Abstract
In Escherichia coli, at least 12 proteins, FtsZ, ZipA, FtsA, FtsE/X, FtsK, FtsQ, FtsL, FtsB, FtsW, FtsI, FtsN, and AmiC, are known to localize to the septal ring in an interdependent and sequential pathway to coordinate the septum formation at the midcell. The FtsEX complex is the latest recruit of this pathway, and unlike other division proteins, it is shown to be essential only on low-salt media. In this study, it is shown that ftsEX null mutations are not only salt remedial but also osmoremedial, which suggests that FtsEX may not be involved in salt transport as previously thought. Increased coexpression of cell division proteins FtsQ-FtsA-FtsZ or FtsN alone restored the growth defects of ftsEX mutants. ftsEX deletion exacerbated the defects of most of the mutants affected in Z ring localization and septal assembly; however, the ftsZ84 allele was a weak suppressor of ftsEX. The viability of ftsEX mutants in high-osmolarity conditions was shown to be dependent on the presence of a periplasmic protein, SufI, a substrate of twin-arginine translocase. In addition, SufI in multiple copies could substitute for the functions of FtsEX. Taken together, these results suggest that FtsE and FtsX are absolutely required for the process of cell division in conditions of low osmotic strength for the stability of the septal ring assembly and that, during high-osmolarity conditions, the FtsEX and SufI functions are redundant for this essential process.
Cell division is an essential and complex process in all organisms that involves the partitioning of the cytoplasm into two daughter cells, each containing a copy of the cell's genetic information. In Escherichia coli, it is known to require the coordinated assembly of at least 12 proteins at the division site forming a multiprotein complex variously called a septalsome or divisome or septal ring (1, 12, 17, 34, 52). The divisome assembly is initiated by the localization of FtsZ at the site of division, followed by the formation of a circumferential ring of FtsZ (Z ring) around the inner surface of the cytoplasmic membrane (reviewed in reference 29). The division site selection is achieved by two overlapping mechanisms: the inhibition of Z ring assembly at the vicinity of the chromosome by nucleoid occlusion protein SlmA and at cell poles by the concerted action of a protein complex, MinCDE (4, 10). The FtsZ ring is stabilized by two other essential division proteins, FtsA and ZipA. Once it is established, the proteins FtsE/X, FtsK, FtsQ, FtsL, FtsB, FtsW, FtsI, FtsN, and AmiC are recruited more or less in a linear fashion to the division site and coordinate the formation of septum leading to the generation of two daughter cells. Of these, FtsQ, FtsL, and FtsB are known to assemble into a complex that is considered to connect the Z ring scaffold (comprising FtsZ-FtsA-ZipA-ZapA) to the septal peptidoglycan synthesis machinery that consists of FtsW and FtsI (7, 49). FtsN is an essential division protein of unknown function identified as a multicopy suppressor of ftsA12 (9). AmiC and EnvC are septal murein hydrolases (2, 3) that facilitate the separation of daughter cells but are not essential for growth, as E. coli has multiple redundant murein hydrolases (18). FtsK is a bifunctional protein that is thought to connect the process of cell division to DNA segregation; its amino-terminal domain is involved in the essential division process, whereas the carboxy-terminal domain, which is dispensable for viability, facilitates chromosome partitioning (28). Most of these division proteins listed above are essential for cell viability. However, a gain-of-function mutation in FtsA (FtsA R286W) can completely bypass the requirement for ZipA or FtsK, suggesting the existence of functional redundancy between various cell division proteins (13, 14). Nevertheless, the biochemical functions of many of these proteins are not known.
The most recently identified component of the septal ring is the predicted ABC transporter complex FtsEX (44), encoded by the second and third genes of the ftsYEX operon located at 77 min on the chromosome of E. coli (16). It has been shown that the recruitment of FtsX to the cell septum is dependent on the presence of early division proteins FtsZ, FtsA, and ZipA, whereas the later proteins FtsK, FtsQ, FtsL, and FtsI are in turn dependent on functional FtsEX for their localizations (44). The results of a bacterial two-hybrid analysis for membrane protein interactions have also shown that FtsX interacts with two other components of the divisome, FtsA and FtsQ (22). Mutations in ftsE were earlier isolated as “filamentation temperature sensitive” (fts), and hence, the locus was implicated in cell division (42).
Nevertheless, the classification of ftsE as a cell division gene has been questioned, as the mutants did not show extensive filamentation in minimal medium even at the restrictive temperature (46). An insertion mutant of ftsE is shown to be filamentous and requiring of high concentrations of salt for its viability. Furthermore, FtsE and FtsX are shown to interact and form a putative ABC-type transporter in the cytoplasmic membrane (11). Therefore, ftsE was considered a conditional salt-dependent essential gene and was implicated in salt transport (11). ftsE(Ts) mutants are also shown to be defective in translocating potassium pump proteins into the cytoplasmic membrane at the restrictive temperature, leading to the proposal that FtsE could participate in protein translocation (47). Interestingly, mutations in either ftsE or ftsX exhibit RecA- and LexA-dependent constitutive SOS induction that is indicative of endogenous DNA damage (35).
As the functions of FtsE and FtsX are not well understood, a genetic analysis of suppressors and of synthetic lethal partners of ftsEX was undertaken in this study. The results provide compelling evidence for the involvement of the FtsEX complex in cell division and also for its functional interactions with other division proteins. In addition, it is shown that the viability of ftsEX mutants is contingent on the presence of a periplasmic protein, SufI, that is implicated in cell division, suggesting the existence of functionally redundant or overlapping pathways in the process of cell septation. In light of these results, the possibility of the divisome assembly being intrinsically sensitive to conditions of low osmotic strength and/or high temperature is discussed.
MATERIALS AND METHODS
Bacterial strains and phages.
All strains used in this study are derivatives of E. coli K-12 and are listed in Table 1. Strain MR2 (MG1655ΔlacI) was used as the wild-type strain. The construction of deletion-insertion mutants of ftsEX or sufI is described below. Null mutations in slmA and minCDE (obtained from Piet de Boer) and other division-defective mutations, ftsZ84, ftsA12, ftsK44, ftsQ1, or ftsI23 (from Jon Beckwith's laboratory), were introduced by P1 phage-mediated transduction into various strain backgrounds with the aid of linked antibiotic markers from strains TB85, TB14, DRC14, EC290, TOE44, JOE86, and LMG64, respectively. Phage P1kc was from laboratory stock. The ordered λ phage library of the E. coli genome was obtained from K. Isono (25).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmida | Relevant genotype or features | Source or reference |
|---|---|---|
| Strains | ||
| MG1655 | Wild-type | CGSCb |
| HME63 | W3110 (λcI857Δcro-bioA) mutS::Amp | D. L. Court |
| JC7623 | recB21 recC22 sbcB15 sbcC201 | CGSC |
| JH39 | sfiA11 thr-1 leu-6 hisG4 argE3 ilv(Ts) galK2 srl (?) rpsL31ΔlacU169 dinD1::MudI (Amprlac) | K. Kreuzer (35) |
| MR2 | MG1655 ΔlacI | This study |
| MR10 | MR2 ΔftsEX210::Kan | This study |
| MR11 | MR10/pMN6 | This study |
| MR12 | MR2 ftsE211::Tn10dCm | This study |
| MR14 | MR12/pMN6 | This study |
| MR19 | MR12/pMN2 | This study |
| MR20 | MR19 tatB::Tn10dKan | This study |
| MR21 | MR2 ΔsufI21::Kan | This study |
| Plasmids | ||
| pACYC184 | p15A-based, Cmr, Tetr | Lab collection |
| pAM34 | pMB1-based, IPTG-dependent replicon, Ampr, Spcr | 15 |
| pCL1920 | pSC101-based; Spcr | 27 |
| pKRP11 | ColEI-based; source of a portable Kanr cassette flanked by multiple cloning sites | 41 |
| pMA2 | pSC101(Ts), Ampr | Japanese cloning vector collection |
| pUC4K | ColE1-based; source of the Kanr cassette | Lab collection |
| pALS10 | pBR322-relA | 45 |
| pALS13 | pBR322-relA′ | 45 |
| pJK537 | pBR322-dksA | 21 |
| pMN2 | pMA2-ftsYEX | This study |
| pMN4 | pCL1920-ftsYEX | This study |
| pMN6 | pAM34-ftsYEX | This study |
| pMN8 | pCL1920-ftsQAZ | This study |
| pMN9 | pCL1920-ftsQA | This study |
| pMN10 | pCL1920-ftsQ | This study |
| pMN11 | pCL1920-ftsA | This study |
| pMN12 | pCL1920-ftsAZ | This study |
| pMN14 | pCL1920-ftsN | This study |
| pMN15 | pCL1920-sdiA | This study |
| pMN16 | pCL1920-sufI | This study |
| pMN17 | pACYC184-relA′ | This study |
| pMN18 | pCL1920 with a 12-kb PstI fragment from λ505 Kohara phage encompassing the sufI gene | This study |
All strains are derivatives of E. coli K-12 and are F−. The mutant allele numbers for ftsEX or sufI are given according to the registry at the CGSC. Strain MR2 was constructed by transducing a deletion of lacI linked to zai-911::Tn10dCm from strain GJ2422-S42 (6) into MG1655 lacZ4525::Tn10dKan (6) and selecting for Lac+ transductants, followed by screening for LacI−, Cms, and Kans colonies.
CGSC, Coli Genetic Stock Center.
Growth media and conditions.
Unless otherwise indicated, Luria-Bertani (LB) medium (that contains 1% NaCl) was used (32), and the growth temperature was 30°C. LBON medium is the same as LB but without NaCl (5). The supplementation of osmolytes to LBON (i.e., glucose, sucrose, glycerol, or NaCl) was at 0.4 M. The following antibiotics were used at the indicated concentrations: ampicillin (Amp), 50 μg/ml; kanamycin (Kan), 50 μg/ml (in LB) and 10 μg/ml (in LBON); tetracycline (Tet), 15 μg/ml; chloramphenicol (Cm), 30 μg/ml; and spectinomycin (Spc), 50 μg/ml. Isopropyl-β-d-thiogalactopyranoside (IPTG) and 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) were used at 0.5 mM and 25 μg/ml, respectively.
Construction of a chromosomal ftsEX deletion-insertion mutant.
A complete deletion of the ftsEX locus was made on the chromosome by recombineering as described previously (53). A pair of primers having homologies at the 5′ end to the flanking region of the ftsEX locus and at 3′ end to the sequences of the kanamycin resistance gene cassette (rpoH-Kan, 5′-CCCTGCTACGGAACCCATTGCAGGGAAAGAGTATAACACGCTTTTATTAGAAAAACTCATCGAG CATCAAATG-3′, and ftsY-Kan, 5′-AGGCGGACGACTTTATAGAGGCACTTTTTGCCCGAGAGGATTAACATTGTGTCTCAAAATCTCTGATG-3′, where italic type indicates the sequences that are homologous to the kanamycin resistance gene of plasmid pUC4K) were employed to amplify the 0.9-kb Kanr cassette using the plasmid pUC4K as the template. The purified linear PCR product with the flanking homologous sequences of ftsEX locus was introduced by electroporation into strain HME63 with or without a plasmid (pMN4) carrying a cloned ftsYEX region. The Kanr transformants were obtained (at 30°C) in both of the strains at equal frequencies (though the Kanr colonies in the strain without the plasmid pMN4 were slow growing and tiny), indicating that this mutant is viable on LB plates at 30°C. The putative ftsEX::Kan deletion-substitution was transferred into a fresh background by P1 transduction. Subsequently, the presence of the deletion was confirmed by PCR amplification using flanking primers (for ftsEXFP, 5′-GGAATTCTTTTTGCCCGAGAGGATTAAC-3′, and for ftsEXRP, 5′-GGAATTCAGACCGTGATTTTATCCAC-3′) and sequencing the junctions of the deletion-insertion. This ftsEX deletion precisely removes the entire intervening sequence from the start codon of ftsE to the stop codon of ftsX and does not appear to affect the expression of either the upstream gene ftsY or the downstream rpoH gene.
Construction of a chromosomal deletion of sufI.
A 1.1-kb KpnI internal fragment that spans the SufI open reading frame was excised from plasmid pMN18, and in its stead, a 1.0-kb KpnI fragment (from plasmid pKRP11) containing the Kanr gene cassette was ligated. The resulting plasmid, pMN19, thus carries an insertion of Kanr and a deletion of an almost complete sufI gene. The fragment carrying the ΔsufI::Kan mutation was recombined into the chromosome of the recBC strain JC7623 by linear transformation. A P1 lysate prepared on one Kanr transformant was used to transduce strain MR2, and the presence of the deletion was confirmed by sequence analysis using flanking primers of the sufI gene.
Identification of synthetic lethal partners of ftsEX using a conditional lethal strategy.
Strain MR19 (MG1655 ΔlacI ftsE211::Tn10dCm/pMN2) was subjected to random insertion mutagenesis with transposon Tn10dKan following infection with phage λ1316, as described previously (32). Plasmid pMN2 is a derivative of pMA2 (that has a temperature-sensitive pSC101 replicon and the gene encoding the LacI repressor) with a cloned ftsYEX operon. Therefore, MR19 is ftsE+ and lacI+ at 30°C, whereas at 42°C, it lacks ftsE and lacI. Consequently, this strain is white at 30°C and blue at 42°C on X-Gal-containing plates and when the strain is grown in the absence of selection at the semipermissive temperature (37°C), the plasmid pMN2 will be rapidly lost from the cells so that the colonies appear like mosaics with white and blue sectors. On the other hand, if any Kanr transposon insertion mutation causes inviability in this background, then that particular colony would remain white as the blue plasmid-cured cells fail to grow. The Kanr transposon-mutagenized library was therefore screened for colonies that were white on LB-X-Gal plates at 37°C. One such mutant clone was designated MR20. The precise location of the transposon in MR20 was identified by cloning the Kanr insertion along with flanking sequences onto the plasmid vector pCL1920, followed by sequencing the gene junctions with the outwardly directed Kan primers 5′-AGCATTACGCTGACTTGACGG-3′ and 5′-GCAATGTAACATCAGAGATT-3′.
Screening for multicopy plasmids that suppress phenotypes of ftsEX mutants.
A multicopy plasmid library carrying overlapping E. coli genomic DNA fragments cloned at the BamHI site in a p15A-based plasmid, pACYC184, was obtained from M. Radman's laboratory. The plasmid pool was introduced into strain MR10, and transformants were plated on LBON-Cm plates at 30°C. Plasmids were isolated from colonies that grew to different extents on these plates, and their abilities to suppress were reconfirmed after an additional round of transformation. Subsequent restriction analysis and sequencing (with the flanking vector primers 184TetA [5′CGCCGAAACAAGCGCTCATGAGCC-3′] and 184TetB [5′CTATGCGCACCCGTTCTCGGAGCAC-3′]) yielded various classes of plasmids that restored the viability of ftsEX on LBON to different extents. The putative candidate genes from the primary suppressor clones (after eliminating the plasmids carrying ftsEX genes) were further subcloned, and these constructions are described below.
Plasmids.
The fragment carrying the wild-type ftsYEX region was cloned from Kohara phage λ612 as a 4.5-kb HindIII fragment into a pSC101-based Spcr plasmid pCL1920 (27) to construct plasmid pMN4. This fragment was also subcloned into an Ampr, pSC101-based, temperature-sensitive plasmid, pMA2, and a pMB1-based, conditional IPTG-dependent, Ampr plasmid, pAM34 (15) to yield pMN2 and pMN6, respectively. The complete ftsQ-ftsA-ftsZ region was subcloned from Kohara phage λ111 as a 5-kb PstI fragment into pCL1920 to create pMN8. A 2.9-kb internal NsiI fragment of pMN8 carrying ftsQ and ftsA was cloned at the PstI site of pCL1920 to make pMN9. Plasmid pMN10 with only ftsQ was obtained by digesting pMN8 with HindIII and religation. The plasmid, pMN11 was generated by BamHI digestion and self-ligation of pMN9, so as to retain only ftsA. The plasmid with the ftsAZ region (pMN12) was made by cloning a 4-kb BamHI fragment from pMN8 at the same site of pCL1920. However, all the plasmid clones obtained had the insert in opposite orientation to that of lac promoter of the vector and the expression of ftsA and ftsZ was poor as seen by the partial complementation of the cognate mutants. All of the other plasmid clones (pMN8 to pMN11) efficiently complemented the thermosensitivity of the corresponding mutant strains.
The complete ftsN gene was cloned between PstI and BamHI sites of pCL1920 as a 1.4-kb NsiI-BclI fragment to create pMN14. The 1.2-kb XmaI-PstI fragment-containing sdiA gene, along with its promoter, was subcloned at the cognate sites in pCL1920 to generate pMN15. The sufI gene was cloned as a 1.7-kb SacI fragment from Kohara phage λ505 at the same site in pCL1920 to generate pMN16. Plasmid pMN17 is a pACYC184 derivative encoding the N-terminal 411 amino acids of the relA gene product. Plasmids pALS10 and pALS13 carrying relA were obtained from M. Cashel (45). Plasmid pJK537, a pBR322 derivative carrying the dksA gene, was obtained from E. Craig via M. Cashel (21).
Molecular and genetic techniques.
Standard protocols were followed for experiments involving recombinant DNA and plasmid manipulations (43). Transpositions, transductions, P1 phage preparations and β-galactosidase assays were performed using standard methods as described previously (32).
Growth and viability measurements.
The viability of each strain was measured by applying 10-μl aliquots of various dilutions (10−2, 10−4, 10−5, 10−6, and 10−7) of overnight cultures onto appropriate plates and incubating them, generally for 20 to 36 h. The relative plating efficiency and growth values were determined for each strain based on a comparison with its control strain on the same plate.
Microscopy.
The strains to be examined by microscopy were diluted into the appropriate medium from fresh overnight cultures and were processed after 6 to 8 h of growth. They were fixed in 2% formaldehyde for 1 h at 37°C, washed twice with phosphate-buffered saline, and suspended in the same buffer containing 50% glycerol. For the DAPI (4,6-diamidino-2-phenylindole dihydrochloride) staining of nucleoids, fixed cells were treated with 0.25 μg/ml DAPI for 15 min at room temperature and mounted on slides. Differential interference contrast (DIC) and fluorescence images were taken using a Zeiss Axioplan fluorescence microscope and processed in Adobe Photoshop.
RESULTS
Construction and characterization of ΔftsEX mutant strain.
In order to study the phenotypes of the ftsEX mutant strain, a complete deletion of the ftsEX region was made on the chromosome of strain MR2 (MG1655 ΔlacI) as described in Materials and Methods. This strain, MR10 (MR2 ΔftsEX210::Kan), grew well on LB medium (with 1% NaCl) forming healthy colonies at 30°C, whereas at 37°C or 42°C, the colonies were very tiny and sick (Fig. 1A). Similarly, it exhibited mild elongation of cells at 30°C, which became very extensive at higher temperatures (Fig. 1B). However, this mutant did not grow on LB without NaCl (LBON) at any temperature, possibly due to excessive lethal filamentation (Fig. 2A, B, and C). Supplementation with any of a variety of osmolytes, such as glucose, sucrose, glycerol, or NaCl, completely rescued the growth of MR10 on LBON medium (Fig. 2A, B, and C). Though the growth of strain MR10 was completely inhibited on LBON plates (Fig. 2A), the cultures grown in LBON broth showed an initial increase in cell mass, as seen by the slow and gradual increase in optical density (optical density at 600 nm of ∼1) before the cessation of growth (Fig. 2B), and at this stage, the culture was extremely filamentous (Fig. 2C). The osmotic requirement did not appear to cause cell death in these mutants, as the addition of the osmolytes restored growth to an inhibited late-stage culture in LBON (data not shown). The filaments of ftsEX mutant grown in LB at 42°C or in LBON at 30°C were very long and appeared to have elongated cells (Fig. 1B and 2C). The visualization of the nucleoids by DAPI staining, followed by fluorescence microscopy, revealed that they are normal and regularly spaced without any significant chromosomal aberrations or partition defects (Fig. 2D).
FIG. 1.
Growth and filamentation of ftsEX mutants. (A) Growth of the wild type (wt) and of ftsEX mutants on LB at different temperatures. (B) DIC micrographs of cells grown in LB broth at different temperatures.
FIG. 2.
Growth and filamentation of ftsEX mutants. (A) Growth of the wild type (wt) and ftsEX mutants on LBON plates supplemented with 0.4 M osmolytes. (B) Growth of MR10 (ΔftsEX210::Kan) in LBON broth (diamonds), LBON supplemented with either 0.4 M glucose (closed circles) or 0.4 M NaCl (open circles) at 30°C. OD600, optical density at 600 nm. (C) DIC micrographs of cells grown in LB without NaCl or with added osmolytes at 30°C. (D) DIC micrograph of a single filament of the ftsEX mutant and the corresponding fluorescence image after DAPI staining.
These data clearly indicate that deletion mutations of ftsEX are suppressed not only by salts but also by any agents that increase the osmolarity of the medium and, therefore, that ftsE and ftsX should be considered conditional osmoremedial essential genes. Single mutants of either ftsE or ftsX generated by transposon insertion mutagenesis (to be described elsewhere) also exhibited characteristics similar to that of an ftsEX deletion mutant (data not shown). As ftsE and ftsX are the second and third genes, respectively, in the ftsYEX operon, insertions in ftsE can be polar on the downstream ftsX gene. However, a plasmid carrying ftsX alone (which is normally capable of complementing an ftsX mutant) did not confer growth to an ftsE insertion mutant on LBON, indicating that both ftsE and ftsX are required for growth on medium with low osmolarity (data not shown).
Identification of multicopy suppressors of ftsEX.
To understand the basis of ftsEX phenotypes, multicopy suppressor plasmids that restored viability to these mutants on low-osmolarity medium were identified (see Materials and Methods). Briefly, strain MR10 was transformed with a multicopy plasmid library, plasmids that conferred growth on LBON were isolated, and the candidate genes responsible for the growth rescue were identified. The extent of suppression by each of these plasmids was rather variable (Fig. 3A and B). Some of the candidate genes that suppressed encode (i) SdiA, a transcriptional activator of the ftsQ-ftsA-ftsZ operon (51); (ii) RelA, a 411-amino-acid, N-terminal fragment of ppGpp synthetase I (8); and (iii) DksA, a potentiator of ppGpp response identified earlier as a multicopy suppressor of dnaK null mutations (21, 36). The suppression shown by plasmids encoding SdiA or DksA was fairly efficient (Fig. 3A) compared to the suppression with RelA plasmid, which was extremely weak (data not shown). The plasmids pALS10 or pALS13, encoding the full length or N-terminal 455 amino acids of the relA product, respectively (45) also partially suppressed the ftsEX phenotypes (data not shown). As all of these factors are implicated in the positive regulation of the expression of the ftsQ-ftsA-ftsZ (ftsQAZ) operon either directly or indirectly (20, 39), the effect of ftsQAZ overexpression on the viability of ftsEX was examined.
FIG. 3.
Effect of multicopy suppressor plasmids on growth of ftsEX. (A) Cultures of ftsEX carrying vector pCL1920 or its derivatives with cloned ftsYEX, ftsN, ftsQAZ, and sdiA genes or plasmid pJK537 (pBR322 with dksA) are grown at 30°C in LB, and 10-μl aliquots of various dilutions (10−2, 10−4, 10−5, 10−6, and 10−7) are applied on LBON plates and grown for 32 h at 30°C. (B) DIC micrographs of the same cells grown in LBON to midexponential phase at 30°C.
Suppression of ftsEX by plasmids carrying ftsQAZ.
The region carrying ftsQAZ was subcloned from λ111 of the Kohara phage library onto plasmid vector pCL1920 to create pMN8. This plasmid abolished the growth defects of ftsEX and made these mutant cells less filamentous but not completely normal in LBON at 30°C (Fig. 3A and B). To identify the division component(s) responsible for the suppression, the QAZ region was further subcloned. A plasmid carrying ftsQ (pMN10) alone did not suppress the defect, whereas plasmids carrying ftsQA (pMN9) or ftsA (pMN11) could not be introduced into the ΔftsEX mutant. A plasmid carrying the ftsAZ region (pMN12) improved the growth of ftsEX on LB at high temperatures marginally but did not support growth on LBON. This could possibly be due to the poor transcription (as this fragment lacks any promoter) of these two genes. Consistent with this possibility, plasmid pMN12 showed weak complementation of ftsA12 and ftsZ84 mutants. The plasmid carrying only ftsZ could not be constructed in this vector, even after repeated attempts. All of these results indicated that the increased coexpression of divisome components FtsQ, FtsA, and FtsZ eliminates the need for FtsEX on medium of low osmolarity.
Multicopy FtsN restores viability to ftsEX.
The screen for the identification of multicopy suppressors yielded a class of plasmids that suppressed the inviability of ftsEX on LBON very effectively. All of these clones had a complete FtsN open reading frame along with some adjoining gene fragments. A plasmid construct of pCL1920 carrying only the subcloned ftsN (pMN14) gene efficiently suppressed the growth defects and filamentation (Fig. 3A and B), demonstrating that FtsN in multicopy can suppress the division defects of ftsEX in addition to partially suppressing the thermosensitivity of other division-defective mutations in ftsA, ftsQ, and ftsI (9). Though all of the above-mentioned multicopy plasmids rescued the growth of ftsEX mutants on LBON at 30°C to variable extents, only pMN14 was effective in restoring viability on LBON at 42°C (data not shown).
ftsEX exacerbates the defects of most division mutants.
The effect of ftsEX deletion on the growth of other temperature-sensitive cell division mutants was examined. Mutations in division genes, such as ftsZ84, ftsA12, ftsK44, ftsQ1, or ftsI23, were introduced into strain MR11 (ΔftsEX210::Kan/pMN6) by phage P1-mediated transductions and selected for the linked leuB::Tn10 antibiotic marker. Plasmid pMN6 is a pAM34 derivative (with an IPTG-dependent replicon) (Table 1) carrying a cloned ftsYEX operon. Hence, with IPTG supplementation, strain MR11 is ftsEX+ and, without IPTG, it lacks ftsEX. The cultures of the double-mutant strains carrying pMN6 were grown with IPTG supplementation, and viability measurements were taken at 30°C (Fig. 4A). On plates without IPTG, the ftsEX single mutant grew well, whereas the double mutants carrying ftsA12, ftsK44, ftsQ1, and ftsI23 alleles showed reduced viability. On the other hand, all of these strains grew well on IPTG-supplemented plates, showing that the single temperature-sensitive division mutants had no growth defects on such plates. Increasing the osmolarity of the medium by the addition of either glucose or NaCl relieved growth inhibition completely in the case of ftsA, ftsK, or ftsQ mutants, but not of ftsI23 mutants, because the growth of the ftsI23 single mutant itself was significantly inhibited on these plates. It was observed during the course of this study that, as opposed to all of the other temperature-sensitive division mutants (such as ftsZ84, ftsA12, ftsQ1, and ftsK44 mutants), ftsI23 mutants grew well on medium lacking NaCl (LBON) at all temperatures and they were extremely sensitive to high osmolarity, even at a permissive temperature (data not shown).
FIG. 4.
Effect of ftsEX deletion on division-defective mutations. (A) Growth of strain MR11 (ΔftsEX210::Kan/pMN6) and its mutant derivatives on LB-Amp-IPTG, LB, or LB supplemented with 0.4 M glucose at 30°C. (B) Growth of ftsEX and ftsEX ftsZ84 mutants. Cultures are grown in LB at 30°C, and 10 μl of various dilutions are spotted on LBON (the plate incubated at 37°C has 10−2, 10−4, and 10−5 dilutions) or LB plates, followed by incubation at 30°C or 37°C for 32 h. (C) DIC micrographs of the cultures grown in LB at 37°C.
However, ftsZ84 ftsEX double mutants were not inhibited on LB plates without IPTG (Fig. 4A); in fact, the ftsZ84 mutation improved the growth of the ftsEX deletion mutant on LB at 37°C (Fig. 4B) or 42°C (data not shown). When these cultures were plated on LBON at 30°C, the ftsEX single mutants grew very poorly with several fast-growing suppressors, whereas the ftsEX ftsZ84 double mutant formed small but discrete colonies (Fig. 4B). These double mutants also showed somewhat reduced filamentation (Fig. 4C), indicating that ftsZ84 mutation is a weak suppressor of ftsEX.
In addition, mutations in ftsE conferred extreme sickness to strains carrying a deletion of the slmA gene that codes for nucleoid occlusion protein and also to a mutant deleted for minCDE. Again, the growth defects of the double mutants were suppressed by elevating the medium osmolarity (Fig. 4A). All of the above inviabilities were also abrogated by increased levels of FtsQAZ or FtsN (data not shown). In summary, the absence of ftsEX is detrimental to the mutants that are already compromised for cell division (except the ftsZ84 mutant) and increasing the medium osmolarity or the amount of other division proteins abolishes the requirement of FtsEX.
Identification of mutations that are synthetically lethal with ftsE.
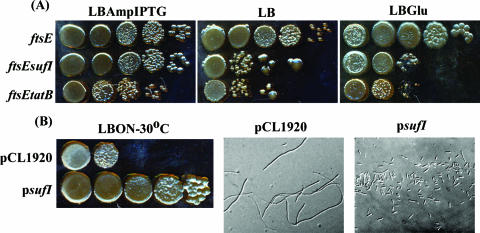
The viability of ftsEX mutants on high-osmolarity medium suggested the possible presence of another division protein with an overlapping function. Therefore, to identify the gene products that are essential for the growth of ftsE mutants on LB plates, a genetic screen for the isolation of synthetic lethal mutations of ftsE was employed as described in Materials and Methods. One Kanr insertion mutation that conferred inviability to the ftsE mutant was identified using this screen. The insertion was found to disrupt the 171-amino-acid-encoding tatB gene after codon 144; thus, it most likely affected the formation of functional TatABC translocase. The tatB::Tn10dKan mutation was transferred to strain MR14 (ftsE211::Tn10dCm/pMN6), and the growth characteristics of the double mutant were compared on medium with or without IPTG. As shown in Fig. 5A, all of the strains grew well on IPTG-supplemented plates, whereas on plates without IPTG, the double mutants did not grow.
FIG. 5.
Interactions of ftsE with sufI. (A) Synthetic lethal mutants of ftsE. Growth of ftsE sufI or ftsE tatB mutants carrying plasmid pMN6. Cultures were grown with IPTG and dilutions were spotted on LB-Amp-IPTG, LB, or LB-glucose plates. (B) Effect of multicopy sufI on the growth and filamentation of ftsEX mutants.
SufI is essential for viability of ftsEX mutants.
The Tat (twin-arginine transport) pathway is a sec-independent translocase that exports prefolded or assembled proteins from the cytoplasm into the periplasm across the inner membrane (reviewed in reference 26). One of its well-studied substrates is a 470-amino-acid periplasmic protein, SufI, that is implicated in cell division, as multiple copies of sufI (suppressor of ftsI), are known to suppress the thermosensitivity of the ftsI23 mutant (23). Therefore, it is predicted that the synthetic lethality of tatB with ftsEX could be due to the inability of SufI to be exported into the periplasm. Hence, a chromosomal deletion-insertion of sufI was made (as described in Materials and Methods) and introduced into strain MR14. Since this double-mutant strain carries the plasmid pMN6, with IPTG supplementation, it is ftsEX+, and without IPTG, it lacks ftsE. As shown in Fig. 5A, this mutant grew well on LB plates containing IPTG, indicating that, in these conditions, the sufI deletion mutant has no obvious growth defects. However, this strain did not grow at all on LB plates without IPTG (Fig. 5A), showing that ftsE sufI double mutants are not viable. The inviability of these double mutants was not restored by either the addition of osmolytes (Fig. 5A) or the increased expression of ftsQAZ or ftsN (data not shown), indicating that the presence of either FtsEX or SufI is absolutely essential for growth.
Division defects of ftsEX mutants are abolished by multicopy SufI.
The reduced viability of sufI ftsE double mutants suggested that both gene products may perform an essential and overlapping function during the process of cell division. Therefore, whether multiple copies of SufI would compensate for the absence of FtsEX was examined. Hence, the wild-type sufI gene, along with its native promoter, was cloned into pCL1920 (pMN16) from Kohara phage λ505 and transformed into strain MR10. As shown in Fig. 5B, strain MR10 with vector alone did not grow on LBON plates, whereas the plasmid pMN16 conferred total viability. The filamentation of strain MR10/pMN16 completely disappeared, and cells regained their shape (Fig. 5B). The plasmid pMN16 also abrogated the temperature sensitivity of the ftsI23 mutant as reported earlier (23; data not shown). To summarize, increased SufI levels conferred growth to ftsEX mutants on LBON, whereas deleting both ftsE and sufI was detrimental to the cell. In addition, the plasmid pMN16 did not suppress the inviability of tatB ftsE double mutants, confirming that a functional Tat transport system is needed for the export of SufI, and it is the periplasmic SufI that is required for the suppression of ftsEX phenotypes (data not shown).
SOS induction of ftsEX mutants.
Insertions in ftsE and ftsX were isolated earlier as weak SOS-inducing mutations in a screen employed for the identification of constitutive SOS-inducing mutations (35). In this study also, it was shown that ftsEX null mutations exhibit a low-level SOS induction (which is dependent on RecA and LexA), as measured by the expression of a β-galactosidase reporter gene fused to a DNA damage-inducible promoter dinD (Table 2). This observation indicates the occurrence of endogenous DNA damage in these mutants that leads to the generation of single-stranded DNA, which is the signal for the induction of SOS response. Interestingly, this induction was not suppressed by either the addition of osmolytes or the introduction of multicopy plasmids encoding FtsQAZ, FtsN, or SufI (Table 2). In an earlier study, it was shown that the inactivation of FtsI either by treatment with β-lactam antibiotics or by temperature upshift of the ftsI23(Ts) allele induces the DNA damage response through the DpiBA two-component signal transduction system (31). The researchers of that study suggested that defective cell wall synthesis is an initiator of SOS response, which is dependent on functional dpiA, recA, or lexA genes. It is tempting to speculate that the SOS induction observed in ftsEX strains could be due to a similar kind of membrane damage. However, in this study, the effect of dpiA or dpiB mutations on the SOS induction of ftsEX mutants was not examined. Though ftsEX mutants exhibit SOS induction and, therefore, would contain induced levels of SulA, which is an inhibitor of FtsZ, the growth or filamentation of ftsEX strains was not affected by a mutation in sulA (data not shown). On the other hand, the sufI deletion strain did not exhibit a significant increase in SOS induction levels (Table 2).
TABLE 2.
β-Galactosidase expression levels
| Strain or characteristica | β-Galactosidase expressionb (Miller units) |
|---|---|
| JH39a | 9 ± 1.2 |
| ΔftsEX210::Kan | 27 ± 5.3 |
| ΔftsEX::Kan (grown in LB with 0.2 M NaCl) | 20 ± 3.5 |
| ΔftsEX::Kan (grown in LB with 0.2 M glucose) | 17 ± 3.8 |
| ftsE::Kana | 26 ± 5.8 |
| ftsX::Kana | 17 ± 3.4 |
| ΔftsEX::Kan lexA3 malB::Tn9 | 11 ± 1.1 |
| ΔftsEX::Kan/pCL1920 | 28 ± 6.2 |
| ΔftsEX::Kan/pMN8 (pftsQAZ) | 25 ± 5.6 |
| ΔftsEX::Kan/pMN14 (pftsN) | 23 ± 3.7 |
| ΔftsEX::Kan/pMN16 (psufI) | 25 ± 4.2 |
| ΔsufI21::Kan | 12 ± 1.3 |
Strain JH39 and its ftsE::Kan or ftsX::Kan mutant derivatives were obtained from K. Kreuzer (35). All the other strains are derivatives of JH39. lexA3 linked to malB::Tn9 was transduced from strain GJ2382 (6).
The specific activity of β-galactosidase was determined (32) in each of the cultures grown to exponential phase in LB, and values are means of at least three independent experiments.
DISCUSSION
In this study, an ftsEX deletion strain was made, and its phenotypes were studied in detail. The growth defects exhibited by this strain were similar to those described earlier for the ftsE::Kan insertion mutant RG60 (11). They reported that the addition of salts, such as NaCl, KCl, or K2SO4, could rescue the viability of RG60 on LBON medium but not the addition of glycerol, sucrose, or compatible solutes, such as glutamate, glutamine, or glycine betaine. Hence, they described ftsE as a conditional salt-dependent essential gene and predicted its possible involvement in salt or ion transport (11). However, in this study, it is observed that the supplementation of LBON with osmolytes, such as glucose, sucrose, glycerol, or NaCl, restores both cell shape and viability to ftsEX deletion mutants (Fig. 2). Although the basis of the discrepancy between these two results is not clearly understood, the most likely reason could be that the concentration of the osmolytes used by de Leeuw et al. (11) was not sufficient for the growth of the RG60 mutant strain. In their assay, the growth was scored after spotting 50 μl of saturating solution of sucrose or 80% glycerol on a filter disc placed on an LBON plate overlaid with RG60 (11), whereas in this study, the concentration of the osmolytes (glucose and sucrose) used for suppression was 0.4 M which is osmotically equivalent to 0.17 M NaCl (the concentration that is normally present in LB). It is evident from this study that ftsEX null mutants are osmotic remedial, i.e., that they are conditionally dependent on high osmolarity for their growth.
Involvement of FtsEX in cell division.
The extreme filamentation of the ftsEX deletion strain in LBON or in LB at higher temperatures (Fig. 1B and 2C) suggests that this mutant is defective in the process of cell division (references 11, 16, 42, and 44 and this study). Similarly, the growth pattern of the ftsEX mutant in LBON broth with an increase in cell mass for a few generations, followed by growth cessation (Fig. 2B), also indicates that FtsEX is required for cell division but not for growth. However, this observation contradicts an earlier study (44), wherein it was shown that the growth of strain RG60 stops immediately and completely after shift to the LBON broth, thereby suggesting that FtsEX is needed for both growth and division. The reason for this variation is not obvious, though it could be due to differences either in the strain background or in the composition of the growth medium that was used. The division phenotypes of ftsEX mutants are partly suppressed by multiple copies of ftsQAZ. There are quite a few instances of ftsQAZ overexpression suppressing the division defects. Recently, it was shown that the deficiency of FtsK could be compensated by increasing the levels of FtsQAZ (13). Similarly, the growth defects of minCDE envC or minCDE slmA double mutants are suppressed by pftsQAZ (3, 4). Null mutations in the cell shape gene pbpA can be obtained in strains with elevated FtsQAZ levels (50). The suppression of ftsEX growth defects by multicopy plasmids encoding SdiA or RelA could also be through the upregulation of ftsQAZ (20, 39, 51). The function of DksA is not very clear, though it is implicated in altering the strength of the promoters that are regulated by ppGpp (36). The preliminary results from this lab show that the suppression by multicopy DksA is not mediated by RelA; introducing a relA251::Kan mutation into an ftsE::Tn10dCm/pdksA strain does not abolish the growth suppression on LBON (data not shown).
The suppression of ftsEX phenotypes by multicopy FtsN strongly argues for the functional involvement of the FtsEX complex in the division process. FtsN is a multicopy suppressor of most of the temperature-sensitive division mutants, such as ftsA12, ftsQ1, ftsI23, and ftsK44, but not of ftsZ84 (9, 17). The biochemical function of FtsN is not clear; in vitro it binds murein sacculus (48) and is presumed to be involved in either the peptidoglycan assembly or the stabilization of the division components.
There is evidence to indicate that there are complex interactions between FtsEX and other division proteins, and it is discussed below. The observation that low-copy-number plasmids carrying ftsA or ftsQA genes could not be introduced into the ftsEX mutant (but which can be transformed into wild-type strains without any difficulty) suggests that there are physical and functional interactions between these division proteins. In the absence of FtsEX, even a subtle increase in FtsA levels may cause an imbalance of the FtsA-to-FtsZ ratio. It is known that the ratio of FtsA to FtsZ is a crucial factor in deciding the fate of Z rings even in normal cells (reviewed in reference 29). There could be a defective assembly of the division components, as more FtsA can cause aberrant Z rings due to the absence of either a coordinate increase in FtsZ levels or a functional FtsEX. Hence, it is possible that FtsEX could be modulating the interaction of the FtsZ-FtsA complex. This model also explains the suppression of ftsEX mutations by ftsZ84 allele. The suppression of ftsEX phenotypes by ftsZ84 is intriguing and implies that functional FtsZ generates a requirement for FtsEX. In other words, the defective ftsZ84 allele can bypass the need for FtsEX, at least partially, most likely by reducing the flux into the division pathway, thus eliminating the requirement for FtsEX in low-osmotic-strength medium. Alternatively, FtsEX may stabilize the interaction between the Z ring scaffold and the FtsQLB complex, particularly in low-osmolarity conditions. This is consistent with the earlier finding that the localization of the FtsEX complex to the septal ring is dependent on the presence of early divisomal proteins, FtsZ and FtsA. Due to this reason, FtsEX was placed between FtsA and FtsQ in the linear recruitment pathway (44). FtsX was also shown to interact with FtsA and FtsQ in two-hybrid studies (22), suggesting that it may bind or connect the Z ring to the periplasmic connector consisting of FtsQLB complex. All of these observations taken together suggest a role for FtsEX in the stabilization of FtsZ ring. It has been proposed earlier (44) that FtsEX is important for the assembly or stability of the septal ring, especially in salt-free medium. Normally, this interaction could be dependent on the osmotic strength of the local environment and, in absence of optimal osmotic conditions, FtsEX would be absolutely required for proper assembly. Since the divisome assembly is based mostly on protein-protein interactions, it is plausible that appropriate osmotic conditions are required for the correct folding of this multiprotein complex. As proposed earlier (13), division proteins may perform two main functions; one is to stabilize the interactions between divisome components, and the second one is to generate the division septum. FtsEX may fall into the former class of divisome proteins. In this context, it is worth noting that organisms of the Mycobacterium tuberculosis complex do not have either zipA, ftsA, or ftsN homologues but have ftsEX genes (30, 33). It would be interesting to examine whether the FtsEX complex would be essential for cell division and growth in these organisms.
It is also feasible that the FtsEX complex could function as an assembly protein; it may facilitate the assembly of division components as well as other proteins that are targeted to the cytoplasmic membrane. This may explain the inability of an ftsE(Ts) mutant to translocate the potassium pump proteins into the inner membrane (47). The ftsX mutants of Flavobacterium johnsoniae are deficient in gliding motility, and this again may reflect their incapability to assemble some essential factors required for motility (24). The expression of ftsE is shown to be upregulated in Corynebacterium glutamicum after treatment with the cell-wall-inhibitory agent ethambutol (40), suggesting that FtsE could be a stress-induced protein. However, in E. coli, the expression of ftsEX (either from the major promoter that is upstream to ftsY or from the weak internal promoter of ftsE and ftsX) does not appear to be transcriptionally regulated either by conditions of stress, such as DNA damage, oxidative stress, altered osmolarity, and high temperature, or by the absence of global regulators like RpoS, H-NS, Fis, or LexA (M. Reddy, unpublished results).
Redundancy of FtsEX and SufI in cell division.
As FtsEX is not essential for growth on medium of high osmolarity, there was a possibility of the existence of another protein performing a related or overlapping function. The identification of sufI as a synthetic lethal mutation of ftsEX permitted the inference that SufI could be such a potential candidate protein. However, the inviability of ftsEX mutants (with a functional copy of SufI) in medium of low osmotic strength raises the question of the role of SufI in these conditions. One explanation for this could be that in low-osmolarity conditions, SufI may not be functional or is limiting. The fact that the increased level of SufI was able to suppress most of the mutant phenotypes of ftsEX favors the latter possibility. Since the sufI mutation is epistatic to multicopy ftsN and ftsQAZ (as sufI ftsE inviability was not abrogated by these multicopy plasmids), it indicates that the latter are acting through the SufI pathway in achieving division in absence of FtsEX.
Is the process of cell division sensitive to conditions of low osmotic strength and/or high temperature?
The fact that most of the point mutations in various division genes are osmoremedial and also that the occurrence of osmoremedial null mutations in genes involved in cell division reflects that perhaps the process of division itself is intrinsically sensitive to conditions of low osmotic strength. ftsZ84, the best-characterized mutation of ftsZ, is osmoremedial at a high temperature (37, 39). In addition, other temperature-sensitive division mutations, such as ftsA12, ftsQ1, ftsK44, and ftsI23, are also sensitive to variations in the osmotic strength of the medium. The existence of osmoremedial null mutations, such as ftsEX, affected in division is also a strong indication that this pathway is dependent on high osmotic strength. In addition to ftsEX mutations, an insertion mutation in ftsK (that disrupts FtsK after codon 233 in the linker region) was found to be osmoremedial (M. Reddy, unpublished results). The phenotypes of this strain were quite similar to that of an ftsEX mutant, i.e., it did not grow on LBON at any temperature but grew normally on LB at 30°C and very poorly at 42°C. In addition, a mutation in the yhhP gene that codes for an 81-amino-acid, putative RNA binding, SirA-like protein was shown to confer extreme filamentation and was also osmoremedial (19). Their results implicate a role for yhhP in division, as its phenotypes were suppressed either by increased osmolarity or by multiple copies of FtsZ or DksA (19). Taken together, all of these findings suggest that the assembly of cell division machinery may require optimal conditions of osmotic strength, as the divisome is essentially a large multiprotein complex tethered by mostly protein-protein interactions. In the absence of optimal osmotic strength, the division components may require additional proteins for their stability or assembly. High temperature may also affect the hydrophobic protein-protein interactions of the divisomal assembly and may therefore alter its stability, particularly in low-osmolar conditions. Consistent with this, the growth patterns of the above-mentioned osmoremedial mutations reflect the increased dependence of osmotic strength at high temperatures (Fig. 1). However, it is also possible that the osmotic strength or temperature may alter the activity of chaperones which facilitate the proper folding of the division proteins and, thus, their effect on division could be indirect. It has been shown earlier that the process of protein export itself is cold sensitive (38). Based on the existence of multiple cold-sensitive mutations in various secretion genes and also cold-sensitive ribosomal null mutations, the cold sensitivity of the secretion process was postulated. The process of cell division could be similarly susceptible to changes in the osmotic conditions of the medium.
Acknowledgments
I thank Jon Beckwith, Mary Berlyn (Coli Genetic Stock Center), Piet de Boer, Mike Cashel, Donald Court, Elizabeth Craig, Kenneth Kreuzer, Seiichi Yasuda, and Miroslav Radman's laboratory for various strains and plasmids; L. Saisree for help in microscopy; and J. Gowrishankar for advice and critical reading of the manuscript.
This work was supported in part by funds from the Department of Biotechnology, Government of India.
Footnotes
Published ahead of print on 27 October 2006.
REFERENCES
- 1.Aarsman, M. E. G., A. Piette, C. Fraipont, T. M. F. Vinkenvleugel, M. Nguyen-Disteche, and T. den Blaauwen. 2005. Maturation of the Escherichia coli divisome occurs in two steps. Mol. Microbiol. 55:1631-1645. [DOI] [PubMed] [Google Scholar]
- 2.Bernhardt, T. G., and P. A. de Boer. 2003. The Escherichia coli amidase AmiC is a periplasmic septal ring component exported via the twin-arginine transport pathway. Mol. Microbiol. 48:1171-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhardt, T. G., and P. A. de Boer. 2004. Screening for synthetic lethal mutants in Escherichia coli and identification of EnvC (YibP) as a periplasmic septal ring factor with murein hydrolase activity. Mol. Microbiol. 52:1255-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernhardt, T. G., and P. A. de Boer. 2005. SlmA, a nucleoid-associated, FtsZ-binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol. Cell 18:555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhandari, P., and J. Gowrishankar. 1997. An Escherichia coli host strain useful for overproduction of cloned gene products with NaCl as the inducer. J. Bacteriol. 179:4403-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharatan, S. M., M. Reddy, and J. Gowrishankar. 2004. Distinct signatures for mutator sensitivity of lacZ reversions and for the spectrum of lacI/lacO forward mutations on the chromosome of nondividing Escherichia coli. Genetics 166:681-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buddelmeijer, N., and J. Beckwith. 2004. A complex of the Escherichia coli cell division proteins FtsL, FtsB and FtsQ forms independently of its localization to the septal region. Mol. Microbiol. 52:1315-1327. [DOI] [PubMed] [Google Scholar]
- 8.Cashel, M., D. R. Gentry, J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 9.Dai, K., Y. Xu, and J. Lutkenhaus. 1993. Cloning and characterization of ftsN, an essential cell division gene in Escherichia coli isolated as a multicopy suppressor of ftsA12(Ts). J. Bacteriol. 175:3790-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer, P. A., R. E. Crossley, and L. I. Rothfield. 1989. A division inhibitor and a topological specificity factor coded for by the mini-cell locus determine proper placement of the division septum in E. coli. Cell 56:641-649. [DOI] [PubMed] [Google Scholar]
- 11.de Leeuw, E., B. Graham, G. J. Phillips, C. M. ten Hagen-Jongman, B. Oudega, and J. Luirink. 1999. Molecular characterization of Escherichia coli FtsE and FtsX. Mol. Microbiol. 31:983-993. [DOI] [PubMed] [Google Scholar]
- 12.Errington, J., R. A. Daniel, and D. J. Scheffers. 2003. Cytokinesis in bacteria. Microbiol. Mol. Biol. Rev. 67:52-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geissler, B., and W. Margolin. 2005. Evidence for functional overlap among multiple bacterial cell division proteins: compensating for the loss of FtsK. Mol. Microbiol. 58:596-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geissler, B., D. Elraheb, and W. Margolin. 2003. A gain-of-function mutation in ftsA bypasses the requirement for the essential cell division gene zipA in Escherichia coli. Proc. Natl. Acad. Sci. USA 100:4197-4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gil, D., and J.-P. Bouche. 1991. ColE1-type vectors with fully repressible replication. Gene 105:17-22. [DOI] [PubMed] [Google Scholar]
- 16.Gill, D. R., G. F. Hatfull, and G. P. C. Salmond. 1986. A new cell division operon in Escherichia coli. Mol. Gen. Genet. 205:134-145. [DOI] [PubMed] [Google Scholar]
- 17.Goehring, N. W., and J. Beckwith. 2005. Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr. Biol. 15:514-526. [DOI] [PubMed] [Google Scholar]
- 18.Heidrich, C., A. Ursinus, J. Berger, H. Schwarz, and J. V. Holtje. 2002. Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli. J. Bacteriol. 184:6093-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii, Y., H. Yamada, T. Yamashino, K. Ohashi, E. Katoh, H. Shindo, T. Yamazaki, and T. Mizuno. 2000. Deletion of yhhP gene results in filamentous cell morphology in Escherichia coli. Biosci. Biotechnol. Biochem. 64:799-807. [DOI] [PubMed] [Google Scholar]
- 20.Joseleau-Petit, D., D. Vinella, and R. D'Ari. 1999. Metabolic alarms and cell division in Escherichia coli. J. Bacteriol. 181:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang, P. J., and E. A. Craig. 1990. Identification and characterization of a new gene that is a dosage-dependent suppressor of a dnaK deletion mutation. J. Bacteriol. 172:2055-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karimova, G., N. Dautin, and D. Ladant. 2005. Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J. Bacteriol. 187:2233-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato, J., Y. Nishimura, M. Yamada, H. Suzuki, and Y. Hirota. 1988. Gene organization in the region containing a new gene involved in chromosome partition in Escherichia coli. J. Bacteriol. 170:3967-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kempf, M. J., and M. J. McBride. 2000. Transposon insertions in the Flavobacterium johnsoniae ftsX gene disrupt gliding motility and cell division. J. Bacteriol. 182:1671-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohara, Y., K. Akiyama, and K. Isono. 1987. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell 50:495-508. [DOI] [PubMed] [Google Scholar]
- 26.Lee, P. A., D. Tullman-Ercek, and G. Georgiou. 2006. The bacterial twin-arginine translocation pathway. Annu. Rev. Microbiol. 60:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lerner, C. G., and M. Inouye. 1990. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 18:4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, G., G. C. Draper, and W. D. Donachie. 1998. FtsK is a bifunctional protein involved in cell division and chromosome localization in Escherichia coli. Mol. Microbiol. 29:893-903. [DOI] [PubMed] [Google Scholar]
- 29.Lutkenhaus, J., and S. G. Addinall. 1997. Bacterial cell division and the Z-ring. Annu. Rev. Biochem. 66:93-116. [DOI] [PubMed] [Google Scholar]
- 30.Margolin, W. 2000. Themes and variations in prokaryotic cell division. FEMS Microbiol. Rev. 24:531-548. [DOI] [PubMed] [Google Scholar]
- 31.Miller, C., L. E. Thomsen, C. Gaggero, R. Mosseri, H. Ingmer, and S. N. Cohen. 2004. SOS response induction by β-lactams and bacterial defense against antibiotic lethality. Science 305:1629-1631. [DOI] [PubMed] [Google Scholar]
- 32.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and other bacteria. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 33.Mir, M. A., H. S. Rajeswari, U. Veeraraghavan, and P. Ajitkumar. 2006. Molecular characterisation of ABC transporter type FtsE and FtsX proteins of Mycobacterium tuberculosis. Arch. Microbiol. 185:147-158. [DOI] [PubMed] [Google Scholar]
- 34.Nanninga, N. 1998. Morphogenesis of Escherichia coli. Microbiol. Mol. Biol. Rev. 62:110-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Reilly, E. K., and K. N. Kreuzer. 2004. Isolation of SOS constitutive mutants of Escherichia coli. J. Bacteriol. 186:7149-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul, B. J., M. B. Berkmen, and R. L. Gourse. 2005. DksA potentiates direct activation of amino acid promoters by ppGpp. Proc. Natl. Acad. Sci. USA 102:7823-7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phoenix, P., and G. R. Drapeau. 1988. Cell division control in Escherichia coli K-12: some properties of the ftsZ84 mutation and suppression of this mutation by the product of a newly identified gene. J. Bacteriol. 170:4338-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pogliano, K. J., and J. Beckwith. 1993. The Cs sec mutants of Escherichia coli reflect the cold sensitivity of protein export itself. Genetics 133:763-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powell, B. S., and D. L. Court. 1998. Control of ftsZ expression, cell division, and glutamine metabolism in Luria-Bertani medium by the alarmone ppGpp in Escherichia coli. J. Bacteriol. 180:1053-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radmacher, E., K. C. Stansen, G. S. Besra, L. J. Alderwick, W. N. Maughan, G. Hollweg, H. Sahm, V. F. Wendisch, and L. Eggeling. 2005. Ethambutol, a cell wall inhibitor of Mycobacterium tuberculosis, elicits l-glutamate efflux of Corynebacterium glutamicum. Microbiology 151:1359-1368. [DOI] [PubMed] [Google Scholar]
- 41.Reece, K. S., and G. J. Philips. 1995. New plasmids carrying antibiotic-resistance cassettes. Gene 165:141-142. [DOI] [PubMed] [Google Scholar]
- 42.Ricard, M., and Y. Hirota. 1973. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J. Bacteriol. 116:314-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 44.Schmidt, K. L., N. D. Peterson, R. J. Kustusch, M. C. Wissel, B. Graham, G. J. Phillips, and D. S. Weiss. 2004. A predicted ABC transporter, FtsEX, is needed for cell division in Escherichia coli. J. Bacteriol. 186:785-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svitil, A. L., M. Cashel, and Z. W. Zyskind. 1993. Guanosine tetraphosphate inhibits protein synthesis in vivo. A possible protection mechanism for starvation stress in Escherichia coli. J. Biol. Chem. 268:2307-2311. [PubMed] [Google Scholar]
- 46.Taschner, P. E. M., P. G. Huls, E. Pas, and C. L. Woldringh. 1988. Division behavior and shape changes in isogenic ftsZ, ftsQ, ftsA, pbpB, and ftsE cell division mutants of Escherichia coli during temperature shift experiments. J. Bacteriol. 170:1533-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ukai, H., H. Matsuzawa, K. Ito, M. Yamada, and A. Nishimura. 1998. ftsE(Ts) affects translocation of K+-pump proteins into the cytoplasmic membrane of the Escherichia coli. J. Bacteriol. 180:3663-3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ursinus, A., F. van den Ent, S. Brechtel, M. de Pedro, J. V. Holtje, J. Lowe, and W. Vollmer. 2004. Murein (peptidoglycan) binding property of the essential cell division protein FtsN from Escherichia coli. J. Bacteriol. 186:6728-6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vicente, M., and A. I. Rico. 2006. The order of the ring: assembly of Escherichia coli cell division components. Mol. Microbiol. 61:5-8. [DOI] [PubMed] [Google Scholar]
- 50.Vinella, D., D. Joseleau-Petit, D. Thevenet, P. Bouloc, and R. D'Ari. 1993. Penicillin-binding protein 2 inactivation in Escherichia coli results in cell division inhibition, which is relieved by FtsZ overexpression. J. Bacteriol. 175:6704-6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, X. D., P. A. de Boer, and L. I. Rothfield. 1991. A factor that positively regulates cell division by activating transcription of the major cluster of essential cell division genes of Escherichia coli. EMBO J. 10:3363-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss, D. S. 2004. Bacterial cell division and the septal ring. Mol. Microbiol. 54:588-597. [DOI] [PubMed] [Google Scholar]
- 53.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]