Abstract
We describe a study on the application of multilocus sequence typing for the analysis of Campylobacter jejuni and C. coli isolates from human domestically acquired infections in the Helsinki-Uusimaa area of Finland in 1996, 2002, and 2003. In addition, isolates from poultry meat and fecal samples of cattle from the seasonal peak (July to September) in 2003 were included in the study. In total, 361 Finnish C. jejuni and C. coli strains were typed. Sequence type 45 (ST-45) (45%), ST-21 (21%), and ST-677 (11%) clonal complexes were the most prevalent. The ST-45 and ST-677 complexes were overrepresented in comparison with previous studies. The longitudinal study revealed an association between C. coli (ST-828 complex) infection and elderly patients (≥60 years). Analysis of exposure factors, determined by a previous case-control study conducted during the seasonal peak in 2002, revealed that the ST-48 complex was significantly (P < 0.05) associated with the tasting or eating of raw minced meat. New and unassigned STs were associated with swimming in natural bodies of water, whereas the ST-677 complex was related to drinking nonchlorinated water from a small water plant or water from natural sources. The ST-45 complex was associated with contact with pet cats and dogs. In 2003, ST-45 occurrence was significantly associated with poultry whereas ST-50 was associated with isolates from humans. In contrast, ST-53, ST-58, ST-61, and ST-883 were significantly associated with isolates from cattle. Further studies are needed to reveal the significance of the observed associations.
Campylobacter jejuni and C. coli are the most frequent causes of bacterial gastroenteritis in Finland (www.ktl.fi/ttr). The number of laboratory-confirmed cases has nearly doubled over a 10-year period (2,197 cases in 1995 to 4,002 cases in 2005). Campylobacter species are frequently isolated from a wide variety of sources, including poultry, cattle, pigs, sheep, cats, dogs, wild birds, and water. Most Campylobacter infections are sporadic, and the relative contributions of different sources of infection remain unknown. Risk factors for Campylobacter infection, as identified by case-control studies, include eating or preparing raw or undercooked meat, especially chicken meat, drinking unpasteurized milk or untreated water, contact with domestic animals and pets, and foreign travel (14, 18, 26). A recent case-control study (19) revealed swimming in natural bodies of water to be a novel risk factor. In addition, eating undercooked meat and drinking from dug wells were independent risk factors for domestically acquired Campylobacter infections in Finland (19).
Discriminatory typing methods for use in the study of the molecular epidemiology and population genetics of Campylobacter isolates are crucial to better understand the epidemiology and ecology of the organism. Once such information is obtained, it could be used to develop intervention strategies to limit the numbers of human infections. Dingle et al. (6) developed a multilocus sequence typing (MLST) scheme for C. jejuni, which has been shown to be a valuable tool for studying the diversity and population genetics of Campylobacter isolates. The advantages of MLST over other molecular methods, such as pulsed-field gel electrophoresis, include transferability, standardized nomenclature, free access to the database on the Internet, and direct comparability of results between different studies. Recently, an MLST scheme utilizing the same loci previously described for typing C. jejuni was also described for C. coli (4).
Studies using molecular methods suggest that some animal host-adapted genotypes or clonal lineages may never or only rarely cause disease in humans whereas others may be common human pathogens with one or several potential food-borne sources (3, 16, 22). Host associations among some C. coli genotypes have also been suggested (17). The impact of the host and that of the bacterial strain on the disease outcome are still poorly understood.
The aims of this study were (i) the longitudinal investigation of the genetic diversity, as measured by MLST, among Finnish C. jejuni and C. coli strains collected from domestically acquired infections in 1996, 2002, and 2003; (ii) the study of the relationships of sequence types (STs) and ST complexes of the infecting strain with demographic characteristics of the patients and serotype and detailed epidemiological data obtained in a case-control study conducted in Helsinki, Finland, in 2002 (19); and (iii) the evaluation of the overlap of MLST profiles between isolates from humans and those from bovine feces and retail poultry meat during the seasonal peak in 2003.
MATERIALS AND METHODS
Bacterial isolates.
C. jejuni and C. coli isolates from domestically acquired human sporadic cases of gastroenteritis were collected at Helsinki University Central Hospital Laboratory from January to December inclusive in 1996 (n = 94), 2002 (n = 112), and 2003 (n = 101). Of these, 94 (C. coli, n = 2), 111 (C. coli, n = 2), and 100 (C. coli, n = 3) isolates, respectively, were successfully typed by MLST. The strains isolated during the seasonal peak from July to September in 2002 (n = 46) had been included in the epidemiological case-control study described in detail by Schönberg-Norio et al. (19, 20). The questionnaire filled in by the patients detailed questions about demographic and clinical data, travel history, recreational water activity, food (>30 exposures), and milk and drinking water consumption as well as contact with pet animals and other domestic animals during the 2-week period prior to falling ill. In addition to isolates from humans, Campylobacter isolates were collected from chicken and turkey retail meat samples from the Helsinki area as well as bovine fecal samples from slaughterhouses all over the country by random sampling during the seasonal peak of 2003. A total of 233 retail meat samples (169 chicken, 58 turkey, and 6 mixed meat) were analyzed, of which 41 chicken and 4 turkey samples were positive for Campylobacter spp. Of these, 36 (C. coli, n = 3) were successfully typed by MLST. In total, 20 bovine fecal isolates were typed by MLST. C. jejuni and C. coli cultures were identified by growth characteristics, microscopic analysis, catalase and oxidase production and hippurate hydrolysis.
DNA isolation.
DNA isolation was carried out as described previously (13). Briefly, cells were harvested from blood agar plates, washed with SET buffer (150 mM NaCl, 15 mM EDTA, 10 mM Tris-HCl, pH 8.0), and lysed with sodium dodecyl sulfate (0.5%) and proteinase K (100 μg/ml) at 50°C for 2 h. Protein removal was done by phenol:chloroform:isoamyl alcohol (25:24:1) extraction followed by extraction with chloroform. DNA was precipitated using 3 M sodium acetate (pH 5.3) and ethanol. The resulting precipitate was washed with 70% ethanol and finally suspended in Tris-EDTA buffer (pH 8), treated with RNase (10 μg/ml), and diluted to approximately 10 ng/μl.
Multilocus sequence typing.
Multilocus sequence typing was performed according to Dingle et al. (6), using additional primers for C. coli as described by Dingle et al. (4) and for C. jejuni as described by Schouls et al. (21), as well as gly-S5 and gly-S7 described at the Campylobacter MLST website (http://pubmlst.org/campylobacter/). PCR amplicons were purified using MultiScreen PCR plates (Millipore, MA) and suspended in 50 μl 0.1× Tris-EDTA buffer. Sequencing reaction mixtures consisted of 0.5 to 2 μl BigDye Terminator v3.1 ready reaction mix (Applied Biosystems, CA), 1.5 μl template, and 1.3 pmol primer per 20-μl reaction mixture. Sequencing reaction products were purified using MultiScreen SEQ plates (Millipore). Alternatively, PCR amplification products were purified by 20% polyethylene glycol-2.5 M NaCl precipitation (8) and sequencing products by sodium acetate-ethanol precipitation (http://pubmlst.org/campylobacter/). Electrophoresis was performed on a 96-capillary Abi 3700 sequencer with POP5 polymer or an ABI PRISM 310 genetic analyzer with a POP6 polymer (Applied Biosystems, CA). Allele sequences were assembled using Staden Package (version 1.6.0) (24).
Data analysis.
Trace files of new allele sequences and new allelic profiles were sent to the PubMLST database for assignment of allele and ST profile numbers as well as clonal complex. Clustering of sequence data was done with BioNumerics v 4.01 software (Applied Maths, Kortrijk, Belgium).
Statistical tests.
Cross-tabulations and statistical tests were performed using SPSS software (version 10.0). Analysis of association of the ST and ST complex with source of isolation and individual exposure factors was carried out using the Pearson chi-square or Fisher's exact two-tailed test, as appropriate. Results were considered statistically significant for P values of ≤0.05.
RESULTS
A total of 305 human (C. coli, n = 7), 20 bovine, 32 chicken (C. coli, n = 3), and four turkey isolates were successfully typed by MLST (Table 1) . These strains represented 19 established ST complexes and 81 STs. Of the 81 STs, 32 were described in the present study for the first time. Isolates belonging to the new STs included 34 human (11%), two chicken (6%), and one bovine (5%) isolate. Eleven (34%) of the new STs were unassigned to a previously identified ST complex. In spite of the high numbers of different STs and ST complexes, three major types, ST-45, ST-50, and ST-677, covered 53% of all isolates, and the three major complexes ST-45, ST-21, and ST-677 covered 76% of all isolates. For the ST-21 complex, ST-50 was the type most prevalent among the Finnish population.
TABLE 1.
ST complex, ST, and allele distribution among C. jejuni and C. coli isolatesa
| ST complex | ST | Allele(s)a
|
Species | No. of isolates
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aspA | glnA | gltA | glyA | pgm | tkt | uncA | Human
|
Cattle yr 2003c | Poultry yr 2003c | |||||
| Yr 1996 | Yr 2002b | Yr 2003c | ||||||||||||
| ST-21 | 21 | 2 | 1 | 1 | 3 | 2 | 1 | 5 | C. jejuni | 1 | 1 | |||
| 50 | 2 | 1 | 12 | 3 | 2 | 1 | 5 | C. jejuni | 6 | 11 (4) | 33 (25) | 5 | ||
| 53 | 2 | 1 | 21 | 3 | 2 | 1 | 5 | C. jejuni | 4 | |||||
| 451 | 2 | 1 | 2 | 3 | 2 | 3 | 5 | C. jejuni | 1 | 1 | ||||
| 883 | 2 | 17 | 2 | 3 | 2 | 1 | 5 | C. jejuni | 1 | 1 | 3 | |||
| 1943 | 2 | 1 | 1 | 3 | 2 | 226 | 5 | C. jejuni | 1 | |||||
| 1948 | 2 | 71 | 12 | 3 | 2 | 1 | 5 | C. jejuni | 1 | |||||
| 1949 | 2 | 1 | 5 | 3 | 2 | 25 | 5 | C. jejuni | 1 | |||||
| 1952 | 2 | 1 | 12 | 3 | 1 | 1 | 5 | C. jejuni | 1 | |||||
| 1969 | 2 | 234 | 2 | 3 | 2 | 1 | 5 | C. jejuni | 1 | 1 | ||||
| ST-22 | 22 | 1 | 3 | 6 | 4 | 3 | 3 | 3 | C. jejuni | 10 | ||||
| 1947 | 1 | 94 | 6 | 4 | 3 | 3 | 3 | C. jejuni | 2 | |||||
| 1966 | 1 | 3 | 6 | 4 | 3 | 276 | 3 | C. jejuni | 1 (1) | |||||
| ST-45 | 11 | 48 | 7 | 10 | 4 | 1 | 7 | 1 | C. jejuni | 3 | 2 (1) | |||
| 45 | 4 | 7 | 10 | 4 | 1 | 7 | 1 | C. jejuni | 23 | 31 (8) | 31 (27) | 2 | 22 | |
| 137 | 4 | 7 | 10 | 4 | 42 | 7 | 1 | C. jejuni | 3 | 8 (3) | 6 (2) | |||
| 230 | 4 | 7 | 41 | 4 | 42 | 7 | 1 | C. jejuni | 3 | 9 (6) | ||||
| 334 | 4 | 7 | 40 | 4 | 42 | 7 | 1 | C. jejuni | 1 (1) | |||||
| 538 | 4 | 7 | 10 | 4 | 42 | 25 | 1 | C. jejuni | 4 (2) | |||||
| 583 | 4 | 7 | 10 | 4 | 42 | 51 | 1 | C. jejuni | 1 | |||||
| 1003 | 8 | 7 | 4 | 4 | 125 | 7 | 1 | C. jejuni | 2 | |||||
| 1326 | 104 | 7 | 10 | 4 | 1 | 7 | 1 | C. jejuni | 2 | |||||
| 1944 | 4 | 7 | 10 | 4 | 320 | 7 | 1 | C. jejuni | 3 | |||||
| 1950 | 4 | 7 | 197 | 4 | 1 | 7 | 1 | C. jejuni | 1 | |||||
| 1958 | 4 | 7 | 41 | 4 | 42 | 7 | 177 | C. jejuni | 1 (1) | |||||
| 1964 | 4 | 7 | 143 | 4 | 1 | 7 | 1 | C. jejuni | 1 (1) | |||||
| 1971 | 4 | 233 | 10 | 4 | 1 | 7 | 1 | C. jejuni | 1 | |||||
| 1973 | 4 | 7 | 10 | 266 | 1 | 7 | 1 | C. jejuni | 1 | |||||
| ST-48 | 48 | 2 | 4 | 1 | 2 | 7 | 1 | 5 | C. jejuni | 5 (3) | ||||
| 475 | 2 | 4 | 1 | 4 | 19 | 62 | 5 | C. jejuni | 2 | 1 (1) | 1 | |||
| 918 | 2 | 4 | 1 | 4 | 19 | 1 | 5 | C. jejuni | 1 | |||||
| ST-52 | 52 | 9 | 25 | 2 | 10 | 22 | 3 | 6 | C. jejuni | 1 | 1 (1) | 1 | 1 | |
| ST-61 | 61 | 1 | 4 | 2 | 2 | 6 | 3 | 17 | C. jejuni | 2 | ||||
| ST-206 | 206 | 2 | 21 | 5 | 37 | 2 | 1 | 5 | C. jejuni | |||||
| 46 | 2 | 21 | 5 | 3 | 2 | 1 | 5 | C. jejuni | 1 | |||||
| 122 | 6 | 4 | 5 | 2 | 2 | 1 | 5 | C. jejuni | 1 | |||||
| 227 | 2 | 4 | 5 | 2 | 2 | 1 | 5 | C. jejuni | 1 | |||||
| 572 | 62 | 4 | 5 | 2 | 2 | 1 | 5 | C. jejuni | 1 | |||||
| ST-257 | 824 | 9 | 2 | 2 | 2 | 11 | 5 | 6 | C. jejuni | 1 | ||||
| ST-283 | 283 | 2 | 7 | 40 | 4 | 42 | 51 | 1 | C. jejuni | |||||
| 267 | 4 | 7 | 40 | 4 | 42 | 51 | 1 | C. jejuni | 2 | 1 | ||||
| 383 | 4 | 7 | 12 | 4 | 42 | 51 | 1 | C. jejuni | 1 | |||||
| ST-353 | 353 | 7 | 17 | 5 | 2 | 10 | 3 | 6 | C. jejuni | 1 | ||||
| 1953 | 7 | 17 | 2 | 2 | 86 | 3 | 1 | C. jejuni | 1 | |||||
| ST-446 | 446 | 47 | 55 | 5 | 10 | 11 | 68 | 8 | C. jejuni | |||||
| 450 | 47 | 55 | 5 | 10 | 11 | 48 | 8 | C. jejuni | 1 | |||||
| ST-574 | 574 | 7 | 53 | 2 | 10 | 11 | 3 | 3 | C. jejuni | |||||
| 305 | 9 | 53 | 2 | 10 | 11 | 3 | 3 | C. jejuni | 1 | |||||
| ST-607 | 607 | 8 | 2 | 5 | 53 | 11 | 3 | 1 | C. jejuni | |||||
| 1716 | 8 | 2 | 5 | 62 | 11 | 5 | 1 | C. jejuni | 1 | |||||
| 1915 | 8 | 2 | 5 | 53 | 11 | 3 | 6 | C. jejuni | 1 | |||||
| Yr 1996 | Yr 2002b | Yr 2003c | ||||||||||||
| ST-658 | 658 | 2 | 4 | 2 | 4 | 19 | 3 | 6 | C. jejuni | 1 (1) | ||||
| 1395 | 1 | 4 | 2 | 4 | 185 | 3 | 6 | C. jejuni | 1 | |||||
| 1967 | 2 | 198 | 2 | 3 | 19 | 68 | 6 | C. jejuni | 2 (2) | |||||
| ST-677 | 677 | 10 | 81 | 50 | 99 | 120 | 76 | 52 | C. jejuni | 12 | 11 (8) | 5 (5) | ||
| 794 | 10 | 81 | 50 | 87 | 120 | 76 | 52 | C. jejuni | 4 | 3 (3) | 2 (1) | 1 | ||
| ST-692 | 692 | 37 | 52 | 57 | 26 | 127 | 29 | 23 | C. jejuni | |||||
| 1954 | 37 | 106 | 4 | 131 | 127 | 29 | 23 | C. jejuni | 1 | |||||
| ST-828 | 828 | 33 | 39 | 30 | 82 | 104 | 43 | 17 | C. coli | |||||
| 854 | 33 | 38 | 30 | 82 | 104 | 43 | 17 | C. coli | 2 | |||||
| 1156 | 33 | 39 | 30 | 79 | 104 | 64 | 17 | C. coli | 1 | |||||
| 1413 | 33 | 38 | 30 | 82 | 104 | 117 | 17 | C. coli | 1 | |||||
| 1585 | 33 | 39 | 30 | 82 | 189 | 43 | 17 | C. coli | 1 | |||||
| 1625 | 33 | 39 | 30 | 82 | 104 | 47 | 139 | C. coli | 1 | |||||
| 1946 | 53 | 39 | 30 | 82 | 104 | 44 | 17 | C. coli | 1 | |||||
| 1957 | 33 | 39 | 65 | 79 | 104 | 35 | 17 | C. coli | 1 | |||||
| 1965 | 159 | 236 | 30 | 78 | 104 | 43 | 17 | C. coli | 1 (1) | |||||
| ST-1034 | 1034 | 2 | 61 | 4 | 64 | 74 | 25 | 23 | C. jejuni | |||||
| 1951 | 2 | 61 | 4 | 64 | 321 | 7 | 23 | C. jejuni | 1 | |||||
| 1956 | 2 | 59 | 4 | 27 | 128 | 25 | 23 | C. jejuni | 1 (1) | |||||
| ST-1287 | 1287 | 84 | 106 | 29 | 28 | 74 | 136 | 90 | C. jejuni | |||||
| 945 | 84 | 106 | 29 | 28 | 136 | 1 | 35 | C. jejuni | 1 (1) | 2 (2) | ||||
| ST-1332 | 1332 | 2 | 1 | 29 | 28 | 58 | 25 | 58 | C. jejuni | 1 | ||||
| Unassigned | 58 | 19 | 24 | 23 | 20 | 26 | 16 | 15 | C. jejuni | 5 | ||||
| 586 | 1 | 2 | 42 | 4 | 98 | 58 | 34 | C. jejuni | 1 | |||||
| 1080 | 10 | 2 | 107 | 137 | 120 | 76 | 1 | C. jejuni | 1 | 1 | ||||
| 1367 | 10 | 119 | 50 | 126 | 171 | 194 | 112 | C. jejuni | 2 (1) | |||||
| 1525 | 2 | 59 | 4 | 27 | 128 | 7 | 23 | C. jejuni | 1 (1) | |||||
| 1759 | 7 | 112 | 5 | 10 | 11 | 67 | 6 | C. jejuni | 2 | |||||
| 1945 | 7 | 78 | 42 | 264 | 106 | 12 | 8 | C. jejuni | 1 | |||||
| 1955 | 18 | 235 | 72 | 100 | 116 | 103 | 6 | C. jejuni | 1 | |||||
| 1959 | 27 | 2 | 22 | 104 | 319 | 86 | 16 | C. jejuni | 1 | |||||
| 1960 | 161 | 33 | 22 | 104 | 134 | 109 | 16 | C. jejuni | 1 | |||||
| 1961 | 76 | 22 | 165 | 98 | 146 | 269 | 16 | C. jejuni | 1 | |||||
| 1962 | 55 | 172 | 21 | 49 | 125 | 83 | 51 | C. jejuni | 1 | |||||
| 1963 | 7 | 2 | 5 | 10 | 22 | 37 | 1 | C. jejuni | 1 (1) | |||||
| 1968 | 33 | 39 | 30 | 79 | 113 | 47 | 178 | C. coli | 1 (1) | |||||
| 1970 | 37 | 29 | 75 | 48 | 126 | 5 | 23 | C. jejuni | 1 | |||||
| 1972 | 7 | 71 | 5 | 62 | 11 | 67 | 1 | C. jejuni | 1 | |||||
| 1974 | 160 | 84 | 27 | 267 | 11 | 3 | 5 | C. jejuni | 1 | |||||
Alleles and STs first described in this study are shown in boldface type.
Numbers in parentheses are numbers of isolates included in the epidemiological case-control study conducted during the seasonal peak in 2002 by Schönberg-Norio et al. (19, 20).
All isolates from cattle and poultry were collected during the seasonal peak from July to September, as were numbers of isolates given in parentheses for isolates collected from humans in 2003.
C. coli strains clearly diverged from those of C. jejuni in the neighbor-joining tree constructed from the combined MLST results (86 to 89% sequence similarity). Intraspecies sequence similarities varied from 99.8 to 100% for C. coli and from 96 to 100% for C. jejuni. In C. jejuni, the ST-61 uncA allele 17, which is typical for C. coli strains (http://eburst.mlst.net/), was present. In contrast, the ST-1367 tkt allele 194 had only 93.5 to 94.6 and 85.6 to 86.3% sequence similarities with other C. jejuni and C. coli strains, respectively.
Fourteen strains (one isolate from chicken and 13 [4%] from humans) were identified as possible mixed C. jejuni/C. jejuni or C. jejuni/C. coli cultures due to the observation of secondary peaks in the chromatograms of one or more of the seven loci. One allelic profile per pure culture was obtained, although several colonies were screened for alleles in which the secondary peaks had been observed. Finally, unambiguous profiles could not be obtained for one isolate from chicken and two from humans.
Diversity of isolates from domestically acquired infections in 1996, 2002, and 2003.
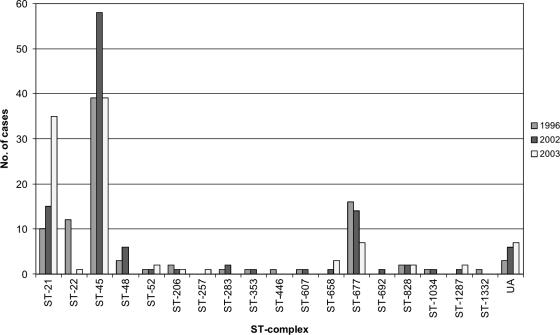
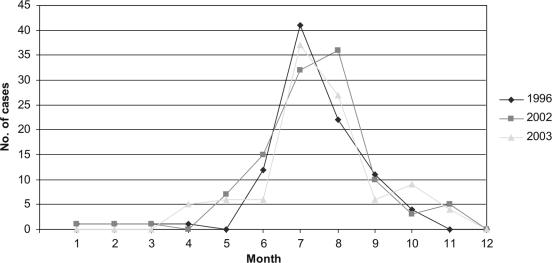
The most common ST complexes among the isolates from humans were ST-45 (44.6%), ST-21 (19.7%), and ST-677 (12.1%) (Table 1 and Fig. 1). The ST-22 complex was more prevalent in 1996 than in other years (P = 0.00001, χ2 test using Yates' correction). In comparison, the ST-45 complex was most prevalent in 2002 (P = 0.042) and the ST-21 complex in 2003 (P < 0.00001). Furthermore, ST-22 (P < 0.00001) and ST-1944 (P = 0.048, χ2 test using Yates' correction) were found only in 1996 whereas ST-48 (P = 0.012) and ST-538 (P = 0.032) were detected only in 2002. ST-50 was detected more often than expected in 2003 (P < 0.00001) but less often than expected in 1996 (P = 0.0016). Similarly, ST-230 was detected more often than expected in 2002 (P = 0.011) but less often than expected in 2003 (P = 0.031). The highest number of isolates was obtained in July and August in each sample year (Fig. 2). However, no clear distinctions between the occurrence of different STs or ST complexes during the different seasons could be made.
FIG. 1.
Numbers of domestically acquired human campylobacteriosis cases per year among different ST complexes. UA, unassigned to a previously described clonal complex.
FIG. 2.
Numbers of domestically acquired human campylobacteriosis cases per month during 1996, 2002, and 2003. Month 1, January; month 2, February; month 3, March; etc.
The association of the age and gender of the patients with the STs and ST complexes was evaluated (data not shown). The ST-828 complex, including six out of seven C. coli isolates, was associated with advanced age (≥60 years; P = 0.002, χ2 test using Yates' correction). The patient age range for all of the clinical C. coli isolates was 19 to 80 years (mean, 55 years). No significant associations between the gender of the patients and either the ST or the ST complex of the isolates were found.
Association of ST and ST complex with epidemiological data and serotype in 2002.
Fisher's exact test was used to evaluate the associations between ST and ST complex and those risk factors given in the questionnaire of Schönberg-Norio et al. (19). The ST-48 complex was significantly associated with the eating or tasting of raw minced meat (Table 2). Additionally, all patients with ST-21 and ST-48 complex isolates reported having eaten minced beef meat whereas only half of the patients with ST-45 (55%) or ST-677 (50%) complex isolates had done so (data not shown). ST-52 (ST-52 complex) was associated with the only reported contact with cattle. Patients who reported drinking unpasteurized milk were infected by ST-52 (ST-52 complex), ST-230 (ST-45 complex), and ST-1958 (ST-45 complex) isolates. Isolates from patients who reported eating or tasting raw or undercooked chicken meat were not associated with any particular ST but belonged to clonal complexes ST-21, ST-45, ST-48, and ST-677.
TABLE 2.
Significant associations between ST or ST complex and epidemiological variablesa
| Variable | No. of positive cases/total no. of casesb | Association (no. of positive cases/total no. of cases)b | P value(s) |
|---|---|---|---|
| Hospitalization | 26/46 | New and unassigned STs (0/4) | 0.030 |
| Swimming in natural bodies of water | 18/46 | New and unassigned STs (4/4) | 0.019 |
| Eating pork chops | 15/36 | ST-45 complex (0/6) | 0.030 |
| Eating minced pork meat | 19/36 | ST-45 complex (5/16) | 0.042 |
| Eating or tasting raw minced meat | 2/38 | ST-48 complex (2/3) | 0.004 |
| Eating boiled or otherwise cooked fish | 21/42 | ST-45 complex (1/8) | 0.045 |
| Eating soft cheese | 15/40 | ST-137 (3/3) | 0.046 |
| Drinking from a dug well | 12/45 | ST-677 complex (0/11) | 0.023 |
| Drinking nonchlorinated water from a small water plant | 6/45 | ST-677 complex (4/11) | 0.025 |
| Drinking water from natural sources | 7/45 | ST-677 complex (4/11) | 0.050 |
| Contact with a dog | 23/44 | ST-677 complex (2/10), ST-45 complex (7/8) | 0.031, 0.048 |
| Contact with a cat | 14/38 | ST-45 complex (10/18) | 0.042 |
Patients infected with new and unassigned ST isolates had been swimming in natural bodies of water more often than expected (Table 2). These patients were less frequently hospitalized. The ST-677 complex was associated with drinking nonchlorinated water from a small water plant and water from natural sources (i.e., lake, spring, stream, etc.). Nine patients (82%) infected with ST-677 complex isolates were hospitalized (data not shown). In comparison, 57% of all patients with C. jejuni and C. coli infections reported having been hospitalized. The difference in frequency of hospitalization among these patients was not statistically significant (P = 0.082). The patients with the ST-677 complex reported a mean length of stay in hospital of 3.9 days (average for all patients, 2.1 days). Among patients with ST-677 complex isolates, drinking from a dug well and contact with pet dogs were reported less frequently than among other patients (Table 2).
The ST-45 complex was significantly associated with contact with cats and associated less frequently than expected with eating minced pork meat (Table 2). In addition, ST-45 was positively associated with contact with pet dogs and negatively associated with eating pork chops or boiled and otherwise cooked fish. On the other hand, ST-137 (ST-45 complex) was associated with eating soft cheese.
During the seasonal peak from July to September in 2002, Penner heat-stable (HS) serotypes of the isolates from humans were associated with the ST complexes as follows: HS1/44 with the ST-21 complex, HS4 complex with ST-48 and ST-677 complexes, HS5 with the ST-52 complex, and HS6/7 with the ST-45 complex. The ST-45 complex included many additional serotypes (HS12, HS21, HS27, HS55, and HS57) that were observed in one to three isolates (data not shown).
Diversity of isolates during summer peak in 2003.
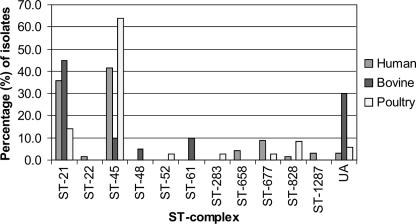
Figure 3 illustrates the percent distribution of isolates from humans, cattle, and poultry among the ST complexes during the seasonal peak from July to September in 2003. The distribution of STs of isolates from humans, cattle, and poultry is shown in Table 1. The levels of overlap of ST complexes between isolates from humans and cattle and between isolates from humans and poultry were 77% and 87%, respectively, whereas the respective levels of overlap of STs were 37 and 74%. ST-50 (ST-21 complex) was significantly associated with isolates from humans (P = 0.00045) and was less common among isolates from cattle than among other sources (P = 0.0035, Fisher's exact test). By comparison, ST-45 (ST-45 complex) was significantly associated with poultry (P = 0.0028, χ2 test) and less common among isolates from cattle (P = 0.002). However, ST-53 (ST-21 complex) (P = 0.00048), ST-58 (P = 0.00006), ST-61 (ST-61 complex) (P = 0.024), and ST-883 (ST-21 complex) (P = 0.0035) were significantly associated with isolates from cattle.
FIG. 3.
Distribution of ST complexes among isolation sources during the seasonal peak from July to September in 2003. UA, unassigned to a previously described clonal complex.
DISCUSSION
Our study is the first to describe, by use of MLST, Finnish C. jejuni and C. coli populations in humans and meat-producing animals. The strains used represented a spatially and temporally restricted collection, and 46 isolates from humans from the seasonal peak in 2002 had a known epidemiological background. The largest clonal complexes (ST-45, ST-21, and ST-677) were prevalent each year of the study and covered 76% of the isolates from humans. The clonal population structure may be due to niche adaptation, geographic isolation, host immune selection, or barriers to genetic exchange (23). Compared to previous studies using collections of strains obtained mainly from humans in the United Kingdom and The Netherlands (5) and also from diverse sources in the United Kingdom (16), we found ST-45 and ST-677 complexes to be more prevalent among the Finnish Campylobacter population. These results together with those of others (7, 10) suggest that different genotypes may be more prevalent in different geographical areas.
The ST-828 complex (C. coli) was associated with patients of ≥60 years. This is in line with a previous report by Gillespie et al. (12), who showed that patients infected with C. coli tended to be older than those infected with C. jejuni. In our study, we found ST-854 (ST-828 complex) isolates in retail chicken meat; however, no overlap between chicken and human C. coli STs was seen. This result supports the view presented in a previous study (4) in which ST-854 was commonly found in chickens (29%) but not in pigs located on the same farm or in humans, suggesting that there might be a host preference for certain C. coli genotypes. The same study revealed a larger ST diversity among C. coli isolates from humans than among those from chickens and pigs. However, no overlap between isolates from these different sources was detected. In contrast, Miller et al. (17) identified most C. coli STs previously identified only in humans (4) also in samples from cattle, pigs, chickens, and/or turkeys, suggesting that many different transmission routes may play a role in the epidemiology of C. coli. Further studies should be conducted in order to understand the epidemiology of C. coli in more detail.
Drinking unpasteurized milk and contact with cattle have been implicated in Campylobacter infections in several studies (9, 18). In our study, ST-53, ST-58, ST-61, and ST-883 were significantly associated with isolates from cattle. In addition, ST-52 was associated with contact with cattle and drinking unpasteurized milk whereas ST-48 was associated with tasting or eating raw minced meat and ST-475 (ST-48 complex) was isolated from bovine feces. These results are in line with previous findings suggesting that ST-48 and ST-61 complexes are overrepresented in isolates from cattle (5, 11, 16). Furthermore, ST-58, which earlier was reported to occur only among cattle in Northern Ireland (http://eburst.mlst.net/), was identified only among isolates from cattle (25%) in our study. Further studies will confirm if ST-58 is associated with cattle and is uncommon in other sources as well as in human infections.
Eating or handling undercooked or raw chicken meat is a well-known risk factor for campylobacteriosis, as shown in several epidemiological studies (9, 14, 15, 18, 19, 26). In Finland, the production and consumption of chicken meat have almost doubled from 1995 to 2003 (http://www.etl.fi/tilastot/pdf/myynti/Kotimaa2005.pdf), and a similar increase has taken place in many other western European countries as well. A recent Danish epidemiological case-control study suggested that the increase in the consumption of fresh, unfrozen chicken meat since the middle of the 1990s has contributed to the increasing number of human Campylobacter infections (26). Our MLST results of isolates collected from humans in 1996, 2002, and 2003 support the expansion of certain clonal lineages, including a slight increase in the numbers of ST-45 and a more pronounced increase in ST-50, both types which often occur also among isolates from chicken. We found that ST-45 was the only type present in all sources studied in 2003. In our study, ST-45 was significantly associated with poultry and humans, in line with the findings of previous studies (2, 5, 16). Although we found that ST-45 was strongly associated with chicken, a variety of other STs were found in chicken meat as well. This result is in line with the finding that tasting or eating raw and undercooked chicken meat was not associated with a particular ST or ST complex in our study.
Healthy cats and dogs are known to carry Campylobacter species, and contact with a pet dog and/or cat has been identified as a risk factor for human campylobacteriosis in previous studies (1, 15, 18, 27). In our study, the most common clonal complex, ST-45, was found to be significantly associated with contact with pet cats and dogs. It has previously been shown (25) that dogs that have regular contact with birds or poultry are more likely to carry C. jejuni. This is in line with our finding that the ST-45 complex was overrepresented among isolates from poultry. However, further studies on the importance of pet dogs and cats in human infections, especially in urban areas, are needed.
The ST-21 complex was the second largest clonal complex found in our study. It was predominated by ST-50 (74%), whereas only 2.7% of the strains represented the founder type, ST-21. Moreover, ST-50 was found to be overrepresented among isolates both from humans and from poultry. In other studies, the most abundant types that belonged to the ST-21 complex were ST-53 in The Netherlands (21), ST-21 in the United Kingdom and the United States (10, 16), and ST-262 and ST-53 in England (2). Although the ST-21 complex included isolates from all sources in our study, different STs were found to be predominant among isolates from cattle (ST-53 and ST-883) and isolates from humans and poultry (ST-50).
Another interesting finding was the association of ST-50 and ST-677 with isolates from humans. ST-677 was not detected among isolates from cattle or poultry in our study. Furthermore, ST-677 was more common among patients requiring hospitalization and a longer stay at the hospital, though further studies are needed to reveal if this particular clonal genotype could cause a more severe disease than others. Schönberg-Norio et al. (20) found that hospitalization due to Campylobacter infections was associated with advanced age. In our study, no statistically significant association was observed between the ST-677 complex and advanced age, although patients with the ST-677 complex had a mean age slightly higher than the overall average age of those with Campylobacter infection.
The occurrence of the ST-677 complex was associated with drinking from natural sources of water and nonchlorinated water from a small water plant. Previous findings of the ST-677 complex from wild birds and natural water sources support the possibility of enhanced survival and adaptation of this particular type for waterborne transmission. However, the negative association of the ST-677 complex with drinking water from a dug well and no association between it and swimming in natural bodies of water remain unexplained. However, the association of new and unassigned STs with swimming in natural bodies of water is in line with the finding of French et al. (11), who reported that the ST-45 complex and uncommon STs were overrepresented in the feces of wildlife and in natural bodies of water. In addition, many uncommon STs previously isolated only from wild birds in Sweden, including ST-1003, ST-1326, ST-1332, and ST-1367, were also isolated from patients with domestically acquired infections in Finland.
Several studies have addressed the questions of whether and to what extent C. jejuni populations in different food production animals and wildlife overlap with isolates from human patients. Many STs have actually been shown to be shared by different sources. On the other hand, certain types that seem to be associated only with a particular source exist. Reasons for this might be due to differences in colonization potential and survival in the food processing chains or in the environment. Studies using MLST have revealed several globally common patterns but also some unique features for certain countries or geographical areas. Comparisons between different MLST types of Campylobacter, achieved utilizing microarrays, stress response analysis, survival, colonization potential, and infectivity, can be expected to reveal factors affecting the ecology and epidemiology of this important pathogen.
Acknowledgments
This publication made use of the Campylobacter jejuni multilocus sequence typing website (http://pubmlst.org/campylobacter/) developed by Keith Jolley and Man-Suen Chan. The development of this site was funded by the Wellcome Trust.
This study was funded by the Academy of Finland, and R. Kärenlampi was supported by funds from the Finnish Graduate School on Applied Bioscience.
The City of Helsinki Environment Centre is acknowledged for providing the chicken and turkey isolates, and the National Veterinary and Food Research Institute of Finland is thanked for providing the bovine fecal samples.
Footnotes
Published ahead of print on 3 November 2006.
REFERENCES
- 1.Carrique-Mas, J., Y. Andersson, M. Hjertqvist, Å. Svensson, A. Torner, and J. Giesecke. 2005. Risk factors for domestic sporadic campylobacteriosis among young children in Sweden. Scand. J. Infect. Dis. 37:101-110. [DOI] [PubMed] [Google Scholar]
- 2.Colles, F. M., K. Jones, R. M. Harding, and M. C. J. Maiden. 2003. Genetic diversity of Campylobacter jejuni isolates from farm animals and the farm environment. Appl. Environ. Microbiol. 69:7409-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devane, M. L., C. Nicol, A. Ball, J. D. Klena, P. Scholes, J. A. Hudson, M. G. Baker, B. J. Gilpin, N. Garrett, and M. G. Savill. 2005. The occurrence of Campylobacter subtypes in environmental reservoirs and potential transmission routes. J. Appl. Microbiol. 98:980-990. [DOI] [PubMed] [Google Scholar]
- 4.Dingle, K. E., F. M. Colles, D. Falush, and M. C. J. Maiden. 2005. Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni. J. Clin. Microbiol. 43:340-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dingle, K. E., F. M. Colles, R. Ure, J. A. Wagenaar, B. Duim, F. J. Bolton, A. J. Fox, D. R. A. Wareing, and M. C. J. Maiden. 2002. Molecular characterization of Campylobacter jejuni clones: a basis for epidemiologic investigation. Emerg. Infect. Dis. 8:949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dingle, K. E., F. M. Colles, D. R. A. Wareing, R. Ure, A. J. Fox, F. E. Bolton, H. J. Bootsma, R. J. L. Willems, R. Urwin, and M. C. J. Maiden. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 39:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duim, B., P. C. R. Godschalk, N. van den Braak, K. E. Dingle, J. R. Dijkstra, E. Leyde, J. van der Plas, F. M. Colles, H. P. Endtz, J. A. Wagenaar, M. C. J. Maiden, and A. van Belkum. 2003. Molecular evidence for dissemination of unique Campylobacter jejuni clones in Curaçao, Netherlands Antilles. J. Clin. Microbiol. 41:5593-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Embley, T. M. 1991. The linear PCR reaction: a simple and robust method for sequencing amplified rRNA genes. Lett. Appl. Microbiol. 13:171-174. [DOI] [PubMed] [Google Scholar]
- 9.Evans, M. R., C. D. Ribiero, and R. L. Salmon. 2003. Hazards of healthy living: bottled water and salad vegetables as risk factors for Campylobacter infection. Emerg. Infect. Dis. 9:1219-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitch, B. R., K. L. Sachen, S. R. Wilder, M. A. Burg, D. W. Lacher, W. T. Khalife, T. S. Whittam, and V. B. Young. 2005. Genetic diversity of Campylobacter sp. isolates from retail chicken products and humans with gastroenteritis in central Michigan. J. Clin. Microbiol. 43:4221-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French, N., M. Barrigas, P. Brown, P. Ribiero, N. Williams, H. Leatherbarrow, R. Birtles, E. Bolton, P. Fearnhead, and A. Fox. 2005. Spatial epidemiology and natural population structure of Campylobacter jejuni colonizing a farmland ecosystem. Environ. Microbiol. 7:1116-1126. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie, I. A., S. J. O'Brien, J. A. Frost, G. K. Adak, P. Horby, A. V. Swan, M. J. Painter, K. R. Neal, et al. 2002. A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerg. Infect. Dis. 8:937-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrington, C. S., F. M. Thomson-Carter, and P. E. Carter. 1997. Evidence for recombination in the flagellin locus of Campylobacter jejuni; implications for the flagellin gene typing scheme. J. Clin. Microbiol. 35:2836-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapperud, G., G. Espeland, E. Wahl, A. Walde, H. Herikstad, S. Gustavsen, I. Tveit, O. Natås, L. Bevanger, and A. Digranes. 2003. Factors associated with increased and decreased risk of Campylobacter infection: a prospective case-control study in Norway. Am. J. Epidemiol. 158:234-242. [DOI] [PubMed] [Google Scholar]
- 15.Kapperud, G., E. Skjerve, N. H. Bean, S. M. Ostroff, and J. Lassen. 1992. Risk factors for sporadic Campylobacter infections: results of a case-control study in southeastern Norway. J. Clin. Microbiol. 30:3117-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning, G., C. G. Dowson, M. C. Bagnall, I. H. Ahmed, M. West, and D. G. Newell. 2003. Multilocus sequence typing for comparison of veterinary and human isolates of Campylobacter jejuni. Appl. Environ. Microbiol. 69:6370-6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, W. G., M. D. Englen, S. Kathariou, I. V. Wesley, G. Wang, L. Pittenger-Alley, R. M. Siletz, W. Muraoka, P. J. Fedorka-Cray, and R. E. Mandrell. 2006. Identification of host-associated alleles by multilocus sequence typing of Campylobacter coli strains from food animals. Microbiology 152:245-255. [DOI] [PubMed] [Google Scholar]
- 18.Neimann, J., J. Engberg, K. Mølbak, and H. C. Wegener. 2003. A case-control study of risk factors for sporadic campylobacter infections in Denmark. Epidemiol. Infect. 130:353-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schönberg-Norio, D., J. Takkinen, M.-L. Hänninen, M.-L. Katila, S.-S. Kaukoranta, L. Mattila, and H. Rautelin. 2004. Swimming and Campylobacter infections. Emerg. Infect. Dis. 10:1474-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schönberg-Norio, D., S. Sarna, M.-L. Hänninen, M.-L. Katila, S.-S. Kaukoranta, and H. Rautelin. 2006. Strain and host characteristics of Campylobacter jejuni infections in Finland. Clin. Microbiol. Infect. 12:754-760. [DOI] [PubMed] [Google Scholar]
- 21.Schouls, L. M., S. Reulen, B. Duim, J. A. Wagenaar, R. J. L. Willems, K. E. Dingle, F. M. Colles, and J. D. A. van Embden. 2003. Comparative genotyping of Campylobacter jejuni by amplified fragment length polymorphism, multilocus sequence typing, and short repeat sequencing: strain diversity, host range, and recombination. J. Clin. Microbiol. 41:15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siemer, B. L., C. S. Harrington, E. M. Nielsen, B. Brock, N. L. Nielsen, J. Engberg, and S. L. W. On. 2004. Genetic relatedness among Campylobacter jejuni serotyped isolates of diverse origin as determined by numerical analysis of amplified fragment length polymorphism (AFLP) profiles. J. Appl. Microbiol. 96:795-802. [DOI] [PubMed] [Google Scholar]
- 23.Smith, J. M., N. H. Smith, M. O'Rourke, and B. G. Spratt. 1993. How clonal are bacteria? Proc. Natl. Acad. Sci. USA 90:4384-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staden, R., K. F. Beal, and J. K. Bonfield. 2000. The Staden package, 1998. Methods Mol. Biol. 132:115-130. [DOI] [PubMed] [Google Scholar]
- 25.Wieland, B., G. Regula, J. Danuser, M. Wittwer, A. P. Burnens, T. M. Wassenaar, and K. D. C. Stärk. 2005. Campylobacter spp. in dogs and cats in Switzerland: risk factor analysis and molecular characterization with AFLP. J. Vet. Med. B 52:183-189. [DOI] [PubMed] [Google Scholar]
- 26.Wingstrand, A., J. Neimann, J. Engberg, E. Møller Nielsen, P. Gerner-Smidt, H. C. Wegener, and K. Mølbak. 2006. Fresh chicken as main risk factor for campylobacteriosis, Denmark. Emerg. Infect. Dis. 12:280-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Workman, S. N., G. E. Mathison, and M. C. Lavoie. 2005. Pet dogs and chicken meat as reservoirs of Campylobacter spp. in Barbados. J. Clin. Microbiol. 43:2642-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]