Abstract
The biotransformation of Hg(II) by cyanobacteria was investigated under aerobic and pH-controlled culture conditions. Mercury was supplied as HgCl2 in amounts emulating those found under heavily impacted environmental conditions where bioremediation would be appropriate. The analytical procedures used to measure mercury within the culture solution, including that in the cyanobacterial cells, used reduction under both acid and alkaline conditions in the presence of SnCl2. Acid reduction detected free Hg(II) ions and its complexes, whereas alkaline reduction revealed that meta-cinnabar (β-HgS) constituted the major biotransformed and cellularly associated mercury pool. This was true for all investigated species of cyanobacteria: Limnothrix planctonica (Lemm.), Synechococcus leopoldiensis (Racib.) Komarek, and Phormidium limnetica (Lemm.). From the outset of mercury exposure, there was rapid synthesis of β-HgS and Hg(0); however, the production rate for the latter decreased quickly. Inhibitory studies using dimethylfumarate and iodoacetamide to modify intra- and extracellular thiols, respectively, revealed that the former thiol pool was required for the conversion of Hg(II) into β-HgS. In addition, increasing the temperature enhanced the amount of β-HgS produced, with a concomitant decrease in Hg(0) volatilization. These findings suggest that in the environment, cyanobacteria at the air-water interface could act to convert substantial amounts of Hg(II) into β-HgS. Furthermore, the efficiency of conversion into β-HgS by cyanobacteria may lead to the development of applications in the bioremediation of mercury.
Mercury in the form of divalent ions constitutes the bulk of that in soils, where it is bound to organic compounds, to clay, and as sulfides (31). Although industrialization in the beginning of the last century is the cause of most of the mercury contamination found in the environment today, rainfall continues to carry mercury [Hg(II)] into aquatic and terrestrial systems worldwide (16, 44). Despite a comprehensive knowledge of the mercury cycle and the aquatic chemistry of its constituents (12, 43, 52), several microbial taxa have not been characterized with respect to their roles in the biotransformation of this heavy metal.
Several mercury biotransformation mechanisms have been described previously (4, 12, 39, 41), and of these, prokaryotic methylation and reduction to Hg(0) may play only limited roles in the biotransformation of Hg(II) in aquatic environments (15, 19). Furthermore, the reduction reaction simply refracts meteorologically precipitated Hg(II) back into the atmosphere. As such, it follows that other processes must make significant contributions to the biogeochemical cycle of Hg even though relative quantitative data are scarce (31). Insight into this area requires an understanding of the major biotransformation processes leading to mercury retention in ecosystems.
The lack of quantitative, mechanistic data behind cellular accumulation versus that for volatilization of Hg is evident even in the eukaryotic models (26) and of particular concern in the cyanobacterial literature.
Environmental pH greatly affects both the aqueous chemistry of Hg and the physiology of organisms. Despite this, much of the present knowledge of Hg is based on biological studies that permit the pH to change through the culture period. In this study, we employ an automated system that maintains a constant pH by the addition of dilute inorganic acids found in natural systems. Chemical buffers were avoided because they can affect the availability of mercury to cells through association with the metal ions.
The present study shows the forms and quantities of Hg in the volatile fraction and that remaining in culture after exposure to Hg(II) supplied as HgCl2 at levels that emulate those found in heavily impacted environments, such as those near and at industrial sources. An environmental isolate of the cyanobacteria Limnothrix planctonica was studied at sublethal (120 ppb) and lethal (200 ppb) exposures. Studies of Synechococcus leopoldiensis provided comparative data on Hg(II) biotransformation within the division Cyanophyta. With representative time course data established, the effects of internal and external thiol modifications as well as temperature were investigated. These approaches provide quantitative analysis of the biosynthesis of β-HgS and its relationship to biotransformation into volatile mercury as well as a possible role for cyanobacteria in mercury bioremediation.
MATERIALS AND METHODS
Culturing medium and cyanobacteria.
Cyanophyte medium was composed of 5 mM (NH4)2SO4, 1.0 mM KH2PO4, 0.5 mM MgSO4 · 7H20, 0.33 mM CaCl2, 10 μM Na2EDTA, 2 μM FeCl3 · 6H2O, 1 μM MnCl2 · 4H2O, 0.2 μM ZnCl2, 0.1 μM Na2MoO4 · 2H2O, and 0.05 μM CoCl2 · 6H2O in deionized H2O. The medium was adjusted to pH 6.5 with 1.0 N H2SO4 and autoclaved.
The filamentous cyanobacteria Limnothrix planctonica (Lemm.), of the family Oscillatoriaceae, was obtained from the surfaces of leaves of bullhead lily (Nuphar variegatum) plants from Tasso Lake, Township of Lake of Bays, Ontario, Canada (45°29′N latitude by 78°55′30′′W longitude). Sections of leaf material were cultured on cyanophyte semisolid medium containing 1% agar. A rapidly growing isolate possessed the following characteristics of cyanobacteria. It was sensitive to penicillin G and streptomycin and contained only one type of photosynthetic pigment, chlorophyll a, as determined chromatographically.
Synechococcus leopoldiensis (Racib.) Komarek (UTEX 625) was obtained from the Culture Collection of Algae, University of Texas at Austin. Phormidium limnetica [formerly identified as Oscillatoria limnetica (Lemm.)] was isolated from Lake Opinicon (Ontario, Canada), in a fashion similar to that for Limnothrix planctonica.
Culture protocol.
The culture apparatus was sterilized by either autoclaving it or treating it with 70% ethanol (EtOH) for 30 min and then rinsing it with deionized water. All cyanobacteria were aseptically inoculated into 250-ml cultures in a laminar flow hood. The pH-stat apparatus and general conditions used were those described by Kelly et al. (24). Under the conditions of our studies at pH 6.5, the speciation of mercury added as 120 μg/liter HgCl2 was 80.5% HgCl2, 15.8% HgClOH, and 3.0% HgCl3−, with only trace amounts of other species, as determined using the freeware program PHREEQC-2 version 2.11 (34). Automatic control maintained pH between 6.45 and 6.55.
The cultures were vigorously bubbled with air, grown to mid-log phase, and used to inoculate new cultures that were in turn grown to mid-log phase before the pH-stat cultures were incubated. Cultures were grown at 25°C, unless otherwise indicated. Chlorophyll content was determined by the method of Marker and colleagues (29), using the following equation: μg chlorophyll/ml = (OD665 − OD720) × 13.42. It follows that optical density at 665 nm (OD665) could be used as an estimate of cyanobacterial biomass, and so, growth was monitored at this wavelength. All additions of HgCl2 to cultures were conducted when the OD665 reached 0.320. Continuous light was provided at saturating conditions for photosynthesis with cool white fluorescent tubes, i.e., 400 micro-Einsteins/m2/s.
Water-jacketed growth columns with 400-ml total culture capacities were constructed from borosilicate glass. A circulating water bath maintained the temperature. Culture uniformity, critical for dispersed-growth conditions and sample homogeneity, was ensured by using a ribbed Teflon magnetic stir disk and air sparging through a port near the base of each column. Applied HgCl2 dispersed to stable concentrations in seconds. A number 8 stopper containing two ports sealed the top of the vessel, with an exhaust tube in one port and a second tube (1 mm inside diameter) for pH titration in the other. A pH electrode, held in a drilled number 5 stopper, was sealed in a port at the base of the vessel just above the turbulence created by the stir bar.
The system used a Bach-Simpson multiport timed switch box capable of passing the sequential input from six Radiometer (GK 2401C) pH electrodes to a Radiometer pH meter and titrator and of relaying titrator output sequentially to six magnetic valves. The valves controlled the flow of titrant to the culture vessels to maintain a preset endpoint (pH 6.5). The titrant employed was 0.02 M HCl, whose concentration permitted pH control without adversely affecting the cyanobacteria while not increasing culture volume by more than 1% over the course of the experiments. The addition of the micronutrient Cl− did not affect growth. All components of the culturing apparatus were either disinfected with 90% EtOH in water or autoclaved, as appropriate. Air was filtered (0.45 μm) to prevent contamination and humidified by sparging it through sterile water prior to column delivery. Electrodes were calibrated and disinfected with 90% EtOH prior to assembly.
All assembly and subsequent manipulations were performed using aseptic techniques as described by Kelly et al. (24). Experimental cultures were inoculated from previously grown mid-log-phase cultures.
HgCl2 (BDH) stocks (100 ppm) were prepared fresh for each experiment in Teflon vials using demineralized H20 to avoid carrier problems (47).
Metabolic inhibitors.
Iodoacetamide at 0.3 mM was used to treat cells at 4°C for 10 min prior to the addition of HgCl2 using the conditions of Flores and colleagues (14). Dimethylfumarate stock solutions (500 mM) were dissolved in EtOH carrier with gentle warming and added to cultures to yield a final concentration of 5 mM. Exposures were for 5 min at 25°C. Controls contained carrier only.
Sample preparation and mercury analysis. (i) Cold vapor atomic absorption of mercury.
Cold vapor atomic absorption spectrometry using an LDC/Milton Roy mercuryMonitor was employed to measure Hg(0) directly or as the product of chemical conversions (2, 18, 21, 27). The detection limit was 0.l ng in 1-ml samples. Analytical methods, including quality assurance and quality control, are described in detail by Kelly and colleagues (24). Briefly, 100-μl samples were reduced under alkaline conditions in 6 ml of 10% SnCl2 dissolved in 10% HNO3 alkalinized to a net equivalent of 10% KOH, giving an estimate for all mercury species in the cyanobacteria and medium. Acid reduction was performed with 6-ml solutions of 10% SnCl2 in 10% HNO3, giving an estimate for all mercury species, excluding meta-cinnabar (β-HgS). The amount of β-HgS is the difference between the amounts of mercury detected by these two methods. Standards for HgCl2 were prepared daily (21).
Daniels and Wigfield (10) found that in the presence of a sulfhydryl reagent (l-cysteine), an acid reduction-resistant mercurial compound or complex was fully reduced to Hg(0) only under alkaline conditions. Our previous studies indicated that this was overcome by increasing incubation periods (24). Extraction followed by either thin-layer or column chromatography of a major mercurial component found in our treated cyanobacteria showed that it comigrated with meta-cinnabar (β-HgS) standards and that it was distinct from all other common mercurials.
(ii) Biological sample preparation.
Samples of between 20 and 100 μl were taken from pH-stat columns and immediately introduced into the reduction vessels, under acid or alkaline conditions as described above.
Acid digestion was used to determine levels of total Hg within samples larger than 0.5 ml (45). Samples were added to equal volumes of concentrated HNO3 and refrigerated until analyzed. Vials were borosilicate glass sealed with solid Teflon-lined caps (catalog number 2-7162/3; Supelco, Canada).
(iii) Culture fractionation for Hg: pellet, medium, and wash.
For the first fractionation method, culture samples (0.5 to 5 ml) were centrifuged at 3,000 × g for 10 min. Medium (supernatant) was sampled as was done for total culture samples, i.e., placed 1:1 in concentrated HN03, and the remaining supernatant removed. The pellet was resuspended to its original density with 5 mM dithioerythritol and recentrifuged. The dithioerythritol wash (supernatant) was then sampled and aspirated as described above prior to resuspension of the pellet in 1 ml HNO3. This was transferred to a borosilicate glass vial as described above for acid digestion.
For the French press method, organisms that had been exposed to Hg(II) (HgCl2) for 1 h during growth in mid-log phase were centrifuged at 3,000 × g, washed with medium, recentrifuged, and made up in half the original volume. This was bubbled with N2 gas for 15 min, extruded through a French press at 20,000 lb/in2, and kept on ice. The resulting suspension was passed through sequentially smaller filters, and the amounts of acid- and alkaline-reducible Hg were determined for each effluent.
All other mercury analytical procedures were performed as described by Kelly and colleagues (24).
Statistics.
All experiments include representative standard errors (SE). Experiments were performed at least three times, and the results presented are those for a representative experiment wherein all Hg determinations were performed in triplicate (i.e., n = 3). SE is represented by bars in the figures. Where the bars are not visible, the SE is smaller than the characters at each point. Where appropriate, Student's t tests were performed.
RESULTS AND DISCUSSION
Justification of mercury exposure levels.
Although average environmental concentrations of mercury in aqueous environments are in the parts-per-trillion range (23), we employed high exposure levels (28, 33, 51) in this study to emulate contaminated sites and effluent from industrial sources. When mercury is supplied as HgCl2, it is absorbed in the form of Hg(II) by aquatic microbes (23). The selected cell culture density (OD665 = 0.32) reflects cyanobacterial population sizes that are typical of eutrophic conditions.
Biotransformation of mercury by cyanobacteria. (i) Limnothrix planctonica.
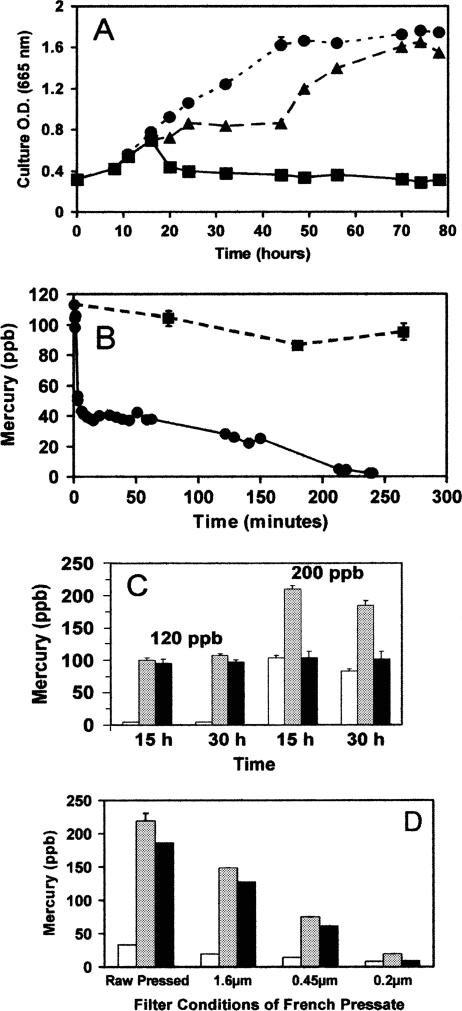
Cultures of Limnothrix planctonica exposed to 120 ppb Hg(II) (HgCl2) at pH 6.5 exhibited a growth lag, followed by recovery, while those exposed to 200 ppb bleached within 30 min and did not recover (Fig. 1A).
FIG. 1.
Effects of HgCl2 on Limnothrix planctonica. (A) Growth of cells in control (•), 120-ppb (▴), and 200-ppb (▪) treatments at pH 6.5. (B) Time course for biotransformation of a sublethal application of Hg(II) (120 ppb HgCl2). Results for total Hg (▪) and acid reduction-detectable Hg (•) are shown. Panel B is adapted from Fig. 1 of a paper by Kelly et al. (24). (C) Biotransformation of 120-ppb and 200-ppb Hg(II) applications after 15 and 30 h. Results for acid-detectable Hg (white), total Hg (gray), and β-HgS (black) are shown. (D) Analysis of French pressed cellular filtrate. Symbols are as defined for panel C (above). Incubation was at 120 ppb Hg(II) for 1 h and extraction as described in Materials and Methods. The particle retention size of the filter is given for each filtrate. All cultures had initial OD665s of 0.32. All values are means ± SE for triplicate determinations.
The sublethal exposure resulted in a rapid decline in acid-reducible Hg within 10 min (Fig. 1B). This decrease was not matched by a similar loss in alkaline-reducible Hg. The difference between alkaline (total inorganic)- and acid-reducible Hg (Hg2+, thiol-complexed Hg2+, and/or nucleoside-bound Hg2+) represents β-HgS, or meta-cinnabar (24). The difference between the initial Hg application and alkaline-reducible Hg represents the maximum for volatile Hg (10 ppb ± 4 ppb; triplicate determinations). In all cases, controls run without cyanobacteria had less than 10% of the Hg volatilization seen with any species of cyanobacteria and they showed no β-HgS production. After 1 h, the 120 ppb of mercury applied to cultures of Limnothrix was composed of 58% β-HgS, 12% Hg(0), and 30% acid-reducible Hg. Because there was no significant volatilization between 1 and 5 h (t test; P > 0.05; triplicate determinations), the decrease in acid-reducible Hg was due to conversion to β-HgS, which constituted 98 ppb ± 11 ppb of the original mercury application. These acid/alkaline reduction data indicate that peptide or protein thiols do not play an important role in long-term sequestration and storage of Hg(II).
Of the volatilized Hg, 98.8% passed through a Carbotrap tube to be trapped by a tube of silvered Chromosorb P. Analysis of cultures using CdCl2-catalyzed alkaline reduction gave no evidence of organomercurials in these aerobic cultures, which therefore appear to volatilize Hg only as Hg(0) (24).
The rapid decline in acid-reducible Hg resembles the Hg(II) assimilation by numerous strains of heterotrophic bacteria (15). Although the microbial biotransformation of Hg(II) to Hg(0) is well known, the role of this pathway in the current study was minor by comparison to that of the transformation to β-HgS. There is some debate as to the degree of Hg(0) production in whole-lake studies that is biotic in origin (3, 52). Application levels in the current study were lower than those in many previous bacterial studies, where less than 18% was transformed to Hg(0) (15). These results bring into question whether biotransformation to Hg(0) is the dominant detoxification mechanism at realistic Hg(II) exposure levels.
At the lethal application of 200 ppb Hg2+, a similarly rapid initial loss of acid-reducible Hg occurred, again with little volatilization (data not shown). However, this fraction of Hg leveled off at 60 ppb ± 4 ppb within 15 min. At the time of chlorophyll bleaching and the loss of cell density (Fig. 1A), acid-reducible Hg increased to 100 ppb. The toxicity seen at 200 ppb Hg2+ is less likely caused by the production of β-HgS, owing to its low solubility (Ksp = l × 10−54), than by the acid-reducible Hg fraction, which includes free ions and potentially deleterious thiol complexing effects. This supports the notion that the amount of total element present is not always the relevant predictor of toxicity (36).
While volatilization of Hg played a similar minor role in the fates of both 120- and 200-ppb applications, significant differences were found for the locations of Hg in fractionated cultures after 15 h. In 120-ppb cultures, 87.5% (90 ppb ± 1 ppb [mean ± SE]; triplicate determinations) of the remaining Hg was associated with the cells, while for cultures exposed to 200 ppb, the frequency was only 62.5% (127 ppb ± 2 ppb [mean ± SE]; triplicate determinations). This suggests that as the ratio of cell density to exposure level increases, typical of realistic environmental exposures, a greater proportion of the applied Hg becomes associated with biomass. This should be considered in the modeling of Hg accumulation by microbial biomass and subsequent sedimentation or grazing effects (7, 35).
The chemical form of Hg was determined after 15 and 30 h of exposure (Fig. 1C). The amount of total Hg in 120-ppb cultures and the amount present as β-HgS were unchanged between 15 and 30 h. A small but significant loss of total Hg was observed in 200-ppb cultures between 15 and 30 h, but again, the β-HgS content was unchanged. Together, these results suggest that volatile-Hg(0) loss came from the acid-reducible and not the β-HgS fraction. Also, conversion to β-HgS may explain the long biological residence time for Hg remaining in culture following initial volatilization. Betz (6) provided evidence that, after a time, cellular Hg becomes unavailable for reduction to Hg(0). He observed that 203Hg-exposed Dunaliella tertiolecta (green alga), transferred after 2 days to a medium containing 197Hg, volatilized only the latter isotope. While similar data for cyanobacteria are unavailable, biotransformation of 203Hg2+ to insoluble β-203HgS would likely make it inaccessible to aqueous mercuric reductase.
Any further loss of Hg could be attributed to slow sublimation. Evidence was sought to support the idea that the acid-reducible mercurials are soluble, while the acid-irreducible/alkali-reducible form identified as β-HgS is filterable and strongly associated with cell matter. Hg-exposed Limnothrix was disrupted with a French Press (20,000 lb/in2) and subjected to sequentially finer filtration steps. Filtration had comparatively little effect on acid-reducible filtrate Hg, as expected for these soluble forms, but a dramatic effect on the alkaline-reducible β-HgS filtrates (Fig. 1D).
meta-Cinnabar has been detected in aquatic liverworts (38), and a number of observations of a range of microbiota have shown mercury metal in their cell walls in a form unavailable for volatilization. In these organisms, the metal was resistant to extraction under all but alkaline conditions and apparently not toxic despite high accumulation (1, 6, 11, 19, 20). In Saccharomyces cerevisiae, differences in total cell wall Hg content are distinctions between Hg-sensitive and -resistant strains (32), but the form of Hg was not identified.
Importantly, both the filtration and the chemical reactivity data are consistent, as they do not support the view that cellular Hg2+ is stored and detoxified in soluble sulfhydryl chelates over long periods. This supposition has long been criticized as lacking any direct demonstration in microbiota as diverse as algae (42) and fungi (17). It remains possible, however, that the synthesis of β-HgS in Limnothrix cultures occurs via thiol-containing intermediates.
(ii) Synechococcus leopoldiensis.
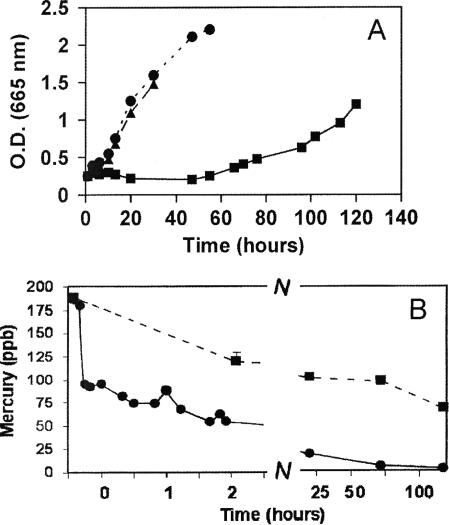
The common research organism Synechococcus leopoldiensis is the only cyanobacterium in which metallothionein has been shown to occur (40, 46). Growth of S. leopoldiensis was less sensitive to Hg than that of Limnothrix planctonica, showing virtually no effect at 120 ppb (Fig. 2A), while 200 ppb Hg2+ induced a growth lag and chlorophyll bleaching. The latter occurred after 10 h and was maximal by 20 h, and recovery began at 50 h. This prolonged period leading to chlorophyll bleaching is distinct from that for Limnothrix, where bleaching occurred in just 30 min with no recovery. As such, Hg tolerance in Synechococcus warranted further study.
FIG. 2.
Effects of HgCl2 on Synechococcus leopoldiensis. (A) Growth of cells in control (•), 120-ppb (▴), and 200-ppb (▪) treatments at pH 6.5. (B) Time course for biotransformation of Hg(II) (200 ppb HgCl2). Results for total Hg (▪) and acid reduction-detectable Hg (•) are shown. All cultures had initial OD665s of 0.32. All values are means ± SE for triplicate determinations.
During the 200-ppb treatment of Synechococcus, acid-reducible Hg decreased rapidly, reaching 150 ppb in 10 min and 100 ppb in 30 min. Concomitant Hg(0) synthesis was also substantial. Then, a slower conversion to β-HgS occurred, during which time Hg(0) synthesis was low but significant (t tests; P < 0.05; triplicate determinations). The proportion of acid-reducible Hg remaining after 30 min was greater than that in Limnothrix under identical conditions. After 1 h, the 200 ppb of mercury applied to cultures of Synechococcus was composed of 35% β-HgS, 25% Hg(0), and 40% acid-reducible Hg. The acid-reducible Hg continued to decline to only 5 ppb (Fig. 2B) in the initial 24 h, when volatilization accounted for half of the lost mercury. Then, a slower process, likely involving conversion from β-HgS, occurred over 4 days.
The primary toxicity of Hg(II) is believed to be caused by chelation to functional sulfhydryl groups of proteins. Hence, the early phase of exposure is probably the most toxic. The time course of recovery from growth inhibition and chlorophyll bleaching raises the possibility that de novo protein synthesis may be required. Our results indicate that lowering of toxicity coincides with the decline in acid-reducible Hg by Hg(0) and β-HgS pathways.
Role of sulfhydryl compounds.
The existence of metal sulfide synthesis has been suspected for some time, and the initial speculation was that direct S2− (H2S) excretion leads to metal precipitation (1). Since then, however, it has been discovered that CdS crystals in yeast are capped by peptides, probably phytochelatins (9). It is, therefore, possible that chelation to this metal-binding sulfhydryl protein is an intermediate step in metal sulfide synthesis.
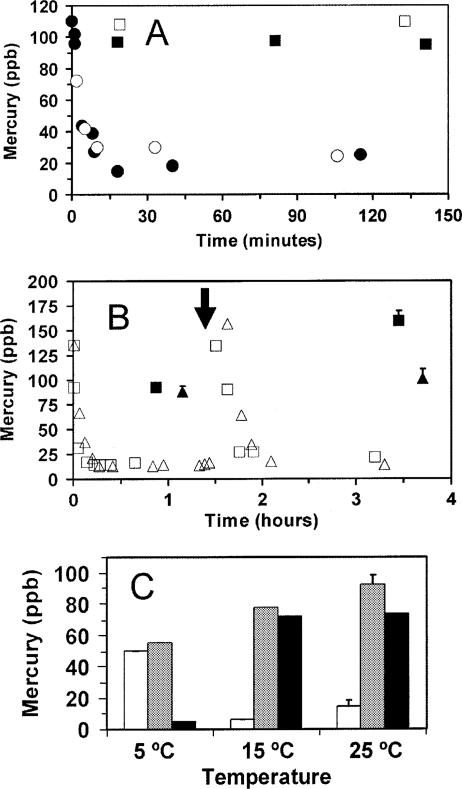
To obtain evidence of a role for sulfhydryl compounds in cyanobacterial β-HgS synthesis, cell surface and intracellular thiols were modified chemically. Iodoacetamide, which can act as a nonpermeant alkylator of sulfhydryl groups (14), did not prevent β-HgS synthesis or the rapid decrease in acid-detectable Hg in Limnothrix (Fig. 3A), although a slight enhancement of initial Hg(0) formation was observed. Therefore, β-HgS formation does not depend on reactions with readily accessible thiol groups on the cell surface.
FIG. 3.
Effects of thiol modification and temperature on the synthesis of βHgS and Hg(0). (A) Extracellular thiol alkylation by iodoacetamide in Limnothrix planctonica was followed by a 120-ppb Hg(II) application (closed symbols). Controls without alkylation are represented by open symbols. Results for total Hg (squares) and acid reduction-detectable Hg (circles) are shown. (B) Intracellular thiol oxidation by dimethylfumarate in Phormidium limnetica. Cultures were exposed to 140 ppb Hg(II). At 1.5 h (↓), half were treated with dimethylfumarate (triangles); untreated controls are represented by squares. An additional 140 ppb Hg(II) was applied at 1.75 h. Results for total Hg (closed symbols) and acid reduction-detectable Hg (open symbols) are shown. (C) Effect of temperature on biotransformation by Limnothrix planctonica. Mercury-free preincubation was for 150 min, followed by 5-h treatments in 120 ppb Hg(II). Results for acid-detectable Hg (white), total Hg (gray), and β-HgS (black) are shown. All cultures had initial OD665s of 0.32. All values are means ± SE for triplicate determinations.
The permeant thiol oxidant dimethylfumarate depletes both surface and internal thiols (22). An experiment was designed to measure Hg components before and after treatment with dimethylfumarate, but this required repeated Hg applications that proved to be lethal to Limnothrix and Synechococcus. Fortunately, another species of cyanobacterium, Phormidium limnetica, could cope with this double treatment. Two sets of cultures of Phormidium were exposed to 140 ppb Hg(II), and biotransformation was monitored for 90 min in the absence of oxidant (Fig. 3B). After 1 h, the mercury was composed of 56% β-HgS, 36% Hg(0), and 8% acid-reducible Hg. Therefore, Phormidium efficiently generated Hg(0) and β-HgS from Hg(II) under the same conditions as those described above for the other two cyanobacterial species (compare Fig. 1B and 2B), thus justifying its use in this comparative study. After this first 90-min period, one culture set was given a short 5-min treatment with dimethylfumarate and then both sets were provided with an additional 140 ppb HgCl2. The amounts of acid-reducible Hg rapidly dropped to 20 ppb Hg in both treated and control cultures for the second time. In the control, there was an approximate doubling of β-HgS that occurred very rapidly, suggesting that a pool of available thiols may be responsible, whereas the treated cultures showed no increase in β-HgS following the second application (Fig. 3B). Interestingly, this removal of intracellular thiols also diverted the applied mercury-to-Hg(0) synthesis. A short exposure time was employed to minimize effects on metabolism, although such effects cannot be entirely ruled out. Nevertheless, the results support a role for internal thiols in the synthesis of β-HgS and do not indicate that its formation is a product of H2S excretion.
Temperature had a similar dramatic effect on biotransformation in cyanobacteria. Cultures of Limnothrix incubated at 5°C for 150 min prior to a 120-ppb Hg2+ application evolved 55% of the application to Hg(0), while just 5% of that remaining in culture was converted to β-HgS after 5 h (Fig. 3C). Cultures grown at 25°C and 15°C evolved about 20% of the initial application to Hg(0), with around 90% conversion to β-HgS.
Biotransformation of Hg(II) by cyanobacteria.
These results show the involvement of metabolism in β-HgS synthesis and, like the thiol depletion data, suggest that Hg(0) synthesis is inversely affected by the capacity for β-HgS synthesis. The relatively temperature insensitive nature of Hg(0) evolution is consistent with the constitutive presence of the enzyme mercuric reductase, requiring only Hg2+ and NADPH for the reaction to proceed. In fact, decreases in temperature-sensitive reductive carbon metabolism may lead to a buildup of photoreduced NADPH.
Cyanobacteria converted Hg(II) (as HgCl2) under pH-controlled and aerated conditions into Hg(0) and β-HgS. Production of Hg(0) was relatively low and exposure level dependent and differed only marginally between the cyanobacterial species. The Hg remaining in culture was virtually all biotransformed to β-HgS at the expense of acid-reducible Hg. This indicates that biotransformation of Hg(II) into β-HgS probably occurs in the epilimnia of lakes receiving contaminated rainfall. Until recently, it was believed to be primarily generated in sediments (31, 52). No evidence of cyanobacterial CH3Hg+ formation was found in cultures or in exhaust gases under the aerobic culture conditions employed in the present study.
Substantial sedimentary potential could be generated through biotransformation by cyanobacteria. This group of organisms is important because it is among the first to encounter mercury that is introduced by rainfall (3). If the biological residence time for β-HgS is longer than the time for complete sedimentation and grazing to occur, then a significant amount of mercury resides in cyanobacterial populations. Based on the results reported by Conover and Mayzaud (8), this may very well be true; however, characteristics of accumulation, metabolism, and subsequent loss to volatilization remain to be determined.
An important role for phytoplankton in the mercury cycle is consistent with ecological findings that total and dissolved organic carbon affect interlake variation in CH3Hg+ levels in both water and fish (13, 30, 49, 50). A possible association of β-HgS with sedimenting biomass may explain why filter-feeding sponges were second only to fish in levels of total Hg (37), offering an origin for the particulate Hg that these researchers surmised to be present.
These findings also suggest that β-HgS, not a chelate of Hg(II), is the relevant dietary mercurial for cyanobacterial grazers. Previous studies which have used HgCl2 as the toxicant should be reexamined (31, 35). In the case of cadmium, sea urchins had higher excretion levels, lower uptake levels, and different tissue distributions of metal after feeding on naturally contaminated than after feeding on cadmium salt-spiked aquatic systems (48). Suggestions that β-HgS formation could play a role in detoxification are not new, but there are few direct quantitative studies. An investigation of the heterotrophic bacterium Klebsiella aerogenes noted parallel increases in cellular inorganic sulfide and cellular cadmium or mercury (1), while Davies (11) surmised that eukaryotic algae accumulated a mercurial with the characteristics of an insoluble compound, possibly a sulfide. However, early studies lacked even a qualitative method for directly demonstrating the presence of this mercurial species. Metal sulfides have also been demonstrated to exist in lower plants (38) and microbiota (9) as well as soil sediments (5) using electron microscopy techniques. We have recently detected mercury sulfide in eukaryotic algae (26) as well as in microfungi (25). How widely this phenomenon occurs in aquatic organisms remains to be determined. In addition, although the mechanism by which β-HgS is formed in cyanobacteria requires further elucidation, the process appears to rely on internal thiols and metabolism, as interference with either favors volatilization.
The rate at which Hg(II) was converted into β-HgS by cyanobacteria indicates their possible utility as organisms for bioremediation. Further studies are required to select the most appropriate species and to optimize environmental conditions for this application.
Acknowledgments
We thank G. vanLoon and D. T. Canvin for advice throughout this research project.
This work was supported by the Natural Sciences and Engineering Research Council of Canada.
Footnotes
Published ahead of print on 27 October 2006.
This paper is dedicated to the memory of K. Budd.
REFERENCES
- 1.Aiking, H., H. Govers, and J. van 't Riet. 1985. Detoxification of mercury, cadmium and lead in Klebsiella aerogenes NCTC 418 growing in continuous culture. Appl. Environ. Microbiol. 50:1262-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, S. E., J. A. Parkinson, and A. P. Rowland. 1989. Pollutants, p. 201-239. In S. E. Allen (ed.), Chemical analysis of ecological materials, 2nd ed. Blackwell Scientific Publishers, Oxford, United Kingdom.
- 3.Amyot, M., G. Mierie, D. R. S. Lean, and D. J. McQueen. 1994. Sunlight-induced formation of dissolved gaseous mercury in lake waters. Environ. Sci. Technol. 28:2366-2371. [DOI] [PubMed] [Google Scholar]
- 4.Baldi, F., M. Pepi, and M. Filippelli. 1993. Methylmercury resistance in Desulfovibrio desulfuricans strains in relation to methylmercury degradation. Appl. Environ. Microbiol. 59:2479-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett, M. O., L. A. Harris, R. R. Turner, R. J. Stevenson, T. J. Henson, R. C. Melton, and D. P. Hoffman. 1997. Formation of mercuric sulfide in soil. Environ. Sci. Technol. 31:3037-3043. [Google Scholar]
- 6.Betz, M. 1977. Investigations on the simultaneous uptake and release of mercury by Dunaliella tertiolecta. Marine Biol. 41:89-97. [Google Scholar]
- 7.Chen, C. Y., and C. L. Folt. 2005. High plankton densities reduce mercury biomagnification. Environ. Sci. Technol. 39:115-121. [PubMed] [Google Scholar]
- 8.Conover, R. J., and P. Mayzaud. 1894. Utilization of phytoplankton by zooplankton during the spring bloom in a Nova Scotia inlet. Can. J. Fish Aquat. Sci. 41:232-252. [Google Scholar]
- 9.Dameron, C. T., R. N. Reese, R. K. Mehra, A. R. Kortan, P. J. Carroll, M. L. Steigerwald, L. E. Brus, and D. R. Winge. 1989. Biosynthesis of cadmium sulphide quantum semiconductor crystallites. Nature 338:596-597. [Google Scholar]
- 10.Daniels, R. S., and D. C. Wigfield. 1989. Cold-vapor mercury atomic absorption spectrometry II. Acidic versus alkaline reduction. Sci. Total Environ. 89:325-329. [Google Scholar]
- 11.Davies, A. G. 1976. An assessment of the basis of mercury tolerance in Dunaliella tertiolecta. J. Mar. Biol. Assoc. U. K. 56:39-57. [Google Scholar]
- 12.Essa, A. M. M., L. E. Macaskie, and N. L. Brown. 2002. Mechanisms of mercury bioremediation. Biochem. Soc. Trans. 30:672-674. [DOI] [PubMed] [Google Scholar]
- 13.Fjeld, E., and S. Rognerud. 1993. Use of path analysis to investigate mercury accumulation in brown trout (Salmo trutta) in Norway and the influence of environmental factors. Can. J. Fish Aquat. Sci. 50:1158-1167. [Google Scholar]
- 14.Flores, B. M., M. A. Batzer, M. A. Stein, C. Petersen, D. L. Diedrich, and B. E. Torian. 1993. Structural analysis and demonstration of the 29 kDa antigen of pathogenic Entamoeba histolytica as the major accessible free thiol-containing surface protein. Mol. Microbiol. 7:755-763. [DOI] [PubMed] [Google Scholar]
- 15.Frischmuth, A., P. Weppen, and W. D. Deckwer. 1993. Microbial transformation of mercury(II). I. Isolation of microbes and characterization of their transformation capabilities. J. Biotechnol. 29:39-55. [Google Scholar]
- 16.Gabriel, M. K., and D. G. Williamson. 2004. Principal biogeochemical factors affecting the speciation and transport of mercury through the terrestrial environment. Environ. Geochem. Health 26:421-434. [DOI] [PubMed] [Google Scholar]
- 17.Galli, U., H. Schuepp, and C. Brunold. 1994. Heavy metal binding by mycorrhizal fungi. Physiol. Plant. 92:364-368. [Google Scholar]
- 18.Gill, U., L. Bigras, and H. Schwartz. 2004. Routine, automated determination of inorganic and total mercury in multimedia using cold vapor atomic absorption spectrometry. Chemosphere 56:1097-1103. [DOI] [PubMed] [Google Scholar]
- 19.Hamdy, M. K., and O. R. Noyes. 1975. Formation of methylmercury by bacteria. Appl. Microbiol. 30:424-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassett, J. M., J. C. Jennett, and J. E. Smith. 1981. Microplate technique for determining accumulation of metals by algae. Appl. Environ. Microbiol. 41:1097-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatch, W. R., and W. L. Ott. 1968. Determination of sub-microgram quantities of mercury by atomic absorption spectrophotometry. Anal. Chem. 40:2085-2087. [Google Scholar]
- 22.Held, K. D., E. R. Epp, S. Awad, and J. E. Biaglow. 1991. Postirradiation sensitization of mammalian cells by the thiol-depleting agent dimethylfumarate. Radiat. Res. 127:75-80. [PubMed] [Google Scholar]
- 23.Kelly, C. A., J. W. M. Rudd, and M. H. Holoka. 2003. Effect of pH on mercury uptake by an aquatic bacterium: implications for Hg cycling. Environ. Sci. Technol. 37:2941-2946. [DOI] [PubMed] [Google Scholar]
- 24.Kelly, D. J. A., K. Budd, and D. D. Lefebvre. 2006. Mercury analysis of acid- and alkaline-reduced biological samples; identification of meta-cinnabar as the major biotransformed compound in algae. Appl. Environ. Microbiol. 72:361-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly, D. J. A., K. Budd, and D. D. Lefebvre. 2006. The biotransformation of mercury in pH-stat cultures of microfungi. Can. J. Bot. 84:254-260. [DOI] [PubMed] [Google Scholar]
- 26.Kelly, D. J. A., K. Budd, and D. D. Lefebvre. 10 October 2006, posting date. Biotransformation of mercury in pH-stat cultures of eukaryotic freshwater algae. Arch. Microbiol. doi: 10.1007/s00203-006-0170-0. [DOI] [PubMed]
- 27.Locatelli, C., and G. Torsi. 2001. Heavy metal determination in aquatic species for food purposes. Ann. Chim. 91:65-72. [PubMed] [Google Scholar]
- 28.Mance, G. 1987. Pollution monitoring series: pollution threat of heavy metals in aquatic environments. Elsevier Applied Science, New York, NY.
- 29.Marker, A. F. H., E. A. Nusch, H. Rai, and B. Riemann. 1980. The measurement of photosynthetic pigments in freshwaters and standardization of methods: conclusions and recommendations. Arch. Hydrobiol. Beihefte. Ergeb. Limnol. 14:91-106. [Google Scholar]
- 30.McMurtry, M. J., D. L. Wales, W. A. Scheider, G. L. Beggs, and P. E. Dimond. 1989. Relationship of mercury concentrations in lake trout (Salvelinus namaycush) and smallmouth bass (Micropterus dolomieui) to the physical and chemical characteristics of Ontario lakes. Can. J. Fish Aquat. Sci. 46:426-434. [Google Scholar]
- 31.Morel, F. M. M., A. M. L. Kraepiel, and M. Amyot. 1998. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 29:543-566. [Google Scholar]
- 32.Ono, B.-I., H. Ohue, and F. Ishihara. 1988. Role of cell wall in Saccharomyces cerevisiae mutants resistant to Hg2+. J. Bacteriol. 170:5877-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panda, K. K., M. Lenka, and B. B. Panda. 1992. Monitoring and assessment of mercury pollution in the vicinity of a chloralkali plant. III. Concentration and genotoxicity of mercury in the industrial effluent and contaminated water of Rushikulya estuary, India. Mutant Res. 280:149-160. [DOI] [PubMed] [Google Scholar]
- 34.Parkhurst, D. L., and C. A. J. Appelo. 1999. User's guide to PHREEQC (version 2)—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. U.S. Geological Survey Water-Resources Investigations Report 99-4259, 312 p. U.S. Geological Survey, Reston, VA.
- 35.Pickhardt, P. C., C. L. Folt, C. Y Chen, B. Klaue, and J. D. Blum. 2002. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. Proc. Natl. Acad. Sci. USA 99:4419-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rayner, M. H., and P. J. Sadler. 1989. Cadmium accumulation and resistance mechanisms in bacteria, p. 39-47. In R. K. Poole and G. M. Gadd (ed.), Special publications of the Society for General Microbiology, vol. 26. Metal-microbe interactions. Oxford University Press, New York, NY. [Google Scholar]
- 37.Riisgard, H. U., and P. S. Larsen. 1995. Filter-feeding in marine macro-invertebrates: Pump characteristics, modeling and energy cost. Biol. Rev. 70:67-106. [DOI] [PubMed] [Google Scholar]
- 38.Satake, K., K. Shibata, and Y. Bando. 1990. Mercury sulphide (β-HgS) crystals in the cell walls of the aquatic bryophytes, Jungermannia vulcanicola Steph. and Scapania undulata (L.) Dum. Aquat. Bot. 36:325-341. [Google Scholar]
- 39.Silver, S., and T. K. Misra. 1988. Plasmid-mediated heavy metal resistances. Annu. Rev. Microbiol. 42:717-743. [DOI] [PubMed] [Google Scholar]
- 40.Silver, S., and L. T. Phung. 1996. Bacterial heavy metal resistance: New surprises. Annu. Rev. Microbiol. 50:753-789. [DOI] [PubMed] [Google Scholar]
- 41.Silver, S., and M. Walderhaug. 1992. Gene regulation of plasmid- and chromosomal-determined inorganic ion transport in bacteria. Microbiol. Rev. 56:195-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stokes, P. M. 1983. Responses of fresh-water algae to metals. p. 87-112. In F. E. Round and D. J. Chapman (ed.), Progress in phycological research, vol. 2. Elsevier, New York, NY. [Google Scholar]
- 43.Stumm, W., and J. J. Morgan. 1981. Aquatic chemistry: an introduction emphasizing chemical equilibria in natural waters, 2nd ed., p. 121-170 and 323-417. John Wiley & Sons, Toronto, Canada.
- 44.Swain, E. B., D. R. Engstrom, M. E. Brigham, T. A. Henning, and P. L. Brezonik. 1992. Increasing rates of atmospheric mercury deposition in midcontinental North America. Science 257:784-787. [DOI] [PubMed] [Google Scholar]
- 45.Tong, S.-L., and W. K. Leow. 1980. Stationary cold-vapor atomic absorption spectrometric attachment for determination of total mercury in undigested fish samples. Anal. Chem. 52:581-583. [DOI] [PubMed] [Google Scholar]
- 46.Turner, J. S., and N. J. Robinson. 1995. Cyanobacterial metallothioneins: biochemistry and molecular genetics. J. Ind. Microbiol. 14:119-125. [DOI] [PubMed] [Google Scholar]
- 47.Walsh, G. E. 1988. Principles of toxicity testing with marine unicellular algae. Environ. Toxicol. Chem. 7:979-987. [Google Scholar]
- 48.Warnau, M., G. Ledent, A. Temara, M. Jangoux, and P. Dubois. 1995. Experimental cadmium contamination of the echinoid Paracentrotus lividus influence of exposure mode and distribution of the metal in the organism. Mar. Ecol. Prog. Ser. 116:117-124. [Google Scholar]
- 49.Watras, C. J., and N. S. Bloom. 1992. Mercury and methylmercury in individual zooplankton: implications for bioaccumulation. Limnol. Oceanogr. 37:1313-1318. [Google Scholar]
- 50.Watras, C. J., K. A. Morrison, J. S. Host, and N. S. Bloom. 1995. Concentration of mercury species in relationship to other site-specific factors in the surface waters of northern Wisconsin lakes. Limnol. Oceanogr. 40:556-565. [Google Scholar]
- 51.Wetzel, R. G., and G. E. Likens. 1991. Limnological analyses, 2nd ed. Springer-Verlag, New York, NY.
- 52.Winfrey, M. R., and J. W. M. Rudd. 1990. Environmental factors affecting the formation of methylmercury in low pH lakes. Environ. Toxicol. Chem. 9:853-869. [Google Scholar]