Abstract
The ability of 76 Bifidobacterium strains to produce folate was investigated. In order to evaluate folic acid productivity, bifidobacteria were cultivated in the folate-free semisynthetic medium SM7. Most of the tested strains needed folate for growth. The production and the extent of vitamin accumulation were not a function of species but were distinctive features of individual strains. Six strains among the 17 that grew without folate produced significantly higher concentrations of vitamin (between 41 and 82 ng ml−1). The effects of exogenous folate and p-aminobenzoic acid (PABA) concentrations on folate production were evaluated. In contrast to most of the other strains, the folate yield of B. adolescentis MB 239 was not negatively affected by either PABA or exogenous folic acid. Folate production by B. adolescentis MB 239 was studied in the pH range of the colonic environment, and a comparison of folate production on raffinose, lactose, and fructo-oligosaccharides, which belong to three important groups of fermentable intestinal carbon sources, was established. Differences in folate biosynthesis by B. adolescentis MB 239 were not observed as a function either of the pH or of the carbon source. Fecal culture experiments demonstrated that the addition of B. adolescentis MB 239 may increase the folate concentration in the colonic environment.
Folate is a vitamin that accepts one-carbon units from donor molecules and is involved in many metabolic pathways, such as methyl group biogenesis and synthesis of nucleotides, vitamins, and some amino acids. The efficiency of DNA replication, repair, and methylation are affected by folate availability; therefore, large amounts of folate are required by rapidly proliferating cells such as leukocytes, erythrocytes, and enterocytes (11). Folate deficiency is often associated with increased cancer risk. Epidemiological studies have indicated that low folate intake is related to increased risk of postmenopausal breast cancer (21) and that low folate homeostasis may induce hypomethylation of DNA, thereby promoting cancer on the proliferating cells of the colonic-rectal mucosa that support rapid and continuous renewal of the epithelium (9, 28). Furthermore, folate supplementation is recommended for patients with inflammatory bowel diseases to contribute to the regulation of rectal cell turnover (2).
Folate is widely distributed in the biological world, intestinal bacteria being one source of this vitamin. It has been demonstrated that folate synthesized by bacteria in the human intestine is absorbed and used by the host (4, 8, 13, 14, 18); however, little is known about folate production by the intestinal microbiota. Some information is available about the parameters, such as external pH, dilution rate, and p-aminobenzoic (PABA) concentration, that influence folate production by lactic acid and starter bacteria used for the production of yogurt, probiotic dairy products, and cheeses (5, 15, 23). Moreover, efforts to increase the production of naturally bioavailable folate by Lactococcus lactis during food fermentation have been successful (22, 24, 25). It has also been shown that increased folate levels in yogurts and fermented milks are possible through judicious selection of the inoculum species; nevertheless, these folate levels remain relatively low in terms of the recommended daily allowance (5).
The present work investigated folate production by bifidobacteria. Bifidobacteria are gram-positive, saccharolytic, intestinal anaerobes. They acidify the large intestine, restricting putrefactive and potentially pathogenic bacteria; produce vitamins and amino acids; stimulate the immune response; repress the conversion of primary bile salts; exert anti-inflammatory activity; and reduce the risk of colon cancer (19, 26, 27). Because of these beneficial health effects, bifidobacteria are generally considered probiotic organisms and are increasingly being used in functional foods and pharmaceutical products. Nevertheless, there is limited information concerning folate production by bifidobacteria; knowledge is limited to the demonstration of diverse folate concentrations after the growth of seven Bifidobacterium strains in skim milk (5).
Most folate absorption occurs through the jejunum, but it has been demonstrated that folate produced by colonic microorganisms can be absorbed across the large intestine (4, 29). The aim of this study was to select bifidobacterial strains that combine the intrinsic probiotic activities of the genus Bifidobacterium with significant production of folic acid, in order to supply proliferating colonocytes with this vitamin.
MATERIALS AND METHODS
Strains.
Bifidobacterium strains were obtained from ATCC (American Type Culture Collection, Manassas, VA), DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany), and the Scardovi Collection (formerly the Institute of Agricultural Microbiology, University of Bologna, Bologna, Italy).
Chemicals and media.
All chemicals were obtained from Sigma-Aldrich (Steinheim, Germany) unless otherwise stated.
Folate production in pure Bifidobacterium cultures was tested in the folate-free semisynthetic medium (SM) SM7. SM7 is based on SM (16) with modifications: Bacto vitamin assay Casamino Acids (Difco) was used, and yeast nitrogen base (Difco) was replaced with pyridoxine (2 mg liter−1), nicotinic acid (2 mg liter−1), thiamine (2 mg liter−1), calcium pantothenate (1 mg liter−1), riboflavin (1 mg liter−1), PABA (0.05 mg liter−1), and biotin (0.05 mg liter−1). The pH was adjusted to 7.0, and the medium was autoclaved for 30 min at 110°C. Glucose was autoclaved separately and added to the sterile basal medium to obtain a concentration of 20 g liter−1. Fecal cultures were carried out in the medium FM7, which is based on the complex medium described by Rycroft et al. (17) with modifications: yeast extract was replaced with PABA (0.45 mg liter−1), biotin (0.45 mg liter−1), pyridoxine (18 mg liter−1), nicotinic acid (18 mg liter−1), thiamine (18 mg liter−1), calcium pantothenate (9 mg liter−1), riboflavin (9 mg liter−1), and fructans (10g liter−1) (Raftilose Synergy, Orafti, Tienen, Belgium) as carbon sources. The pH was adjusted to 7.0, and the medium was autoclaved for 30 min at 110°C.
Culture conditions.
Bifidobacterium strains were subcultured in Lactobacilli MRS broth (Difco) containing 0.5 g liter−1 l-cysteine · HCl and were anaerobically incubated at 37°C for 24 h. Cells from the MRS cultures were inoculated (5%, vol/vol) into 10 ml of SM7; SM7 cultures were incubated anaerobically at 37°C for 48 h.
The growth of Bifidobacterium strains and production of intracellular and extracellular folate were assayed in liquid cultures of SM7. To determine whether exogenous folate down-regulated production, the folate concentration was determined in cultures grown in SM7 supplemented with 10, 25, 50, and 100 μg liter−1 vitamin. In order to evaluate the effect of PABA concentration on folate biosynthesis, the folate concentration was determined in cultures grown in SM7 containing 0, 0.1, 1, 10, or 100 μM PABA. Cultures were always propagated at least seven times in the same medium before the measurement of folate concentrations in the supernatants or cell extracts. Growth was determined by measuring the final pH and optical density at 600 nm (OD600).
pH-controlled batch cultures were performed in SM7 in a 2-liter-working-volume BM-PPS3 bioreactor (Bioindustrie Mantovane, Porto Mantovano, Italy). The temperature was kept constant at 37°C. The pH was continuously measured (Mettler Toledo InPro 3030/325) and kept constant by the automatic addition of 4 M NaOH. Anaerobiosis (<5 ppm oxygen) was maintained by sparging of the culture with 0.05 vol/vol/min filter-sterilized nitrogen (Millex filter type GS, 33 mm). The culture was constantly stirred at 300 rpm. The fermenter was inoculated (10%, vol/vol) with exponential-phase precultures grown in the same medium. Samples were periodically collected for dry weight measurement and folate analysis.
Anaerobic chemostat cultivation was carried out in 1 liter of SM7 at a dilution rate of 0.075 h−1 without control of the pH. The fresh medium was anaerobically maintained by the flushing of filter-sterilized CO2 into the headspace of the feeding tank. The fermentation was initiated batchwise by directly inoculating the grown seed culture in the fermenter. At minimum, five residence times were allowed to elapse, and steady state was considered attained when the pH and biomass concentration remained constant for at least two residence times. Steady-state samples were collected for biomass measurement and folate analysis. The effect of different carbon sources on folate production was studied in continuous fermentations wherein the glucose of SM7 was replaced by lactose, fructose, raffinose, or fructans. All fermentations were carried out in duplicate.
To prepare fecal cultures, fresh feces were obtained from seven healthy volunteers (four men and three women) who had followed a pre/probiotic-free diet for 1 month and had not been treated with antibiotics for at least 3 months. All preparations were done in an anaerobic cabinet (Anaerobic System; Forma Scientific Co., Marietta, GA) under an atmosphere of 85% N2, 10% CO2, and 5% H2. Fecal samples were anaerobically resuspended (10%, wt/vol) in 0.1 M phosphate buffer, pH 7.0, and pasteurized at 80°C for 15 min. Fecal suspension was added (10%, vol/vol) to FM7 anaerobic serum bottles. Then, fecal cultures were inoculated (5%, vol/vol) with a 24-h culture of B. adolescentis MB 239 grown in SM7 or, as a negative control, with the same volume of sterile SM7. Fecal cultures were incubated at 37°C, and samples were collected for folate analysis at 0 and 48 h.
Folate analysis.
The folate concentration was assayed on cell extracts and culture supernatants. Thirty-milliliter cultures were centrifuged at 13,000 × g for 10 min at 0°C. The supernatant was filtered through a 0.22-μm-pore-size filter. The biomass was washed with 0.05 M K-phosphate buffer, pH 6.5, and the wet pellet was resuspended 1:1 (wt/vol) in the same buffer. Then, 0.5-g glass beads (≤106 μm) (Sigma-Aldrich) were added to a 1-ml suspension, and cells were disrupted at 1,800 rpm for 10 min at 4°C in a vibration homogenizer (MS1; IKA, Wilmington, NC). The cell extract was heated at 100°C for 3 min to release folate from folate binding proteins and to precipitate proteins, and then it was centrifuged (13,000 × g, 15 min, 4°C) and filtered (0.22-μm-pore-size filter).
The folate concentration was analyzed with a microbiological bioassay (7, 12). For the bioassay, Bacto folic acid assay medium (Difco) was used with Enterococcus hirae ATCC 8043 as the test organism, according to the protocol described by the medium manufacturer. The total folate concentration, including polyglutamyl folate, was analyzed after the samples were treated with human plasma (Sigma-Aldrich), as a source of γ-glutamyl hydrolase activity, at 37°C and pH 4.8 for 4 h. Microbiological assay measurements were replicated at least 10 times.
RESULTS
Screening.
Seventy-six strains belonging to the genus Bifidobacterium, of human or animal origin, were screened for the ability to grow in the folate-free synthetic medium SM7, which contained all nutrients for the growth of bifidobacteria except for folic acid. Growth, pH, and folate concentration were evaluated after at least seven subcultures in SM7. All 76 strains grew well in SM7 medium supplemented with 1 μg/liter folic acid (data not shown). In the absence of folate, 59 strains died within three passages and 17 grew abundantly for seven passages or more (Table 1). These 17 strains were all of human origin; 6 belonged to the species B. adolescentis (B. adolescentis DSMZ 20086, MB 114, MB 115, MB 227, and MB 239), 3 belonged to B. breve (B. breve MB 234, MB 235, and MB 622), and 3 belonged to B. pseudocatenulatum (B. pseudocatenulatum MB 116, MB 237, and MB 264). Other single strains that grew in the absence of folate belonged to six different Bifidobacterium species (B. animalis F 200, B. bifidum MB 106, B. catenulatum DSMZ 16992, B. dentium MB 117, B. infantis ATCC 15697, and B. longum MB 214).
TABLE 1.
Species and origins of 76 Bifidobacterium strains screened for the ability to grow in SM7a
| Strain (originb) | OD600 | pH | Folate concnc (ng ml−1) | Strain (originb) | OD600 | pH | Folate concnc (ng ml−1) | |
|---|---|---|---|---|---|---|---|---|
| B. adolescentis ATCC 15703 (h) | — | — | — | B. catenulatum DSMZ 16992 (h) | 0.4 | 5.3 | 3.0 | |
| B. adolescentis DSMZ 20086 (h) | 0.4 | 5.3 | 1.4 | B. cuniculi RA 94 (ra) | — | — | — | |
| B. adolescentis B 752 (h) | — | — | — | B. cuniculi RA 98 (ra) | — | — | — | |
| B. adolescentis B 788 (h) | — | — | — | B. cuniculi RA 99 (ra) | — | — | — | |
| B. adolescentis B 792 (h) | — | — | — | B. dentium MB 117 (h) | 0.5 | 5.3 | 29.0 | |
| B. adolescentis B 841 (h) | — | — | — | B. globosum RU 809 (br) | — | — | — | |
| B. adolescentis MB 114 (h) | 0.7 | 4.9 | 44.0 | B. globosum T 18 (rt) | — | — | — | |
| B. adolescentis MB 115 (h) | 0.8 | 4.8 | 65.0 | B. infantis ATCC 15697 (h) | 1.3 | 4.5 | 27.0 | |
| B. adolescentis MB 227 (h) | 1.0 | 4.7 | 54.0 | B. infantis ATCC 27920 (h) | — | — | — | |
| B. adolescentis MB 239 (h) | 1.5 | 4.4 | 54.0 | B. infantis B 625 (h) | — | — | — | |
| B. animalis DSMZ 20104 (rt) | — | — | — | B. infantis BI USA (h) | — | — | — | |
| B. animalis F 200 (h) | 0.4 | 5.8 | 26.0 | B. infantis MB 256 (h) | — | — | — | |
| B. animalis MB 238 (h) | — | — | — | B. lactis DSMZ 10140 (h) | — | — | — | |
| B. animalis RU 224 (br) | — | — | — | B. longum ATCC 15707 (h) | — | — | — | |
| B. animalis T 160 (rt) | — | — | — | B. longum ATCC 15708 (h) | — | — | — | |
| B. animalis T 27 (rt) | — | — | — | B. longum DSMZ 20097 (h) | — | — | — | |
| B. animalis T 6 (rt) | — | — | — | B. longum B 2055 (h) | — | — | — | |
| B. bifidum DSMZ 200456 (h) | — | — | — | B. longum B 612 (h) | — | — | — | |
| B. bifidum DSMZ 20082 (h) | — | — | — | B. longum B 923 (h) | — | — | — | |
| B. bifidum DSMZ 20239 (h) | — | — | — | B. longum BL USA (h) | — | — | — | |
| B. bifidum MB 106 (h) | 0.6 | 5.1 | 0.6 | B. longum MB 214 (h) | 0.4 | 5.7 | 1.8 | |
| B. bifidum MB 110 (h) | — | — | — | B. longum MB 225 (h) | — | — | — | |
| B. bifidum MB 254 (h) | — | — | — | B. longum MB 226 (h) | — | — | — | |
| B. breve B 2036 (h) | — | — | — | B. longum MB 228 (h) | — | — | — | |
| B. breve B 2409 (h) | — | — | — | B. longum MB 236 (h) | — | — | — | |
| B. breve B 2456 (h) | — | — | — | B. longum MB 246 (h) | — | — | — | |
| B. breve B 622 (h) | 0.6 | 5.0 | 1.3 | B. longum MB 247 (h) | — | — | — | |
| B. breve B 637 (h) | — | — | — | B. longum MB 248 (h) | — | — | — | |
| B. breve B 941 (h) | — | — | — | B. longum MB 249 (h) | — | — | — | |
| B. breve DSMZ 20091 (h) | — | — | — | B. longum subsp. animalis MB 6 (c) | — | — | — | |
| B. breve MB 202 (h) | — | — | — | B. magnum RA 3 (ra) | — | — | — | |
| B. breve MB 233 (h) | — | — | — | B. pseudocatenulatum MB 116 (h) | 1.3 | 4.4 | 82.0 | |
| B. breve MB 234 (h) | 0.4 | 5.3 | 2.5 | B. pseudocatenulatum MB 237 (h) | 0.9 | 4.7 | 41.0 | |
| B. breve MB 235 (h) | 0.4 | 5.2 | 2.3 | B. pseudocatenulatum MB 264 (h) | 1.1 | 4.5 | 12.0 | |
| B. breve MB 251 (h) | — | — | — | B. suis SU 895 (s) | — | — | — | |
| B. breve MB 252 (h) | — | — | — | B. thermophilus ATCC 25866 (br) | — | — | — | |
| B. breve MB 262 (h) | — | — | — | Bifidobacterium sp. strain B 860 (h) | — | — | — | |
| B. breve MB 265 (h) | — | — | — | Bifidobacterium sp. strain F 92 (h) | — | — | — |
Values are means from three experiments (SDs were always less than 0.17 for OD600, 0.15 for pH, and 4.0 ng ml−1 for folate concentration). —, strain died within three passages.
Abbreviations: h, human; rt, rat; ra, rabbit; br, bovine rumen; c, calf; s, swine.
Extracellular concentration.
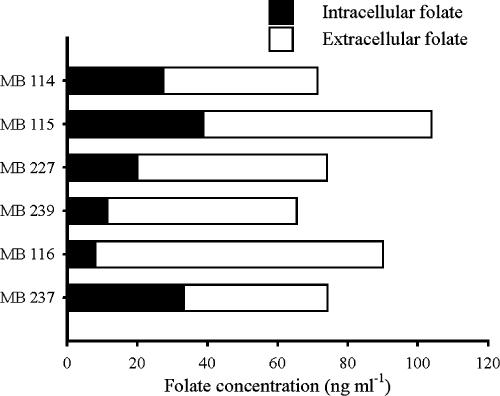
The folate concentration in cell-free supernatants was measured by microbiological assay of 48-h cultures. Folate was found in the supernatants of all 17 strains that grew in SM7. Substantial strain-to-strain differences in concentration, ranging between 0.6 and 82 ng ml−1, were observed. No correlation was observed between the final turbidity and the folate released into SM7 (R2 = 0.43). The intracellular folate concentration was determined for the strains that yielded more than 40 ng ml−1 in the supernatant (B. adolescentis MB 114, MB 115, MB 227, and MB 239; B. pseudocatenulatum MB 116 and MB 237) to determine the total amount of folate synthesized and to establish in which measure the vitamin was accumulated or excreted. Intracellular and extracellular folate concentrations in 48-h Bifidobacterium cultures are reported in Fig. 1. All of the selected strains accumulated folate at a concentration that ranged between 9 and 38% of the total biosynthesized vitamin.
FIG. 1.
Intracellular and extracellular folate concentrations in 48-h cultures of B. adolescentis MB 114, MB 115, MB 227, and MB 239 and B. pseudocatenulatum MB 116 and MB 237. Mean values from three separate experiments are reported; SDs were always less than 3.0 ng ml−1.
Effects of cultivation conditions on productivity.
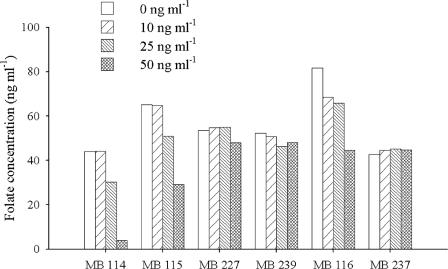
To determine whether the folate yield was affected by the exogenous vitamin concentration, B. adolescentis MB 114, MB 115, MB 227, and MB 239 and B. pseudocatenulatum MB 116 and MB 237 were cultured in SM7 supplemented with 0, 10, 25, or 50 ng ml−1 folate. Folate was measured in the cell-free supernatants of fifth-passage 48-h cultures, and the de novo synthesized folate was calculated as the difference between total and exogenous folate. Figure 2 reports the net folate production in Bifidobacterium strains as a function of the initial vitamin concentration. Folate at 50 ng ml−1 reduced by 91, 55, and 46% the net vitamin production in B. adolescentis MB 114, MB 115, and MB 116. In B. adolescentis MB 227 and MB 239 and B. pseudocatenulatum MB 237, the folate yield was not dependent on the vitamin concentration.
FIG. 2.
Net extracellular folate production in 48-h cultures of B. adolescentis MB 114, MB 115, MB 227, and MB 239 and B. pseudocatenulatum MB 116 and MB 237 in SM7 supplemented with 0, 10, 25, or 50 ng ml−1 folate. Mean values from three separate experiments are reported; SDs were always less than 4.0 ng ml−1.
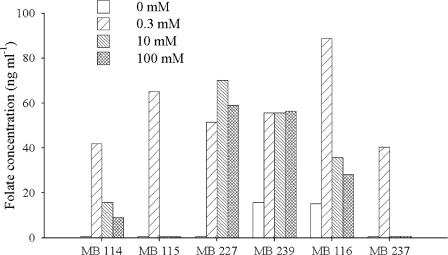
PABA is a precursor of folate biosynthesis. To study the effects of PABA concentration on folate productivity in bifidobacteria, B. adolescentis MB 114, MB 115, MB 227, and MB 239 and B. pseudocatenulatum MB 116 and MB 237 were cultured in SM7 containing 0, 0.3, 10, or 100 μM PABA. For all strains tested, the highest biomass yield was obtained at 0.3 μM PABA, and growth was inhibited at 100 μM PABA. Folate was determined in the cell-free supernatants of seventh-passage cultures (Fig. 3). For each strain, in the absence of PABA the production of folate was strongly reduced or suppressed, though growth was not inhibited. Great variability in folate production was observed among the tested strains with increasing PABA concentrations. The PABA concentration did not affect the final folate concentration in B. adolescentis MB 239, whereas productivity was highest at 0.3 μM PABA and was lower at higher concentrations of PABA in B. adolescentis MB 114, B. adolescentis MB 115, B. pseudocatenulatum MB 116, and B. pseudocatenulatum MB 237. Otherwise, B. breve MB 227 showed the highest productivity at 10 μM PABA.
FIG. 3.
Extracellular folate concentrations in 48-h cultures of B. adolescentis MB 114, MB 115, MB 227, and MB 239 and B. pseudocatenulatum MB 116 and MB 237 in SM7 supplemented with 0, 0.3, 10, or 100 μM PABA. Mean values from three separate experiments are reported; SDs were always less than 3.0 ng ml−1.
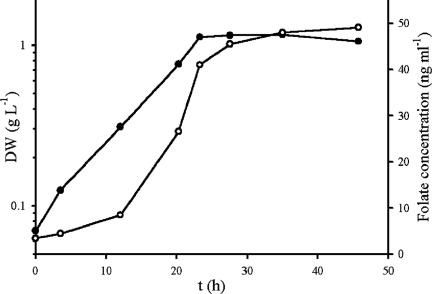
The kinetics of folate production by B. adolescentis MB239 was investigated by means of pH-controlled batch fermentations. Production was growth associated and occurred mostly during the exponential phase (Fig. 4). The effect of pH on folate production was studied in batch experiments with the pH kept constant at 5.7, 6.0, 6.3, 6.6, or 6.9. The folate concentration was measured by microbiological bioassay after 24 h of anaerobic cultivation. Average yields ranged between 49.5 and 53.1 ng ml−1, and no statistically significant differences were observed as a function of the pH (P ≤ 0.05).
FIG. 4.
Batch fermentation of B. adolescentis MB 239 in SM7 with controlled pH (6.5). Symbols: ○, DW, (dry weight); •, extracellular folate concentration.
To study whether folate production in B. adolescentis MB 239 can be affected by the carbon source, chemostat experiments were carried out in SM7 modified by the presence of different carbohydrates as the sole carbon source. Steady-state values of folate/biomass yield coefficients with growth on glucose, fructose, lactose, raffinose and fructo-oligosaccharides were 0.067, 0.064, 0.060, 0.068, and 0.051 mg g−1, respectively. No statistically significant differences were observed (P ≤ 0.05), excluding any relationship between carbon source and folate productivity.
Production of folate by B. adolescentis MB 239 in fecal cultures.
Fecal cultures were inoculated with 24-h cultures of B. adolescentis MB 239 in order to study whether the strain could grow and produce folate in a medium that resembled fecal composition. The folate concentration was measured after inoculation of B. adolescentis MB 239 at 0 and 48 h of anaerobic incubation at 37°C. Uninoculated fecal cultures were used as negative controls. To evaluate the net contribution of B. adolescentis MB 239 to folate concentration, fecal material was pasteurized in order to prevent fecal bacteria from producing or absorbing folate.
Fecal bottles that were not inoculated with B. adolescentis MB 239 showed no change in folate concentration (27.0 ng ml−1 [standard deviation {SD}, 8.2 ng ml−1] and 28.3 ng ml−1 [SD, 11.9 ng ml−1] at 0 and 48 h, respectively), whereas the presence of B. adolescentis MB 239 led to a significant increase in vitamin concentration, from 28.3 ng ml−1 (SD, 11.9 ng ml−1) to 52.8 ng ml−1 (SD, 1.8 ng ml−1).
DISCUSSION
With the aim of developing a probiotic that would provide proliferating colonocytes with folic acid, 76 wild-type Bifidobacterium strains were screened for folate production. The results of this investigation demonstrated that supplementation of folate was necessary for the growth of most of the screened strains. The ability to grow on SM7 and to produce folate was found in 17 human strains belonging to nine different species. Folate production and the extent of vitamin accumulation were not common characteristics of the species but seemed to be traits of single strains. The annotated genome sequence of B. longum NCC2705 reveals the existence of all genes encoding the folate biosynthesis pathway (20). In the present study, 15 strains of B. longum were screened, but only one of them grew without this vitamin. This confirms that the presence of gene sequences does not necessarily ensure that the corresponding enzymes are actually functional. This may be due to the presence of nonactive remnant genes, evolutionary pressures leading to loss of function, inactivating mutations, the lack of transcription/translation, and posttranslational processing. Furthermore, the nutritional requirements of single strains may differ and cannot be assumed to be homogeneous within a species. Nevertheless, the highest folate levels were produced by four strains of B. adolescentis (MB 114, MB 115, MB 227, and MB 239) and two strains of B. pseudocatenulatum (MB 116 and MB 237). To clarify whether the bifidobacteria accumulated folate or excreted it into the medium, the intracellular folate concentrations were determined for these strains. Intracellular folate accumulation was also strongly heterogeneous and strain dependent, as it ranged between 9 and 38% of total vitamin production.
Our study provided results rather different from those of previous studies. Deguchi et al. actually regarded B. adolescentis and B. longum as low-folic-acid producers and B. bifidum and B. infantis as high-folic-acid producers (6). In other studies, the highest folate accumulations in reconstituted skim milk differed after incubation with strains of B. breve and B. infantis (6) or B. longum (15). Such discrepancies may be due both to strain-to-strain differences and to different experimental designs. Folate-free media had never been used in previous studies; in this work, cultures were passaged seven times in folate-free medium to evaluate growth and folate production.
In the human intestinal tract, bifidobacteria are exposed to exogenous folate, the concentration range of which can be rather large depending on vitamin intake and absorption and excretion from urine, skin, and bile (3). To clarify whether exogenous vitamin affected productivity, bifidobacteria were cultivated in the presence of folate in a range between 0 and 50 ng ml−1. Folate production by B. adolescentis MB 114 and MB 115 and B. pseudocatenulatum MB 116 was negatively affected by high exogenous folate concentrations, whereas no feedback effects were observed for B. adolescentis MB 227, B. adolescentis MB 239, or B. pseudocatenulatum MB 237. It is conceivable that folate biosynthesis by these strains is not regulated, considering that the final concentrations were at least 50-fold higher than the requirement of all strains.
GTP and PABA are the building blocks of folate biosynthesis. The former comes from the biosynthesis of purines, while the latter is produced through the shikimate pathway (10, 24). It has already been demonstrated that the addition of PABA at concentrations ranging between 1 and 100 μM results in an increase in folate production by Lactobacillus casei and Lactococcus lactis (23). In this study, bifidobacteria were cultured with different PABA concentrations, in a range between 0 and 100 mM, in order to evaluate the effects on growth and folate yield. For all tested bifidobacteria, the highest biomass yield was obtained at 0.3 mM PABA, while growth was inhibited when the PABA concentration was increased to 100 mM. Furthermore, folate production was generally maximal at 0.3 mM PABA and decreased with increasing PABA concentrations. In contrast to the other strains tested, increased PABA concentrations did not significantly decrease folate biosynthesis by B. adolescentis MB 239.
Folate production by B. adolescentis MB 239 was studied in depth with batch and chemostat experiments. As expected, production was growth associated, in agreement with previous studies (15). Batch fermentations were carried out at different pHs, in a range from 5.7 to 6.9, chosen to resemble the pH range of the colonic environment. No statistically significant differences in folate production were observed as a function of the pH. Hence, the probiotic strain B. adolescentis MB 239 may be effective as a folate producer in the intestinal pH range.
Oligosaccharides are effective energy and carbon sources for the growth of intestinal bifidobacteria (19). Previous studies demonstrated that B. adolescentis MB 239 grows well on the most important prebiotic carbohydrates, including α- and β-galactosides and β-fructosides (1). A comparison of folate production on raffinose, lactose, fructo-oligosaccharides, and their constitutive moieties was established. Folate was produced on all of the carbohydrates tested, and yields were not affected by the carbon sources. Therefore, the contribution of this strain to intestinal folate bioavailability may further increase in symbiotic nutritional supplements comprised of prebiotics.
The contribution of B. adolescentis MB 239 to folate concentration in the colonic environment was simulated in fecal cultures. As the folate concentration increased from 27 to 54 ng ml−1 after a 48-h incubation, the potential efficacy of B. adolescentis MB 239 as a folate producer was demonstrated. This result suggests that in-depth in vivo studies are required, because in our experimental design fecal material was pasteurized in order to prevent folate production and absorption by other fecal bacteria and to evaluate the net contribution of B. adolescentis MB 239.
The observation that the level of folate produced by B. adolescentis MB 239 is not influenced by the exogenous folate or PABA concentrations, pH, or carbohydrate, as well as the folate production observed in fecal cultures, suggests that B. adolescentis MB 239 is an excellent prospect for use as a folate-producing strain in probiotic and symbiotic supplements. The presence in the colon of a such probiotic strain producing 50 ng/ml of folic acid, independently of the external concentration, could continuously and contiguously supply the proliferating colonocytes with this vitamin.
Our study provides new perspectives on the specific uses of probiotics, such as to prevent the localized folate deficiency that is associated with premalignant changes in the colonic epithelia. The oral administration of folate-producing probiotic strains may more efficiently confer protection against inflammation and cancer, both by exerting the beneficial effects of bifidobacteria and by delivering folate to colonic-rectal cells.
Acknowledgments
This study was partially supported by a grant from Anidral/Probiotical Ltd., Novara, Italy.
Footnotes
Published ahead of print on 27 October 2006.
REFERENCES
- 1.Amaretti, A., E. Tamburini, T. Bernardi, A. Pompei, S. Zanoni, G. Vaccari, D. Matteuzzi, and M. Rossi. Substrate preference of Bifidobacterium adolescentis MB 239: compared growth on single and mixed carbohydrates. Appl. Microbiol. Biotechnol., in press. [DOI] [PubMed]
- 2.Biasco, G., U. Zannoni, G. M. Paganelli, R. Santucci, P. Gionchetti, G. Rivolta, R. Miniero, L. Pironi, C. Calabrese, G. Di Febo, and M. Miglioli. 1997. Folic acid supplementation and cell kinetics of rectal mucosa in patients with ulcerative colitis. Cancer Epidemiol. Biomarkers Prev. 6:469-471. [PubMed] [Google Scholar]
- 3.Birn, H. 2006. The kidney in vitamin B12 and folate homeostasis: characterization of receptors for tubular uptake of vitamins and carrier proteins. Am. J. Physiol. Renal Physiol. 291:22-36. [DOI] [PubMed] [Google Scholar]
- 4.Camilo, E., J. Zimmerman, J. B. Mason, B. Golner, R. Russell, J. Selhub, and I. H. Rosenberg. 1996. Folate synthesized by bacteria in the human upper small intestine is assimilated by the host. Gastroenterology 110:991-998. [DOI] [PubMed] [Google Scholar]
- 5.Crittenden, R. G., N. R. Martinez, and M. J. Playne. 2003. Synthesis and utilisation of folate by yoghurt starter cultures and probiotic bacteria. Int. J. Food Microbiol. 80:217-222. [DOI] [PubMed] [Google Scholar]
- 6.Deguchi, Y., T. Morishita, and M. Mutai. 1985. Comparative studies of water soluble vitamins among human species of bifidobacteria. Agric. Biol. Chem. 49:13-19. [Google Scholar]
- 7.Difco Laboratories. 1998. Bacto folic acid assay medium, p. 200-201. In Difco Laboratories (ed.), Difco manual, 11th ed. Becton Dickinson and Company, Sparks, MD.
- 8.Dudeja, P. K., S. A. Torania, and H. M. Said. 1997. Evidence for the existence of a carrier-mediated folate uptake mechanism in human colonic luminal membranes. Am. J. Physiol. 272:G1408-G1415. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs, C. S., W. C. Willett, G. A. Colditz, D. J. Hunter, M. J. Stampfer, F. E. Speizer, and E. L. Giovannucci. 2002. The influence of folate and multivitamin use on the familial risk of colon cancer in women. Cancer Epidemiol. Biomarkers Prev. 11:227-234. [PubMed] [Google Scholar]
- 10.Green, J., B. P. Nichols, and R. G. Matthews. 1996. Folate biosynthesis, reduction, and polyglutamylation, p. 665-673. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 11.Jacob, R. A. 2000. Folate, DNA methylation, and gene expression: factors of nature and nurture. Am. J. Clin. Nutr. 72:903-904. [DOI] [PubMed] [Google Scholar]
- 12.Keagy, P. M., and S. M. Oace. 1989. Rat bioassay of wheat bran folate and effects of intestinal bacteria. J. Nutr. 119:1932-1939. [DOI] [PubMed] [Google Scholar]
- 13.Krause, L. J., C. W. Forsberg, and D. L. O'Connor. 1996. Feeding human milk to rats increases Bifidobacterium in the cecum and colon which correlates with enhanced folate status. J. Nutr. 126:1505-1511. [DOI] [PubMed] [Google Scholar]
- 14.Kumar, C. K., M. P. Moyer, P. K. Dudeja, and H. M. Said. 1997. A protein-tyrosine kinase-regulated, pH-dependent, carrier-mediated uptake system for folate in human normal colonic epithelial cell line NCM460. J. Biol. Chem. 272:6226-6231. [DOI] [PubMed] [Google Scholar]
- 15.Lin, M. Y., and C. M. Young. 2000. Folate levels in cultures of lactic acid bacteria. Int. Dairy J. 10:409-413. [Google Scholar]
- 16.Rossi, M., C. Corradini, A. Amaretti, M. Nicolini, A. Pompei, S. Zanoni, and D. Matteuzzi. 2005. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study in pure and fecal cultures. Appl. Environ. Microbiol. 71:6150-6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rycroft, C. E., M. R. Jones, G. R. Gibson, and R. A. Rastall. 2001. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J. Appl. Microbiol. 91:878-887. [DOI] [PubMed] [Google Scholar]
- 18.Said, H. M., and Z. M. Mohammed. 2006. Intestinal absorption of water-soluble vitamins: an update. Curr. Opin. Gastroenterol. 22:140-146. [DOI] [PubMed] [Google Scholar]
- 19.Salminen, S., C. Bouley, M. C. Boutron-Ruault, J. H. Cummings, A. Franck, G. R. Gibson, E. Isolauri, M. C. Moreau, M. Roberfroid, and I. Rowland. 1998. Functional food science and gastrointestinal physiology and function. Br. J. Nutr. 80:S147-S171. [DOI] [PubMed] [Google Scholar]
- 20.Schell, M. A., M. Karmirantzou, B. Snel, D. Vilanova, B. Berger, G. Pessi, M. C. Zwahlen, F. Desiere, P. Bork, M. Delley, R. D. Pridmore, and F. Arigoni. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 99:14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sellers, T. A., L. H. Kushi, J. R. Cerhan, R. A. Vierkant, S. M. Gapstur, C. M. Vachon, J. E. Olson, T. M. Therneau, and A. R. Folsom. 2001. Dietary folate intake, alcohol, and risk of breast cancer in a prospective study of postmenopausal women. Epidemiology 12:420-428. [DOI] [PubMed] [Google Scholar]
- 22.Siezen, R. J., B. Renckens, I. van Swam, S. Peters, R. van Kranenburg, M. Kleerebezem, and W. M. de Vos. 2005. Complete sequences of four plasmids of Lactococcus lactis subsp. cremoris SK11 reveal extensive adaptation to the dairy environment. Appl. Environ. Microbiol. 71:8371-8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sybesma, W., M. Starrenburg, L. Tijsseling, M. H. N. Hoefnagel, and J. Hugenholtz. 2003. Effects of cultivation conditions on folate production by lactic acid bacteria. Appl. Environ. Microbiol. 69:4542-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sybesma, W., M. Starrenburg, M. Kleerebezem, I. Mierau, W. M. de Vos, and J. Hugenholtz. 2003. Increased production of folate by metabolic engineering of Lactococcus lactis. Appl. Environ. Microbiol. 69:3069-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sybesma, W., E. van den Born, M. Starrenburg, I. Mierau, M. Kleerebezem, W. M. de Vos, and J. Hugenholtz. 2003. Controlled modulation of folate polyglutamyl tail length by metabolic engineering of Lactococcus lactis. Appl. Environ. Microbiol. 69:7101-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tannock, G. V. 1999. Probiotics: a critical review. Horizon Scientific Press, Norfolk, United Kingdom.
- 27.Tannock, G. V. 2002. Probiotics and prebiotics: where are we going? Caister Academic Press, Norfolk, United Kingdom.
- 28.Terry, P., M. Jain, A. B. Miller, G. R. Howe, and T. E. Rohan. 2002. Dietary intake of folic acid and colorectal cancer risk in a cohort of women. Int. J. Cancer 97:864-867. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman, J. 1990. Folic acid transport in organ-cultured mucosa of human intestine: evidence for distinct carriers. Gastroenterology 99:964-972. [DOI] [PubMed] [Google Scholar]