Abstract
Unsaturated fatty acids play an essential role in the biophysical characteristics of cell membranes and determine the proper function of membrane-attached proteins. Thus, the ability of cells to alter the degree of unsaturation in their membranes is an important factor in cellular acclimatization to environmental conditions. Many eukaryotic organisms can synthesize dienoic fatty acids, but Saccharomyces cerevisiae can introduce only a single double bond at the Δ9 position. We expressed two sunflower (Helianthus annuus) oleate Δ12 desaturases encoded by FAD2-1 and FAD2-3 in yeast cells of the wild-type W303-1A strain (trp1) and analyzed their effects on growth and stress tolerance. Production of the heterologous desaturases increased the content of dienoic fatty acids, especially 18:2Δ9,12, the unsaturation index, and the fluidity of the yeast membrane. The total fatty acid content remained constant, and the level of monounsaturated fatty acids decreased. Growth at 15°C was reduced in the FAD2 strains, probably due to tryptophan auxotrophy, since the trp1 (TRP1) transformants that produced the sunflower desaturases grew as well as the control strain did. Our results suggest that changes in the fluidity of the lipid bilayer affect tryptophan uptake and/or the correct targeting of tryptophan transporters. The expression of the sunflower desaturases, in either Trp+ or Trp− strains, increased NaCl tolerance. Production of dienoic fatty acids increased the tolerance to freezing of wild-type cells preincubated at 30°C or 15°C. Thus, membrane fluidity is an essential determinant of stress resistance in S. cerevisiae, and engineering of membrane lipids has the potential to be a useful tool of increasing the tolerance to freezing in industrial strains.
Tolerance to freezing is an essential trait influencing the viability and leavening capacity of baker's yeast in frozen dough (4, 42). The so-called frozen-dough technology has been widely accepted by consumers and bakers due to several advantages, which include supplying oven-fresh bakery products and improving labor conditions. However, no appropriate industrial strain with a high tolerance to freezing is available, and it is unlikely that the classical breeding program could significantly improve this trait. Freezing is a complex and multifaceted stress, in which different stressors and stress responses appear to play important roles. Cells exposed to subzero temperatures are injured by the formation of ice crystals and crystal growth during frozen storage (33). At a slow freezing rate, cells are exposed to hyperosmotic solutions and equilibrate by movement of water across the membranes (65). Finally, during the thawing process, cells can suffer biochemical damage by oxidative stress (16). It is not surprising, therefore, that tolerance to freezing involves several mechanisms working in concert.
Biological membranes are the first barrier that separates cells from their environment and are a primary target for damage during environmental stress. Sudden changes in environmental conditions cause alterations in the organization and dynamic structure of membrane lipids (62) and alter the function of many cellular activities. For example, reduced incubation temperatures increase the molecular order of membrane lipids, i.e., rigidification (29), and alter the activity of membrane-associated enzymes and transporters (5). Other stresses, e.g., heat shock, freezing, and osmotic stress, induce dramatic changes in the organization and dynamic properties of membrane lipids (12, 27). To date, most of the research in this field has focused on the connections between the physical state of the membrane and cold tolerance.
Many organisms have developed mechanisms to maintain the appropriate fluidity of membrane lipids regardless of ambient temperature. These mechanisms include changes in the proportions of types of lipid and alterations in the lipid/protein ratio (26). The most widely recognized change in cell membranes at low temperatures is the unsaturation of lipid acyl chains (44, 54). Phospholipids with unsaturated fatty acids have a lower melting point and more flexibility than do phospholipids with saturated acyl chains (35). Such adaptation involves the induction of fatty acid desaturases (55, 63), which incorporate unsaturated bonds at defined positions in fatty acids that are linked to membrane glycerolipids.
In Saccharomyces cerevisiae, exposure to low temperature increases the expression of OLE1 (36), which encodes the only known desaturase in this yeast (52, 53). Ole1p, a Δ9 fatty acid desaturase, converts palmitic (16:0) and stearic (18:0) fatty acids into their corresponding monounsaturated fatty acids, palmitoleic acid (16:1Δ9) and oleic acid (18:1Δ9), respectively. Consistent with the increased expression of OLE1, there is an increase in the degree of unsaturation of total fatty acids when yeast cells are shifted from 30°C to 10°C (36). Nevertheless, OLE1 does not appear to be essential in acclimation to low temperature, since its overexpression does not confer growth advantages at 10°C (20). Overexpression of OLE1 is toxic and reduces growth at 30°C (52). Thus, the functional role of OLE1 is unclear, and the mechanism by which S. cerevisiae alters membrane fluidity in response to different types of stress remains unknown.
Unlike S. cerevisiae, many organisms synthesize polyunsaturated fatty acids de novo through the activity of specific desaturase enzymes. Genes encoding a range of different fatty acid desaturases (FAD) have been cloned from microorganisms, animals, and plants, and expressed in heterologous hosts (28, 37, 38), including S. cerevisiae (14, 19, 40, 43). FAD2-expressing yeast cells contained high levels of diunsaturated fatty acids. However, the physiological significance of this change in fatty acid composition has only rarely been studied in detail. S. cerevisiae cells overexpressing the Arabidopsis FAD2 gene were more resistant to ethanol than were control cells (19). Production of a Δ12 desaturase from Caenorhabditis elegans in yeast increased the growth rate at low temperatures and enhanced resistance to ethanol and H2O2 (40).
Cultivated sunflower (Helianthus annuus L.) seeds are rich in unsaturated fatty acids 18:1Δ9 and 18:2Δ9,12 (linoleic acid). The enzyme responsible for synthesizing linoleic acid is the microsomal oleate desaturase (1-acyl-2-oleoyl-sn-glycero-3-phosphocholine Δ12 desaturase). Three different cDNA sequences, FAD2-1, FAD2-2, and FAD2-3, encoding the sunflower microsomal oleate desaturases (fatty acid desaturase 2 [FAD2]) have been isolated and characterized (31). FAD2 activity in developing sunflower seeds is regulated by temperature and oxygen availability (32). FAD2 activity levels were similar at 10°C and 20°C and decreased at 30°C, which is consistent with a hypothesis that FAD2 activity is decreased by high temperatures. This phenomenon has been mainly attributed to the relatively low thermal stability of the major and seed-specific desaturase isoform FAD2-1 (45).
Our objective in this study was to alter the unsaturation index of the S. cerevisiae membrane through the heterologous expression of the sunflower desaturase genes, FAD2-1 and FAD2-3. We hypothesized that gene-engineered fluidization of the membrane lipids might play a protective role upon freezing. The results may have practical applications in increasing yeast resistance to freezing.
MATERIALS AND METHODS
Strains, culture media, and general methods.
S. cerevisiae W303-1A (MATα leu2-3,112 ura3-1 trp1-1 his3-11,15 ade2-1 can1-100 GAL mal SUC2) wild-type strain (57) was used throughout this work. Escherichia coli strain DH10B was used as the host for plasmid construction. Yeast cells were cultured at 30°C in YPD (1% yeast extract, 2% peptone, 2% glucose) or SD [0.2% yeast nitrogen base without amino acids (DIFCO, BD Diagnostics, Sparks, MD), 0.5% (NH4)2SO4, 2% glucose] supplemented with the appropriate auxotrophic requirements (49). In some experiments, the concentration of tryptophan was increased by supplementing the medium with 200 μg/ml of this amino acid. E. coli was grown in Luria-Bertani (LB) medium (1% peptone, 0.5% yeast extract, 0.5% NaCl) supplemented with ampicillin (50 mg/liter). Yeast cells were transformed by the lithium acetate method (18). E. coli was transformed by using an Eppendorf electroporator 2510 (Eppendorf AG, Hamburg, Germany).
Stress sensitivity tests.
Cells were grown at 30°C to midexponential phase, collected by centrifugation (3,000 × g, 2 min, 4°C), transferred to fresh medium, and incubated at various temperatures, after which growth was monitored. Doubling times (g) were calculated from the formula g = ln 2/μ, where μ is the specific growth rate constant of the culture. μ was calculated from the slope of the line obtained after plotting ln X versus t, where X is the cell density of the culture, measured as optical density at 600 nm (OD600), at multiple time points (t) during logarithmic growth. For plate phenotype experiments, cultures were diluted to an OD600 of 0.3, and 10-fold serial dilutions were spotted (3 μl) onto SD or YPD agar solid medium containing sorbitol or NaCl at different concentrations. Unless otherwise indicated, colony growth was inspected after 2 to 4 days of incubation at 30°C.
For freezing tolerance assays, cells were grown in SD medium at 30°C or 15°C, harvested (OD600 of 3), and resuspended in YPD (OD600 of 10), and 10-μl aliquots were shifted to −20°C. At various times, samples were thawed at 30°C for 10 min and diluted, and cells were plated onto solid YPD. After 2 days at 30°C, colonies were counted. Viability is expressed as the percentage of viable cells relative to unfrozen control samples.
Plasmids.
Plasmids for expressing FAD2 desaturases were pVTHaFAD2-1 and pVTHaFAD2-3 (45), which contain the sunflower (H. annuus) genes FAD2-1 (GenBank accession number AF251842) and FAD2-3 (GenBank accession number AF251844), respectively, flanked by the S. cerevisiae ADH1 promoter in the URA3-based multicopy vector pVT102-U (60).
Northern blot.
Total RNA was prepared as previously described (49). Equal amounts of RNA (10 μg) were separated in 1% (wt/vol) agarose gels containing formaldehyde (2.5% vol/vol), transferred to a nylon membrane, and hybridized with a 32P-labeled probe of the FAD2-1 and FAD2-3 genes. Probes were obtained by restriction with BamHI of plasmids pVTHaFAD2-1 and pVTHaFAD2-3. A PCR-generated fragment of the S. cerevisiae ACT1 gene (+10 to +1,066) was used as the loading control. Probes were radiolabeled with the random primer Ready-to-Go kit (Amersham Biosciences, Chalfont-St. Giles, England) and [α-32P]dCTP (Amersham Biosciences). Hybridization was carried out under standard conditions (49). Filters were exposed to a high-resolution BAS-MP 2040S imaging plate (Fuji, Kyoto, Japan) for 24 h and scanned in a phosphorimager (FLA-3000; Fuji).
Lipid analysis.
Cells were grown to stationary phase (OD600 of ∼3) in SD at 30°C or 15°C. Culture samples of 50 ml corresponding to approximately 45 mg (dry weight) of cells (OD600 of 1 equals 0.3 mg [dry weight] of cells/ml), were centrifuged (3,000 × g, 2 min, 4°C) and washed twice with ultrapure water (MilliQ RO 10 Plus; Millipore, Bedford, MA). Total lipid content and fatty acid composition of whole yeast cells were determined by using the one-step method of Garcés and Mancha (15). Briefly, following the addition of 3.3 ml methanol-toluene-dimetoxypropane-H2SO4 (39:20:5:2, vol/vol/vol/vol) and 1.7 ml heptane to the yeast pellet, the mixture was incubated at 80°C for 1 h, forming a single phase. After the mixture was cooled, the upper phase containing the fatty acid methyl esters was separated, washed with 5 ml 6.7% Na2SO4, and evaporated to dryness under nitrogen. The methyl esters were dissolved in an appropriate volume of heptane and analyzed by gas-liquid chromatography using a HP-5890 (Hewlett-Packard, Palo Alto, CA) fitted with a capillary column (30-m length; 0.25-mm inner diameter; 0.20-μm film thickness) of fused silica (Supelco, Bellefonte, PA) and a flame ionization detector. Hydrogen was used as the carrier gas with a linear rate of 28 cm s−1 and a split ratio of 1/50. The temperature of the injector and detector was 220°C, and the oven temperature was 170°C. Heptadecanoic acid was used as an internal standard.
The yeast lipid composition was determined from yeast cells harvested and washed as described above. After the cell pellet was heated at 100°C for 10 min to stop any enzymatic reaction, the fresh weight of the cell pellet was measured, and the yeast cells were resuspended with distilled water. Lipids were extracted from 1-ml aliquots with 3.75 ml of chloroform-methanol (1:2, vol/vol) according to the method of Bligh and Dyer (9) with the modifications of Kates (24), and the lower phase was separated and evaporated to dryness with nitrogen. Fatty acid composition of the different lipid classes was determined as described previously (45).
Membrane fluidity determination.
Membrane fluidity was measured as previously described (27) by using the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene as a reporter. The degree of fluorescence polarization was calculated as described by Ansari et al. (3). In these experiments, decreases in the degree of fluorescence polarization reflect increases of the fluidity of the lipid bilayer, which controls or affects the mobility of DHP on the membrane.
Statistical analysis.
The significance of variations in the unsaturation index among strains was determined by a global analysis of variance (available at www.physics.csbsju.edu/stats/anova.html).
RESULTS
Heterologous expression of sunflower FAD2 desaturases enhances unsaturation of yeast lipids.
S. cerevisiae W303FAD2-1 and W303FAD2-3 strains, which express the sunflower genes FAD2-1 and FAD2-3, respectively, were analyzed for FAD2 mRNA levels. Northern blot analysis showed a single hybridizing band that was absent in samples of the control strain transformed with an empty plasmid (data not shown). Differences in the expression levels of FAD2-1 and FAD2-3 were insignificant in cells growing either actively or in the diauxic shift. Moreover, none of the transformant strains had abnormal-sized desaturase mRNAs (data not shown).
Heterologous expression of microsomal oleate desaturase-encoding genes resulted in the production of palmitolinoleic (16:2Δ9,12) and linoleic acid (18:2Δ9,12), and the reduction of the amount of fatty acids 16:1Δ9 and 18:1Δ9, thus altering the fatty acid profile and increasing the unsaturation index of yeast lipids (Table 1). This result indicates that the sunflower desaturases are active and act on endogenous yeast monounsaturated fatty acids, preferentially converting oleic acid to linoleic acid (Table 1). As in a previous study (45), the dienoic acids were almost evenly distributed among the different lipid classes analyzed, including polar (phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol) and neutral lipids (diacylglycerol, triacylglycerol, and sterol ester), although a higher percentage was detected in phosphatidylethanolamine and neutral lipids, two of the most prominent components of the yeast plasma membrane lipids (8). As previously reported (32, 45), the incubation temperature altered the amount of unsaturated fatty acids present and the monoenoic/dienoic acid ratio of the transformed cells. Cells incubated at 15°C contained higher levels of dienoic acids than did those cultivated at 30°C (Table 1). This difference was statistically significant (P < 0.01) in cells expressing the FAD2-1 gene, in which the percentages of 16:2 and 18:2 fatty acids increased four- and threefold, respectively, at 15°C relative to levels at 30°C. However, the activity of FAD2 desaturases did not alter the total amount of fatty acids, except at 15°C, where a moderate increase was observed (Table 1).
TABLE 1.
Fatty acid composition of total lipids in S. cerevisiae FAD2 transformants grown at different temperaturesa
| Strain | Temp (°C) | Fatty acid composition (mol%)b
|
UIc | Total wt of fatty acidsd | |||||
|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 16:1 | 16:2 | 18:0 | 18:1 | 18:2 | ||||
| trp1 strains | |||||||||
| W303-1A | 30 | 20 ± 0.5 | 34 ± 1.2 | UDe | 11 ± 0.3 | 36 ± 1.4 | UD | 0.69 A | 21 |
| 15 | 20 ± 0.1 | 35 ± 0.7 | UD | 8.9 ± 0.2 | 37 ± 0.6 | UD | 0.71 A | 22 | |
| W303FAD2-1 | 30 | 20 ± 0.4 | 30 ± 1.4 | 1.6 ± 0.4 | 11 ± 1.7 | 29 ± 0.6 | 8.0 ± 0.1 | 0.79 B | 18 |
| 15 | 20 ± 1.3 | 23 ± 1.1 | 6.0 ± 0.3 | 12 ± 0.9 | 18 ± 0.2 | 20 ± 2.8 | 0.94 C | 26 | |
| W303FAD2-3 | 30 | 22 ± 0.8 | 27 ± 0.8 | 5.2 ± 0.0 | 12 ± 1.1 | 16 ± 0.4 | 19 ± 1.0 | 0.90 C | 20 |
| 15 | 20 ± 0.2 | 27 ± 0.5 | 5.1 ± 0.1 | 11 ± 0.1 | 15 ± 0.2 | 22 ± 0.6 | 0.97 C | 26 | |
| trp1 TRP1 strains | |||||||||
| W303-1A | 30 | 21 ± 0.5 | 31 ± 0.5 | UD | 11 ± 1.6 | 37 ± 1.4 | UD | 0.68 A | 39 |
| 15 | 19 ± 0.9 | 39 ± 0.4 | UD | 9.5 ± 1.4 | 33 ± 0.4 | UD | 0.72 AB | 49 | |
| W303FAD2-1 | 30 | 20 ± 0.9 | 30 ± 0.9 | UD | 11 ± 0.7 | 33 ± 0.5 | 6.0 ± 0.9 | 0.74 B | 36 |
| 15 | 19 ± 0.3 | 27 ± 0.6 | 7.2 ± 1.2 | 11 ± 0.2 | 16 ± 1.9 | 20 ± 1.2 | 0.97 C | 45 | |
| W303FAD2-3 | 30 | 22 ± 0.9 | 23 ± 2.8 | 5.4 ± 1.7 | 12 ± 0.8 | 16 ± 3.3 | 21 ± 2.7 | 0.92 C | 34 |
| 15 | 19 ± 0.5 | 27 ± 2.0 | 6.6 ± 1.3 | 11 ± 1.9 | 16 ± 3.5 | 20 ± 1.5 | 0.96 C | 48 | |
Tryptophan auxotrophic (trp1) and prototrophic (trp1 TRP1) cells of the S. cerevisiae W303-1A wild-type strain expressing the sunflower genes FAD2-1 and FAD2-3 were analyzed.
Values are the means ± standard errors of the means for at least three independent experiments with duplicate determinations of fatty acid composition.
UI, unsaturation index. The unsaturation index was defined as follows: [(percentage of 16:1 + percentage of 18:1) + 2(percentage of 16:2 + percentage of 18:2)]/100. Values with different letters are significantly different at a P of 0.01 (analysis of variance).
In micrograms per milligram of cells (dry weight).
UD, undetected.
Effects of heterologous FAD2 desaturases on membrane fluidity and growth.
Membrane fluidity in whole cells of wild-type and FAD2-overexpressing strains was estimated by measuring the fluorescence polarization of the lipophilic membrane probe 1,6-diphenyl-1,3,5-hexatriene. In both strains expressing FAD2, W303FAD2-1 and W303FAD2-3, the fluorescence polarization values at 25°C were significantly lower, 0.116 ± 0.033 and 0.125 ± 0.020, respectively, than those of the control cells (0.160 ± 0.016). Thus, the fluidity of yeast membrane lipids increases when monoenoic fatty acids are converted to dienoic fatty acids.
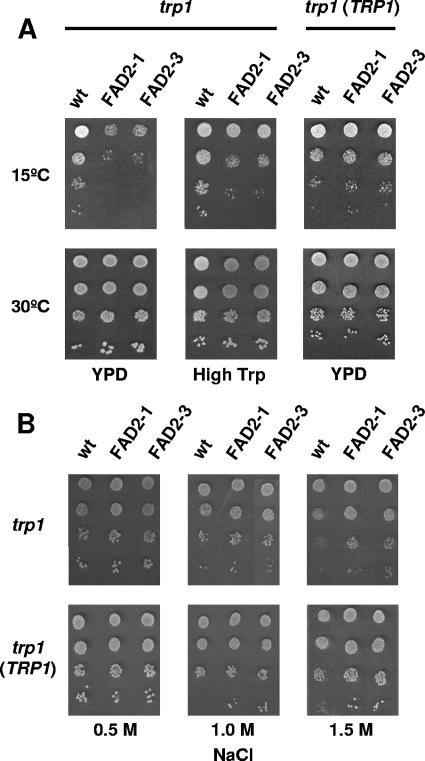
Overproduction of FAD2 desaturases increased growth at 30°C and decreased growth of wild-type cells cultivated in liquid SD medium at 10 or 15°C (Table 2), with FAD2-overproducing cells not producing visible colonies on solid YPD at 15°C (Fig. 1A). Similar phenotypes were observed when cells were plated on solid media, both SD (data not shown) and YPD (Fig. 1A), but the effect of FAD2 on growth at low temperature was more pronounced.
TABLE 2.
Growth of FAD2 transformants at different temperaturesa
| Strain | Doubling time (h) atb:
|
||
|---|---|---|---|
| 30°C | 15°C | 10°C | |
| W303-1A | 2.2 ± 0.1 | 11.5 ± 0.2 | 89 ± 5.3 |
| W303FAD2-1 | 1.7 ± 0.1 | 13.3 ± 0.2 | 107 ± 5.2 |
| W303FAD2-3 | 1.7 ± 0.1 | 12.8 ± 0.1 | 107.3 ± 12 |
Tryptophan auxotrophic (trp1) cells of the S. cerevisiae W303-1A wild-type strain expressing the sunflower genes FAD2-1 and FAD2-3 were analyzed. Cells were grown in liquid SD medium.
Values represent the means ± standard errors of the means for at least two independent experiments.
FIG. 1.
FAD2-overexpressing yeast cells display trp1-dependent cold sensitivity and enhanced NaCl tolerance. (A) Tryptophan auxotrophic (trp1) cells of the S. cerevisiae W303-1A wild-type strain (wt) were transformed with plasmids pVTHaFAD2-1 (FAD2-1) and pVTHaFAD2-3 (FAD2-3), which contain the sunflower (H. annuus) genes FAD2-1 and FAD2-3, respectively, and transformants were assayed for growth at 15°C and 30°C. Wild-type and FAD2-overexpressing strains transformed with the integrative plasmid YIplac112, carrying the wild-type TRP1 gene (trp1 TRP1), were also tested. Cells were grown in SD liquid medium at 30°C until early exponential phase and adjusted to an OD600 of 0.3. Serial 10-fold dilutions (to 10−3) of the adjusted cultures were spotted (3 μl) onto standard YPD agar plates (YPD) or YPD plates containing excess tryptophan (200 μg per ml) (High Trp). Plates were incubated for 2 (30°C) or 7 (15°C) days. (B) The same strains were cultivated at 30°C on YPD plates containing NaCl at the indicated concentrations and inspected after 5 days. Cultures were pregrown and diluted, and cells were spotted as described above. In all cases, a representative experiment is shown.
Nutrient uptake may be sensitive to changes in the fatty acid composition of the yeast membrane. Tryptophan uptake can affect the growth temperature profile of S. cerevisiae (1) and is impaired in cells exposed to low temperatures (1), and the strain used in this study was a trp1 strain. Yeast growth was stimulated by increasing tryptophan availability at low temperatures (Fig. 1A), although the growth reduction was still detectable under these conditions. The addition of excess tryptophan did not affect yeast cell growth at 30°C. No growth differences at either 15 or 30°C were observed between wild-type and FAD2-overexpressing strains transformed with the integrative plasmid YIplac112, carrying the wild-type TRP1 gene (Fig. 1A). This result was not due to a lack of activity of the heterologous desaturases in the Trp+ background, since both S. cerevisiae W303FAD2-1 and W303FAD2-3 accumulated dienoic fatty acids (Table 1) in a manner similar to that observed in the corresponding tryptophan auxotrophic strains (Table 1).
Membrane fluidity and tolerance to ion stress and freezing injury.
Production of dienoic fatty acids did not alter the growth of yeast cells, either Trp+ or Trp−, exposed to different concentrations of sorbitol (data not shown). However, overexpression of FAD2 genes did increase Na+ tolerance relative to strains harboring an empty plasmid (Fig. 1B).
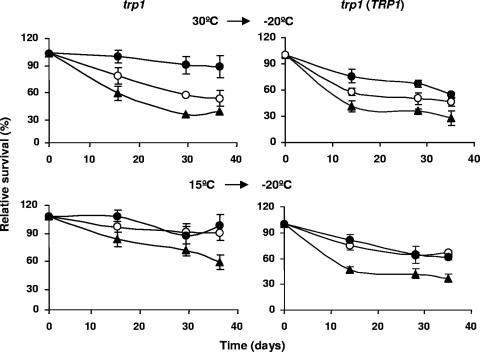
Cultures of wild-type and FAD2-expressing cells were transferred from 30 to −20°C and analyzed for cell viability after different periods of frozen storage. The viability of wild-type W303-1A (trp1) cells, in which no detectable dienoic fatty acids were produced, was less than that of Trp− transformed cells carrying a high-copy-number expression cassette of FAD2 (Fig. 2). The extent of unsaturation of lipids at 30°C was critical for cell survival below 0°C. Cells expressing FAD2-1 died more rapidly when frozen than did the cells producing the FAD2-3 enzyme, which corresponds with their dienoic acid content (Table 1). Similar results were also observed for the Trp+ strains, although the effects on tolerance to freezing were less pronounced (Fig. 2).
FIG. 2.
Fluidization of yeast lipids enhances tolerance to freezing. Cell cultures of the trp1 and trp1 TRP1 S. cerevisiae strains, wild-type W303-1A (▴) and transformants overexpressing FAD2-1 (○) and FAD2-3 (•), were grown at 30°C (OD600 of 0.4 to 0.6) and transferred directly to −20°C or preincubated at 15°C for 24 h, prior the shift to −20°C. After 14, 28, and 35 days, cell samples were thawed at 30°C for 30 min, diluted, and plated onto solid YPD, and the percentage of survival was determined. Values represent the means of at least three independent experiments. The error associated with the points was calculated by using the formula: (1.96 × SD)/√n, where SD is the standard deviation and n is the number of measurements.
Yeast cell survival following freezing and frozen storage increased if the cells were grown at 15°C instead of 30°C (Fig. 2), confirming that death during freezing can be prevented or alleviated by growth at low temperatures (23, 39). Again, the loss of viability following freezing was significantly larger for the control strain. In a representative experiment, ∼50% of wild-type cells and ∼90% of the FAD2-3-overproducing cells survived for 35 days at −20°C (Fig. 2). The differences in tolerance to freezing of FAD2-3 and FAD2-1-producing cells grown at 15°C were less evident than those observed in cells grown at 30°C, probably because the two FAD2-expressing strains produce similar amounts of dienoic fatty acids at low temperatures (Table 1). These observations were further confirmed in Trp+ strains (Fig. 2).
DISCUSSION
Heterologous expression of sunflower microsomal oleate desaturases in yeast cells enabled us to evaluate the functional role of dienoic fatty acids. Cells of S. cerevisiae into which either the FAD2-1 or FAD2-3 gene for Δ12 desaturases from H. annuus were introduced could efficiently convert palmitoleic and oleic acid into palmitolinoleic and linoleic acid, respectively. The extent of accumulation of dienoic acids was similar to that obtained by overexpression of other plant FAD2 genes, which range from 10 to 50% (11, 13, 19, 25). More diunsaturated fatty acids were produced at 15°C than at 30°C, especially in FAD2-1 transformants. In this respect, reports of the expression of Arabidopsis FAD2 in yeast have yielded contradictory results, in which the amounts of 16:2 and 18:2 could increase at low temperatures (13) or remain constant (11). We found that the unsaturation index of lipids in both Trp+ and Trp− W303FAD2-1 cells varied from 0.74 to 0.79 at 30°C to 0.94 to 0.97 at 15°C, a difference that has been attributed to the low thermal stability of the FAD2-1 isoform (45). However, the overexpression of the sunflower FAD2 genes did not increase the total unsaturated fatty acid content. This fact could be explained by possible feedback inhibition of Ole1p, since exogenous 18:2 in the culture medium could repress transcription of OLE1 (10).
Increasing the unsaturation index of yeast lipids fluidized the yeast membrane and altered the stress response of yeast cells. The finding that biosynthesis of 16:2Δ9,12 and 18:2Δ9,12 fatty acids increased tolerance to salt stress and freezing was not completely unexpected. There are previous reports that suggest that membrane lipid composition is correlated with tolerance to different stresses, including heat shock (51), heavy metals (17), and exposure to the herbicide 2,4-dichlorophenoxyacetic acid (61). Survival following freezing/thawing might also depend on the physical properties of the membrane. Below 0°C, cells are injured by the formation of ice crystals, which results in macromolecule and membrane denaturation (33). The growth of ice crystals during frozen storage could further degrade the plasma membrane (65). Thus, changing the fluidity of the lipid bilayer may help prevent or alleviate membrane damage due to freezing and contribute to cell survival.
The same protective mechanism may also operate in cells exposed to hyperosmolarity, since osmotic stress reduces cell membrane fluidity (27), which influences membrane permeabilization and cell death (41). We found that fluidization of the yeast membrane produced a moderate increase in Na+ tolerance but had no effects on resistance to pure osmotic stress. In S. cerevisiae, Na+ is extruded by both the P-type ATPase Ena1p (30) and the H+-antiporter Nha1p (21). In addition, Na+ compartmentation is mediated by vacuolar antiporters (48). All of these mechanisms of ion homeostasis depend on membrane proteins, whose activity might be affected by changes in membrane fluidity. Phospholipid composition of the membrane affects membrane-associated processes, such as plasma membrane ATPase activity (47), the higher proton motive force (34), and the transport of various amino acids (58).
Increased unsaturation of yeast lipids altered the growth profile of W303-1A (trp1) cells in response to ambient temperature. Unlike previous reports (40), dienoic fatty acid-enriched yeast cells grew slightly faster at 30°C than did wild-type cells. Similar results were observed at 37°C (data not shown). Their growth at low temperatures was also greatly reduced, but the cold sensitivity phenotype was alleviated by increasing tryptophan availability and completely overcome by tryptophan prototrophy. No phenotypic differences were detected in Trp+ transformants grown at 30°C or 37°C (data not shown).
Tryptophan uptake has been termed the Achilles' heel of yeast physiology (2), since under a variety of stress conditions, it becomes a limiting factor for cell growth. The sensitivity of tryptophan permeases to changes in membrane fluidity may also determine the growth temperature profile of S. cerevisiae (1). Trp− yeast cells that overproduce Tat2p, a high-affinity tryptophan transporter (46), grow better than the wild-type cells at 10 or 15°C, while their growth at 37°C is relatively reduced (1). Thus, Tat2p activity may be sensitive to the changes in fatty acid composition resulting from overexpression of sunflower FAD2 desaturases.
Increased unsaturation of yeast lipids may also affect the protein sorting mediated by lipid rafts and the final destination of key membrane proteins. Association with lipid rafts, a sphingolipid- and sterol-rich membrane domain (50), plays an essential role in the correct localization of proteins such as Tat2p (59), Ole1p (56), or Pma1p (6, 7), the major plasma membrane H+-ATPase in S. cerevisiae. Mutations in PMA1 result in cold sensitivity and Na+ resistance (22, 64), phenotypes shared by Trp− FAD2-overexpressing cells. Thus, changes in the unsaturation index of lipids could affect the localization of key proteins and thereby cause pleiotropic phenotypes.
The data presented here provide direct evidence that overexpression of desaturases can be used to increase the tolerance of yeast cells to freezing. This trait is critical in determining an optimal leavening of lean and sweet frozen-dough products, and increasing the resistance of industrial yeasts to freezing is one of the most important biotechnological challenges in this field (42). Altering fatty acid composition and content by expressing heterologous desaturases could also be used to modify lipid microdomains and to study their function in cell signaling, polarity, and sorting. Thus, the results reported in this study have both basic and applied significance.
Acknowledgments
We thank A. Blasco for technical assistance.
This research was funded by the CICYT projects (AGL2001-1203, AGL2004-00462, AGL2001-1060, and AGL2004-02060) of the Ministry of Education and Science (MEC, Spain). S.R.-V. was supported by a CSIC-EPO fellowship. A.S.-G. was supported by an FPI predoctoral fellowship, and J.M.M.-R. was the recipient of a postdoctoral contract within the “Ramón y Cajal” Program, both from MEC.
Footnotes
Published ahead of print on 27 October 2006.
REFERENCES
- 1.Abe, F., and K. Horikoshi. 2000. Tryptophan permease gene TAT2 confers high-pressure growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 20:8093-8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe, F., and H. Iida. 2003. Pressure-induced differential regulation of the two tryptophan permeases Tat1 and Tat2 by ubiquitin ligase Rsp5 and its binding proteins, Bul1 and Bul2. Mol. Cell. Biol. 23:7566-7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansari, S., P. Gupta, S. K. Mahanty, and R. Prasad. 1993. Uptake of amino acids by erg mutants of Candida albicans. J. Med. Vet. Mycol. 31:377-386. [Google Scholar]
- 4.Attfield, P. V. 1997. Stress tolerance: the key to effective strains of industrial baker's yeast. Nat. Biotechnol. 15:1351-1357. [DOI] [PubMed] [Google Scholar]
- 5.Avery, S. V., D. Lloyd, and J. L. Harwood. 1995. Temperature-dependent changes in plasma-membrane lipid order and the phagocytotic activity of the amoeba Acanthamoeba castellanii are closely correlated. Biochem. J. 312:811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagnat, M., S. Keränen, A. Shevchenko, A. Shevchenko, and K. Simons. 2000. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 97:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagnat, M., A. Chang, and K. Simons. 2001. Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol. Biol. Cell 12:4129-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blagovic, B., J. Rupcic, M. Mesaric, and V. Maric. 2005. Lipid analysis of the plasma membrane and mitochondria of brewer's yeast. Folia Microbiol. (Praha) 50:24-30. [DOI] [PubMed] [Google Scholar]
- 9.Bligh, E., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 10.Bossie, M. A., and C. E. Martin. 1989. Nutritional regulation of yeast Δ-9 fatty acid desaturase activity. J. Bacteriol. 171:6409-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, A. P., R. Dann, S. Bowra, and M. J. Hills. 1998. Characterization of expression of a plant oleate desaturase in yeast. J. Am. Oil Chem. Soc. 75:77-82. [Google Scholar]
- 12.Carratù, L., S. Franceschelli, C. L. Pardini, G. S. Kobayashi, I. Horvath, L. Vigh, and B. Maresca. 1996. Membrane lipid perturbation modifies the set point of the temperature of heat shock response in yeast. Proc. Natl. Acad. Sci. USA 93:3870-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Covello, P. S., and D. W. Reed. 1996. Functional expression of the extraplastidial Arabidopsis thaliana oleate desaturase gene (FAD2) in Saccharomyces cerevisiae. Plant Physiol. 111:223-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyer, J. M., D. C. Chapital, J. W. Cary, and A. B. Pepperman. 2001. Chilling-sensitive, post-transcriptional regulation of a plant fatty acid desaturase expressed in yeast. Biochem. Biophys. Res. Commun. 282:1019-1025. [DOI] [PubMed] [Google Scholar]
- 15.Garcés, R., and M. Mancha. 1993. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem. 211:139-143. [DOI] [PubMed] [Google Scholar]
- 16.Hermes-Lima, M., and K. B. Storey. 1993. Antioxidant defences in the tolerance of freezing and anoxia by garter snakes. Am. J. Physiol. 265:R646-R652. [DOI] [PubMed] [Google Scholar]
- 17.Howlett, N. G., and S. V. Avery. 1997. Induction of lipid peroxidation during heavy metal stress in Saccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturation. Appl. Environ. Microbiol. 63:2971-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito, H., K. Jukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kajiwara, S., A. Shirai, T. Fujii, T. Toguri, K. Nakamura, and K. Ohtaguchi. 1996. Polyunsaturated fatty acid biosynthesis in Saccharomyces cerevisiae: expression of ethanol tolerance and the FAD2 gene from Arabidopsis thaliana. Appl. Environ. Microbiol. 62:4309-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajiwara, S., T. Aritomi, K. Suga, K. Ohtaguchi, and O. Kobayashi. 2000. Overexpression of the OLE1 gene enhances ethanol fermentation by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 53:568-574. [DOI] [PubMed] [Google Scholar]
- 21.Kamauchi, S., K. Mitsui, S. Ujike, M. Haga, N. Nakamura, H. Inoue, S. Sakajo, M. Ueda, A. Tanaka, and H. Kanazawa. 2002. Structurally and functionally conserved domains in the diverse hydrophilic carboxy-terminal halves of various yeast and fungal Na+/H+ antiporters (Nha1p). J. Biochem. (Tokyo) 131:821-831. [DOI] [PubMed] [Google Scholar]
- 22.Kaminska, J., A. Tobiasz, M. Gniewosz, and T. Zoladek. 2000. The growth of mdp1/rsp5 mutants of Saccharomyces cerevisiae is affected by mutations in the ATP-binding domain of the plasma membrane H+-ATPase. Gene 242:133-140. [DOI] [PubMed] [Google Scholar]
- 23.Kandror, O., N. Bretschneider, E. Kreydin, D. Cavalieri, and A. L. Goldberg. 2004. Yeast adapt to near-freezing temperatures by STRE/Msn2,4-dependent induction of trehalose synthesis and certain molecular chaperones. Mol. Cell 13:771-781. [DOI] [PubMed] [Google Scholar]
- 24.Kates, M. 1986. Lipid extraction procedures, p. 100-111. In M. Kates (ed.), Techniques of lipidology. Elsevier, Amsterdam, The Netherlands.
- 25.Kirsch, C., K. Hahlbrock, and I. Somssich. 1997. Rapid and transient induction of a parsley microsomal Δ12 fatty acid desaturase mRNA by fungal elicitor. Plant Physiol. 115:283-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein, W., M. H. W. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laroche, C., L. Beney, P. A. Marechal, and P. Gervais. 2001. The effect of osmotic pressure on the membrane fluidity of Saccharomyces cerevisiae at different physiological temperatures. Appl. Microbiol. Biotechnol. 56:249-254. [DOI] [PubMed] [Google Scholar]
- 28.Los, D. A., and N. Murata. 1998. Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta 1394:3-15. [DOI] [PubMed] [Google Scholar]
- 29.Los, D. A., and N. Murata. 2004. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 1666:142-157. [DOI] [PubMed] [Google Scholar]
- 30.Marquez, J. A., and R. Serrano. 1996. Multiple transduction pathways regulate the sodium-extrusion gene PMR2/ENA1 during salt stress in yeast. FEBS Lett. 382:89-92. [DOI] [PubMed] [Google Scholar]
- 31.Martínez-Rivas, J. M., P. Sperling, W. Lühs, and E. Heinz. 2001. Spatial and temporal regulation of three different microsomal oleate desaturase genes (FAD2) from normal-type and high-oleic varieties of sunflower (Helianthus annuus L.). Mol. Breed. 8:159-168. [Google Scholar]
- 32.Martínez-Rivas, J. M., A. Sánchez-García, M. D. Sicardo, M. T. García-Díaz, and M. Mancha. 2003. Oxygen-independent temperature regulation of the microsomal oleate desaturase (FAD2) activity in developing sunflower (Helianthus annuus) seeds. Physiol. Plant 117:179-185. [Google Scholar]
- 33.Morris, G. J., G. E. Coulson, and K. J. Clarke. 1988. Freezing injury in Saccharomyces cerevisiae. The effects of growth conditions. Cryobiology 25:471-472. [Google Scholar]
- 34.Morsomme, P., and M. Boutry. 2000. The plant plasma membrane H+-ATPase: structure, function and regulation. Biochim. Biophys. Acta 1465:1-16. [DOI] [PubMed] [Google Scholar]
- 35.Murata, N., and H. Wada. 1995. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem. J. 308:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa, Y., N. Sakumoto, Y. Kaneko, and S. Harashima. 2002. Mga2p is a putative sensor for low temperature and oxygen to induce OLE1 transcription in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 291:707-713. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura, M. T., and T. Y. Nara. 2002. Gene regulation of mammalian desaturases. Biochem. Soc. Trans. 30:1076-1079. [DOI] [PubMed] [Google Scholar]
- 38.Napier, J. A., L. V. Michaelson, and A. K. Stobart. 1999. Plant desaturases: harvesting the fat of the land. Curr. Opin. Plant Biol. 2:123-127. [DOI] [PubMed] [Google Scholar]
- 39.Panadero, J., C. Pallotti, S. Rodríguez-Vargas, F. Randez-Gil, and J. A. Prieto. 2006. A downshift in temperature activates the high osmolarity glycerol (HOG) pathway, which determines freeze tolerance in Saccharomyces cerevisiae. J. Biol. Chem. 281:4638-4645. [DOI] [PubMed] [Google Scholar]
- 40.Peyou-Ndi, M. M., J. L. Watts, and J. Browse. 2000. Identification and characterization of an animal Δ12 fatty acid desaturase gene by heterologous expression in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 376:399-408. [DOI] [PubMed] [Google Scholar]
- 41.Poirier, I., P. A. Marechal, S. Richard, and P. Gervais. 1999. Saccharomyces cerevisiae viability is strongly dependent on rehydration kinetics and the temperature of dried cells. J. Appl. Microbiol. 86:87-92. [DOI] [PubMed] [Google Scholar]
- 42.Randez-Gil, F., J. Aguilera, A. Codón, A. M. Rincón, F. Estruch, and J. A. Prieto. 2003. Baker's yeast: challenges and future prospects, p. 57-97. In J. H. de Winde (ed.), Functional genetics of industrial yeasts. Springer-Verlag, Heidelberg, Germany.
- 43.Reed, D. W., U. A. Schafer, and P. S. Covello. 2000. Characterization of the Brassica napus extraplastidial linoleate desaturase by expression in Saccharomyces cerevisiae. Plant Physiol. 122:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakamoto, T., G. Shen, S. Higashi, N. Murata, and D. A. Bryant. 1998. Alteration of low-temperature susceptibility of the cyanobacterium Synechococcus sp. PCC 7002 by genetic manipulation of membrane lipid unsaturation. Arch. Microbiol. 169:20-28. [DOI] [PubMed] [Google Scholar]
- 45.Sánchez-García, A., M. Mancha, E. Heinz, and J. M. Martínez-Rivas. 2004. Differential temperature regulation of three sunflower microsomal oleate desaturase (FAD2) isoforms overexpressed in Saccharomyces cerevisiae. Eur. J. Lipid Sci. Technol. 106:583-590. [Google Scholar]
- 46.Schmidt, A., M. N. Hall, and A. Koller. 1994. Two FK506 resistance-conferring genes in Saccharomyces cerevisiae, TAT1 and TAT2, encode amino acid permeases mediating tyrosine and tryptophan uptake. Mol. Cell. Biol. 14:6597-6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrano, R., C. Montesinos, and J. Sanchez. 1988. Lipid requirements of the plasma membrane ATPases from oat roots and yeast. Plant Sci. 56:117-122. [Google Scholar]
- 48.Serrano, R., and A. Rodriguez-Navarro. 2001. Ion homeostasis during salt stress in plants. Curr. Opin. Cell Biol. 13:399-404. [DOI] [PubMed] [Google Scholar]
- 49.Sherman, F., G. R. Fink, and J. B. Hicks. 1986. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 50.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 51.Steels, E. L., R. P. Learmonth, and K. Watson. 1994. Stress tolerance and membrane lipid unsaturation in Saccharomyces cerevisiae grown aerobically or anaerobically. Microbiology 140:569-576. [DOI] [PubMed] [Google Scholar]
- 52.Stukey, J. E., V. M. McDonough, and C. E. Martin. 1989. Isolation and characterization of OLE1, a gene affecting fatty acid desaturation from Saccharomyces cerevisiae. J. Biol. Chem. 264:16537-16544. [PubMed] [Google Scholar]
- 53.Stukey, J. E., V. M. McDonough, and C. E. Martin. 1990. The OLE1 gene of Saccharomyces cerevisiae encodes the delta 9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J. Biol. Chem. 265:20144-20149. [PubMed] [Google Scholar]
- 54.Szalontai, B., Y. Nishiyama, Z. Gombos, and N. Murata. 2000. Membrane dynamics as seen by Fourier transform infrared spectroscopy in a cyanobacterium, Synechocystis PCC 6803. The effects of lipid unsaturation and the protein-to-lipid ratio. Biochim. Biophys. Acta 1509:409-419. [DOI] [PubMed] [Google Scholar]
- 55.Tasaka, Y., Z. Gombos, Y. Nishiyama, P. Mohanty, T. Ohba, K. Ohki, and N. Murata. 1996. Targeted mutagenesis of acyl-lipid desaturases in Synechocystis: evidence for the important roles of polyunsaturated membrane lipids in growth, respiration and photosynthesis. EMBO J. 15:6416-6425. [PMC free article] [PubMed] [Google Scholar]
- 56.Tatzer, V., G. Zellnig, S. D. Kohlwein, and R. Schneiter. 2002. Lipid-dependent subcellular relocalization of the acyl chain desaturase in yeast. Mol. Biol. Cell 13:4429-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas, B. J., and R. Rothstein. 1989. Elevated recombination rates in transcriptionally active DNA. Cell 56:619-630. [DOI] [PubMed] [Google Scholar]
- 58.Trivedi, A., G. S. Singhal, and R. Prasad. 1983. Effect of phosphatidylserine enrichment on amino acid transport in yeast. Biochim. Biophys. Acta 729:85-89. [DOI] [PubMed] [Google Scholar]
- 59.Umebayashi, K., and A. Nakano. 2003. Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J. Cell Biol. 161:1117-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vernet, T., D. Dignard, and D. Y. Thomas. 1987. A family of yeast expression vectors containing the phage f1 intergenic region. Gene 52:225-233. [DOI] [PubMed] [Google Scholar]
- 61.Viegas, C. A., M. G. Cabral, M. C. Teixeira, G. Neumann, H. J. Heipieper, and I. Sa-Correia. 2005. Yeast adaptation to 2,4-dichlorophenoxyacetic acid involves increased membrane fatty acid saturation degree and decreased OLE1 transcription. Biochem. Biophys. Res. Commun. 330:271-278. [DOI] [PubMed] [Google Scholar]
- 62.Vigh, L., B. Maresca, and J. L. Harwood. 1998. Does the membrane's physical state control the expression of heat shock and other genes? Trends Biochem. Sci. 23:369-374. [DOI] [PubMed] [Google Scholar]
- 63.Weber, M. H., W. Klein, L. Muller, U. M. Niess, and M. A. Marahiel. 2001. Role of the Bacillus subtilis fatty acid desaturase in membrane adaptation during cold shock. Mol. Microbiol. 39:1321-1329. [DOI] [PubMed] [Google Scholar]
- 64.Withee, J. L., R. Sen, and M. S. Cyert. 1998. Ion tolerance of Saccharomyces cerevisiae lacking the Ca2+/CaM-dependent phosphatase (calcineurin) is improved by mutations in URE2 or PMA1. Genetics 149:865-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolfe, J., and G. Bryant. 1999. Freezing, drying, and/or vitrification of membrane-solute-water systems. Cryobiology 39:103-129. [DOI] [PubMed] [Google Scholar]