, Escherichia hermanii, Escherichia coli, Hafnia alvei, Klebsiella oxytoca, Klebsiella pneumoniae, Klebsiella ozaenae, Raoultella terrigena, Kluyvera sp., Leclercia adecarboxylata, Pantoea sp., Proteus vulgaris, Providencia rettgeri, Salmonella enterica serovar Enteritidis, Serratia marcescens, and Serratia ficaria.
Growth media.
The following media were prepared according to the manufacturers' instructions: buffered peptone water (BPW) (CM0509; Oxoid, Basingstoke, United Kingdom), EE (CM0317; Oxoid), modified LST (mLST) (CM0451; Oxoid) with 0.5 M NaCl and 10 mg l−1 vancomycin) (6), violet red bile glucose agar (VRBGA) (CM0485; Oxoid), violet red bile lactose agar (VRBL) (CM0107; Oxoid), E. sakazakii chromogenic agar (Druggan-Forsythe-Iversen [DFI] formulation) (CM1055; Oxoid) (11), and tryptone soya agar (TSA) (CM0131; Oxoid). E. sakazakii isolation agar (ESIA) (AEB520010; AES Laboratoire) (8) and E. sakazakii selective broth (ESSB) (AEB611448; AES Laboratoire) were purchased as prepared media. Milk agar was prepared as follows: bacteriological agar (3.0 g) (LP0011; Oxoid) and ammonium sulfate were dissolved in 40 ml of distilled water. After autoclaving, 200 ml of warm (55°C) liquid IFM was added and the mixture was dispensed into petri dishes.
E. sakazakii enrichment broth (ESE) was composed of disodium hydrogen phosphate (6.5 g), potassium dihydrogen phosphate (2.0 g), yeast extract (1.5 g), neutralized peptone (4.0 g), base tryptone (12.0 g), sodium chloride (4.0 g), sucrose (100.0 g), and sodium deoxycholate (0.5 g) dissolved in distilled water (1,000 ml). The complete medium (pH 7.0 ± 0.1) was autoclaved at 121°C for 15 min.
Growth measurement.
Enrichment broths were inoculated (1 × 104 CFU ml−1) with overnight cultures diluted in sterile saline. Initially, growth was determined by measuring the change in optical density at 590 nm (OD590) at 37°C and 44°C with a TECAN SPECTRA Fluor instrument (TECAN United Kingdom Ltd., Reading, United Kingdom). Due to the inability to detect growth of some E. sakazakii strains in selective media with the OD measurements, 10 ml of EE, ESSB, and mLST were inoculated (1 × 107 CFU ml−1) from overnight cultures into BPW (18 E. sakazakii strains and 21 strains of other Enterobacteriaceae). After a 24-h incubation, the viable counts were determined by decimal dilutions on TSA incubated at 37°C.
Recovery of desiccated E. sakazakii from powdered IFM containing competing organisms.
Three strains of E. sakazakii (NCTC 11467T, ATCC 12868, and SK90) were grown overnight on milk agar at 37°C. These strains are the species type strain, the ATCC Perceptrol quality control strain, and a clinical strain kindly supplied by Franco Pagotto (19), respectively. The cells were harvested from the plates and resuspended in sterile infant formula to give cell densities of ca. 1011 CFU/ml prior to freeze-drying. The freeze-dried samples were stored for 4 weeks prior to use for the bacterial concentrations to stabilize. The bacterial viable cell counts in the desiccated samples were estimated by a most-probable-number technique (n = 8) in BPW prior to inoculation of powdered IFM. Appropriate quantities of desiccated cells were used to inoculate triplicate 25-g quantities of commercial milk-based powdered infant formula (Cow & Gate Premium Stage 1) at 0.2 to 2,000 CFU 25g−1. The IFM contained endogenous Bacillus spp., and one aliquot contained endogenous Raoultella terrigena. All aliquots were additionally inoculated with yellow-pigmented Enterobacteriaceae isolates comprising an α-glucosidase-positive strain and an α-glucosidase-negative Pantoea strain at 0.4 CFU g−1.
Four recovery methods were compared: FDA (http://www.cfsan.fda.gov/∼comm/mmesakaz.html), DFI (11; also this study), mLST (6), and AES (http://www.aeslaboratoire.com/). For convenience, the preenrichment, enrichment, primary isolation, and presumptive identification steps for each of these methods are summarized in Table 1. Up to five presumptive isolates were selected and identified by biochemical profiles with an API20E instrument (bioMérieux United Kingdom Ltd.) according to the manufacturer's instructions.
TABLE 1.
Methods for the recovery of desiccated E. sakazakii from powdered IFM
| Method | Preenrichment | Enrichment | Primary isolation | Presumptive identification |
|---|---|---|---|---|
| FDA (21) | BPW (37°C) | EE broth (37°C) | VRBGA (37°C) | Yellow pigment on TSA at 25°C for 48-72 h |
| DFI (11) | BPW (37°C) | ESE broth (37°C) | DFI (37°C) | Blue-green colonies |
| mLST (6) | BPW (37°C) | mLST + vancomycin (45°C) | TSA (37°C with enhanced light exposure) | Yellow, α-glucosidase-positive colonies |
| AES | ESSB broth (37°C) | ESIA (44°C) | Blue-green colonies |
RESULTS
Enrichment broth evaluation.
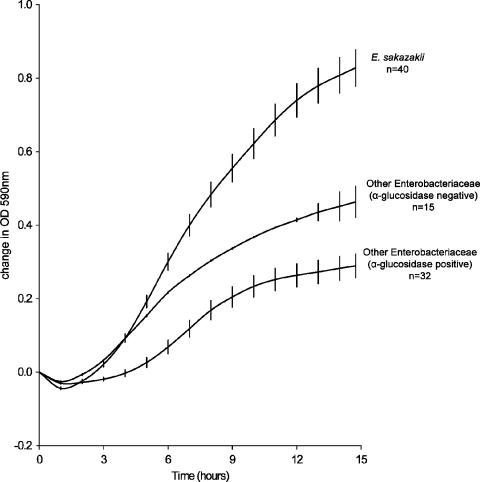
Preliminary experiments had shown that a number of E. sakazakii strains were sensitive to brilliant green, lauryl sulfate, crystal violet, and/or novobiocin and that all E. sakazakii strains studied were able to ferment sucrose. In contrast, the majority of non-E. sakazakii α-glucosidase-positive Enterobacteriaceae did not utilize sucrose. Therefore, with sucrose in place of dextrose and/or lactose, E. sakazakii was able to outgrow other Enterobacteriaceae in ESE (Fig. 1). Sodium deoxycholate was included as a selective agent to suppress gram-positive organisms.
FIG. 1.
Comparative growth of E. sakazakii and other organisms in ESE. Error bars represent the mean ± the standard error of the sample mean, calculated from the standard deviation of the sample mean divided by √n. A difference in OD590 of 0.5 is equivalent to ca. 0.5 log10 CFU ml−1.
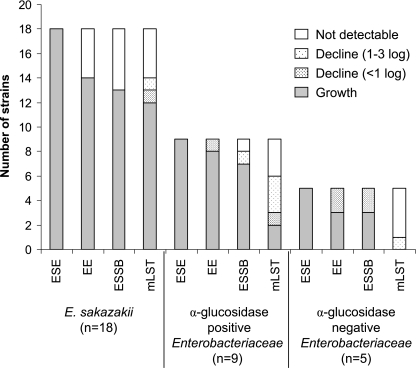
All 177 E. sakazakii strains grew well in ESE at 37°C (Table 2). In contrast, growth was not detected for 3 to 13% (n = 177) of E. sakazakii strains in EE, mLST, or ESSB at 37°C and 44°C. The viable cell counts after a 24-h incubation in selective broths are presented in Fig. 2. All Enterobacteriaceae strains grew in ESE. In the three selective enrichment broths—EE, mLST, and ESSB—the viability of four to six E. sakazakii strains decreased and some were unrecoverable (>6 log decline). mLST was the most selective broth, with only two non-E. sakazakii strains able to grow. Bacillus cereus (n = 1), Bacillus subtilis (n = 2), Staphylococcus aureus (n = 2), and Lactobacillus spp. (n = 2) were not recoverable from any broths (data not shown).
TABLE 2.
Strains showing increases in OD after 24 h of incubation in enrichment media
| Organism (no. of strains) | % of strainsa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 37°C
|
44°C
|
|||||||
| ESE | EE | mLST | ESSB | ESE | EE | mLST | ESSB | |
| E. sakazakii (177) | 100 | 97 | 96 | 96 | 99 | 95 | 94 | 87 |
| α-Glucosidase-positive Enterobacteriaceae (40) | 100 | 100 | 95 | 93 | 98 | 95 | 78 | 65 |
| α-Glucosidase-negative Enterobacteriaceae (34) | 100 | 100 | 100 | 97 | 100 | 100 | 91 | 88 |
Values are percentages of strains showing increases in OD after 24 h of incubation at the indicated temperatures. See Materials and Methods for enrichment medium manufacturers' details.
FIG. 2.
Growth, persistence, and death of E. sakazakii and competitive organisms in enrichment broths.
Selective agar assessment.
Table 3 shows that 2% (n = 177) of E. sakazakii strains did not produce yellow pigmentation on TSA after 3 days of incubation at 25°C, a criterion which has been recommended for the presumptive identification of E. sakazakii (8, 21). All of the E. sakazakii strains grew at 37°C and produced characteristic colonies on the Enterobacteriaceae (VRBGA) and coliform (VRBL) agars; however, one strain (NTU 531) grew very poorly on these media. This isolate was also the only E. sakazakii strain in this study that did not grow and produced characteristic (blue-green) colonies on DFI after 24 h of incubation at 37°C. NTU 531 appears to be more sensitive to sodium deoxycholate than the other isolates tested; decreasing the concentration of this compound in the media to 0.3g l−1 improved the growth of this strain. At 44°C, 1% (n = 177) of E. sakazakii strains did not grow on TSA, whereas on ESIA and DFI, three and five E. sakazakii strains (respectively) were unable to grow. In addition, rather than blue-green colonies, eight strains produced white or partially colored colonies on DFI and four strains produced mauve, rather than blue-green, colonies on ESIA at this temperature.
TABLE 3.
Growth of E. sakazakii and other Enterobacteriaceae on various selective and differential agars
| Organism (no. of strains) | % of strainsa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Yellow pigment production 25°C (TSA, 48-72 h) | Incubation temp for 24 h
|
|||||||
| 37°C
|
44°C
|
|||||||
| TSA | DFI | VRBGA | VRBL | TSA | DFI | ESIA | ||
| E. sakazakii (177) | 98 | 100 | 99 | 100 | 100 | 99 | 93 | 96 |
| α-Glucosidase-positive Enterobacteriaceae (40) | 93 | 100 | 100 | 100 | 92 | 95 | 45 | 63 |
| α-Glucosidase-negative Enterobacteriaceae (34) | 32 | 100 | 0 | 100 | 96 | 100 | 0 | 0 |
Values are percentages of strains showing the following growth parameters: TSA, growth; DFI, blue-green colonies; VRBGA, red colonies; VRBL, red colonies. See Materials and Methods for agar manufacturers' details.
Recovery of desiccated E. sakazakii from powdered IFM.
The numbers of samples positive for recovery of three desiccated E. sakazakii strains from powdered IFM are presented in Table 4. There were three replicates per strain at each inoculation level (2,000 to 0.2 CFU/25 g). The AES method recovered only the E. sakazakii type strain (NCTC 11467T). Endogenous R. terrigena was the only organism recovered by the FDA method (21) for one of the samples inoculated with 2,000 CFU 25 g−1 E. sakazakii; therefore, R. terrigena had overgrown E. sakazakii. The mLST method recovered both R. terrigena and E. sakazakii in the corresponding sample, whereas the DFI method recovered only E. sakazakii. Fewer presumptive positive isolates were found to be false positive with the mLST method than with the other methods.
TABLE 4.
Recovery of E. sakazakii from powdered IFM in the presence of competing flora by four isolation methods
| E. sakazakii concn (CFU 25 g−1) | No. of samples positive for E. sakazakii recovery (n = 9) by the indicated methoda
|
|||
|---|---|---|---|---|
| DFI (11) | FDA (21) | mLST (6) | AESb | |
| 2,000 | 9 | 7 | 9 | 3 |
| 200 | 8 | 6 | 8 | 1 |
| 20 | 6 | 3 | 3 | 1 |
| 2 | 3 | 1 | 0 | 0 |
| 0.2 | 0 | 0 | 0 | 0 |
See Table 1 for details of the four methods.
Only E. sakazakii NCTC 11467T was recovered by this method.
DISCUSSION
ESE broth was developed to facilitate comparison of the performance of E. sakazakii selective enrichment broths. Preliminary experiments had shown that all E. sakazakii strains were able to ferment sucrose, whereas the majority of non-E. sakazakii α-glucosidase-positive Enterobacteriaceae did not. Therefore, ESE broth was formulated to support good growth of E. sakazakii compared with competing organisms (Fig. 1). Although other Enterobacteriaceae also utilize sucrose, these are mainly α-glucosidase-negative organisms and so can be differentiated on current chromogenic media. As E. sakazakii has been shown to have greater desiccation tolerance than most other Enterobacteriaceae (1; J. Caubilla-Baron and S. J. Forsythe, submitted for publication), a high concentration of sucrose was incorporated into the broth to act as a humectant, lowering the available water. Sodium deoxycholate was incorporated to suppress the growth of gram-positive bacteria.
All E. sakazakii isolates grew at 37°C in ESE, but 2 to 4% (n = 177) of the strains were undetected in EE, mLST, or ESSB. Assessment of the viability of these strains by standard plate counts showed that, for five strains, one or more of the selective media were bactericidal. There have been previous reports of E. sakazakii strains failing to grow in mLST (15), as well as in LST and brilliant green bile broth (12). Other α-glucosidase-positive organisms lost their viability in mLST to a greater extent than did E. sakazakii. Therefore, the selectivity of this medium is not necessarily a result of the increased growth of E. sakazakii but of the greater die-off of nontarget cells.
All strains produced characteristic red colonies on VRBGA and VRBLA. However, these media are selective only for Enterobacteriaceae and coliforms, respectively, and are not specific for E. sakazakii. They are therefore of use with respect to general hygiene monitoring but not for detection of specific pathogens, such as Salmonella and E. sakazakii. At the recommended incubation temperatures of 37 and 44°C, respectively, 99% of E. sakazakii strains grew on DFI agar but only 96% on ESIA. Incubation of DFI at 44°C (above the manufacturer's recommendation of 37°C) resulted in 7% of strains not showing the characteristic blue-green colony morphology. As 1% of E. sakazakii strains were unable to grow on nonselective medium (TSA) at 44°C, incubation at this temperature may not ensure the recovery of E. sakazakii.
Detection methods should be evaluated with desiccated E. sakazakii cells in the presence of competing flora to mimic environmental samples from manufacturing facilities. Comparison of four methods for the recovery of desiccated E. sakazakii from IFM indicated that the most sensitive method was preenrichment in BPW, followed by enrichment in ESE and plating on DFI agar (Table 4). However, at the lower inoculum levels, this method produced a large number of false-positive colonies on DFI. The presence of competing organisms reduced the sensitivity of the FDA method, and the AES method recovered only the E. sakazakii type strain. The mLST method was not as sensitive as the DFI method at low inoculum levels but produced fewer presumptive false positives. As one of the strains used in this experiment was sensitive to lauryl sulfate, the ability of the mLST method to recover it at the higher inoculum levels suggests that this method works better in the presence of the sample matrix (IFM) than when used for pure cultures. This may be due to divalent cations in the IFM counteracting the effects of the lauryl sulfate. Therefore, the performance of the mLST method may be reduced if used for other sample matrices.
This study has used a large number (n = 177) of E. sakazakii strains to demonstrate that the levels of selective agents such as crystal violet, sodium lauryl sulfate, brilliant green, and sodium deoxycholate in media need to be reassessed to ensure the recovery of the organism, especially from mixed cultures. The use of sucrose (100 g/liter) in ESE promoted the growth of E. sakazakii relative to other α-glucosidase-positive Enterobacteriaceae. However, this broth is not selective enough to be considered a viable alternative enrichment method, and further development of effective media for the isolation of E. sakazakii is needed.
Acknowledgments
We thank Oxoid Ltd. (United Kingdom) and Nottingham Trent University for their support of this project.
We are particularly grateful to Patrick Druggan for expert advice on medium composition.
Footnotes
Published ahead of print on 27 October 2006.
REFERENCES
- 1.Breeuwer, P., A. Lardeau, M. Peterz, and H. Joosten. 2003. Desiccation and heat tolerance of Enterobacter sakazakii. J. Appl. Microbiol. 95:967-973. [DOI] [PubMed] [Google Scholar]
- 2.European Commission. 2005. Commission regulation (EC) number 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Official J. Eur. Union L338:1-26. [Google Scholar]
- 3.Farmer, J. J., M. A. Asbury, F. W. Hickman, D. J. Brenner, and the Enterobacteriaceae Study Group (USA). 1980. Enterobacter sakazakii, new species of Enterobacteriaceae isolated from clinical specimens. Int. J. Syst. Bacteriol. 30:569-584. [Google Scholar]
- 4.Food and Agriculture Organization-World Health Organization. 2004. Enterobacter sakazakii and other microorganisms in powdered infant formula: meeting report, MRA series 6. WHO, Geneva, Switzerland.
- 5.Food and Agriculture Organization-World Health Organization. 2006. Enterobacter sakazakii and Salmonella in powdered infant formula. Second Risk Assessment Workshop, 16-20 January 2006. WHO, Rome, Italy.
- 6.Guillaume-Gentil, O., V. Sonnard, M. C. Kandhai, J. D. Marugg, and H. Joosten. 2005. A simple and rapid cultural method for detection of Enterobacter sakazakii in environmental samples. J. Food Prot. 68:64-69. [DOI] [PubMed] [Google Scholar]
- 7.Himelright, I., E. Harris, V. Lorch, and M. Anderson. 2002. Enterobacter sakazakii infections associated with the use of powdered infant formula—Tennessee, 2001. JAMA 287:2204-2205. [PubMed] [Google Scholar]
- 8.ISO DTS 22964. 2005. Milk and milk products—detection of Enterobacter sakazakii. ISO TC 34/SC 5.
- 9.Iversen, C., and S. J. Forsythe. 2003. Risk profile of Enterobacter sakazakii, an emergent pathogen associated with infant milk formula. Trends Food Sci. Technol. 14:443-454. [Google Scholar]
- 10.Iversen, C., and S. J. Forsythe. 2004. Isolation of Enterobacter sakazakii and other Enterobacteriaceae from powdered infant formula milk and related products. Food Microbiol. 21:771-776. [Google Scholar]
- 11.Iversen, C., P. Druggan, and S. J. Forsythe. 2004. A selective differential medium for Enterobacter sakazakii. Int. J. Food Microbiol. 96:133-139. [DOI] [PubMed] [Google Scholar]
- 12.Iversen, C., M. Lane, and S. J. Forsythe. 2004. The growth profile, thermotolerance and biofilm formation of Enterobacter sakazakii grown in infant formula milk. Lett. Appl. Microbiol. 38:378-382. [DOI] [PubMed] [Google Scholar]
- 13.Iversen, C., P. Druggan, and S. Forsythe. 2005. Comparison of methods for the isolation of Enterobacter sakazakii, abstr. P54, p. 152. Abstr. 105th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.
- 14.Iversen, C., M. Waddington, S. L. On, and S. Forsythe. 2004. Identification and phylogeny of Enterobacter sakazakii relative to Enterobacter and Citrobacter species. J. Clin. Microbiol. 42:5368-5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehner, A., S. Nitzsche, P. Breeuwer, B. Diep, K. Thelen, and R. Stephan. 2006. Comparison of two chromogenic media and evaluation of two molecular based identification systems for Enterobacter sakazakii detection. BMC Microbiol. 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muytjens, H. L., J. van Spaceder Ros-van de Repeqq, and H. A. M. van Druten. 1984. Enzymatic profiles of Enterobacter sakazakii and related species with special reference to the α-glucosidase reaction and reproducibility of the test system. J. Clin. Microbiol. 20:684-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muytjens, H. L., H. Roelofs-Willemse, and G. H. Jaspar. 1988. Quality of powdered substitutes for breast milk with regard to members of the family Enterobacteriaceae. J. Clin. Microbiol. 26:743-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nazarowec-White, M., and J. M. Farber. 1997. Incidence, survival, and growth of Enterobacter sakazakii in infant formula. J. Food Prot. 60:226-230. [DOI] [PubMed] [Google Scholar]
- 19.Pagotto, F. J., M. Nazarowec-White, S. Bidawid, and J. M. Farber. 2003. Enterobacter sakazakii: infectivity and enterotoxin production in vitro. J. Food Prot. 66:370-375. [DOI] [PubMed] [Google Scholar]
- 20.Simmons, B. P., M. S. Gelfand, M. Haas, L. Metts, and J. Ferguson. 1989. Enterobacter sakazakii infections in neonates associated with intrinsic contamination of a powdered infant formula. Infect. Control Hosp. Epidemiol. 10:398-401. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration. 2002. Isolation and enumeration of Enterobacter sakazakii from dehydrated powdered infant formula. http://www.cfsan.fda.gov/∼comm/mmesakaz.html.
- 22.van Acker, J., F. de Smet, G. Muyldermans, A. Bougatef, A. Naessens, and S. Lauwers. 2001. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J. Clin. Microbiol. 39:293-297. [DOI] [PMC free article] [PubMed] [Google Scholar]