Abstract
The potential of Bacillus thuringiensis Cry proteins to control the grape pest Lobesia botrana was explored by testing first-instar larvae with Cry proteins belonging to the Cry1, Cry2, and Cry9 groups selected for their documented activities against Lepidoptera. Cry9Ca, a toxin from B. thuringiensis, was the protein most toxic to L. botrana larvae, followed in decreasing order by Cry2Ab, Cry1Ab, Cry2Aa, and Cry1Ia7, with 50% lethal concentration values of 0.09, 0.1, 1.4, 3.2, and 8.5 μg/ml of diet, respectively. In contrast, Cry1Fa and Cry1JA were not active at the assayed concentration (100 μg/ml). In vitro binding and competition experiments showed that none of the toxins tested (Cry1Ia, Cry2Aa, Cry2Ab, and Cry9C) shared binding sites with Cry1Ab. We conclude that either Cry1Ia or Cry9C could be used in combination with Cry1Ab to control this pest, either as the active components of B. thuringiensis sprays or expressed together in transgenic plants.
Most Bacillus thuringiensis strains that have been identified contain a mixture of up to eight different Cry proteins (26), which provides a great diversity of insecticidal toxins (2, 14), representing an important toxin reservoir for the development of numerous pest control products.
The grape moth (Lobesia botrana) is the most important component of the lepidopteran pest complex attacking vineyards in Europe (4, 27, 28). This pest has been successfully controlled using certain B. thuringiensis strains (3, 34). However, the effects of single Cry proteins against this pest have not been tested systematically, with the exception of a single study (30) that reported Cry1Ab to be the most active of a series of Cry1 proteins tested.
Reduced binding of toxin to the insect midgut target sites represents a well-known mechanism of resistance to B. thuringiensis toxins (7). Alterations and modifications in certain insect gut molecules that could be associated with resistance to B. thuringiensis toxins have been also reported (9, 16, 17, 20, 38).
In the present study, we have evaluated the potential of B. thuringiensis toxins for the control of L. botrana. We determined the insecticidal activities of some of the most common lepidopteran-active Cry proteins (selected from the Cry1, Cry2, and Cry9 classes) and investigated their binding site relationships to determine their suitability for combined use in pest management programs.
For this, seven single Cry toxins, purified from recombinant B. thuringiensis strains, were tested. Strains EG7077 (Cry1Ab), Cry1Ja (EG11096), and Cry1Fa (EG7279) were obtained from Ecogen Inc. (Langhorne, PA) and EG7543 (Cry2Aa) and EG7699 (Cry2Ab) from Monsanto Co. (Chersterfield, MO), and these strains were grown in casein hydrolysate-yeast extract medium (36), supplemented with the appropriate antibiotic (3 μg/ml of chloramphenicol for EG11069, EG7543, and EG7699; 10 μg/ml of tetracycline for EG7077; and 4 μg/ml of chloramphenicol for EG7279), and further activated by trypsin treatment, as described previously (6). Trypsin-activated proteins used for binding assays were purified by fast protein liquid chromatography (Pharmacia, Uppsala, Sweden). Cry1Ia7 (Cry1Ia) was produced in recombinant BL21(DE3) Escherichia coli cultures. Cells were grown overnight in LB with kanamycin (50 μg/ml) at 37°C and used to inoculate 750 ml of 2× TY (tryptone-yeast extract) culture medium (23). The culture was grown at 37°C until the optical density at 600 nm was 0.5 to 0.6 and then incubated at 25°C for 45 min. Isopropyl-β-d-thiogalactopyranoside (IPTG) (1 mM) was added and the incubation continued for 2 h at 25°C. Cold phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.5) (0.5 volume) was added to the culture, and cells were recovered by centrifugation (16,000 × g, 15 min). The pellet was resuspended in cold PBS (1/10 volume), centrifuged, and stored at −80°C. Cells were thawed on ice with a 1/33 volume of cold binding buffer (40 mM imidazole, 4 M NaCl, 160 mM Tris-HCl, pH 7.9; Novagen, Darmstadt, Germany) and sonicated for 60 s in 15-s pulses. Protein was expressed with a histidine residue, and purification was performed using an affinity nickel column (His Bind purification kit; Novagen). Finally, the buffer was changed to carbonate (50 mM NaCO3, 100 mM NaCl, pH 11.3) with a Sephadex G-25 prepacked column (Amersham Biosciences, Uppsala, Sweden) and the protein solution stored at 4°C until use. Cry1Ia was trypsin activated for binding assays but used as a protoxin for insect bioassays. Purified and activated Cry9Ca (the Lys mutant) (22) was obtained from Bayer CropScience (Gent, Belgium). The protein concentration was determined as described by Bradford (1), using bovine serum albumin (BSA) as a standard. Strain HD-1, obtained from the Bacillus Genetic Stock Center (BGSC, OH), was used as a standard in bioassays for activity comparisons. Spore-crystal suspensions were obtained, and the crystals were purified as described previously (33) and administrated to larvae without any previous treatment.
The toxicities of individual purified toxins and HD-1 were determined by incorporating the proteins into an artificial diet (3.9% wheat germ, 3.25% brewer's yeast, 0.1% corn oil, 0.1% nipagin, 2.5% agar, 3.25% corn flour, 0.32% ascorbic acid, and 0.1% benzoic acid [wt/vol]) (25). Initially, the differential susceptibilities of L. botrana larvae to individual Cry toxins at a relatively high concentration (100 μg of toxin/ml of diet) were tested. In a second experiment, bioassays involving a series of five toxin concentrations of each Cry protein, ranging from 0.012 to 30 μg of toxin/ml of diet, were used to determine the concentration-response relationship. For this, a solidified diet containing the appropriate toxin was offered individually to L. botrana larvae. Twenty-five neonate larvae were tested for each toxin concentration as well as for the control. The whole bioassay was performed three times. Larvae in the bioassays were maintained under constant conditions (22 ± 1°C, 65% ± 5% relative humidity, and a 16:8 [light/dark {h}]) photoperiod). Mortality was recorded after 5 days. Control experiments were performed under the same conditions but without any Cry protein in the diet. Concentration-mortality data were subjected to probit analysis (8), and the 50% lethal concentration (LC50) values were calculated using the POLO-PC program (24).
Brush border membrane vesicles (BBMV) of L. botrana were prepared as described previously (33). Activated and purified Cry1Ab was labeled with 125I by the chloramine T method (37), and the specific activity of the radio-iodinated toxin was analyzed by a sandwich enzyme-linked immunosorbent assay (37). The specific activity for 125I-Cry1Ab was 2.9 mCi/mg. Cry1Ia was trypsin activated, and Cry1Ab and Cry9Ca were trypsinized and further purified by fast protein liquid chromatography (as performed for radio iodination). Each protein was then biotinylated using a kit, following the manufacturer's instructions (Amersham Biosciences, Uppsala, Sweden). Binding experiments with L. botrana BBMV and 125I-Cry1Ab were performed as described previously (6). The appropriate conditions for time of incubation, concentration of labeled toxin, and concentration of BBMV were determined in preliminary tests using the grape moth. These conditions were 1.2 ng of 125I-Cry1Ab, 0.15 mg/ml BBMV, and 1 h incubation at room temperature in a binding buffer (PBS [1 mM KH2PO4, 10 mM Na2HPO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4], 0.1% BSA; final volume, 0.1 ml). Competition experiments were performed by increasing concentrations of nonlabeled Cry1Ab, Cry1Ia, Cry2Aa, Cry2Ab, and Cry9Ca. 125I-Cry1Ab bound to the BBMV after the assay was measured using a gamma counter (Compugamma 1282; LKB). All the experiments were performed at least twice. Quantitative analyses for obtaining the dissociation constants and the binding site concentrations were performed with the LIGAND program (29), using data from the homologous-competition experiment. Graphical representations and curve fittings were plotted using Graphpad Prism version 4.0 for Windows (Graphpad Software, San Diego, CA). Binding assays with biotinylated toxins were carried out with a binding buffer (PBS, 0.1% BSA; final volume, 0.1 ml) by incubating the biotinylated protein with the appropriate amount of BBMV (25 μg) for 1 h at room temperature. The amount of biotinylated protein was 30 ng of Cry1Ab, 140 ng of Cry1Ia, and 20 ng of Cry9Ca. The same binding conditions described for Cry1Ab were used for competition binding assays, but a ≥400-fold excess of unlabeled protein was also included. Toxin bound to the BBMV after incubation was recovered by centrifugation at 11,000 × g for 10 min at 4°C, followed by two washes with 0.5 ml of cold binding buffer as described elsewhere (11). The final pellet was suspended with 10 μl electrophoresis sample buffer (21) and boiled for 10 min, and the proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Control lanes with biotinylated toxins were run using 8 to 10 ng of Cry1Ab, Cry1I, and Cry9C. Proteins were electrotransferred onto a nitrocellulose membrane (Hybond ECL; Amersham Biosciences) and blocked with a 3% enhanced-chemiluminescence blocking agent (Amersham Biosciences), 0.1% BSA, and 0.1% Tween 20 in PBS. The membrane was incubated for 1 h with streptavidin conjugated to alkaline phosphatase (Roche Diagnosis, IN) and washed three times with 0.1% BSA, 0.1% Tween 20 in PBS in order to detect the amount of biotinylated toxin bound to the BBMV. The final detection was carried out with a nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) solution (Roche Diagnosis), following the manufacturer's instructions.
Cry1Fa did not have any effect on mortality or larval growth at the concentration of 100 μg/ml, whereas Cry1Ja caused less than 10% mortality, but a reduction in the size of larvae was observed. Low toxicities for Cry1Fa and Cry1Ja were previously reported by Herrero et al. (10, 11) with Cacyreus marshalli and L. botrana. In contrast, at this high concentration, all the other five Cry toxins tested (Cry1Ab, Cry1Ia, Cry2Aa, Cry2Ab, and Cry9C) resulted in the mortality of all the treated larvae. The most insecticidal toxins were Cry9C, Cry2Ab, and Cry1Ab, with LC50 values of 0.09, 0.1, and 1.4 μg/ml, respectively, followed by Cry2Aa and Cry1Ia (3.2 and 8.5 μg/ml, respectively) (Table 1) . The only previous study with L. botrana involved assays with a limited number of Cry1 toxins: Cry1Aa, Cry1Ab, Cry1Ac, Cry1B, Cry1C, Cry1D, and Cry1E (30). That study reported that Cry1Ab was the protein most active against the grape moth, whereas the results for the present work revealed that L. botrana was 9- and 16-fold more sensitive to the Cry2Ab and Cry9C proteins, respectively, than to Cry1Ab (Table 1). Our experimental results are in agreement with the observation that commercial B. thuringiensis insecticides containing Cry1Ab protein are toxic to the grape moth (32, 34). Other proteins present in commercial strains whose crystal compositions include Cry1Aa, Cry1Ac, Cry1D (30), and Cry2Aa could contribute to the total activity of these strains.
TABLE 1.
Toxicity and relative potencies of the active Cry proteins to neonate Lobesia botrana larvaea
| Cry protein | Regression value ± SE
|
LC50 (μg/ml) | Goodness of fit value
|
Relative potency | 95% CI
|
|||
|---|---|---|---|---|---|---|---|---|
| Slope | ab | χ2 | df | Lower | Upper | |||
| Cry1Ab | 1.9 ± 0.2 | 4.7 ± 0.1 | 1.4 | 0.3 | 3 | 1 | ||
| Cry1Ia | 2.9 ± 0.2 | 2.3 ± 0.2 | 8.5 | 0.7 | 3 | 0.2 | 0.1 | 0.3 |
| Cry2Aa | 2.6 ± 0.3 | 3.7 ± 0.3 | 3.2 | 1.8 | 3 | 0.4 | 0.3 | 0.6 |
| Cry2Ab | 1.7 ± 0.2 | 6.3 ± 0.2 | 0.1 | 1.3 | 3 | 9.0 | 6.3 | 12.9 |
| Cry9Ca | 2.6 ± 0.3 | 7.7 ± 0.3 | 0.09 | 0.1 | 3 | 16.1 | 11.6 | 22.3 |
| HD-1c | 1.9 ± 0.2 | 5.7 ± 0.1 | 0.44 | 0.9 | 3 | 3.3 | 2.3 | 4.7 |
Parameters were obtained from the POLO-PC program (24). The χ2 value was not significant (P > 0.05) by a goodness of fit test for each regression. Slopes could not be fitted in parallel. The relative potencies were calculated with respect to that for Cry1Ab, and the values were expressed as the ratio of the LC50 value for each Cry protein to the LC50 value for Cry1Ab (31).
a, intercept of the regression line.
HD-1 means the entire content of the Cry proteins obtained from the B. thuringiensis kurstaki HD-1 strain.
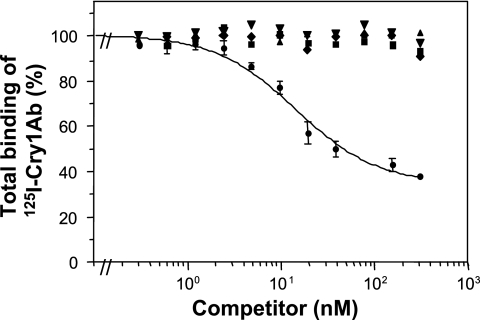
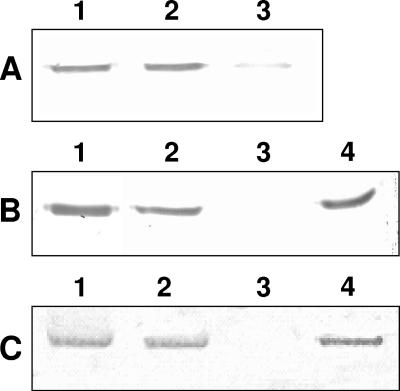
Radio-iodinated Cry1Ab bound to L. botrana BBMV in vitro, and its binding was specific (Fig. 1). Quantitative analysis of the homologous competition data gave a dissociation constant (± standard error of the mean) of 6.5 ± 1.5 nM and a binding site concentration (± standard error of the mean) of 8 ± 2 pmol/mg for Cry1Ab binding sites. Heterologous competition experiments with excesses of unlabeled Cry1Ia, Cry2Aa, Cry2Ab, and Cry9Ca showed that none of them shared common binding sites with 125I-Cry1Ab (Fig. 1). Biotinylated Cry1Ia, biotinylated Cry9Ca, and also biotin-labeled Cry1Ab, used as a control, bound to L. botrana BBMV, and their binding was specific (Fig. 2, lanes 1 to 3). Nonlabeled Cry1Ab used as a competitor in the assays did not displace biotin-Cry1Ia (Fig. 2B, lane 4) or biotin-Cry9Ca (Fig. 2C, lane 4). In vitro binding experiments were performed to determine whether the assayed Cry proteins could be used either as substitutes for the most frequently used proteins in pest management programs or in combination with Cry proteins produced by B. thuringiensis strains or other recombinant microorganisms. Our competition experiments with labeled Cry1Ab showed that Cry1Ia, Cry2Aa, Cry2Ab, and Cry9Ca did not share Cry1Ab binding sites. Similarly, Cry1Ab was shown not to share binding sites with biotinylated Cry1Ia and Cry9Ca in L. botrana BBMV. The fact that Cry1Ia did not share common binding sites with Cry1A toxins has recently been reported for the cotton pest Earias insulana (13). Cry2A toxin-specific binding could not be demonstrated in this study by using the same conditions as those used for Cry1A toxins, but this is not surprising, as these toxins seem to have different modes of action (5, 18, 19).
FIG. 1.
Binding of 125I-Cry1Ab to L. botrana BBMV at increasing concentrations of unlabeled Cry1Ab (•), Cry1Ia (⧫), Cry2Aa (▾), Cry2Ab (▴), and Cry9Ca (▪). Experiments were repeated three times, and error bars represent the standard errors of the means.
FIG. 2.
Binding of biotinylated toxins to Lobesia botrana BBMV. (A) Biotinylated Cry1Ab (lane 1), binding of biotin-Cry1Ab alone (lane 2), and binding of biotin-Cry1Ab in the presence of an excess of unlabeled Cry1Ab (lane 3). (B) Biotinylated Cry1Ia (lane 1), binding of biotin-Cry1Ia alone (lane 2), and binding of biotin-Cry1Ia in the presence of an excess of unlabeled Cry1Ia (lane 3) or unlabeled Cry1Ab (lane 4). (C) Biotinylated Cry9Ca (lane 1), binding of biotin-Cry9Ca alone (lane 2), and binding of biotin-Cry9Ca in the presence of an excess of unlabeled Cry9Ca (lane 3) or Cry1Ab (lane 4).
To date, L. botrana control has been performed using some commercial products based on the HD-1 strain (e.g., Dipel; Abbot Laboratories, Chicago) or other strains belonging to serovars kurstaki or aizawai (Delfin and Xentari, respectively), which have been found to be effective against L. botrana under laboratory or field conditions (15, 32, 34). The activities of these strains have been associated with a group of proteins belonging to the Cry1 and Cry2 classes (35). Our results with the HD-1 strain indicated an LC50 value lower than those for the Cry1Ab and Cry2Aa toxins, probably due to a synergic action among the proteins that constitute the crystal (12, 39). No commercial strain used for L. botrana control contains all of the most active proteins, Cry1Ab, Cry2Ab, and Cry9C. In addition, Cry1Ab and Cry9C do not share binding sites. Consequently, this protein combination in a particular strain is likely to prove highly effective as a bioinsecticide for use in the control of the grape berry moth, together or in combination with Cry1Ab. Moreover, Cry9Ca is of particular interest because of its high potency against the grape berry moth and its broad host range, which includes other important pests, like Ostrinia nubilalis and Agrotis segetum, that are difficult to control with current B. thruringiensis-based products (22).
Acknowledgments
We are grateful to V. Marco (Universidad de La Rioja) for supplying insects, Jeroen Van Rie for the Cry9C protein, Jim Baum (Monsanto, Chesterfield, MO) for the Cry2Aa and Cry2Ab recombinant clones, and Noelia Gorría for insect rearing. We thank Trevor Williams for critically reading the manuscript.
This study was supported by the Spanish Ministry of Science and Technology (AGL2000-0840-C03 and AGL2003-09282-CO3). I.R.D.E., A.E., and B.E. received support from the Government of Navarre, fellowship FP2000-5497, and the Ramón y Cajal program, respectively.
Footnotes
Published ahead of print on 3 November 2006.
REFERENCES
- 1.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 2.Bravo, A., S. Sarabia, L. Lopez, H. Ontiveros, C. Abarca, A. Ortiz, M. Ortiz, L. Lina, F. J. Villalobos, G. Pena, M. E. Nunez-Valdez, M. Soberon, and R. Quintero. 1998. Characterization of cry genes in a Mexican Bacillus thuringiensis strain collection. Appl. Environ. Microbiol. 64:4965-4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coscollá, R., V. Beltrán, M. Fabra, A. Ribesi, and R. Laborda. 1990. Utilisation du fenoxycarbe et du Bacillus thuringiensis Berl. dans la lutte contre Lobesia botrana Den et Schiff. Bull. OILB-SROP 13:68-71. [Google Scholar]
- 4.Charmillot, P. J., and D. Pasquier. 2000. Vers de la grappe: technique de confusion, lutte clasique et dynamique des populations. Rev. Suisse Vitic. Arboric. Hortic. 32:315-320. [Google Scholar]
- 5.English, L., H. L. Robbins, M. A. Vontersch, C. A. Kulesza, D. Ave, D. Coyle, C. S. Jany, and S. L. Slatin. 1994. Mode of action of CryIIA: a Bacillus thuringiensis delta-endotoxin. Insect Biochem. Mol. Biol. 24:1025-1035. [Google Scholar]
- 6.Estela, A., B. Escriche, and J. Ferré. 2004. Interaction of Bacillus thuringiensis toxins with larval midgut binding sites of Helicoverpa armigera (Lepidoptera: Noctuidae). Appl. Environ. Microbiol. 70:1378-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferré, J., M. D. Real, J. Vanrie, S. Jansens, and M. Peferoen. 1991. Resistance to the Bacillus thuringiensis bioinsecticide in a field population of Plutella xylostella is due to a change in a midgut membrane receptor. Proc. Natl. Acad. Sci. USA 88:5119-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finney, D. J. 1971. Probit analysis. Cambridge University Press, Cambridge, United Kingdom.
- 9.Gahan, L. J., F. Gould, and D. G. Heckel. 2001. Identification of a gene associated with Bt resistance in Heliothis virescens. Science 293:857-860. [DOI] [PubMed] [Google Scholar]
- 10.Herrero, S., M. Borja, and J. Ferré. 2002. Extent of variation of the Bacillus thuringiensis toxin reservoir: the case of the geranium bronze, Cacyreus marshalli Butler (Lepidoptera: Lycaenidae). Appl. Environ. Microbiol. 68:4090-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero, S., J. González-Cabrera, B. E. Tabashnik, and J. Ferré. 2001. Shared binding sites in Lepidoptera for Bacillus thuringiensis Cry1Ja and Cry1A toxins. Appl. Environ. Microbiol. 67:5729-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes, P. A., M. M. Stevens, H. W. Park, B. A. Federici, E. S. Dennis, and R. Akhurst. 2005. Response of larval Chironomus tepperi (Diptera:Chironomidae) to individual Bacillus thuringiensis var. israelensis toxins and toxin mixtures. J. Invertebr. Pathol. 88:34-39. [DOI] [PubMed] [Google Scholar]
- 13.Ibargutxi, M. A., A. Estela, J. Ferré, and P. Caballero. 2006. Bacillus thuringiensis toxins for the control of the cotton pest Earias insulana (Boisd.) (Lepidoptera: Noctuidae). Appl. Environ. Microbiol. 72:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibarra, J. E., M. C. del Rincon, S. Orduz, D. Noriega, G. Benintende, R. Monnerat, L. Regis, C. M. F. de Oliveira, H. Lanz, M. H. Rodriguez, J. Sanchez, G. Pena, and A. Bravo. 2003. Diversity of Bacillus thuringiensis strains from Latin America with insecticidal activity against different mosquito species. Appl. Environ. Microbiol. 69:5269-5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ifoulis, A. A., and M. Savopoulou-Soultani. 2004. Biological control of Lobesia botrana (Lepidoptera: Tortricidae) larvae by using different formulations of Bacillus thuringiensis in 11 vine cultivars under field conditions. J. Econ. Entomol. 97:340-343. [DOI] [PubMed] [Google Scholar]
- 16.Jurat-Fuentes, J. L., and M. J. Adang. 2004. Characterization of a Cry1Ac-receptor alkaline phosphatase in susceptible and resistant Heliothis virescens larvae. Eur. J. Biochem. 271:3127-3135. [DOI] [PubMed] [Google Scholar]
- 17.Jurat-Fuentes, J. L., L. J. Gahan, F. L. Gould, D. G. Heckel, and M. J. Adang. 2004. The HevCaLP protein mediates binding specificity of the Cry1A class of Bacillus thuringiensis toxins in Heliothis virescens. Biochemistry 43:14299-14305. [DOI] [PubMed] [Google Scholar]
- 18.Jurat-Fuentes, J. L., F. L. Gould, and M. J. Adang. 2003. Dual resistance to Bacillus thuringiensis Cry1Ac and Cry2Aa toxins in Heliothis virescens suggests multiple mechanisms of resistance. Appl. Environ. Microbiol. 69:5898-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karim, S., S. Riazuddin, F. Gould, and D. H. Dean. 2000. Determination of receptor binding properties of Bacillus thuringiensis delta-endotoxins to cotton bollworm (Helicoverpa zea) and pink bollworm (Pectinophora gossypiella) midgut brush border membrane vesicles. Pestic. Biochem. Physiol. 67:198-216. [Google Scholar]
- 20.Knight, P. J., N. Crickmore, and D. J. Ellar. 1994. The receptor for Bacillus thuringiensis CrylA(c) delta-endotoxin in the brush border membrane of the lepidopteran Manduca sexta is aminopeptidase N. Mol. Microbiol. 11:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Lambert, B., L. Buysse, C. Decock, S. Jansens, C. Piens, B. Saey, J. Seurinck, K. Van Audenhove, J. Van Rie, A. Van Vliet, and M. Peferoen. 1996. A Bacillus thuringiensis insecticidal crystal protein with a high activity against members of the family Noctuidae. Appl. Environ. Microbiol. 62:80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lech, K., and R. Brent. 1992. Escherichia coli, plasmids and bacteriophages, p. 1-51. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Short protocols in molecular biology. Harvard Medical School. John Wiley & Sons, New York, NY.
- 24.LeOra. 1987. Polo-PC: a user's guide to probit or logit analysis. LeOra Software, Berkeley, CA.
- 25.MacIntosh, S. C., T. B. Stone, S. R. Sims, P. L. Hunst, J. T. Greenplate, P. G. Marrone, F. J. Perlak, D. A. Fischhoff, and R. L. Fuchs. 1990. Specificity and efficacy of purified Bacillus thuringiensis proteins against agronomically important insects. J. Invertebr. Pathol. 56:258-266. [DOI] [PubMed] [Google Scholar]
- 26.Martínez, C., M. Porcar, A. López, I. Ruiz de Escudero, F. J. Pérez-Llarena, and P. Caballero. 2004. Characterization of a Bacillus thuringiensis strain with a broad spectrum of activity against lepidopteran insects. Entomol. Exp. Appl. 111:71-77. [Google Scholar]
- 27.Moschos, T. 2005. Yield loss quantification and assessment of economic injury level for the anthophagous generation of the European grapevine moth Lobesia botrana Den. et Schiff. (Lepidoptera: Tortricidae). Int. J. Pest Manag. 51:81-89. [Google Scholar]
- 28.Moschos, T. 2006. Yield loss quantification and economic injury level estimation for the carpophagous generation of the European grapevine moth Lobesia botrana Den. et Schiff. (Lepidoptera: Tortricidae). Int. J. Pest Manag. 52:141-147. [Google Scholar]
- 29.Munson, P. J., and D. Rodbard. 1980. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 107:220-239. [DOI] [PubMed] [Google Scholar]
- 30.Piedrafita, A. C. 1996. Estudio de los mecanismos de toxicidad y adquisición de resistencias a delta-endotoxinas de Bacillus thuringiensis en insectos plaga. Ph.D. thesis. Universidad de Valencia, Valencia, Spain.
- 31.Robertson, J. L., and H. K. Preisler. 1992. Pesticide bioassay with arthropods. CRC Press, Boca Raton, FL.
- 32.Roditakis, N. E. 1986. Effectiveness of Bacillus thuringiensis Berliner var. kurstaki on the grape berry moth Lobesia botrana Den. and Shiff. (Lepidoptera: Tortricidae) under field and laboratory conditions in Crete. Entomol. Hell. 4:31-35. [Google Scholar]
- 33.Ruiz de Escudero, I., A. Estela, M. Porcar, C. Martinez, J. A. Oguiza, B. Escriche, J. Ferre, and P. Caballero. 2006. Molecular and insecticidal characterization of a Cry1I protein toxic to insects of the families Noctuidae, Tortricidae, Plutellidae, and Chrysomelidae. Appl. Environ. Microbiol. 72:4796-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scalco, A., P. J. Charmillot, D. Pasquier, and P. Antonin. 1997. Comparaison de produits à base de Bacillus thuringiensis dans la lutte contre les vers de la grappe: du laboratoire au vignoble. Rev. Suisse Vitic. Arboric. Hortic. 29:345-350. [Google Scholar]
- 35.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart, G. S. A., K. Johnstone, E. Hagelberg, and D. J. Ellar. 1981. Commitment of bacterial spores to germinate. Biochem. J. 198:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Rie, J., S. Jansens, H. Höfte, D. Degheele, and H. Van Mellaert. 1989. Specificity of Bacillus thuringiensis delta-endotoxins. Importance of specific receptors on brush border membrane of the mid-gut of target insects. Eur. J. Biochem. 186:239-247. [DOI] [PubMed] [Google Scholar]
- 38.Xu, X. J., L. Y. Yu, and Y. D. Wu. 2005. Disruption of a cadherin gene associated with resistance to Cry1Ac delta-endotoxin of Bacillus thuringiensis in Helicoverpa armigera. Appl. Environ. Microbiol. 71:948-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue, J. L., Q. X. Cai, D. S. Zheng, and Z. M. Yuan. 2005. The synergistic activity between Cry1Aa and Cry1C from Bacillus thuringiensis against Spodoptera exigua and Helicoverpa armigera. Lett. Appl. Microbiol. 40:460-465. [DOI] [PubMed] [Google Scholar]