Abstract
Latent membrane protein 2A (LMP2A) and LMP2B are viral proteins expressed during Epstein-Barr virus (EBV) latency in EBV-infected B cells both in cell culture and in vivo. LMP2A has important roles in modulating B-cell receptor (BCR) signal transduction by associating with the cellular tyrosine kinases Lyn and Syk via specific phosphotyrosine motifs found within the LMP2A N-terminal tail domain. LMP2A has been shown to alter normal BCR signal transduction in B cells by reducing levels of Lyn and by blocking tyrosine phosphorylation and calcium mobilization following BCR cross-linking. Although little is currently known about the function of LMP2B in B cells, the similarity in structure between LMP2A and LMP2B suggests that they may localize to the same cellular compartments. To investigate the function of LMP2B, B-cell lines expressing LMP2A, LMP2B, LMP2A/LMP2B, and the relevant vector controls were analyzed. As was previously shown, cells expressing LMP2A had a dramatic block in normal BCR signal transduction as measured by calcium mobilization and tyrosine phosphorylation. There was no effect on BCR signal transduction in cells expressing LMP2B. Interestingly, when LMP2B was expressed in conjunction with LMP2A, there was a restoration of normal BCR signal transduction upon BCR cross-linking. The expression of LMP2B did not alter the cellular localization of LMP2A but did bind to and prevent the phosphorylation of LMP2A. A restoration of Lyn levels, but not a change in LMP2A levels, was also observed in cells coexpressing LMP2B with LMP2A. From these results, we conclude that LMP2B modulates LMP2A activity.
Epstein-Barr virus (EBV) is a member of the gammaherpesvirus family which establishes a latent, persistent infection in B cells. EBV has been associated with several human cancers, including African Burkitt's lymphoma, Hodgkin's disease, adult T-cell leukemia, nasopharyngeal carcinoma, and some gastric cancers. It has been proposed that EBV uses different programs of gene expression to artificially drive B-cell development to the long-lived memory B-cell compartment and thereby establish latency (3, 4, 39, 46, 47, 88, 90). In this model, EBV infects naive B cells that traffic in close proximity to the oral mucosal epithelium. The resulting infected cells express the latency III or growth program in which all of the EBV nuclear antigens (EBNAs; EBNA1, EBNA2, EBNA3A, EBNA3B, EBNA3C, and EBNALP), LMP1, and LMP2 are expressed (5, 46, 81, 84, 90). This program of gene expression activates B cells, which migrate into follicles to form germinal centers. These cells are in a state that resembles antigen- and CD40-driven B-cell activation and proliferation (90, 91). Once the B cell has moved into the follicle, the virus switches to the latency II or default program of gene expression to deliver signals normally supplied by antigen and T-helper cells. In this program, EBNA1, LMP1, and LMP2A are expressed (6, 11, 27, 83, 100). LMP2A has been shown to deliver survival signals to B cells, as well as drive B cells to go through isotype switching and form germinal centers (13, 14, 41). LMP1 acts as a constitutively active CD40 receptor to deliver signals that would normally be delivered by T-helper cells (33, 37, 40, 52, 73, 94, 97). Once the infected B cells exit to the peripheral circulation, EBV gene expression switches to a nearly quiescent state. EBV transcripts have been detected in infected peripheral B cells, but few if any viral proteins are expressed to allow the latently infected cells to avoid immune surveillance (5, 16, 17, 24, 38, 72, 80, 92).
LMP2A is one of two isoforms transcribed by the LMP2 gene and functions to deliver development and survival signals to B cells even in the absence of normal BCR signals (13, 14). LMP2B, the other isoform of the LMP2 gene, has not been well studied, and as a result the function of LMP2B in B cells is largely unknown. LMP2A and LMP2B are transcribed under the control of two separate promoters separated by 3 kb and differ only in their first exons; exon 1 of LMP2A encodes a 119-amino-acid N-terminal tail, while exon 1 of LMP2B is noncoding (7, 54-56, 60, 82, 86, 93, 101). The promoter of LMP2A lies directly upstream of the first exon, while LMP2B shares a bidirectional promoter with LMP1 (55, 86). Both are transcribed across the fused terminal repeats of the EBV episome.
LMP2A has important roles in modulating B-cell receptor (BCR) signal transduction by associating with the cellular tyrosine kinases Lyn and Syk via specific phosphotyrosine motifs found within the LMP2A N-terminal tail domain (12, 29, 30, 59). In uninfected primary B cells, cross-linking of the BCR by antigen aggregates BCRs into glycosphingolipid-rich microdomains (lipid rafts) of the plasma membrane (19). These lipid rafts contain an increased concentration of Src family protein tyrosine kinases such as Lyn which interact with BCR immunoglobulin alpha (Igα) and Igβ subunits and mediate the phosphorylation of the BCR immunoreceptor tyrosine activation motifs (ITAMs) (45, 51, 77). ITAMs with both tyrosine residues phosphorylated become a binding site for the dual SH2 domains of the tyrosine kinase Syk (85). Binding of Syk to the ITAM results in its phosphorylation and activation via Lyn and activation of subsequent signaling events including protein phosphorylation and calcium mobilization. LMP2A has been shown to alter normal BCR signal transduction in B cells by reducing levels of Lyn and consequently downregulating tyrosine phosphorylation and calcium mobilization following BCR cross-linking (68-70).
Mutational analysis has identified residues important in mediating the effects of LMP2A. Mutation of residue Y112 of LMP2A results in the inability of LMP2A to bind the SH2 domain of Lyn with high affinity in order to facilitate the phosphorylation of Lyn, Syk, and LMP2A (30). Cells expressing Y112 mutants were also able to readily mobilize calcium and exhibit tyrosine phosphorylation upon BCR cross-linking. Y74 and Y85 of LMP2A form a putative ITAM, which has been demonstrated to be important in recruiting and constitutively phosphorylating Syk (29). Mutation of either of these tyrosine residues restores calcium mobilization upon BCR cross-linking. Additionally, the dual PPPPY proline motifs found in the N-terminal tail of LMP2A have been shown to recruit Nedd4 ubiquitin ligases (42, 98). Mutations of these motifs result in the restoration of Lyn to wild-type levels and an increase in the steady-state phosphorylation of a number of cellular proteins, including LMP2A (43). Interestingly, there was induction of tyrosine phosphorylation upon BCR cross-linking in the dual PPPPY motif mutants.
Although both LMP2A and LMP2B have been shown to promote motility and cell spread in epithelial cells, little is known about the function of LMP2B in B cells (2, 18, 61, 75). The sequence homology of LMP2B to LMP2A suggests that both proteins may localize to similar cellular compartments and may have some functions in common. The lack of the amino-terminal domain found in LMP2A has led us to hypothesize that LMP2B may negatively regulate LMP2A activity.
MATERIALS AND METHODS
Cell lines and cell culture.
BJAB cell lines were maintained in RPMI 1640 medium containing 10% fetal bovine serum, 1,000 U/ml penicillin, 1,000 μg/ml streptomycin, 400 U/ml hygromycin, and 210 mg/ml neomycin. Previously described LMP2A+ and LMP2A− cell lines were used in the construction of the four cell lines (42). The complete LMP2B coding sequence, as described previously (86), was ligated into the pLXSN neomycin selectable retroviral vector with a hemagglutinin (HA) tag on the C-terminal tail, and retrovirus stocks were made by transient transfection of the GP293 cell line. The resulting stocks were used to infect BJAB cells, which were then selected for in 210 mg/ml neomycin. Cells were also plated in 96-well plates 48 h postinfection to derive clonal cell lines in parallel with the batch lines. Three clonal lines were derived for each of the batch lines.
Cell fractionation.
Fractionation of cellular lysates was performed with discontinuous sucrose gradients as described previously (20). Cells (107) were electroporated with 10 μg of RL34 (LMP2A) or 10 μg of RL34 and 10 μg of RL169 (HA-tagged LMP2B) and incubated for 18 h at 37°C. These cells were lysed in 1 ml of 1% Triton X-100 lysis buffer in TNE (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA) with protease inhibitors (1 μM pepstatin, 1 μM leupeptin, and 0.5 mM phenylmethylsulfonyl fluoride) at 4°C for 30 min. Lysates were further homogenized with 10 strokes of a Wheaton Dounce homogenizer and spun down to remove debris. Lysates were diluted 1:1 with 1 ml of cold 85% sucrose in TNE in the bottom of a Beckman centrifuge tube (14 by 89 mm). The lysate mixture was then overlaid with 6 ml of 35% sucrose in TNE, followed by 3.5 ml of 5% sucrose in TNE. Gradients were centrifuged at 200,000 × g in an SW41 rotor for 18 h at 4°C. One-milliliter fractions were collected from the top of the gradient and diluted 4:1 with 5× sodium dodecyl sulfate (SDS) sample buffer. Fractions 4 and 11 were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotted with a rat anti-LMP2A antibody (14B7) and a mouse anti-CD45 antibody. One-quarter of the volume of fraction 11 was loaded into the SDS-PAGE gel, compared to fraction 4, to equalize the protein concentration. Fractionation of cellular lysates was also performed on the 2A and 2A2B cell lines described above.
Calcium mobilization.
Cells were incubated in 1 ml serum-free RPMI 1640 medium and loaded with the calcium-binding dye Indo-1 (Invitrogen) at 3 μM at 37°C for 30 min. After loading, cells were washed twice and resuspended in 1 ml serum-free medium. Cells were allowed to incubate for an additional 30 min at 37°C and then analyzed with a BD LSR II fluorescence-activated cell sorter (FACS). A baseline was established for approximately 30 s before 10 μg of goat anti-human cross-linking antibody per ml of cell suspension was added. Calcium flux was measured for an additional 4 min after the addition of cross-linking antibodies. The percentage of cells responding was calculated by dividing the number of cells responding (cells above the baseline) by the total number of cells. Percent calcium flux was calculated by dividing the ratio of bound (405 nm) to unbound (485 nm) dye in cells responding (cells above the baseline) by the ratio of bound to unbound dye in all cells. For experiments with untagged LMP2B, 107 BJAB cells were transiently transfected with a plasmid expressing red fluorescent protein (RFP), with plasmids expressing LMP2A and RFP, or with plasmids expressing LMP2A, RFP, and untagged LMP2B with a Bio-Rad Gene Pulser. The cells were incubated for 18 h at 37°C and then examined by flow cytometry analysis. Cells were gated on RFP to exclude untransfected cells, and the calcium flux stimulated by BCR cross-linking was examined as described above.
Tyrosine phosphorylation assay.
Two samples of each cell line were incubated in 0.5 ml serum-free medium at 37°C for 90 min. Five microliters of goat anti-human cross-linking antibody was added to one of the two samples of each cell line and allowed to incubate at 37°C for 5 min. Triton X-100 lysates were then prepared from both the cross-linked and uncross-linked samples and immunoprecipitated with anti-pY antibody and protein G beads overnight. Immunoprecipitates were washed three times in Triton X-100 buffer, resuspended in 2× SDS buffer, heated to 70°C for 5 min, separated by 10% SDS-PAGE, and immunoblotted with a horseradish peroxidase (HRP)-conjugated anti-pY antibody. Proteins were detected with ECL Plus.
Antibodies.
Rat monoclonal antibodies 14B7, 8C3, 14E6, and 15F9 have been previously described (28). All HRP-linked secondary antibodies were purchased from Amersham (Arlington Heights, IL). The mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was purchased from Abcam. The anti-HA epitope mouse monoclonal antibody (HA11) and rat monoclonal antibody (3F10) were purchased from Babco and Boehringer-Mannheim, respectively. Goat anti-human Ig (IgM plus IgG plus IgA, heavy and light chains) for cross-linking surface Ig experiments and fluorescein isothiocyanate (FITC)-conjugated anti-human Ig antibody were purchased from Southern Biotechnology Associates (Santa Cruz, CA). The mouse monoclonal antibody to Syk (4D10), the anti-phosphotyrosine antibody (PY20), and the anti-phosphotyrosine antibody conjugated to HRP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse CD45 monoclonal antibody was purchased from Transduction Laboratories. The Cy3-conjugated donkey anti-rat secondary antibody and FITC-conjugated goat anti-mouse antibody used for immunofluorescence were purchased from Jackson and Invitrogen, respectively. Microtubule-binding peripheral Golgi membrane protein 58k was purchased from Sigma. NeutrAvidin-HRP was purchased from Pierce. The Lyn mouse monoclonal antibody was purchased from Transduction Laboratories.
Immunofluorescence.
To examine colocalization of LMP2A with IgM, cell lines were fixed to a glass slide with methanol at −20°C for 5 min, blocked for 30 min in 20% goat serum, and incubated with anti-LMP2A antibodies (14B7, 8C3, 14E6, and 15F9) for 2 h at a 1:8,000 dilution. Cells were washed with phosphate-buffered saline (PBS), incubated with a Cy3-conjugated donkey anti-rat secondary antibody at a 1:500 dilution and an FITC-conjugated goat anti-IgM antibody at a 1:2,000 dilution for 30 min, and examined with a Leica DMIRE2 inverted microscope. To examine colocalization of LMP2A with the Golgi apparatus, cell lines were fixed to a glass slide with methanol at −20°C for 5 min, blocked for 30 min in 20% goat serum, and incubated with anti-LMP2A antibodies at a 1:8,000 dilution and mouse 58k antibody (microtubule-binding peripheral Golgi membrane protein 58k) at a 1:200 dilution for 2 h. Cells were washed with PBS, incubated with a Cy3-conjugated donkey anti-rat secondary antibody at a 1:500 dilution and an FITC-conjugated anti-mouse antibody at a 1:400 dilution for 30 min, stained with 4′,6′-diamidino-2-phenylindole (DAPI; Vectashield), and examined with a Leica DMIRE2 inverted microscope.
Immunoprecipitation and immunoblotting.
Lysates were cleared and incubated with either protein G or protein A beads and with the appropriate antibodies (indicated in the figure legends) overnight at 4°C. Immunoprecipitates were washed three times in Triton X-100 buffer, resuspended in 2× SDS buffer, heated to 70°C for 5 min, and separated by 12% SDS-PAGE.
For immunoblotting, cell lysates and immunoprecipitates were electrophoresed and transferred to Immobilon membranes. Membranes were blocked in Tris-buffered saline-Tween (TBST) containing 5% milk for 1 h at room temperature, incubated for 1 h in milk containing the appropriate primary antibodies at room temperature, washed three times in TBST, and incubated with secondary antibodies in milk for 30 min at room temperature. For detection, membranes were washed three times in TBST and detected with ECL Plus (Pierce, Rockford, IL).
RESULTS
Construction of LMP2A, LMP2B, LMP2A/LMP2B, and vector control BJAB cell lines.
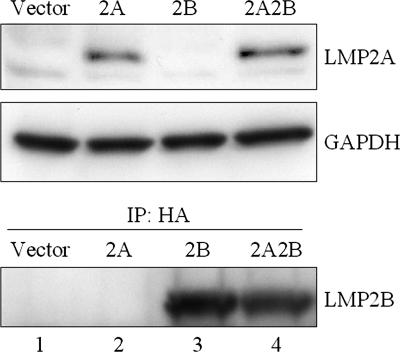
In order to investigate the effects of LMP2B on LMP2A signaling, previously constructed LMP2A-expressing BJAB cell lines were used (42). An HA-tagged LMP2B (2B) retroviral construct encoding a neomycin resistance gene and a vector control were used to infect the LMP2A and vector control hygromycin-resistant BJAB cells. Infected cells were selected in neomycin and hygromycin and generated four different cell lines. Because of the lack of LMP2B-specific antibodies, LMP2B was HA tagged at the C terminus to facilitate detection. In addition to the 4 polyclonal batch cell lines, 12 monoclonal cell lines (3 clonal cell lines for each of the batch lines) were expanded and tested. The results for the polyclonal cell populations are shown in Fig. 1. Similar data were also observed for the clonal populations. LMP2A was detected in both the batch and clonal 2A and 2A2B cell lines, and LMP2B was detected in the batch and clonal 2B and 2A2B cell lines. LMP2A and LMP2B were not detected in any of the vector batch or clonal cell lines. Similar expression levels of LMP2A and LMP2B were observed in both batch and clonal cell lines irrespective of whether the lines expressed LMP2A, LMP2B, or both LMP2A and LMP2B. BCR expression was examined in batch-expressing lines by FACS analysis monitoring surface Ig expression, and no significant differences were observed for any of the cell lines tested (data not shown).
FIG. 1.
Expression of LMP2A and LMP2B in BJAB cell lines. (A) Triton X-100 lysates of the four separate BJAB cell lines were separated by SDS-PAGE, transferred to Immobilon, blocked for 1 h, immunoblotted with anti-LMP2A antibody 14B7, and incubated with an anti-rat HRP-conjugated secondary antibody. Proteins were detected with ECL Plus. The blot was stripped and reprobed for GAPDH as a loading control. Levels of LMP2A expression were similar in the 2A and 2A2B cell lines. (B) Lysates were immunoprecipitated with rat anti-HA antibody, separated by SDS-PAGE, transferred to Immobilon, blocked for 1 h, immunoblotted with mouse anti-HA antibody, incubated with an anti-mouse HRP-conjugated secondary antibody, and detected with ECL Plus. LMP2B was detected in both the 2B and 2A2B cell lines. IP, immunoprecipitate.
Coexpression of LMP2B does not alter the cellular localization of LMP2A.
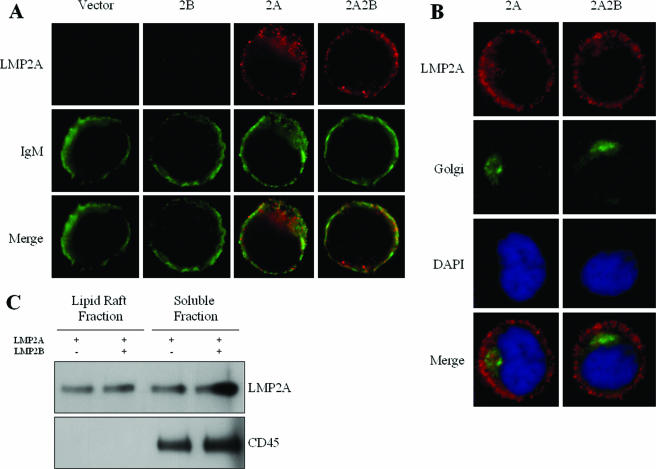
It has been previously suggested that LMP2A localizes to the plasma membrane of B cells, colocalizes with cell surface-associated proteins, and excludes BCRs from translocating to lipid rafts upon BCR cross-linking (25, 59, 60). To investigate if expression of LMP2B alters the localization of LMP2A, immunofluorescence microscopy was used to detect LMP2A in the four BJAB cell lines (Fig. 2). LMP2A was detected in both the 2A and 2A2B cell lines with rat anti-LMP2A primary antibodies and goat anti-rat Cy3-conjugated secondary antibodies. Cells were also stained with antibodies against the microtubule-binding peripheral Golgi membrane protein 58k and against IgM.
FIG. 2.
Coexpression of LMP2B does not affect LMP2A localization. (A and B) Stably transfected BJAB cells were fixed to a glass slide with methanol, blocked for 30 min in 20% goat serum, and incubated with anti-LMP2A antibody for 2 h at a 1:8,000 dilution. Cells were washed with PBS, incubated with an FITC-conjugated anti-rat secondary antibody for 30 min, and examined with a microscope. Cells were also stained with FITC-conjugated antibodies against the BCR (A) or primary antibodies against the microtubule-binding peripheral Golgi membrane protein 58k and FITC-conjugated secondary antibodies and DAPI (B). Coexpression of LMP2B does not seem to alter the localization of LMP2A with IgM or the Golgi, as demonstrated by the 2A and 2A2B cell lines. (C) Lysates were prepared from equal numbers of cells transiently transfected with either LMP2A or LMP2A and LMP2B and lysed in 1% Triton X-100 in TNE with protease inhibitors at 4°C for 30 min. Lysates were subjected to discontinuous sucrose gradient centrifugation, and fractions were collected. Fractions 4 and 11, which have been previously shown to correspond to the membrane raft fraction and soluble fraction, were separated by SDS-PAGE and immunoblotted with a rat anti-LMP2A antibody (14B7). Fractions were also probed for CD45. One-quarter of the volume of fraction 11 was loaded, compared to fraction 4, to equalize the protein concentration. Similar levels of LMP2A were detected in the membrane raft fractions of both lysates, as well as the soluble fractions.
When LMP2A staining is merged with the IgM staining, a modest overlap is observed, compatible with the observation that LMP2A excludes at least a portion of IgM from lipid rafts but is also localized with IgM in the plasma membrane (Fig. 2A). LMP2A was also detected in the Golgi apparatus (Fig. 2B). LMP2A localization with IgM and the Golgi did not appear to change when LMP2B was coexpressed. From these results, we concluded that the coexpression of LMP2B does not alter the localization of LMP2A.
Fluorescently conjugated antibodies against the Flu tag of LMP2B were used to detect the localization of LMP2B with LMP2A. However, because of the low-affinity interaction of the HA antibodies with the single HA tag expression of LMP2B, the protein was not detected. Transient transfections in BJAB cells were used to express larger amounts of LMP2B. LMP2B was readily detected in these cells and localized with LMP2A (data not shown).
Localization of LMP2A to both soluble and lipid raft fractions was also examined to determine if the coexpression of LMP2B alters the localization of LMP2A in lipid rafts. Lysates were prepared from equal numbers of cells transfected with a vector expressing LMP2A or both LMP2A and LMP2B. Lysates were subjected to discontinuous sucrose gradient centrifugation, and fractions were collected. Fractions 4 and 11, which have been previously determined to correspond to the membrane raft fraction and soluble fraction, respectively (20, 25, 48), were separated by SDS-PAGE and probed for LMP2A (Fig. 2C). Similar amounts of LMP2A were detected in the membrane raft fractions of both lysates, as well as the soluble fractions. The presence of the phosphatase CD45, which is excluded from lipid rafts, was also examined in the fractions to rule out contamination. CD45 was detected in the soluble fractions but not in the lipid raft fractions. Soluble and lipid raft fractions of lysates prepared from the 2A and 2A2B cell lines were also examined to confirm that LMP2B did not change the localization of LMP2A in stably expressing cell lines. Similar levels of LMP2A were detected in the membrane raft fractions of both lysates, as well as the soluble fractions (data not shown). From these results, we concluded that the coexpression of LMP2B does not affect the localization of LMP2A in lipid rafts.
Coexpression of LMP2B with LMP2A restores BCR signaling upon cross-linking.
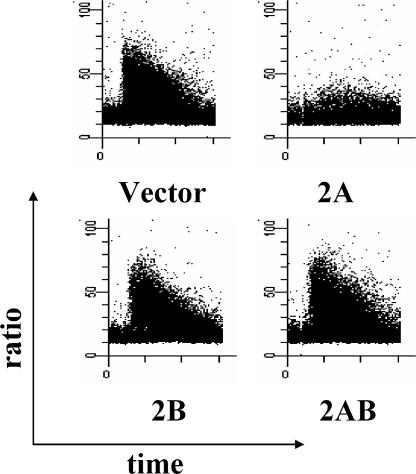
It has been previously shown that the expression of LMP2A in B cells blocks calcium mobilization and tyrosine phosphorylation induced by BCR cross-linking (68-70). To investigate a role for LMP2B in modulating LMP2A activity, changes in intracellular free calcium induced by BCR cross-linking were examined by FACS analysis with flow cytometry with the radiometric fluorescent dye Indo-1 in the 4 batch cell lines and 12 clonal cell lines constructed. Cells were measured for changes in intracellular free calcium for approximately 30 s to establish a baseline and then measured for an additional 4 min after being treated with BCR cross-linking antibodies. Calcium mobilization was measured on three separate occasions for the batch and clonal cell lines, and the results are shown in Table 1. Representative graphs of the calcium flux in the batch cell lines are shown in Fig. 3.
TABLE 1.
Calcium mobilization in batch and clonal cell lines
| Cell linea | % Change in calciumb | % of cells respondingc |
|---|---|---|
| Vector batch | 71 | 78 |
| Vector 1 | 77.3 | 79.2 |
| Vector 2 | 84.0 | 85.5 |
| Vector 3 | 73.8 | 76.7 |
| 2A batch | 10 | 17.5 |
| 2A 1 | 11.2 | 13.7 |
| 2A 2 | 10.7 | 14.8 |
| 2A 3 | 12.9 | 16.4 |
| 2B batch | 72 | 81 |
| 2B 1 | 85.6 | 87.3 |
| 2B 2 | 63.9 | 66.4 |
| 2B 3 | 67.2 | 83.7 |
| 2A2B batch | 61 | 71 |
| 2A2B 1 | 78.4 | 81.9 |
| 2A2B 2 | 85.0 | 87.3 |
| 2A2B 3 | 77.4 | 80.0 |
Relevant BJAB cell lines were constructed as detailed in Materials and Methods.
Percent change in intracellular calcium level was determined by comparing mean fluorescence levels before and after antibody treatment.
Percent cells responding to anti-Ig treatment was determined by comparing the mean numbers of cells responding before and after antibody treatment. The threshold for responding cells was determined as described in Materials and Methods.
FIG. 3.
Coexpression of LMP2B with LMP2A restores calcium flux. Cells were analyzed by flow cytometry with the calcium-sensitive dye Indo-1. The x axis represents time in minutes, while the y axis represents intracellular free-calcium change as measured by the ratio of calcium-bound (405 nm) to unbound (485 nm) dye. A baseline was established for approximately 30 s before 10 μg of goat anti-human cross-linking antibody per ml of cell suspension was added. Calcium flux was truncated considerably in the 2A cell line compared to the vector and 2B cell lines. Coexpression of LMP2B with LMP2A in the 2A2B cell line restored calcium flux levels.
Normally, B cells exhibit a transient increase in intracellular calcium following addition of cross-linking antibodies. This transient increase was readily observed in the vector cells. LMP2B-expressing cells had a flux in calcium similar to that of vector control cells, indicating that LMP2B does not alter calcium mobilization. This result is not entirely unexpected, since previous studies have indicated that domains contained in the LMP2A N terminus are essential for the block in calcium mobilization (28-30). As previously shown, the LMP2A-expressing cell line demonstrated significantly lower levels of cells responding and calcium mobilization. Interestingly, the coexpression of LMP2B with LMP2A in the 2A2B cell line completely restored the ability of the cells to mobilize calcium upon BCR cross-linking, indicating that LMP2B alters this LMP2A function.
To confirm that the HA tag on the C terminus of LMP2B did not alter LMP2B function, BJAB cells were transiently transfected with a plasmid expressing RFP, with plasmids expressing LMP2A and RFP, or with plasmids expressing LMP2A, RFP, and untagged LMP2B. The cells were then examined by FACS analysis and gated on RFP to exclude untransfected cells, and the calcium flux stimulated by BCR cross-linking was examined. The number of cells that were able to flux when transfected with LMP2A dropped significantly compared to cells transfected only with RFP (68.3% RFP-only cells responding compared to 26.3% RFP and LMP2A cells responding; data not shown). When an equimolar amount of LMP2B was cotransfected with LMP2A, a significant number of the transfected cells was then able to flux (42.0% of cells responding; data not shown). From these results, we concluded that the HA tag had no effect on the ability of LMP2B to restore calcium flux in LMP2A-expressing cells.
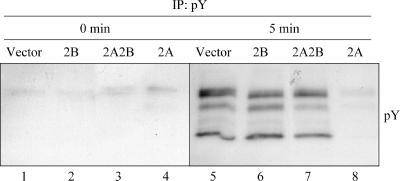
Induction of tyrosine phosphorylation following BCR cross-linking was also measured in the four batch cell lines (Fig. 4). Similar to calcium mobilization, tyrosine phosphorylation of BCR-associated proteins is one of the earliest events following BCR activation. As may have been expected from the calcium mobilization experiments, the expression of LMP2B did not affect tyrosine phosphorylation levels compared to the vector cell line following BCR cross-linking (Fig. 4, lanes 2 and 6). In contrast to this, the expression of LMP2A in the 2A cell line drastically reduced the induction of tyrosine phosphorylation, as has been previously shown (lanes 4 and 8) (68). Coexpression of LMP2B with LMP2A in the 2A2B cell line restored tyrosine phosphorylation levels induced by BCR cross-linking (lanes 3 and 7). From these results, we conclude that coexpression of LMP2B with LMP2A completely restores BCR signaling capacity as measured by tyrosine phosphorylation and calcium mobilization.
FIG. 4.
Coexpression of LMP2B with LMP2A restores tyrosine phosphorylation. BJAB cell lines were incubated in 0.5 ml serum-free medium at 37°C for approximately 90 min. Five microliters of goat anti-human cross-linking antibody was added, and the cell suspension was allowed to incubate at 37°C for 5 min. Triton X-100 lysates were then prepared from both cross-linked and uncross-linked samples, immunoprecipitated with anti-pY antibody, separated by SDS-PAGE, and immunoblotted with an HRP-conjugated anti-pY antibody. The expression of LMP2A in the 2A cell line decreased tyrosine phosphorylation upon BCR cross-linking compared to the vector and 2B cell lines. Coexpression of LMP2B with LMP2A in the 2A2B cell line restored tyrosine phosphorylation. IP, immunoprecipitate.
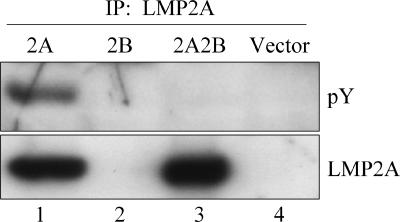
Coexpression of LMP2B blocks phosphorylation of LMP2A.
Tyrosine phosphorylation of the N-terminal cytoplasmic tail of LMP2A is essential for mediating the association of LMP2A with the SH2 domains of Lyn and Syk. Mutation of the tyrosine residues of the ITAM, as well as mutation of Y112, has been shown to restore calcium mobilization upon BCR cross-linking (29, 30). On the basis of the calcium mobilization and tyrosine phosphorylation results obtained (Fig. 3 and 4), we hypothesized that coexpression of LMP2B with LMP2A restores calcium mobilization and tyrosine phosphorylation by blocking the phosphorylation of LMP2A tyrosine residues and subsequent recruitment of tyrosine kinases. In order to investigate this, lysates of the four cell lines were prepared, immunoprecipitated with anti-LMP2A antibody, separated by SDS-PAGE, and probed for tyrosine phosphorylation (Fig. 5). Tyrosine phosphorylation was detected in lysates from the 2A cell line (lane 1) at the position at which LMP2A normally runs (∼55 kDa) but not in any of the other lanes. To confirm that the phosphorylated protein was LMP2A, the blot was stripped and reprobed. LMP2A was detected in lysates from both the 2A and the 2A2B cell lines (lanes 1 and 3), but only LMP2A from the 2A cell lines was phosphorylated. From these results, we concluded that coexpression of LMP2B with LMP2A blocks the tyrosine phosphorylation of LMP2A.
FIG. 5.
LMP2A is not constitutively phosphorylated when coexpressed with LMP2B. Triton X-100 lysates of the BJAB cell lines were immunoprecipitated with rat anti-LMP2A antibody 14B7, separated by SDS-PAGE, and immunoblotted with HRP-conjugated anti-pY antibody. The blot was stripped and reprobed with biotin-conjugated primary antibody 14B7 and streptavidin conjugated to HRP as a secondary antibody. LMP2A is constitutively phosphorylated in the 2A cell line, but coexpression of LMP2B in the 2A2B cell line abrogates this phosphorylation. IP, immunoprecipitate.
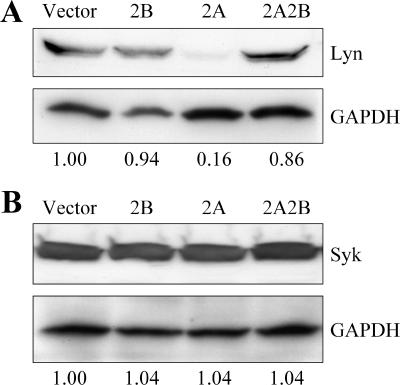
Coexpression of LMP2B with LMP2A restores Lyn levels.
Expression of LMP2A results in decreased constitutive levels of Lyn but not Syk (30, 42, 43, 98). Nedd4 ubiquitin ligases associate with the PY motifs found in the cytoplasmic N-terminal domain of LMP2A and mediate the ubiquitination and subsequent degradation of Lyn (42, 43, 98). Mutation of the PY motifs of LMP2A results in an increase in constitutive levels of Lyn and LMP2A, as well as hyperphosphorylation of LMP2A. We hypothesized that coexpression of LMP2B blocks the phosphorylation of LMP2A and subsequent recruitment of Lyn tyrosine kinase, which would result in restoration of Lyn levels. To examine this, we probed lysates prepared from the four batch cell lines for Lyn, Syk, and LMP2A on an SDS-PAGE protein gel. GAPDH was used as a protein loading control to normalize Lyn, Syk, and LMP2A levels quantified by densitometric analysis. As previously described, the expression of LMP2A in BJAB cells resulted in a decrease in constitutive Lyn levels but not in Syk levels (Fig. 6). Expression of LMP2B did not affect Lyn levels compared to the vector. However, coexpression of LMP2B with LMP2A resulted in almost complete restoration of Lyn levels (Fig. 6, 2A2B lane). Levels of LMP2A were not significantly affected by the coexpression of LMP2B (Fig. 1). From these data, we conclude that coexpression of LMP2B with LMP2A restores Lyn levels but does not affect levels of LMP2A or Syk.
FIG. 6.
Coexpression of LMP2B with LMP2A restores Lyn levels. (A) Triton X-100 lysates of the BJAB cell lines were separated by SDS-PAGE and immunoblotted with anti-Lyn antibody. The blot was stripped and reprobed for GAPDH as a loading control, and densitometric analysis was used to normalize Lyn levels with loading controls. Levels of Lyn were lower in the 2A cell line compared to the EV and 2B cell lines. Lyn levels were almost completely restored from the 2A cell line upon coexpression of LMP2B. (B) Syk Western blot assay with GAPDH loading controls. The expression of LMP2A, LMP2B, or both does not affect Syk levels in BJAB cells.
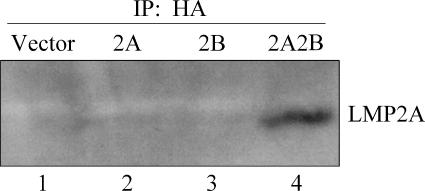
LMP2A associates with LMP2B.
The structural homology of LMP2A and LMP2B suggests that they may localize to similar cellular compartments and interact. In addition, LMP2A and LMP2B have been previously shown to at least partially colocalize in transient-transfection experiments (62) similar to our studies indicated above as data not shown. To verify that the two proteins physically interact, coimmunoprecipitation studies were performed. Lysates prepared from the four cell lines were used to immunoprecipitate LMP2B, and immunoprecipitates were run on an SDS gel and probed for LMP2A expression (Fig. 7). LMP2A was readily detected in the 2A2B lane but not in the 2A lane or the 2B lane. From these results, we conclude that LMP2B and LMP2A associate.
FIG. 7.
LMP2A immunoprecipitates with LMP2B. Radioimmunoprecipitation assay buffer lysates of the BJAB cell lines were immunoprecipitated with mouse anti-HA antibody, separated by SDS-PAGE, and immunoblotted with rat anti-LMP2A antibody. LMP2A protein was detected in the 2A2B coimmunoprecipitation but not in the 2A or 2B cell line. IP, immunoprecipitate.
DISCUSSION
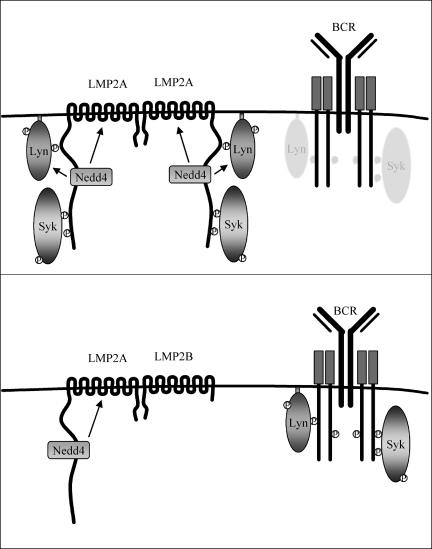
Our present findings indicate that LMP2B physically associates with LMP2A in B cells and modulates LMP2A activity. Interestingly, LMP2B blocks one of the earliest events important for LMP2A function, namely, LMP2A phosphorylation. We had shown previously that tyrosines phosphorylated in the N-terminal LMP2A tail sequester Lyn and Syk to modulate normal BCR signaling (28-30). By blocking LMP2A phosphorylation, LMP2B prevents the recruitment of these kinases to LMP2A. Also as a result of LMP2B expression, normal BCR signal transduction is restored as monitored by calcium mobilization and the induction of tyrosine phosphorylation. Finally, Lyn protein levels were restored to wild-type levels in LMP2A/LMP2B-expressing cells. Notably, we were unable to discern any effects of LMP2B expression on BCR signaling, indicating that a major function of LMP2B may be the regulation of LMP2A activity.
Although the exact mechanism of LMP2B regulation of LMP2A function cannot be entirely discerned from our present studies, we are proposing the model shown in Fig. 8. Our previous studies have shown that LMP2A functions as a BCR mimic by constitutively assembling as a signalosome in lipid rafts (12, 25, 26, 28-31, 59, 60, 66, 69, 70, 74, 78, 79, 89). Lipid rafts are rich in Src family tyrosine kinases such as Lyn, and the aggregation of LMP2A in lipid rafts allows Lyn to transiently interact with LMP2A. This may be mediated by a DQSL sequence found within the LMP2A ITAM, as a similar sequence (DCSM) is found in the BCR Igα subunit and is important for recruiting Lyn to BCR complexes (21). This recruitment mediates the phosphorylation of LMP2A, followed by binding of the Lyn SH2 domain to LMP2A tyrosine 112 and the dual SH2 domains of Syk to the LMP2A ITAM at tyrosines 74 and 85. This complex, along with other proteins recruited to the LMP2A signalosome, transduces a signal to the B cell. Additionally, Nedd4 ubiquitin ligases also recruited by LMP2A mediate the ubiquitination of LMP2A and Lyn. This allows LMP2A to alter normal BCR signaling transduced through Lyn and Syk and replace these signals with its own. In our model, coexpression of LMP2B interferes with the phosphorylation of LMP2A residues important in the recruitment of Lyn and Syk by physically associating with LMP2A, thus blocking LMP2A function at an early step.
FIG. 8.
Proposed model for LMP2B modulation of LMP2A signaling. (A) LMP2A aggregates in lipid rafts and weakly and transiently associates with resident Src family protein tyrosine kinases such as Lyn. The aggregation of LMP2A in lipid rafts mediates the cross-phosphorylation of tyrosine residues on the N-terminal tail of LMP2A. The phosphorylated LMP2A is then able to strongly bind to the SH2 domain of Lyn and further mediate phosphorylation of the LMP2A ITAM. Once phosphorylated, the ITAM is able to recruit Syk, which is used to transduce tonic signals to the cell. Lyn recruited to LMP2A is ubiquitinated by Nedd4 ubiquitin ligases recruited by LMP2A and is subsequently degraded. This allows LMP2A to block normal BCR signaling transduced through Lyn and Syk and replace these signals with its own. (B) Coexpression of LMP2B interferes with the phosphorylation of LMP2A residues important in recruiting Lyn and Syk by physically associating with LMP2A, which restores normal Lyn levels and BCR signaling.
Previous studies have shown that LMP2A contains a clustering signal found in the C-terminal tail (63). We hypothesize that LMP2B, which has an identical C-terminal tail, may associate with LMP2A and prevent homodimerization of LMP2A, which would in turn prevent aggregation and phosphorylation of LMP2A. Other shared domains of LMP2A and LMP2B are also likely to be important for mediating complex formation, in particular the shared 12-transmembrane domains. Previous studies have shown that the LMP1 transmembrane domains are important for many biological aspects related to LMP1 function (9, 22, 35, 49, 57, 95, 96, 99). Particularly important to our present studies is the observation that a critical four-amino-acid sequence (FWLY) found in an LMP1 transmembrane domain is important for LMP1 intermolecular interaction, raft localization, and signaling (99). In addition to the C-terminal tail clustering domain, we suggest that the transmembrane domains of LMP2A and LMP2B have features that are important for function. We hypothesize that this is in part due to the fact that the effect of LMP2B expression on LMP2A function is so dramatic. Simple heterodimerization does not appear to adequately explain the almost total loss of LMP2A function since a portion of LMP2A would still form LMP2A homodimers. Thus, it seems that higher-order complexes are likely formed so that LMP2B inhibition is complete. Further study of the interactions of the domains required for the interactions of LMP2A and LMP2B is clearly warranted.
Two earlier studies have shown the importance of the clustering of the LMP2A N-terminal domain in its signaling capacity, indicating that any alteration by LMP2B could have dramatic effects on LMP2A function. By making chimeric proteins with the N-terminal domain of LMP2A fused to the extracellular domain of CD8, it was shown that aggregation by the addition of cross-linking antibody was required for LMP2A function (1, 8). Thus, any disruption of LMP2A aggregation, such as that caused by the expression of LMP2B, may block the cross phosphorylation of the LMP2A N-terminal tail and prevent the high-affinity binding of Lyn and Syk to LMP2A tyrosines, thus blocking LMP2A phosphorylation and function as we observed in the present studies.
A splice variant of a gene that generates an alternative form of the protein product to modulate signaling is not without precedent. A splice variant of the human growth hormone-releasing hormone (GHRH) receptor was shown to act as a dominant inhibitor of receptor signaling (65). The GHRH receptor is a seven-transmembrane G protein-coupled receptor that localizes to the plasma membrane of cells. Binding of GHRH to its receptor leads to an influx of calcium, changes in gene transcription, and phosphorylation of downstream proteins (10, 15, 34, 44, 58, 64, 71). Expression of the naturally occurring splice variant in humans encodes a truncated receptor which acts as a dominant inhibitor of receptor signaling. Also, the exon 7-skipped variant (ERΔE7) of the estrogen receptor has been shown to suppress estrogen-dependent transcriptional activation (32). The inhibitory effects of this splice variant are due to the formation of heterodimers with wild-type ERα and ERβ and inhibition of binding of wild-type receptors to their responsive elements.
Although we believe that LMP2A signaling likely occurs at the plasma membrane, we cannot exclude the possibility that LMP2A signaling occurs intracellularly, as has been reported for LMP1 (53). As expected, like LMP1, LMP2A can be detected in a variety of cellular compartments. Studies utilizing transiently transfected cells have suggested that LMP2A and LMP2B localize to perinuclear regions of epithelial and BJAB cells (62). A significant proportion of both LMP2A and LMP2B was shown to colocalize with γ-adaptin, a component of the trans-Golgi network. However, the overexpression of LMP2A and LMP2B in these transient-expression experiments may have resulted in endoplasmic-reticulum-associated protein degradation. Previous studies have shown that overexpression of membrane or secreted proteins can result in misfolding and induction of the endoplasmic reticulum-associated protein degradation system, causing a portion of the overexpressed protein to become mislocalized (50, 67). We have found that the levels of LMP2A and LMP2B expressed from the retroviral constructs used in the present study are quite similar to the amount of LMP2A expressed in EBV-transformed B cells grown in culture (42, 70). Our results indicated that LMP2A primarily localizes to the plasma membrane of B cells, although some LMP2A was detected in the Golgi apparatus. Coexpression of LMP2B did not significantly alter the cellular localization of LMP2A in either case.
The restoration of calcium flux and protein phosphorylation upon coexpression of LMP2B led us to hypothesize that LMP2B may be preventing LMP2A-mediated degradation of Lyn. It has been shown previously that the proline motifs found in the N-terminal tail of LMP2A associate with Nedd4 ubiquitin ligases (42, 98). The expression of LMP2A in B cells results in a decreased amount of Lyn but does not change Syk levels (30, 42, 43, 68, 98). Mutation of the LMP2A proline motifs results in an increase in LMP2A levels, as well as a restoration of Lyn levels and protein phosphorylation upon BCR cross-linking (43). Our results indicate that Lyn levels are restored to wild-type levels with the coexpression of LMP2B with LMP2A. This suggests that LMP2B may be preventing the association of Lyn, Nedd4 ubiquitin ligases, or both with LMP2A. LMP2A levels have been shown to increase when the proline motifs found in the N-terminal tail are mutated, whereas with the coexpression of LMP2B, LMP2A levels did not increase. Densitometric analysis of the LMP2A expressed in the 2A and2A2B cell lines shows that the amounts of LMP2A expressed in these two cell lines are similar (2A = 1 and 2A2B = 1.02 when normalized to GAPDH controls; Fig. 1). This suggests that the expression of LMP2B has no impact on the ability of Nedd4 ubiquitin ligases to target LMP2A for degradation. Since LMP2A is not tyrosine phosphorylated when LMP2B is expressed, the likely block of LMP2A function is a result of the lack of LMP2A phosphorylation as in the Y112 mutant (30). LMP2B may function to downregulate the ability of LMP2A to block B-cell activation but leave other signaling capabilities intact. Just as LMP2B did not affect the Nedd4 ubiquitin ligase-mediated degradation of LMP2A, it may also not affect the association of LMP2A with other cellular proteins, such as mitogen-activated protein kinase (74). LMP2A has also been demonstrated to augment signals delivered by LMP1 (23). This signaling pathway is particularly interesting, as LMP2B shares a bidirectional promoter with LMP1 that is differentially expressed from the LMP2A promoter (55, 76). It is possible that LMP2B works in concert with LMP1; the expression of LMP2B downregulates the LMP2A-mediated block of B-cell signaling, while LMP1 activates the B cell through the NF-κB, AP-1, and JAK/STAT pathways (36).
LMP2B may also function in the initial EBV infection of B cells. The latency III or growth program of gene expression drives B-cell activation and proliferation by expressing all of the EBNAs, LMP1, and LMP2. The expression of LMP2B may be essential at this phase of the virus life cycle to regulate LMP2A activity to ensure that cells do not undergo lytic replication. In particular, previous studies have suggested that LMP2A expression can activate virus lytic replication (87). Thus, the strength of the LMP2A signal may have very diverse effects. In this case, the resulting LMP2A signal would resemble the signal generated by an activated BCR and induce lytic replication whereas lower levels may provide a signal that more closely resembles the tonic signal provided by an unactivated BCR. Thus, LMP2B may be important in regulating the activation of lytic virus production from latently infected memory B cells. In this case, prior to induction of lytic replication, memory B cells would be triggered to proliferate by changes in viral gene expression. LMP2B expression would be coordinated with LMP1 expression to allow for the expansion of cells harboring EBV by preventing LMP2A-induced lytic replication. Lytic replication would then be allowed to be triggered by normal B-cell signal transduction following expansion of the EBV-infected cells. Further studies are clearly needed to further delineate the role of LMP2B in LMP2A signaling and determine if LMP2B alone may alter the normal B-cell phenotype.
Acknowledgments
R.L. is a Stohlman Scholar of the Leukemia and Lymphoma Society of America and is supported by Public Health Service grants CA62234, CA73507, and CA93444 from the National Cancer Institute. M.R. is supported by the National Cancer Institute Training Program in Carcinogenesis (NIH T32 AR07593).
Footnotes
Published ahead of print on 11 October 2006.
REFERENCES
- 1.Alber, G., K. M. Kim, P. Weiser, C. Riesterer, R. Carsetti, and M. Reth. 1993. Molecular mimicry of the antigen receptor signalling motif by transmembrane proteins of the Epstein-Barr virus and the bovine leukaemia virus. Curr. Biol. 3:333-339. [DOI] [PubMed] [Google Scholar]
- 2.Allen, M. D., L. S. Young, and C. W. Dawson. 2005. The Epstein-Barr virus-encoded LMP2A and LMP2B proteins promote epithelial cell spreading and motility. J. Virol. 79:1789-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babcock, G. J., L. L. Decker, R. B. Freeman, and D. A. Thorley-Lawson. 1999. Epstein-Barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J. Exp. Med. 190:567-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babcock, G. J., L. L. Decker, M. Volk, and D. A. Thorley-Lawson. 1998. EBV persistence in memory B cells in vivo. Immunity 9:395-404. [DOI] [PubMed] [Google Scholar]
- 5.Babcock, G. J., D. Hochberg, and A. D. Thorley-Lawson. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13:497-506. [DOI] [PubMed] [Google Scholar]
- 6.Babcock, G. J., and D. A. Thorley-Lawson. 2000. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proc. Natl. Acad. Sci. USA 97:12250-12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baer, R., A. T. Bankier, M. D. Biggin, P. L. Deininger, P. J. Farrell, T. J. Gibson, G. Hatfull, G. S. Hudson, S. C. Satchwell, C. Seguin, et al. 1984. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310:207-211. [DOI] [PubMed] [Google Scholar]
- 8.Beaufils, P., D. Choquet, R. Z. Mamoun, and B. Malissen. 1993. The (YXXL/I)2 signalling motif found in the cytoplasmic segments of the bovine leukaemia virus envelope protein and Epstein-Barr virus latent membrane protein 2A can elicit early and late lymphocyte activation events. EMBO J. 12:5105-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blake, S. M., A. G. Eliopoulos, C. W. Dawson, and L. S. Young. 2001. The transmembrane domains of the EBV-encoded latent membrane protein 1 (LMP1) variant CAO regulate enhanced signalling activity. Virology 282:278-287. [DOI] [PubMed] [Google Scholar]
- 10.Bodner, M., J. L. Castrillo, L. E. Theill, T. Deerinck, M. Ellisman, and M. Karin. 1988. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell 55:505-518. [DOI] [PubMed] [Google Scholar]
- 11.Brooks, L., Q. Y. Yao, A. B. Rickinson, and L. S. Young. 1992. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J. Virol. 66:2689-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkhardt, A. L., J. B. Bolen, E. Kieff, and R. Longnecker. 1992. An Epstein-Barr virus transformation-associated membrane protein interacts with src family tyrosine kinases. J. Virol. 66:5161-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell, R. G., R. C. Brown, and R. Longnecker. 2000. Epstein-Barr virus LMP2A-induced B-cell survival in two unique classes of EmuLMP2A transgenic mice. J. Virol. 74:1101-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caldwell, R. G., J. B. Wilson, S. J. Anderson, and R. Longnecker. 1998. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 9:405-411. [DOI] [PubMed] [Google Scholar]
- 15.Chen, C., J. M. Israel, and J. D. Vincent. 1989. Electrophysiological responses of rat pituitary cells in somatotroph-enriched primary culture to human growth-hormone releasing factor. Neuroendocrinology 50:679-687. [DOI] [PubMed] [Google Scholar]
- 16.Chen, F., J. Z. Zou, L. di Renzo, G. Winberg, L. F. Hu, E. Klein, G. Klein, and I. Ernberg. 1995. A subpopulation of normal B cells latently infected with Epstein-Barr virus resembles Burkitt lymphoma cells in expressing EBNA-1 but not EBNA-2 or LMP1. J. Virol. 69:3752-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, H., P. Smith, R. F. Ambinder, and S. D. Hayward. 1999. Expression of Epstein-Barr virus BamHI-A rightward transcripts in latently infected B cells from peripheral blood. Blood 93:3026-3032. [PubMed] [Google Scholar]
- 18.Chen, S. Y., J. Lu, Y. C. Shih, and C. H. Tsai. 2002. Epstein-Barr virus latent membrane protein 2A regulates c-Jun protein through extracellular signal-regulated kinase. J. Virol. 76:9556-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng, P. C., B. K. Brown, W. Song, and S. K. Pierce. 2001. Translocation of the B cell antigen receptor into lipid rafts reveals a novel step in signaling. J. Immunol. 166:3693-3701. [DOI] [PubMed] [Google Scholar]
- 20.Cheng, P. C., M. L. Dykstra, R. N. Mitchell, and S. K. Pierce. 1999. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med. 190:1549-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark, M. R., S. A. Johnson, and J. C. Cambier. 1994. Analysis of Ig-α-tyrosine kinase interaction reveals two levels of binding specificity and tyrosine phosphorylated Ig-α stimulation of Fyn activity. EMBO J. 13:1911-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coffin, W. F., III, T. R. Geiger, and J. M. Martin. 2003. Transmembrane domains 1 and 2 of the latent membrane protein 1 of Epstein-Barr virus contain a lipid raft targeting signal and play a critical role in cytostasis. J. Virol. 77:3749-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawson, C. W., J. H. George, S. M. Blake, R. Longnecker, and L. S. Young. 2001. The Epstein-Barr virus encoded latent membrane protein 2A augments signaling from latent membrane protein 1. Virology 289:192-207. [DOI] [PubMed] [Google Scholar]
- 24.Decker, L. L., L. D. Klaman, and D. A. Thorley-Lawson. 1996. Detection of the latent form of Epstein-Barr virus DNA in the peripheral blood of healthy individuals. J. Virol. 70:3286-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dykstra, M. L., R. Longnecker, and S. K. Pierce. 2001. Epstein-Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity 14:57-67. [DOI] [PubMed] [Google Scholar]
- 26.Engels, N., M. Merchant, R. Pappu, A. C. Chan, R. Longnecker, and J. Wienands. 2001. Epstein-Barr virus latent membrane protein 2A (LMP2A) employs the SLP-65 signaling module. J. Exp. Med. 194:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fåhraeus, R., H. L. Fu, I. Ernberg, J. Finke, M. Rowe, G. Klein, K. Falk, E. Nilsson, M. Yadav, P. Busson, et al. 1988. Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal carcinoma. Int. J. Cancer 42:329-338. [DOI] [PubMed] [Google Scholar]
- 28.Fruehling, S., S. K. Lee, R. Herrold, B. Frech, G. Laux, E. Kremmer, F. A. Grasser, and R. Longnecker. 1996. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J. Virol. 70:6216-6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fruehling, S., and R. Longnecker. 1997. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology 235:241-251. [DOI] [PubMed] [Google Scholar]
- 30.Fruehling, S., R. Swart, K. M. Dolwick, E. Kremmer, and R. Longnecker. 1998. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J. Virol. 72:7796-7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuda, M., and R. Longnecker. 2005. Epstein-Barr virus (EBV) latent membrane protein 2A regulates B-cell receptor-induced apoptosis and EBV reactivation through tyrosine phosphorylation. J. Virol. 79:8655-8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García Pedrero, J. M., P. Zuazua, C. Martinez-Campa, P. S. Lazo, and S. Ramos. 2003. The naturally occurring variant of estrogen receptor (ER) ERΔE7 suppresses estrogen-dependent transcriptional activation by both wild-type ERα and ERβ. Endocrinology 144:2967-2976. [DOI] [PubMed] [Google Scholar]
- 33.Gires, O., U. Zimber-Strobl, R. Gonnella, M. Ueffing, G. Marschall, R. Zeidler, D. Pich, and W. Hammerschmidt. 1997. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 16:6131-6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez, G. A., and M. R. Montminy. 1989. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59:675-680. [DOI] [PubMed] [Google Scholar]
- 35.Hatzivassiliou, E., W. E. Miller, N. Raab-Traub, E. Kieff, and G. Mosialos. 1998. A fusion of the EBV latent membrane protein-1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor receptor expression, nuclear factor-κB, and stress-activated protein kinase. J. Immunol. 160:1116-1121. [PubMed] [Google Scholar]
- 36.Hatzivassiliou, E., and G. Mosialos. 2002. Cellular signaling pathways engaged by the Epstein-Barr virus transforming protein LMP1. Front. Biosci. 7:d319-d329. [DOI] [PubMed] [Google Scholar]
- 37.He, B., N. Raab-Traub, P. Casali, and A. Cerutti. 2003. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J. Immunol. 171:5215-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hochberg, D., J. M. Middeldorp, M. Catalina, J. L. Sullivan, K. Luzuriaga, and D. A. Thorley-Lawson. 2004. Demonstration of the Burkitt's lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proc. Natl. Acad. Sci. USA 101:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hochberg, D., T. Souza, M. Catalina, J. L. Sullivan, K. Luzuriaga, and D. A. Thorley-Lawson. 2004. Acute infection with Epstein-Barr virus targets and overwhelms the peripheral memory B-cell compartment with resting, latently infected cells. J. Virol. 78:5194-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huen, D. S., S. A. Henderson, D. Croom-Carter, and M. Rowe. 1995. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-κB and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene 10:549-560. [PubMed] [Google Scholar]
- 41.Ikeda, A., M. Merchant, L. Lev, R. Longnecker, and M. Ikeda. 2004. Latent membrane protein 2A, a viral B cell receptor homologue, induces CD5+ B-1 cell development. J. Immunol. 172:5329-5337. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda, M., A. Ikeda, L. C. Longan, and R. Longnecker. 2000. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology 268:178-191. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda, M., A. Ikeda, and R. Longnecker. 2001. PY motifs of Epstein-Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins. J. Virol. 75:5711-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ingraham, H. A., R. P. Chen, H. J. Mangalam, H. P. Elsholtz, S. E. Flynn, C. R. Lin, D. M. Simmons, L. Swanson, and M. G. Rosenfeld. 1988. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell 55:519-529. [DOI] [PubMed] [Google Scholar]
- 45.Johnson, S. A., C. M. Pleiman, L. Pao, J. Schneringer, K. Hippen, and J. C. Cambier. 1995. Phosphorylated immunoreceptor signaling motifs (ITAMs) exhibit unique abilities to bind and activate Lyn and Syk tyrosine kinases. J. Immunol. 155:4596-4603. [PubMed] [Google Scholar]
- 46.Joseph, A. M., G. J. Babcock, and D. A. Thorley-Lawson. 2000. Cells expressing the Epstein-Barr virus growth program are present in and restricted to the naive B-cell subset of healthy tonsils. J. Virol. 74:9964-9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joseph, A. M., G. J. Babcock, and D. A. Thorley-Lawson. 2000. EBV persistence involves strict selection of latently infected B cells. J. Immunol. 165:2975-2981. [DOI] [PubMed] [Google Scholar]
- 48.Katzman, R. B., and R. Longnecker. 2004. LMP2A does not require palmitoylation to localize to buoyant complexes or for function. J. Virol. 78:10878-10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaykas, A., K. Worringer, and B. Sugden. 2002. LMP-1's transmembrane domains encode multiple functions required for LMP-1's efficient signaling. J. Virol. 76:11551-11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim, P. S., and P. Arvan. 1998. Endocrinopathies in the family of endoplasmic reticulum (ER) storage diseases: disorders of protein trafficking and the role of ER molecular chaperones. Endocrinol. Rev. 19:173-202. [DOI] [PubMed] [Google Scholar]
- 51.Kurosaki, T. 1999. Genetic analysis of B cell antigen receptor signaling. Annu. Rev. Immunol. 17:555-592. [DOI] [PubMed] [Google Scholar]
- 52.Lam, N., and B. Sugden. 2003. CD40 and its viral mimic, LMP1: similar means to different ends. Cell Signal. 15:9-16. [DOI] [PubMed] [Google Scholar]
- 53.Lam, N., and B. Sugden. 2003. LMP1, a viral relative of the TNF receptor family, signals principally from intracellular compartments. EMBO J. 22:3027-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laux, G., F. Dugrillon, C. Eckert, B. Adam, U. Zimber-Strobl, and G. W. Bornkamm. 1994. Identification and characterization of an Epstein-Barr virus nuclear antigen 2-responsive cis element in the bidirectional promoter region of latent membrane protein and terminal protein 2 genes. J. Virol. 68:6947-6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laux, G., A. Economou, and P. J. Farrell. 1989. The terminal protein gene 2 of Epstein-Barr virus is transcribed from a bidirectional latent promoter region. J. Gen. Virol. 70(Pt. 11):3079-3084. [DOI] [PubMed] [Google Scholar]
- 56.Laux, G., M. Perricaudet, and P. J. Farrell. 1988. A spliced Epstein-Barr virus gene expressed in immortalized lymphocytes is created by circularization of the linear viral genome. EMBO J. 7:769-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liebowitz, D., J. Mannick, K. Takada, and E. Kieff. 1992. Phenotypes of Epstein-Barr virus LMP1 deletion mutants indicate transmembrane and amino-terminal cytoplasmic domains necessary for effects in B-lymphoma cells. J. Virol. 66:4612-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin, C., S. C. Lin, C. P. Chang, and M. G. Rosenfeld. 1992. Pit-1-dependent expression of the receptor for growth hormone releasing factor mediates pituitary cell growth. Nature 360:765-768. [DOI] [PubMed] [Google Scholar]
- 59.Longnecker, R., B. Druker, T. M. Roberts, and E. Kieff. 1991. An Epstein-Barr virus protein associated with cell growth transformation interacts with a tyrosine kinase. J. Virol. 65:3681-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Longnecker, R., and E. Kieff. 1990. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J. Virol. 64:2319-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu, J., W. H. Lin, S. Y. Chen, R. Longnecker, S. C. Tsai, C. L. Chen, and C. H. Tsai. 2006. Syk tyrosine kinase mediates Epstein-Barr virus latent membrane protein 2A-induced cell migration in epithelial cells. J. Biol. Chem. 281:8806-8814. [DOI] [PubMed] [Google Scholar]
- 62.Lynch, D. T., J. S. Zimmerman, and D. T. Rowe. 2002. Epstein-Barr virus latent membrane protein 2B (LMP2B) co-localizes with LMP2A in perinuclear regions in transiently transfected cells. J. Gen. Virol. 83:1025-1035. [DOI] [PubMed] [Google Scholar]
- 63.Matskova, L., I. Ernberg, T. Pawson, and G. Winberg. 2001. C-terminal domain of the Epstein-Barr virus LMP2A membrane protein contains a clustering signal. J. Virol. 75:10941-10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCormick, A., H. Brady, L. E. Theill, and M. Karin. 1990. Regulation of the pituitary-specific homeobox gene GHF1 by cell-autonomous and environmental cues. Nature 345:829-832. [DOI] [PubMed] [Google Scholar]
- 65.McElvaine, A. T., and K. E. Mayo. 2006. A dominant-negative human growth hormone-releasing hormone (GHRH) receptor splice variant inhibits GHRH binding. Endocrinology 147:1884-1894. [DOI] [PubMed] [Google Scholar]
- 66.Merchant, M., and R. Longnecker. 2001. LMP2A survival and developmental signals are transmitted through Btk-dependent and Btk-independent pathways. Virology 291:46-54. [DOI] [PubMed] [Google Scholar]
- 67.Meusser, B., C. Hirsch, E. Jarosch, and T. Sommer. 2005. ERAD: the long road to destruction. Nat. Cell Biol. 7:766-772. [DOI] [PubMed] [Google Scholar]
- 68.Miller, C. L., A. L. Burkhardt, J. H. Lee, B. Stealey, R. Longnecker, J. B. Bolen, and E. Kieff. 1995. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity 2:155-166. [DOI] [PubMed] [Google Scholar]
- 69.Miller, C. L., J. H. Lee, E. Kieff, and R. Longnecker. 1994. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc. Natl. Acad. Sci. USA 91:772-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller, C. L., R. Longnecker, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J. Virol. 67:3087-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller, T. L., P. A. Godfrey, V. I. Dealmeida, and K. E. Mayo. 1999. The rat growth hormone-releasing hormone receptor gene: structure, regulation, and generation of receptor isoforms with different signaling properties. Endocrinology 140:4152-4165. [DOI] [PubMed] [Google Scholar]
- 72.Miyashita, E. M., B. Yang, G. J. Babcock, and D. A. Thorley-Lawson. 1997. Identification of the site of Epstein-Barr virus persistence in vivo as a resting B cell. J. Virol. 71:4882-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mosialos, G., M. Birkenbach, R. Yalamanchili, T. VanArsdale, C. Ware, and E. Kieff. 1995. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell 80:389-399. [DOI] [PubMed] [Google Scholar]
- 74.Panousis, C. G., and D. T. Rowe. 1997. Epstein-Barr virus latent membrane protein 2 associates with and is a substrate for mitogen-activated protein kinase. J. Virol. 71:4752-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pegtel, D. M., A. Subramanian, T. S. Sheen, C. H. Tsai, T. R. Golub, and D. A. Thorley-Lawson. 2005. Epstein-Barr-virus-encoded LMP2A induces primary epithelial cell migration and invasion: possible role in nasopharyngeal carcinoma metastasis. J. Virol. 79:15430-15442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng, R., S. C. Moses, J. Tan, E. Kremmer, and P. D. Ling. 2005. The Epstein-Barr virus EBNA-LP protein preferentially coactivates EBNA2-mediated stimulation of latent membrane proteins expressed from the viral divergent promoter. J. Virol. 79:4492-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pleiman, C. M., C. Abrams, L. T. Gauen, W. Bedzyk, J. Jongstra, A. S. Shaw, and J. C. Cambier. 1994. Distinct p53/56lyn and p59fyn domains associate with nonphosphorylated and phosphorylated Ig-α. Proc. Natl. Acad. Sci. USA 91:4268-4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Portis, T., and R. Longnecker. 2004. Epstein-Barr virus (EBV) LMP2A alters normal transcriptional regulation following B-cell receptor activation. Virology 318:524-533. [DOI] [PubMed] [Google Scholar]
- 79.Portis, T., and R. Longnecker. 2004. Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene 23:8619-8628. [DOI] [PubMed] [Google Scholar]
- 80.Qu, L., and D. T. Rowe. 1992. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J. Virol. 66:3715-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, PA. [Google Scholar]
- 82.Rowe, D. T., L. Hall, I. Joab, and G. Laux. 1990. Identification of the Epstein-Barr virus terminal protein gene products in latently infected lymphocytes. J. Virol. 64:2866-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rowe, M., A. L. Lear, D. Croom-Carter, A. H. Davies, and A. B. Rickinson. 1992. Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J. Virol. 66:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rowe, M., D. T. Rowe, C. D. Gregory, L. S. Young, P. J. Farrell, H. Rupani, and A. B. Rickinson. 1987. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 6:2743-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rowley, R. B., A. L. Burkhardt, H. G. Chao, G. R. Matsueda, and J. B. Bolen. 1995. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Igα/Igβ immunoreceptor tyrosine activation motif binding and autophosphorylation. J. Biol. Chem. 270:11590-11594. [DOI] [PubMed] [Google Scholar]
- 86.Sample, J., D. Liebowitz, and E. Kieff. 1989. Two related Epstein-Barr virus membrane proteins are encoded by separate genes. J. Virol. 63:933-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schaadt, E., B. Baier, J. Mautner, G. W. Bornkamm, and B. Adler. 2005. Epstein-Barr virus latent membrane protein 2A mimics B-cell receptor-dependent virus reactivation. J. Gen. Virol. 86:551-559. [DOI] [PubMed] [Google Scholar]
- 88.Souza, T. A., B. D. Stollar, J. L. Sullivan, K. Luzuriaga, and D. A. Thorley-Lawson. 2005. Peripheral B cells latently infected with Epstein-Barr virus display molecular hallmarks of classical antigen-selected memory B cells. Proc. Natl. Acad. Sci. USA 102:18093-18098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swart, R., I. K. Ruf, J. Sample, and R. Longnecker. 2000. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 74:10838-10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thorley-Lawson, D. A. 2001. Epstein-Barr virus: exploiting the immune system. Nat. Rev. Immunol. 1:75-82. [DOI] [PubMed] [Google Scholar]
- 91.Thorley-Lawson, D. A., and A. Gross. 2004. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 350:1328-1337. [DOI] [PubMed] [Google Scholar]
- 92.Tierney, R. J., N. Steven, L. S. Young, and A. B. Rickinson. 1994. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J. Virol. 68:7374-7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsang, S. F., F. Wang, K. M. Izumi, and E. Kieff. 1991. Delineation of the cis-acting element mediating EBNA-2 transactivation of latent infection membrane protein expression. J. Virol. 65:6765-6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uchida, J., T. Yasui, Y. Takaoka-Shichijo, M. Muraoka, W. Kulwichit, N. Raab-Traub, and H. Kikutani. 1999. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science 286:300-303. [DOI] [PubMed] [Google Scholar]
- 95.Wang, D., D. Liebowitz, and E. Kieff. 1988. The truncated form of the Epstein-Barr virus latent-infection membrane protein expressed in virus replication does not transform rodent fibroblasts. J. Virol. 62:2337-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang, D., D. Liebowitz, F. Wang, C. Gregory, A. Rickinson, R. Larson, T. Springer, and E. Kieff. 1988. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J. Virol. 62:4173-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang, F., C. Gregory, C. Sample, M. Rowe, D. Liebowitz, R. Murray, A. Rickinson, and E. Kieff. 1990. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J. Virol. 64:2309-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Winberg, G., L. Matskova, F. Chen, P. Plant, D. Rotin, G. Gish, R. Ingham, I. Ernberg, and T. Pawson. 2000. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol. Cell. Biol. 20:8526-8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yasui, T., M. Luftig, V. Soni, and E. Kieff. 2004. Latent infection membrane protein transmembrane FWLY is critical for intermolecular interaction, raft localization, and signaling. Proc. Natl. Acad. Sci. USA 101:278-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Young, L. S., C. W. Dawson, D. Clark, H. Rupani, P. Busson, T. Tursz, A. Johnson, and A. B. Rickinson. 1988. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J. Gen. Virol. 69(Pt. 5):1051-1065. [DOI] [PubMed] [Google Scholar]
- 101.Zimber-Strobl, U., K. O. Suentzenich, G. Laux, D. Eick, M. Cordier, A. Calender, M. Billaud, G. M. Lenoir, and G. W. Bornkamm. 1991. Epstein-Barr virus nuclear antigen 2 activates transcription of the terminal protein gene. J. Virol. 65:415-423. [DOI] [PMC free article] [PubMed] [Google Scholar]