Abstract
Dendritic cells (DCs) are an important early target cell for many mosquito-borne viruses, and in many cases mosquito-cell-derived arboviruses more efficiently infect DCs than viruses derived from mammalian cells. However, whether mosquito-cell-derived viruses differ from mammalian-cell-derived viruses in their ability to induce antiviral responses in the infected dendritic cell has not been evaluated. In this report, alphaviruses, which are mosquito-borne viruses that cause diseases ranging from encephalitis to arthritis, were used to determine whether viruses grown in mosquito cells differed from mammalian-cell-derived viruses in their ability to induce type I interferon (IFN) responses in infected primary dendritic cells. Consistent with previous results, mosquito-cell-derived Ross River virus (mos-RRV) and Venezuelan equine encephalitis virus (mos-VEE) exhibited enhanced infection of primary myeloid dendritic cells (mDCs) compared to mammalian-cell-derived virus preparations. However, unlike the mammalian-cell-derived viruses, which induced high levels of type I IFN in the infected mDC cultures, mos-RRV and mos-VEE were poor IFN inducers. Furthermore, the poor IFN induction by mos-RRV contributed to the enhanced infection of mDCs by mos-RRV. These results suggest that the viruses initially delivered by the mosquito vector differ from those generated in subsequent rounds of replication in the host, not just with respect to their ability to infect dendritic cells but also in their ability to induce or inhibit antiviral type I IFN responses. This difference may have an important impact on the mosquito-borne virus's ability to successfully make the transition from the arthropod vector to the vertebrate host.
Mosquito-borne viruses, which include a wide range of human pathogens, including flaviviruses, alphaviruses, and bunyaviruses, cause diseases ranging from arthritis to hemorrhagic fever or encephalitis (10, 31, 38, 42, 43). A number of mosquito-borne viruses are thought to initially interact with and replicate in myeloid dendritic cells (mDCs) in the skin following delivery from the mosquito vector (26, 36, 46), and it is likely that early interactions with mDCs play an important role in determining whether the virus successfully establishes infection and ultimately causes disease.
One of the most important obstacles that an invading virus has to overcome is the host type I interferon (IFN) system, which plays an essential role in the early control of many viral infections, modulates downstream aspects of other components of the innate immune response, and helps orchestrate the adaptive immune response (reviewed in reference 3). This may be particularly important for mosquito-borne pathogens that initially replicate in dendritic cells of myeloid origin, since mDCs are capable of mounting robust type I interferon responses (7). Several arthropod-borne viruses, including members of Flaviviridae and Bunyaviridae, encode antagonists of the type I IFN system that can block multiple components in the type I IFN pathway (33, 44). However, mDCs are different from many other cell types, such as fibroblasts, since they can rapidly respond to the incoming virus and do not require viral replication to initiate a type I IFN response (15), which may make these cells less susceptible to virally encoded interferon antagonists than other cell types. Therefore, other factors, such as immune-suppressive components of mosquito saliva, may also promote transmission to the vertebrate host (23, 24, 37). However, whether the virus derived from the mosquito differs from virus derived from mammalian cells in its ability to induce an antiviral response in mDCs or other cell types has not been evaluated.
Alphaviruses are single-stranded, positive-sense RNA viruses that are transmitted by mosquitoes and cause human diseases ranging from infectious arthritis to lethal encephalitis (reviewed in reference 42). Alphaviruses are extremely amenable to genetic manipulation and, in addition, well-established cell culture and animal pathogenesis models exist for several of these viruses (31, 42, 43, 45). Furthermore, several alphaviruses target to dendritic cells in vivo (26, 36). Additionally, mosquito-cell-derived Sindbis virus has been shown to preferentially infect human mDCs due to interactions between the dendritic-cell-specific C-type lectin DC-SIGN and high-mannose glycans on the mosquito-cell-derived virion (18). Therefore, alphaviruses represent an extremely useful set of viruses for studying how mosquito-borne viruses interact with mDCs.
In this study, Ross River virus (RRV) and other alphaviruses were grown in either mosquito or mammalian cell cultures (mam- or mos-RRV) and evaluated for their ability to infect murine or human mDCs and induce an antiviral type I interferon response. These studies demonstrate that for several alphaviruses, the mosquito-cell-derived virus was able to efficiently infect the mDCs but was an extremely poor inducer of type I IFN, while the same virus derived from mammalian cells was a potent IFN inducer. These results strongly suggest that alphaviruses delivered from mosquito vectors are able to infect mDCs while simultaneously avoiding the type I IFN response.
MATERIALS AND METHODS
Virus stocks and cell lines.
Ross River virus (T48 strain) was produced from the pRR64 infectious clone (generously provided by Richard Kuhn, Purdue University), which includes the full-length T48 cDNA clone (21) originally isolated from Aedes vigilax mosquitoes in Queensland, Australia (8). The RR64-GFP virus, which expresses green fluorescent protein (GFP) from a second viral subgenomic promoter, was constructed as described previously (31). Briefly, genome-length viral RNA was transcribed using mMessage mMachine SP6 in vitro transcription kits (Ambion), and the RNA was electroporated into baby hamster kidney-21 cells (BHK-21; ATCC CCL-10). Twenty-four hours postelectroporation, supernatants were harvested, the virus was pelleted through a 20% (wt/vol) sucrose cushion by ultracentrifugation at 72,000 × g, and the virus was resuspended in phosphate-buffered saline (PBS). Venezuelan equine encephalitis virus (VEE), which had been engineered to express GFP from a second subgenomic promoter, was generated from clone dpV3000-GFP in a similar manner as described previously (45). To generate mos-RRV or mos-VEE, virus was passaged a single time at a multiplicity of infection (MOI) of 0.1 in C6/36 Aedes albopictus, and input virus was washed off after a 1-hour infection. Alternatively, C6/36 cells were electroporated with in vitro-transcribed RR64 RNA using the same protocol to generate BHK-cell-derived virus. Both passaged and electroporated mos-RRV showed equivalent levels of IFN induction (data not shown). At 18 to 24 h, supernatant was collected and concentrated. RRV generated from Chinese hamster ovary (CHO) cells was obtained by a single passage of mam-RRV through either the parental Pro-5 (ATCC CRL-1781) or mutant Lec-1 (ATCC CRL-1735) CHO cells. Virus stocks were titrated on BHK-21 cells by standard plaque assay. Barmah Forest virus (BFV; strains 10E101SC and BH2193) (22) was grown in BHK-21 cells to generate mam-BFV. To generate mos-BFV, virus was passaged a single time in C6/36 Aedes albopictus cells for 18 to 24 h, and supernatant was collected and concentrated by ultracentrifugation the same way as for mam-BFV. Both virus stocks were reconstituted in phosphate-buffered saline, and titers were determined on BHK-21 cells by standard plaque assay.
BHK-21 cells were grown in alpha minimal essential medium (Gibco) with 10% fetal bovine serum (FBS), 10% tryptose phosphate broth, and 0.29 mg of l-glutamine per ml. C6/36 cells were grown in minimal essential medium with Earl's salts and supplemented with 5% FBS, nonessential amino acids, penicillin, and streptomycin.
Bone marrow dendritic cell cultures and virus infection conditions.
Murine bone marrow-derived dendritic cells were generated from 129 Sv/Ev mice and 129 Sv/Ev αβ RKO bone marrow as described previously (39). Bone marrow was harvested from the femur and tibia of 1- to 3-month-old mice, red blood cells were lysed, and cells were resuspended in RPMI 1640, 10% FBS, penicillin, streptomycin, and 2-mercaptoethanol. Cells were grown in suspension in the presence of recombinant granulocyte-macrophage colony stimulating factor and interleukin-4 for 7 days at 20 ng/ml (days 0 to 3), 10 ng/ml (days 3 to 5), and 5 ng/ml (days 5 to 7) in ultra-low-cluster six-well Costar plates (Costar) to obtain an immature dendritic cell phenotype, which was confirmed by staining with antibodies to CD11b, CD11c, B220, CD80, CD86, and CD40 (eBioscience). At day 7 post-bone marrow harvest, cells were infected with either RRV or VEE for 2 hours in a total volume of 0.2 ml. Following infection, 0.5 ml of medium was added back to the culture. At various times postinfection, supernatants were removed and assayed for type I IFN levels, while the cells were fixed in 4% paraformaldehyde and analyzed by flow cytometry for GFP expression to quantify the percentage of infected cells within the culture.
Generation of human monocyte-derived DCs.
Peripheral blood was obtained from healthy volunteers and diluted 1/2 in PBS. The peripheral blood mononuclear cells were isolated following separation over lymphocyte separation medium (ICN Biomedicals), washed in PBS, and resuspended in RPMI 1640 medium as described for murine DCs. Cells were plated in culture flasks and allowed to adhere for 2 h, at which time nonadherent peripheral blood mononuclear cells were removed and discarded. Cells were cultured in the presence of 800 IU/ml granulocyte-macrophage colony-stimulating factor and 500 IU/ml interleukin-4 (Peprotech) for 6 days, with fresh cytokines added every 48 to 72 h. On day 6, cells were resuspended, phenotyped by flow cytometry staining with anti-CD11c, HLA-DR, DC-SIGN, and CD14 (BD Biosciences Pharmingen), and infected in the same manner as the murine mDCs. Experiments were performed on blood from two separate donors.
IFN bioassay.
Type I interferon levels in cell culture supernatants were measured by interferon bioassay as described previously (2, 45). Briefly, L929 mouse fibroblasts (ATCC CCL-1), human lung carcinoma A549 cells (CCL-185), or Vero cells (CRL-1586) were seeded in 96-well plates. All samples were acidified to a pH of 2.0 for 24 h and then were neutralized to pH 7.4 and further inactivated by UV light for 10 min prior to titration by twofold dilutions down the plate. Twenty-four hours after the addition of the supernatant, interferon-sensitive encephalomyocarditis virus or Sindbis virus was added to each plate. At 18 to 24 h postinfection (hpi), 3-[4,5-dimethylthylthiazol-2yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma), an indicator of viable cells, was added to each well in the murine bioassay only. The MTT product was then dissolved in isopropanol-0.4% HCl, and absorbance was read on a microplate reader at 570 nm. Alternatively, human bioassay plates were scored for 50% cytopathic effect as an indicator of IFN concentration. Each plate contained an IFN standard (Chemicon or R&D Systems) to determine the international units (IU/ml) of type I IFN in each culture.
Real-time PCR and semiquantitative PCR assays.
Total cellular RNA was isolated with the Ultraspec RNA isolation reagent (Biotex), and total RNA was reverse transcribed with a cDNA archive kit (Applied Biosystems). TaqMan real-time PCR was performed with primer probe sets for specific genes of interest (Applied Biosystems) and analyzed on the Prism 7000 machine (Applied Biosystems). For all samples, an equivalent amount of RNA was reverse transcribed and an internal reference control of 18s rRNA or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was included. For studies with Barmah Forest virus, total RNA was isolated from cells by standard methods using RNAwiz (Ambion). Reverse transcriptase PCR (RT-PCR) was then carried out as previously described (28). Primer sequences for hypoxanthine phosphoribosyltransferase and IFN-β have been described previously (27). For RRV genome analysis, primers for sequences specific to the NSP1 and NSP4 regions of RRV were used. The primer sequences were the following: for NSP1, forward, 5′-AGAGTGCGGAAGACCCAGAG-3′, and reverse, 5′-CCGTGATCTTACCGGACACA-3′; for NSP4, forward, 5′-ACCCGACAGTGGCTAGTTAC-3′, and reverse, 5′ CGGTTGGTGGTAAGCATGAT-3′). Purified virions were isolated with the MagMax viral RNA extraction kit (Ambion) and reverse transcribed using the cDNA archive kit (Applied Biosystems), and cDNA was used for quantitative real-time PCR using Sybr Green (Applied Biosystems). A standard curve was generated to ensure optimal primer efficiency, and relative differences between virus stocks were calculated.
Specific infectivity assays.
The specific infectivity of RRV produced in BHK-21 cells or C6/36 was performed as previously described (19). In brief, at 4 hpi culture medium was replaced with methonine-free medium. At 8 hpi, virus growth medium was radiolabeled with [35S]methionine at 10 μCi/ml. At 24 hpi, virus was harvested and banded twice over two 20/60% discontinuous sucrose gradients in PBS and pelleted over a 20% sucrose cushion at 70,000 × g. Virus was reconstituted in PBS, total counts per minute (cpm) were measured, and equal counts per minute were used for a standard plaque assay on BHK-21 cells. The cpm/PFU ratio was then calculated to determine particle/PFU ratios for each virus preparation.
Western blotting.
Purified RRV virions were analyzed on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, and Western blot assays were performed using an anti-RRV mouse hyperimmune ascites fluid. Total fluorescence was detected by ECL Plus immunofluorescence (Amersham), and fluorescence was measured on a phosphorimager (Storm Scanner) and quantified with ImageQuant 5.0.
Statistics.
All groups were compared using a two-tailed unpaired Student t test or a one-way analysis of variance (ANOVA) where indicated using GraphPad InStat software.
RESULTS
Mosquito- and mammalian-cell-derived RRV differ in their ability to induce type I interferon in bone marrow-derived dendritic cells.
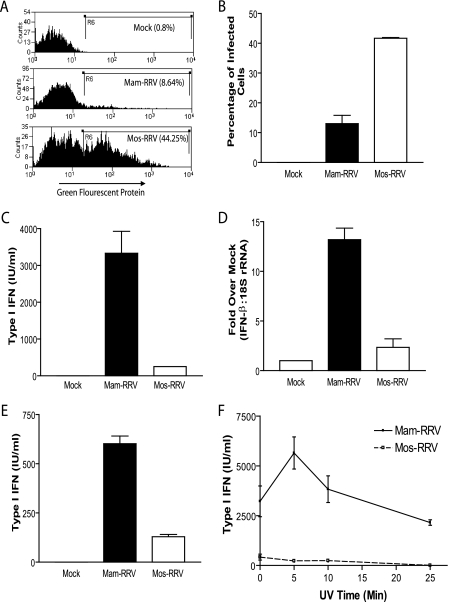
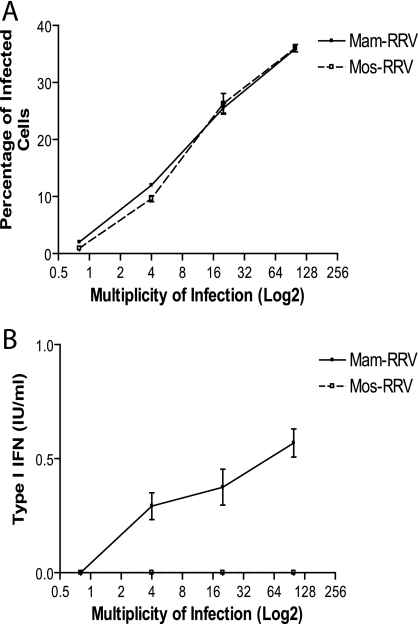
In order to assess the ability of mammalian- and mosquito-cell-derived virions to infect murine mDCs, RRV expressing GFP was grown in either BHK-21 cells or C6/36 Aedes Albopictus cells, and GFP was measured as an indicator of infection. Consistent with previous reports with Sindbis virus and West Nile virus infection of human dendritic cells (6, 18), mos-RRV infected a higher percentage of mDCs than mam-RRV (Fig. 1A and B) at 12 hpi. However, although mos-RRV infected a higher percentage of the mDCs, mos-RRV-infected cultures produced little type I IFN, while mam-RRV induced a robust IFN response (Fig. 1C) as measured in a type I IFN bioassay. Similar results were obtained when RRV without the GFP transgene was used (data not shown). These results were confirmed by quantitative RT-PCR to measure IFN-β mRNA levels in the virally infected cultures. Consistent with the bioassay results, mam-RRV induced an approximately 13-fold increase in IFN-β message over mock-infected cultures, compared to an approximately 2-fold induction by mos-RRV (Fig. 1D). These results strongly suggest that mos-RRV, but not mam-RRV, was able to suppress or avoid the induction of type I IFN following infection of mDCs.
FIG. 1.
mos-RRV infects myeloid bone marrow-derived dendritic cells more efficiently than mam-RRV but induces less type I interferon. A. mos-RRV infected a higher percentage of mDCs than mam-RRV, as shown in representative histograms measuring GFP expression in infected mDCs at 12 h postinfection by flow cytometry. B. Data from panel A, displaying the mean and standard error of the mean (SEM) of infection percentages from three independent samples. C. Type I IFN responses measured from the same cultures as in panel B. Each bar represents the mean and SEM of three independent samples. D. Myeloid DCs were infected with either mam- or mos-RRV, and RNA was isolated at 2 hpi and analyzed by real-time RT-PCR for IFN-β message. Data are presented as fold induction over mock and normalized to 18S rRNA. E. mam-RRV induces more type I IFN than mos-RRV in human monocyte-derived dendritic cells. Immature human DCs were generated and infected with either mam- or mos-RRV at an MOI of 20. At 18 h postinfection, type I IFN responses were measured by IFN bioassay. F. UV-inactivated mam-RRV induces type I IFN, while UV-inactivated mos-RRV does not induce type I IFN. mam- or mos-RRV was inactivated by UV light, and type I IFN responses were measured from infected mDCs by IFN bioassay. All data are representative of at least two to three independent experiments. Differences between mam- and mos-RRV were statistically significant (P < 0.05) for panels B to E as determined by one-way ANOVA. mam- and mos-RRV corresponding time points in panel F were statistically different as determined by Student's unpaired t test.
To determine if type I IFN differences between the mammalian- and mosquito-cell-derived viruses extended beyond murine DCs, human monocyte-derived DCs were infected with either mam- or mos-RRV and assessed for infectivity and IFN production. When these cells were infected with mam-RRV or mos-RRV, little productive infection was observed with either virus as determined by GFP expression (data not shown). However, when type I IFN levels in these cultures were assessed by bioassay, significantly higher levels of type I IFN were observed in supernatants from mam-RRV-infected DCs compared to mos-RRV-infected cells (Fig. 1E). These results suggest that the differences in type I IFN induction between the mosquito- and mammalian-cell-derived viruses also extend to human DCs, which further underscores the potential relevance of this phenotype to alphavirus-induced disease in humans.
Another important question was whether type I IFN induction by the mammalian-derived virus was replication dependent, since UV-inactivated Semliki Forest virus (SFV) is a potent inducer of type I IFN in mDCs. Consistent with the SFV findings, UV-inactivated RRV was able to induce type I IFN (Fig. 1F). However, heat inactivation of the virus ablated type I IFN induction and IFN induction copurified with the viral particle (data not shown). These data suggest that an intact particle, but not productive replication, is required for IFN induction by mam-RRV. This also suggests that endotoxin, which previously has been shown to be heat stable (29) and is a potent inducer of type I IFN (17), did not contribute to the type I IFN induction by the mammalian-cell-derived virus. Similar to infectious mos-RRV, UV-inactivated mos-RRV did not induce type I IFN, suggesting that the lack of type I IFN induction by mos-RRV was not dependent on viral replication, which indicated that a virally encoded de novo-synthesized factor was not required for this effect.
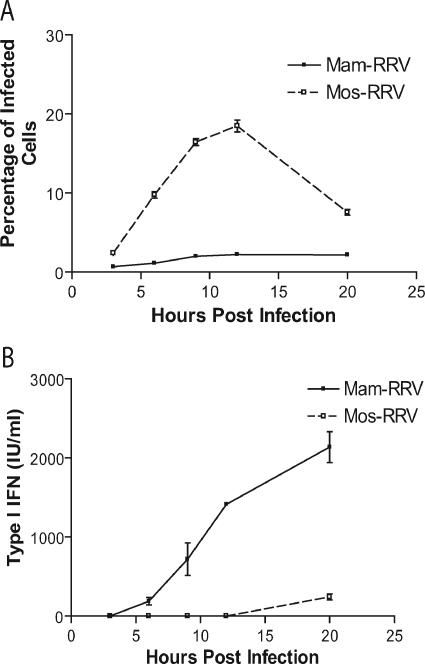
The differences in type I IFN induction between mam- and mos-RRV might simply reflect a difference in the kinetics of type I IFN induction. Therefore, mDC cultures were infected with mam-RRV or mos-RRV and both the percentage of infected cells and the levels of type I IFN produced in the cultures were evaluated at several times postinfection. As shown in Fig. 2, mos-RRV infected a higher percentage of mDCs than mam-RRV at all time points, with peak infection at 12 hpi (Fig. 2A). However, the levels of type I IFN in the supernatants were significantly lower from the mosquito virus-infected cultures compared to the mammalian virus-infected cultures at the 9-, 12-, and 20-h time points (Fig. 2B). Only at 20 hpi was IFN detectable by bioassay following mos-RRV infection; however, this response was approximately 10% of the IFN induction in mam-RRV-infected cultures (242 IU/ml compared to 2,138 IU/ml in mam-RRV-infected cultures) in a representative study. The low level of IFN in mos-RRV cultures at 20 hpi may reflect secondary infection in the culture where virus produced by the infected mDCs would have properties of mammalian-cell-derived virus.
FIG. 2.
mos-RRV infects more mDCs yet induces less type I IFN than mam-RRV over time. A. Immature murine mDCs were infected with either mos- or mam-RRV at a multiplicity of infection of 20, and wells were harvested at the indicated times and assessed for GFP-positive cells by flow cytometry. B. Supernatants from panel A were collected, and type I IFN was measured by IFN bioassay. Solid lines represent mam-RRV, and dotted lines represent mos-RRV. Each data point represents the mean and standard error of the mean of three samples. Data are representative of at least three independent experiments.
Particle differences do not explain the differential type I IFN induction by mam- and mos-RRV.
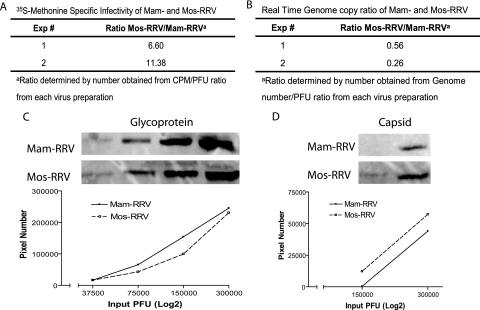
Since alphavirus defective interfering particles have been shown to induce type I IFN (11, 12) and inactivated RRV and SFV particles induce type I IFN responses in mDCs (Fig. 1f) (15), particle-to-PFU ratios for mam- and mos-RRV were calculated. If the mam-RRV preparations contained a larger number of particles per PFU than mos-RRV, excess particles might be responsible for the difference in type I IFN induction between the two RRV preparations. However, when the ratio of viral particles per PFU was determined for mam- and mos-RRV stocks, mos-RRV had 6.6- to 11-fold more particles/PFU than the mammalian-cell-derived virus stocks (Fig. 3). To confirm these results, two additional assays were used to evaluate the ratio of particles or genomes per PFU for both viruses.
FIG. 3.
Comparison of particle-to-PFU ratios for mam-RRV and mos-RRV. A. The total counts per minute/PFU ratio for both mam- and mos-RRV were determined by [35S]methonine labeling of virus particles followed by a plaque assay. The data are represented as the fold difference in particle-to-PFU ratio between mos-RRV and mam-RRV. B. The relative number of genomes for both mos- and mam-RRV was determined using a virus-specific real-time PCR assay. Data are presented as the fold difference in genome-to-PFU ratio between mos- and mam-RRV. C. Equal PFU of mos- and mam-RRV contain similar amounts of glycoprotein, as shown by Western blotting of serial twofold dilutions of mam- and mos-RRV probed with a polyclonal anti-RRV antibody. D. Fluorescent signal quantified for capsid bands from either mos- or mam-RRV presented as in panel C.
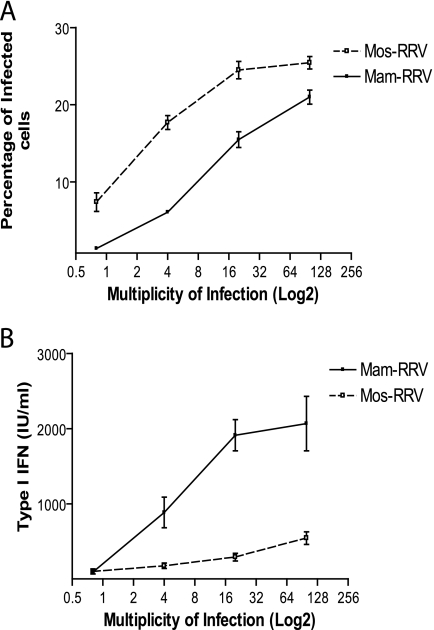
Quantitative real-time PCR was used to measure the relative numbers of RRV genomes between mam- and mos-RRV. This assay indicated that there were two- to fourfold more genomes/PFU of mam-RRV compared to mos-RRV (Fig. 3). Additionally, semiquantitative Western blot assays were performed to measure relative glycoprotein and capsid content of both mam- and mos-RRV (Fig. 3C and D). This assay, where equivalent PFU of each stock were analyzed by Western blotting with polyclonal antiserum recognizing the viral E2 glycoprotein and capsid, showed some variation where matched mam- and mos-RRV virus preparations ranged from equivalent levels of glycoprotein and capsid signal to either virus stock having up to two- to sixfold more glycoprotein- and capsid-specific signal than the corresponding virus stock from the other cell type. However, we saw no differences in the type I IFN induction phenotype for mam- and mos-RRV virus stocks, regardless of whether the virus stocks had equivalent capsid/glycoprotein signal or if mos-RRV had more or less glycoprotein-specific signal than the matched mam-RRV stock. This suggests that higher particle/PFU ratios of mam-RRV do not explain the difference in type I IFN induction. However, the higher ratio of genomes/PFU of mam-RRV compared to mos-RRV detected by the real-time PCR assay (Fig. 3B) could result in enhanced IFN induction. Therefore, we performed dose-response experiments to determine whether small differences in genome-to-PFU ratios between the mammalian- and mosquito-derived viruses could contribute to the differential IFN induction. Myeloid DCs derived from wild-type 129Sv/Ev mice were infected with mam-RRV or mos-RRV over a range of MOI (0.8 to 100.0 PFU/cell), and the percentage of infected cells and levels of type I IFN were determined at 12 hpi. As shown in Figure 4A, mos-RRV infected a higher percentage of cells than mam-RRV at all MOIs tested; however, mam-RRV infection induced higher levels of type I IFN at MOIs from 4 to 100 (Fig. 4B). The fact that mam-RRV induced higher IFN levels than mos-RRV over a 25-fold range of input virus, where mam-RRV induced more type I IFN at an MOI of 4.0 than mos-RRV at an MOI of 100.0, supports the idea that differences in particle number between the virus stocks are not solely responsible for the differences in IFN induction.
FIG. 4.
mam-RRV induces more type I IFN than mos-RRV over a broad range of viral doses. A. Infection percentages of immature mDCs from 129 Sv/ev mice infected with either mam- or mos-RRV ranging from 100 to 0.8 at 12 h postinfection. B. Type I IFN responses measured from the same cultures as in panel A. Solid lines represent mam-RRV, and dotted lines represent mos-RRV. Each point represents the mean and standard error of the mean of three samples. Differences between mam- and mos-RRV were statistically significant (P < 0.05) for all input doses except 0.8, as determined by a two-tailed unpaired Student's t test.
Differential type I IFN induction enhances mDC infection by mosquito-cell-derived RRV.
The observation that mos-RRV infected a higher percentage of mDCs than mam-RRV but was a poor inducer of type I IFN compared to mam-RRV raised the question of whether enhanced DC infection by mos-RRV was due to the viral effects on type I IFN induction. Therefore, mDCs were generated from mice lacking a functional type I IFN receptor (32) and infected with mosquito- or mammalian-cell-derived RRV at the same range of MOIs utilized in wild-type mDCs as in the experiment shown in Fig. 4. In contrast to the wild-type 129 mDCs, where mos-RRV showed enhanced infection, the type I IFN receptor-deficient mDCs showed equivalent levels of infection by viruses derived from either mammalian or mosquito cells (Fig. 5A). This result strongly suggests that the ability of mos-RRV to avoid or suppress the induction of the type I IFN response in infected cultures enhances that virus's ability to infect wild-type mDCs.
FIG. 5.
mam-RRV induces more type I IFN than mos-RRV in 129 αβ receptor-deficient mDCs. A. Infection percentages of immature mDCs from type I IFN receptor-deficient mice infected with mam- or mos-RRV at 12 h postinfection. B. IFN responses measured from the same samples as in panel A. Solid lines represent mam-RRV, and dotted lines represent mos-RRV. Each point represents the mean and standard error of the mean of three samples. Data are representative of at least three independent experiments.
Another important question was whether the differences in type I IFN induction between mos- and mam-RRV were due to a block of the initial IFN induction or an inhibition of amplification of the IFN response within the cultures. In most cells, including mDCs, the type I IFN response is an amplification loop, where a subset of early IFN genes (e.g., IFN-β and IFN-α4) are induced early in the virus infection, and these proteins then signal through the type I IFN receptor in an autocrine or paracrine manner to induce much higher levels of their own expression as well as the expression of late interferon genes (reviewed in reference 3). The absence of signaling from the receptor significantly reduces the levels of IFN induced both at the protein and mRNA level. The quantitative RT-PCR results, which showed significant differences in IFN-β mRNA induction between mos- and mam-RRV-infected cultures at 2 hpi (Fig. 1D) strongly suggested that the differences in type I IFN production between the mammalian and mosquito virus-infected cultures were at the level of the initial IFN induction. However, to test this further, IFN levels were measured in mDC cultures derived from the type I IFN receptor-deficient mice, as these cells are able to induce early IFN gene expression but the lack of type I IFN receptor signaling in these cells prevents amplification of the response (32). As shown in Fig. 5B, mam-RRV was able to induce type I IFN in the interferon receptor-deficient mDCs, while mos-RRV failed to induce detectable type I IFN responses as measured by bioassay. These results indicate that the mosquito-cell- and mammalian-cell-derived viruses differ in their ability to induce type I IFN, though an effect on the amplification step cannot be ruled out.
A single passage of Mos-RRV through BHK-21 cells restores the ability of the virus to induce type I IFN in mDC cultures.
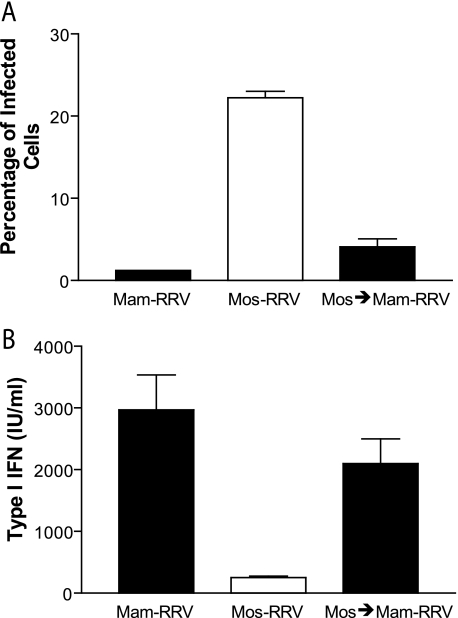
It is possible that genetic differences between mam- and mos-RRV explain the enhanced infection and decreased IFN production on mDCs. To test the stability of the mos-RRV phenotype, the mosquito-cell-derived virus, which was a poor type I IFN inducer, was passaged once at an MOI of 1 through BHK-21 cells, and the resulting mam-RRV virions were placed on mDCs (Fig. 6). A single passage of mos-RRV in mammalian cells resulted in a reduction of infection efficiency (Fig. 6A) on mDC cultures as well as an increase in type I IFN induction (Fig. 6B). This suggests that the lack of IFN induction by mos-RRV is likely not due to a coding change(s) in the viral genome, as genetic variation would not be predicted to consistently occur after just a single passage. Furthermore, these results strongly suggest that evasion of type I IFN induction by mos-RRV in the vertebrate host would only apply to the first infected cells, since progeny virions would have the properties of mammalian-cell-derived virus.
FIG. 6.
A single passage of mosquito-cell-derived virus (mos-RRV) through mammalian cells (generating mam-RRV) restores type I IFN induction in mDCs. A. Myeloid DCs were infected with mam-RRV, mos-RRV, or mos-RRV passaged a single time back through BHK-21 cells. Infection percentages were calculated for GFP-positive cells by flow cytometry at 12 h postinfection. B. Supernatants from each of the corresponding samples were evaluated by type I IFN bioassay. Data are from a representative experiment and are presented as the mean and standard error of the mean of three independent samples. mam-RRV compared to mos-RRV and mos-RRV compared to mos-RRV passaged through mammalian cells (mos-RRV → mam-RRV) were statistically significant (P < 0.05) as determined by one-way ANOVA for the experiments in both panels A and B. mam-RRV compared to mos-RRV → mam-RRV was not statistically significant as determined by one-way ANOVA for both panels A and B.
Differential type I interferon responses between mosquito- and mammalian-cell-derived viruses occur with other alphaviruses.
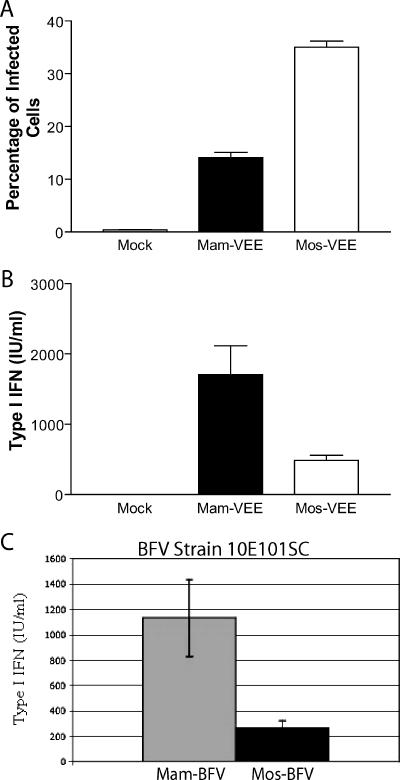
To evaluate whether type I IFN differences on primary mDCs were specific to RRV or applied to other alphaviruses, similar experiments were performed with VEE and BFV. Previous studies have demonstrated that VEE infects human dendritic cells in vitro (30) as well as murine mDCs in vitro (T. P. Moran, M. Pressley, and R. E. Johnston, unpublished data) and targets murine dendritic cells in vivo (26). Consistent with previous data, VEE was able to infect mDCs in vitro and, similar to RRV, mos-VEE infected more mDCs at 12 hpi than mam-VEE at 12 hpi (Fig. 7A), yet mos-VEE induced less type I IFN than mam-VEE (Fig. 7B). Similar results were observed following infection of mDCs with mosquito- and mammalian-cell-derived Barmah Forest virus (strain 10E101SC) in mDCs at 12 hpi by IFN bioassay (Fig. 7C). These results suggest that the differential type I IFN induction by the mosquito- and mammalian-cell-derived viruses is not specific to RRV and in fact may be a general trait of alphaviruses.
FIG. 7.
Mosquito-cell-derived VEE virus and BFV induce less type I IFN than mammalian-cell-derived viruses on mDCs. A. Myeloid DCs were infected with mam- and mos-VEE at an MOI of 8.0, and infectivity was calculated by flow cytometry based on GFP expression at 12 h postinfection. B. Supernatants from the same samples as in panel A were analyzed by type I IFN bioassay. C. mos-BFV (strain 10E101SC) with E2 N-linked glycans induces less type I IFN than mam-BFV at 12 h postinfection by IFN bioassay. Data in panels A and B represent the mean and standard error of the mean of three independent samples and are representative of results from two independent experiments. Differences between mam- and mos-VEE were statistically significant (P < 0.05) as determined by one-way ANOVA (A and B).
Differential N-linked glycosylation contributes to the differences in type I IFN induction by mosquito- and mammalian cell-derived alphaviruses.
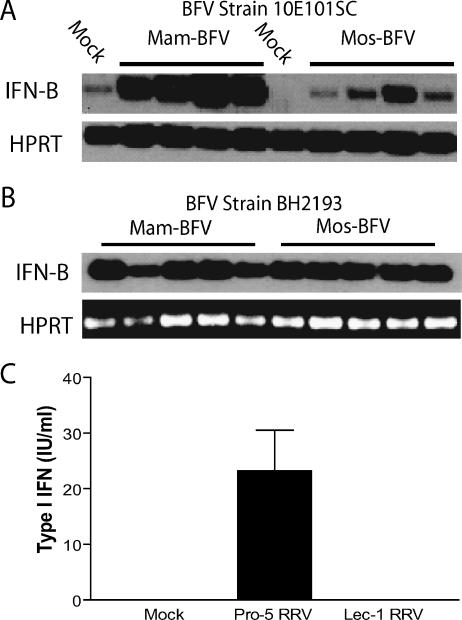
The 10E101SC strain of BFV initially used in these studies differs from most BFV strains in that it contains N-linked glycans on its E2 glycoprotein (S. Mahalingam, unpublished data). The IFN bioassay results shown in Fig. 7C were confirmed using a semiquantitative RT-PCR assay for IFN-β transcripts (Fig. 8A) and we next assessed whether classical strains of BFV, which do not have N-linked glycans on the E2 glycoprotein (22), also exhibited the same phenotype. When the BH2193 strain of BFV, which lacks N-linked E2 glycans, was assessed for type I IFN induction, there were no significant differences in type I IFN responses between mam- and mos-BFV by semiquantitative RT-PCR (Fig. 7E). While there are likely to be other differences between the two virus strains, these results strongly suggest that the N-linked glycans on the E2 glycoprotein contribute to the differential type I IFN induction by the mosquito- and mammalian-cell-derived viruses.
FIG. 8.
Glycosylation differences between mammalian- and mosquito-cell-derived virions of BFV and RRV contribute to differential type I IFN induction in myeloid DCs. A. Analysis of IFN induction by mam- or mos-BFV (strain 10E101SC), which has N-linked glycans on the E2 glycoprotein. mos-10E101SC BFV induced less type I IFN than mam-10E101SC BFV at 12 h postinfection as measured by RT-PCR for IFN-β transcripts. B. RT-PCR analysis for IFN-β message in mDCs following infection with BFV strain BH2193, which lacks glycosylation sites on the E2 glycoprotein. Both mos- and mam-BH2193 BVF induced IFN-β mRNA in infected mDCs. Each lane represents an independent sample. C. Virus derived from wild-type Pro-5 CHO cells (complex carbohydrates) induced more type I IFN in infected mDC cultures than virus derived from mutant Lec-1 CHO cells (high-mannose N-linked glycans) as measured by bioassay.
We therefore tested whether the presence of high-mannose N-linked glycans on mos-RRV were contributing to the poor IFN induction phenotype. RRV stocks were generated in parental CHO cells (Pro-5) or mutant CHO cells (Lec-1). Lec-1 CHO cells lack GlcNAc glycosyl transferase and glycans are blocked at the Man5-GlcNAC2-Asn intermediate. Therefore, viruses generated in Lec-1 cells have high mannose but no complex glycans on the virion (similar to mos-RRV), while the parental Pro-5 RRV contains complex glycans (similar to mam-RRV) (40, 41). Like mos-RRV generated from C6/36 cells, Lec-1 RRV was sensitive to endo-β-N-acetylglucosaminidase H (endo H) digestion, which is indicative of high-mannose glycans on the virion, while virus grown in BHK and Pro-5 cells was endo H resistant (data not shown). Furthermore, virus derived from Pro-5 cells (complex glycans) induced type I IFN in infected mDC cultures, while IFN levels in cultures infected with Lec-1-derived virus (high mannose) were below the limit of detection (Fig. 8C). Taken together, these data indicate that the presence of complex versus high-mannose glycans on the mammalian- and mosquito-derived viruses contributes to their differential ability to induce type I IFN responses in infected dendritic cells.
DISCUSSION
The transition from the arthropod vector to the vertebrate host is a critical step that is likely to play a major role in determining whether arboviruses successfully establish infection and disseminate throughout the vertebrate host. Therefore, mechanisms that would enable the newly delivered arbovirus to subvert the type I IFN response in mDCs may provide an advantage to the virus as it establishes infection in the vertebrate host. A number of factors, including virally encoded interferon antagonists, (1, 4, 33, 44) and immunosuppressive components of the arthropod saliva (23, 24, 37), are thought to modulate the host antiviral response against arthropod-borne viruses. However, the question of whether viruses delivered by the mosquito vector differ from mammalian-cell-derived viruses with respect to their ability to induce type I interferon responses has not previously been addressed.
In this report, we demonstrate that mammalian-cell-derived alphaviruses are potent inducers of type I IFN in mDCs, while mosquito-cell-derived viruses are poor inducers of type I IFN. This suggests that the virus initially delivered by the mosquito vector is able to suppress or avoid the induction of type I IFN in the initially infected dendritic cell. It is possible that this intrinsic ability of the mosquito-cell-derived virus to avoid the induction of a type I IFN response acts in concert with immunosuppressive factors present in arthropod saliva and/or virally encoded interferon antagonists to promote the successful transition of the virus from the arthropod vector to the vertebrate host.
Recent work with several mosquito-borne viruses, including dengue virus and VEE, have demonstrated that dendritic cells and/or Langerhans cells are an early target of viral replication following delivery into the skin (6, 26, 35, 46). Furthermore, the presence of high-mannose glycans on mosquito-derived Sindbis and West Nile virions has been shown to enhance viral infection of dendritic cells via interactions with the dendritic cell-specific mannose binding lectins DC-SIGN (CD209) (6, 18). The findings presented in this paper indicate that mosquito-derived viruses not only have an enhanced ability to infect murine dendritic cells, compared to mammalian-cell-derived viruses, but that these viruses also suppress or avoid the induction of type I IFN responses in infected dendritic cell cultures. Furthermore, this effect on interferon induction is likely to contribute to the enhanced infection efficiency of mDCs by the mosquito-derived virus, as evidenced by the decrease in infection differences between mam- and mos-RRV in type I IFN receptor-deficient DCs compared to wild-type DCs (Fig. 5B).
Myeloid DCs are capable of quickly responding to the incoming virus by mounting a very rapid antiviral response. This response has been shown to be independent of MyD88 for Semliki Forest virus, another alphavirus (15), and preliminary experiments with RRV and VEE indicate type I IFN induction in mDCs is MyD88 and Toll-like receptor 3 independent (R. S. Shabman, T. Morrison, and M. Heise, unpublished results). Furthermore, several studies have shown that mDCs rely on the RIG-I/Mda5 pathway to sense incoming viruses and induce type I IFN responses (16). Although a growing body of evidence indicates that mDCs can rapidly respond to viral infections and that they play a central role in regulating the host immune response to incoming pathogens, these cell types are also an early target for arbovirus replication. Our results suggest that the virus initially delivered by the mosquito vector differs from the virus subsequently produced in mammalian cells, and this difference may allow the mosquito-derived virus to avoid induction of an antiviral response in the initially infected DC.
One of the major differences between the mosquito- and mammalian-cell-derived viruses is the exclusive presence of mannose glycans (high mannose and paucimannose) on the mosquito-derived viruses and complex/hybrid/high-mannose glycans on the mammalian-cell-derived virus (25). A role for N-linked glycans in mediating the differential type I IFN induction by the mammalian- and mosquito-derived viruses is supported by the finding that the 10E101SC strain of Barmah Forest virus, which has N-linked glycans on its E2 glycoprotein, exhibited the differential type I IFN induction, while the BH2193 strain, which lacks N-linked glycans on its E2 glycoprotein, showed no difference in type I IFN induction between the mammalian- and mosquito-derived virus preparations (Fig. 8A and B). Furthermore, RRV generated in Pro-5 cells with complex glycan additions induced more type I IFN on mDCs than RRV generated in Lec-1 cells, which contain exclusively mannose glycans (Fig. 8C). Therefore, additional studies are needed to directly assess the role of N-linked glycans in this phenotype, since it is possible that high-mannose glycans on the mosquito-derived virus interact with C-type lectins on dendritic cells, resulting in an inhibition of type I IFN from the mDC cultures. This is intriguing, since previous data indicate that ligation of DC-SIGN, the mannose receptor, or other C-type lectin receptors suppresses or modifies Toll-like receptor signaling in human mDCs and pDCs (5, 9, 14, 20). Therefore, additional studies on the role of high-mannose glycans on the mosquito-cell-derived virus in blocking type I IFN induction by the virus are required.
Experiments presented here were designed to explore several potential mechanisms other than the presence of high-mannose species on the mosquito-cell-derived viruses, including nonviral factors, differences in the number of defective interfering particles, which are known to be potent inducers of type I IFN (11, 12), or genetic differences in the viruses derived from the mammalian versus mosquito cells.
Several lines of evidence argue against nonviral factors being responsible for the differing type I IFN induction between the mosquito- and mammalian-cell-derived viruses. First, the results are highly reproducible between multiple virus preparations and with several different alphaviruses. Secondly, the type I IFN induction by mam-RRV appears to be virus specific, since the IFN inductive capacity separated with the ultracentrifuged virus pellet. Boiling, which would denature the virus, but not lipopolysaccharide, which can also induce type I IFN (13), abolished the ability of the virus preparation to induce type I IFN.
The finding that multiple assays comparing mos- and mam-RRV preparations vary in their particle-to-PFU ratio by only 2- to 11-fold, while the difference in type I IFN induction in mDCs occurs over a 25-fold range of input virus, argues against a role for defective interfering particles being responsible for the type I IFN induction differences. A role for genetic differences between the mosquito- and mammalian-derived viruses cannot be ruled out. Strong selection can rapidly select for adaptive mutations in alphaviruses (19, 34); however, the fact that a single pass through mosquito or mammalian cells reproducibly results in a switch in the interferon induction phenotype argues against this possibility or suggests that an extremely strong selection event is occurring that selects for a genetic change leading to poor type I IFN induction. Finally, it is possible that in addition to differential glycosylation, differences in viral cholesterol content, or modifications to the viral nucleic acid in a cell-type-specific manner could affect the ability of the virus to induce type I IFN responses in mDCs but not other cell types. Therefore, additional studies are needed to address these possibilities.
The poor IFN induction by mosquito-cell-derived viruses was observed for several different alphavirus family members. These included RRV, VEE, and BFV, suggesting that poor IFN induction by mosquito-derived virus may be a general trait of alphaviruses. Whether this effect is specific to alphaviruses or reflects a more general trait of arthropod-borne viruses is an important question that remains to be determined. However, these studies demonstrate that at least a subset of mosquito-derived viruses are able to infect dendritic cells without initiating a potent antiviral response. Though this effect is likely to only occur during the initial round of replication, since the subsequent mammalian-cell-derived virus would be expected to induce an IFN response (Fig. 6), this may be sufficient to give the incoming virus an advantage that permits the establishment of infection. Therefore, further study of the mechanisms underlying the ability of the mosquito-cell-derived alphaviruses to avoid or suppress type I IFN induction in mDCs is likely to significantly enhance our understanding of how mosquito-borne viruses initially establish infection and interact with the host innate immune system.
Acknowledgments
We thank Nancy Davis, Robert Johnston, Joseph Thompson, Clayton Beard, Aravinda deSilva, and Kristen Bernard for helpful scientific discussions and/or critical reading of the manuscript. We thank Alan Whitmore for assistance with murine dendritic cell phenotyping as well as helpful scientific discussions. We also thank Timothy Moran and Jon Serody for assistance in generating murine and human myeloid dendritic cells and Kenya Madric and Wrennie Edwards for assistance with cell culture.
The work was supported by research grant R01 AR47190-05 to M.T.H. and NHMRC grant no. 303404 to S.M. T.E.M. was supported by F32AR052600-02. S.M. is a recipient of an NHMRC RD Wright Fellowship.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA 101:17264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antalis, T. M., M. La Linn, K. Donnan, L. Mateo, J. Gardner, J. L. Dickinson, K. Buttigieg, and A. Suhrbier. 1998. The serine proteinase inhibitor (serpin) plasminogen activation inhibitor type 2 protects against viral cytopathic effects by constitutive interferon alpha /beta priming. J. Exp. Med. 187:1799-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biron, C. A., and G. C. Sen. 2001. Interferons and other cytokines, p. 321-351. In B. N. Fields and D. M. Knipe (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 4.Cardenas, W. B., Y.-M. Loo, M. Gale, Jr., A. L. Hartman, C. R. Kimberlin, L. Martinez-Sobrido, E. O. Saphire, and C. F. Basler. 2006. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J. Virol. 80:5168-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chieppa, M., G. Bianchi, A. Doni, A. Del Prete, M. Sironi, G. Laskarin, P. Monti, L. Piemonti, A. Biondi, A. Mantovani, M. Introna, and P. Allavena. 2003. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J. Immunol. 171:4552-4560. [DOI] [PubMed] [Google Scholar]
- 6.Davis, C. W., H.-Y. Nguyen, S. L. Hanna, M. D. Sanchez, R. W. Doms, and T. C. Pierson. 2006. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 80:1290-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. R. E. Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. [DOI] [PubMed] [Google Scholar]
- 8.Doherty, R. L., R. H. Whitehead, B. M. Gorman, and A. K. O'Gower. 1963. The isolation of a third group A arbovirus in Australia, with preliminary observations on its relationship to epidemic polyarthritis. Aust. J. Sci. 26:183-184. [Google Scholar]
- 9.Dzionek, A., Y. Sohma, J. Nagafune, M. Cella, M. Colonna, F. Facchetti, G. Gunther, I. Johnston, A. Lanzavecchia, T. Nagasaka, T. Okada, W. Vermi, G. Winkels, T. Yamamoto, M. Zysk, Y. Yamaguchi, and J. Schmitz. 2001. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J. Exp. Med. 194:1823-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott, R. 1997. Emerging viruses: the Bunyaviridae. Mol. Med. 3:572-577. [PMC free article] [PubMed] [Google Scholar]
- 11.Fuller, F. J., and P. I. Marcus. 1980. Interferon induction by viruses. IV. Sindbis virus: early passage defective-interfering particles induce interferon. J. Gen. Virol. 48:63-73. [DOI] [PubMed] [Google Scholar]
- 12.Fuller, F. J., and P. I. Marcus. 1980. Interferon induction by viruses. Sindbis virus: defective-interfering particles temperature-sensitive for interferon induction. J. Gen. Virol. 48:391-394. [DOI] [PubMed] [Google Scholar]
- 13.Gauzzi, M. C., I. Canini, P. Eid, F. Belardelli, and S. Gessani. 2002. Loss of type I IFN receptors and impaired IFN responsiveness during terminal maturation of monocyte-derived human dendritic cells. J. Immunol. 169:3038-3045. [DOI] [PubMed] [Google Scholar]
- 14.Geijtenbeek, T. B., S. J. Van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. Vandenbroucke-Grauls, B. Appelmelk, and Y. Van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidmark, A. S., G. M. McInerney, E. K. L. Nordstrom, I. Douagi, K. M. Werner, P. Liljestrom, and G. B. K. Hedestam. 2005. Early alpha/beta interferon production by myeloid dendritic cells in response to UV-inactivated virus requires viral entry and interferon regulatory factor 3 but not MyD88. J. Virol. 79:10376-10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19-28. [DOI] [PubMed] [Google Scholar]
- 17.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131-137. [DOI] [PubMed] [Google Scholar]
- 18.Klimstra, W. B., E. M. Nangle, M. S. Smith, A. D. Yurochko, and K. D. Ryman. 2003. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J. Virol. 77:12022-12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klimstra, W. B., K. D. Ryman, and R. E. Johnston. 1998. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72:7357-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koppel, E. A., I. S. Ludwig, M. S. Hernandez, T. L. Lowary, R. R. Gadikota, A. B. Tuzikov, C. M. Vandenbroucke-Grauls, Y. van Kooyk, B. J. Appelmelk, and T. B. Geijtenbeek. 2004. Identification of the mycobacterial carbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology 209:117-127. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn, R. J., H. G. Niesters, Z. Hong, and J. H. Strauss. 1991. Infectious RNA transcripts from Ross River virus cDNA clones and the construction and characterization of defined chimeras with Sindbis virus. Virology 182:430-441. [DOI] [PubMed] [Google Scholar]
- 22.Lee, E., C. Stocks, P. Lobigs, A. Hislop, J. Straub, I. Marshall, R. Weir, and L. Dalgarno. 1997. Nucleotide sequence of the Barmah Forest virus genome. Virology 227:509-514. [DOI] [PubMed] [Google Scholar]
- 23.Limesand, K. H., S. Higgs, L. D. Pearson, and B. J. Beaty. 2003. Effect of mosquito salivary gland treatment on vesicular stomatitis New Jersey virus replication and interferon alpha/beta expression in vitro. J. Med. Entomol. 40:199-205. [DOI] [PubMed] [Google Scholar]
- 24.Limesand, K. H., S. Higgs, L. D. Pearson, and B. J. Beaty. 2000. Potentiation of vesicular stomatitis New Jersey virus infection in mice by mosquito saliva. Parasite Immunol. 22:461-467. [DOI] [PubMed] [Google Scholar]
- 25.Lozach, P.-Y., L. Burleigh, I. Staropoli, E. Navarro-Sanchez, J. Harriague, J.-L. Virelizier, F. A. Rey, P. Despres, F. Arenzana-Seisdedos, and A. Amara. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J. Biol. Chem. 280:23698-23708. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald, G. H., and R. E. Johnston. 2000. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 74:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahalingam, S., G. Chaudhri, C. Ling Tan, A. John, P. S. Foster, and G. Karupiah. 2001. Transcription of the interferon γ (IFN-γ)-inducible chemokine Mig in IFN-γ-deficient mice. J. Biol. Chem. 276:7568-7574. [DOI] [PubMed] [Google Scholar]
- 28.Mahalingam, S., and B. A. Lidbury. 2002. Suppression of lipopolysaccharide-induced antiviral transcription factor (STAT-1 and NF-kappa B) complexes by antibody-dependent enhancement of macrophage infection by Ross River virus. Proc. Natl. Acad. Sci. USA 99:13819-13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majde, J. A. 1993. Microbial cell-wall contaminants in peptides: a potential source of physiological artifacts. Peptides 14:629-632. [DOI] [PubMed] [Google Scholar]
- 30.Moran, T. P., M. Collier, K. P. McKinnon, N. L. Davis, R. E. Johnston, and J. S. Serody. 2005. A novel viral system for generating antigen-specific T cells. J. Immunol. 175:3431-3438. [DOI] [PubMed] [Google Scholar]
- 31.Morrison, T. E., A. C. Whitmore, R. S. Shabman, B. A. Lidbury, S. Mahalingam, and M. T. Heise. 2006. Characterization of Ross River virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis. J. Virol. 80:737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 33.Munoz-Jordan, J. L., M. Laurent-Rolle, J. Ashour, L. Martinez-Sobrido, M. Ashok, W. I. Lipkin, and A. Garcia-Sastre. 2005. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 79:8004-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olmsted, R. A., R. S. Baric, B. A. Sawyer, and R. E. Johnston. 1984. Sindbis virus mutants selected for rapid growth in cell culture display attenuated virulence in animals. Science 225:424-427. [DOI] [PubMed] [Google Scholar]
- 35.Ryman, K. D., K. C. Meier, E. M. Nangle, S. L. Ragsdale, N. L. Korneeva, R. E. Rhoads, M. R. MacDonald, and W. B. Klimstra. 2005. Sindbis virus translation is inhibited by a PKR/RNase L-independent effector induced by alpha/beta interferon priming of dendritic cells. J. Virol. 79:1487-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryman, K. D., L. J. White, R. E. Johnston, and W. B. Klimstra. 2002. Effects of PKR/RNase L-dependent and alternative antiviral pathways on alphavirus replication and pathogenesis. Viral Immunol. 15:53-76. [DOI] [PubMed] [Google Scholar]
- 37.Schneider, B. S., L. Soong, N. S. Zeidner, and S. Higgs. 2004. Aedes aegypti salivary gland extracts modulate anti-viral and TH1/TH2 cytokine responses to Sindbis virus infection. Viral Immunol. 17:565-573. [DOI] [PubMed] [Google Scholar]
- 38.Sejvar, J. J., M. B. Haddad, B. C. Tierney, G. L. Campbell, A. A. Marfin, J. A. Van Gerpen, A. Fleischauer, A. A. Leis, D. S. Stokic, and L. R. Petersen. 2003. Neurologic manifestations and outcome of West Nile virus infection. JAMA 290:511-515. [DOI] [PubMed] [Google Scholar]
- 39.Serody, J. S., E. J. Collins, R. M. Tisch, J. J. Kuhns, and J. A. Frelinger. 2000. T cell activity after dendritic cell vaccination is dependent on both the type of antigen and the mode of delivery. J. Immunol. 164:4961-4967. [DOI] [PubMed] [Google Scholar]
- 40.Stanley, P., V. Caillibot, and L. Siminovitch. 1975. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cell. Cell 6:121-128. [DOI] [PubMed] [Google Scholar]
- 41.Stanley, P., S. Narasimhan, L. Siminovitch, and H. Schachter. 1975. Chinese hamster ovary cells selected for resistance to the cytotoxicity of phytohemagglutinin are deficient in a UDP-N-acetylglucosamine-glycoprotein N-acetylglucosaminyltransferase activity. Proc. Natl. Acad. Sci. USA 72:3323-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strauss, J. H., and E. G. Strauss. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58:491-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suthar, M. S., R. Shabman, K. Madric, C. Lambeth, and M. T. Heise. 2005. Identification of adult mouse neurovirulence determinants of the Sindbis virus strain AR86. J. Virol. 79:4219-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber, F., A. Bridgen, J. K. Fazakerley, H. Streitenfeld, N. Kessler, R. E. Randall, and R. M. Elliott. 2002. Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J. Virol. 76:7949-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White, L. J., J.-G. Wang, N. L. Davis, and R. E. Johnston. 2001. Role of alpha/beta interferon in Venezuelan equine encephalitis virus pathogenesis: effect of an attenuating mutation in the 5′ untranslated region. J. Virol. 75:3706-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu, S.-J. L., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]