Abstract
Cellular immune responses to influenza virus infection and influenza virus vaccination have not been rigorously characterized. We quantified the effector and memory B-cell responses in children and adults after administration of either live attenuated (LAIV) or inactivated (TIV) influenza virus vaccines and compared these to antibody responses. Peripheral blood mononuclear cells were collected at days 0, 7 to 12, and 27 to 42 after immunization of younger children (6 months to 4 years old), older children (5 to 9 years old), and adults. Influenza virus-specific effector immunoglobulin A (IgA) and IgG circulating antibody-secreting cells (ASC) and stimulated memory B cells were detected using an enzyme-linked immunospot assay. Circulating influenza virus-specific IgG and IgA ASC were detected 7 to 12 days after TIV and after LAIV immunization. Seventy-nine percent or more of adults and older children had demonstrable IgG ASC responses, while IgA ASC responses were detected in 29 to 53% of the subjects. The IgG ASC response rate to LAIV immunization in adults was significantly higher than the response rate measured by standard serum antibody assays (26.3% and 15.8% by neutralization and hemagglutination inhibition assays, respectively). IgG ASC and serum antibody responses were relatively low in the younger children compared to older children and adults. TIV, but not LAIV, significantly increased the percentage of circulating influenza virus-specific memory B cells detected at 27 to 42 days after immunization in children and adults. In conclusion, although both influenza vaccines are effective, we found significant differences in the B-cell and antibody responses elicited after LAIV or TIV immunization in adults and older children and between young children and older age groups.
Influenza virus is a common respiratory pathogen that causes substantial morbidity and mortality every year. In the United States, 226,000 hospitalizations and 36,000 deaths are associated with influenza virus infection annually, as estimated by the Centers for Disease Control and Prevention (43, 44). While influenza virus causes disease in all ages, influenza-related hospitalizations occur at a higher rate in very young children (less than 5 years old) and the elderly. Adults (65 years old and above) account for most influenza-related deaths, although significant mortality is also encountered in very young children (23, 31). While influenza disease is not generally associated with hospitalizations or death in healthy adults and older children, worker productivity is often affected due to the infection itself or illness in family members, and utilization of health care services is considerable by these population groups (37). Preschool- and school-age children have the highest influenza virus infection rates and are the major source of spread of influenza virus in human populations (8, 22, 37, 42).
Although several antiviral drugs against influenza are currently available, annual vaccination before the influenza season remains the most effective method to prevent the disease. Induction of humoral immunity to the influenza hemagglutinin (HA) molecule is extremely important and is key to protection against subsequent infection. Antigenic drifts in influenza viruses have tracked with changes in the amino acid residing in the antigenic sites for the HA molecule (39). The inactivated influenza virus vaccine, used since 1945, has been generally well tolerated and has been reported to induce substantial levels of protection, in the range of 70 to 90% when the vaccine and circulating wild-type strains are antigenically similar (7). However, recent studies suggest limited effectiveness of the inactivated vaccine in several settings, including vaccination of the elderly and vaccination in the face of antigenic mismatches between the vaccine and the wild-type strain (6, 24). Antigenic mismatch has occurred relatively frequently in recent years. At present, the trivalent inactivated influenza virus vaccine (TIV), produced by several manufacturers, is licensed worldwide and recommended for many populations, including children 6 months to 5 years, adults over the age of 50, people with a variety of chronic illnesses, and health care workers. A new live, attenuated, cold-adapted, trivalent influenza virus vaccine (LAIV) was licensed in the United States in 2003 for use in healthy children and adults ages 5 through 49 (3, 8, 38). The TIV and the LAIV vaccines have been shown to be immunogenic and efficacious, but there are no published studies directly comparing the immunogenicity of these two formulations of influenza vaccine in children and adults (7, 34, 38, 46). Intramuscular vaccination with TIV generally induces significant serum hemagglutination inhibition (HAI) antibody responses, particularly in older children and adults who have significant immunological memory to influenza virus. In contrast, intranasal administration of LAIV induces robust serum and mucosal antibody responses, especially in young, nonimmune children; however, the magnitude of influenza virus-specific antibody responses in the serum of older children and adults is generally substantially decreased compared to TIV (3, 7, 20). Despite the lower serum antibody response in older children and adults following LAIV immunization, vaccine efficacy appears to be at least comparable to TIV (4, 38, 46). Because serum and mucosal antibodies, as well as T-cell responses, induced by LAIV are likely to contribute to protection against influenza virus infection, the serum antibody titers following LAIV vaccination might not fully reflect the vaccine immunogenicity (3, 13). Of note, reliable immunologic markers of “take” following LAIV vaccination in adults are lacking (3, 30, 45).
The purpose of vaccination is to provide protection from disease via induction and recall of immunological memory responses. Antigen-specific antibodies in the serum are mainly produced by effector B cells (antibody-secreting cells [ASC]) that are differentiated from naïve and memory B cells upon stimulation by their specific antigen (16, 32). Very low quantities (0 to 4 cells per 1 million peripheral blood mononuclear cells [PBMC]) of influenza ASC have been detected in PBMC from healthy children and adults who had been previously infected or vaccinated (9, 15, 21). After TIV vaccination, the quantity of ASC in the circulation increases rapidly and peaks at 6 to 8 days postvaccination; this population then declines to very low quantities by 28 to 42 days postvaccination (15, 21). The timing and magnitude of the appearance of ASC in the circulation after influenza virus infection or LAIV administration is less well characterized. Regarding the memory B-cell response, the existence of influenza virus-specific memory B cells in PBMC was first reported in 1979, but methods to directly detect antigen-specific memory B cells in the circulation were not available (11). New assays to quantify antigen-specific memory B cells in the PBMC have been developed recently (5, 17). The new memory B-cell assays use a polyclonal B-cell stimulation cocktail containing a CpG motif to stimulate B cells via Toll-like receptor 9, thus causing their differentiation to ASC. Thus, the new memory B-cell assays do not rely on specific antigen stimulation, but rather a polyclonal stimulation, which permits the direct comparison of memory B-cell responses generated by different antigens or vaccination schemes, since the stimulation of memory B cells is antigen independent.
The present study is part of a larger comprehensive project in which the B-cell, T-cell, and NK cell responses to influenza virus vaccination are being studied in children and adults before and after vaccination with either TIV or LAIV over several influenza seasons. To evaluate the B-cell responses induced by these two different influenza vaccines, we measured the serum antibody response, the effector B-cell (ASC) response, and the memory B-cell response in adults, younger children (0.5 to 4 years old) who had never received influenza vaccine, and older children (5 to 9 years old) before and after TIV or LAIV vaccination. Our data show significant differences in the B-cell response elicited after LAIV or TIV immunization in adults and older children based on serum antibody titers, the acute ASC response, and the memory B-cell response. These new data provide a more complete picture of the host response to these two vaccines and suggest that measuring effector and memory B-cell responses, as well as serum antibody levels, may help in understanding differences in efficacy of the two vaccines or variations in the efficacy of one or both of the vaccine preparations during a particular influenza season.
MATERIALS AND METHODS
Human subjects and vaccination protocols.
Forty-four adults (age range, 21 to 48 years), 39 older children (age range, 5 to 9 years), and 25 younger children (age range, 6 months to 4 years) were enrolled in this study between September and early December 2004. Each individual was immunized with either TIV (Fluzone; Aventis-Pasteur) or LAIV (FluMist; MedImmune) following current guidelines for influenza vaccination. All adult subjects were participants in a study in the previous (2003-2004) influenza season, in which they were randomized in a 1:1 ratio to receive either TIV or LAIV. The adults were immunized in 2004-2005 with one dose of the same vaccine that they received in the previous season (2003-2004). The older children were randomized at a 1:1 ratio to receive either TIV or LAIV. For the influenza vaccine-naïve children in this group, a second dose of the same vaccine was given at approximately 28 days (for TIV) or 42 days (for LAIV) after the first dose. Of note, 19 of the 39 older children were also previously immunized with either TIV or LAIV in 2003-2004. Children in the youngest group were all influenza vaccine naïve and received two doses of TIV by intramuscular injection approximately 28 days apart. No subjects in this age group received LAIV. All adult subjects and parents of the children gave written informed consent, and children older than 7 years also completed an assent form. None of the subjects had serious health problems (autoimmune disease, immunosuppressive, immunodeficiency, or impairment of immunologic function), allergies to any component of the vaccine (including thimerosal), or a history of allergy to eggs or egg products. None of the adults or older children had chronic cardiovascular or pulmonary disorders or a history of asthma or reactive airway disease.
Three blood samples were obtained from adults and older children: prior to vaccination, on day 9 (range, day 7 to 12 in adults and day 9 to 11 in older children), and on day 30 (range, day 27 to 42) after vaccination. Two blood samples were obtained from the youngest children: the first, for the entire group, was obtained before TIV vaccination, and for the second sample these children were randomly assigned and tested on day 9 (range, day 9 to 11) after either the first or second dose of TIV.
The 2004-2005 TIV and LAIV vaccines both contained A/New Caledonia/20/99 (H1N1) and A/Wyoming/03/2003 (H3N2) strains. The third strain in TIV was B/Jiangsu/10/2003, while LAIV contained B/Jilin/20/2003. Both 2003-2004 formulations contained A/New Caledonia/20/99 (H1N1) and A/Panama/2007/99 (H3N2). The third strain in TIV was B/Hong Kong/1434/2002, while LAIV contained B/Hong Kong/330/2001. All the immunologic assays were carried out on coded specimens that did not indicate the vaccine group to which the subject had been randomized.
Two-color ELISPOT assay.
PBMC for the enzyme-linked immunospot (ELISPOT) assay were isolated by Ficoll gradient centrifugation of heparinized whole blood and resuspended in RPMI 1640 (Gibco, Grand Island, NY) containing 10% fetal calf serum (HyClone, South Logan, UT). Total and influenza virus-specific ASC were measured by ELISPOT assay. Ninety-six-well plates (Immobilon P membrane; MAIPN4510; Millipore, Billerica, MA) were coated with TIV influenza vaccine at a concentration of 9 μg/ml in phosphate-buffered saline (PBS; mixture of 4.5 μg of TIV 2002-2003 and 4.5 μg of TIV 2004-2005) or with affinity-purified goat anti-human immunoglobulin A (IgA) plus IgG plus IgM (H+L; Kirkegaard & Perry, Gaithersburg, MD) at a concentration of 4 μg/ml in PBS. Plates were coated with PBS as a negative control. Plates were incubated overnight at 4°C and blocked for 2 h at 37°C prior to use with RPMI 1640 containing 10% fetal calf serum, 100 units/ml of penicillin G, and 100 μg/ml of streptomycin (Gibco), referred to as complete medium. Freshly isolated PBMC or cultured PBMC (see below) were suspended in complete medium containing 6.3 μg/ml of peroxidase-conjugated goat anti-human IgA antibody (Sigma, St. Louis, MO) and 0.5 μg/ml of phosphatase-conjugated goat anti-human IgG (H+L) antibody (Kirkegaard & Perry), distributed into ELISPOT plates, and incubated overnight at 37°C in a CO2 incubator. Plates were washed with PBS and first developed with an AEC substrate kit for peroxidase (Vector, Burlingame, CA) and subsequently developed with a Blue alkaline phosphatase substrate kit (Vector). Human IgA ASC were visualized as red spots, and IgG ASC were visualized as blue spots in the same wells. The quantity of ASC per well was determined by counting the spots under a dissecting microscope, and the quantity reported is the average counts of two independent readers. Background (nonspecific) ASC detected in the wells coated with PBS were subtracted from the quantity of influenza virus-specific and total ASC.
Memory B-cell assay.
A memory B-cell assay, in which memory B cells are stimulated to differentiate into ASC, was performed essentially as described by Crotty et al. (17). PBMC were distributed into 24-well plates (Costar, Corning, NY) at 5 × 105 cells/well in complete medium with 55 μM of 2-mercaptoethanol and a mix of polyclonal mitogens: 1/100,000 dilution of pokeweed mitogen extract (kindly provided by R. Ahmed, Emory University, Atlanta, GA), 6 μg/ml of CpG oligonucleotide ODN-2006 (Invivogen, San Diego, CA), and 1/10,000 dilution of fixed Staphylococcus aureus Cowan (Sigma). Duplicate wells were cultured per individual, and negative control wells were cultured in complete medium, including 2-mercaptoethanol but without CpG, S. aureus Cowan cells, or pokeweed mitogen. Cells were cultured for 5 to 6 days at 37°C in a CO2 incubator. The cultured PBMC were washed twice with complete medium and placed in ELISPOT plates as described above. Total and influenza virus-specific ASC detected in unstimulated samples (negative controls) were considered indicative of additional background ASC. The number of influenza virus-specific or total ASC observed following stimulation was adjusted for both this background and that observed in the ELISPOT assay, as described above. Data are presented as the percentage of influenza virus-specific ASC per total ASC per culture well (17).
Serum neutralization and hemagglutination inhibition assays.
Serum samples were pretreated with receptor-destroying enzyme (Denka Seiken, Tokyo, Japan) overnight at 37°C and subsequently heated at 56°C for 45 min. Neutralization antibody titers were determined for 100% inhibition of cytopathic effect (CPE) in Madin-Darby canine kidney cells (European Collection of Animal Cell Cultures, Wiltshire, United Kingdom). Serially diluted serum specimens were incubated with 50 50% tissue culture infective doses of the indicated influenza virus for 1 h at 33°C and transferred to the Madin-Darby canine kidney cell monolayers in 96-well culture plates (Costar). After 6 days of culture at 33°C in a CO2 incubator, CPE in the wells was examined under a light microscope. The serum neutralizing antibody titer of a given sample was defined as the reciprocal of the last serum dilution with no observed CPE (3). A titer of 10 was assigned to all samples in which the first dilution (1:20) was negative.
The HAI assay was performed using a standard technique (12); serially diluted 25-μl aliquots of serum samples in PBS were mixed with 25-μl aliquots of virus, corresponding to four HA units, in V-bottom 96-well plates (Nunc, Rochester, NY) and incubated for 30 min at room temperature. At the end of the incubation, 50 μl of 0.5% chicken (for influenza H1 and B viruses) or turkey (for influenza H3 viruses) red blood cells was added and incubated for a minimum of 30 min before reading for HAI activity. The serum HAI antibody titer of a given sample was defined as the reciprocal of the last serum dilution with no HA activity. A titer of 2 was assigned to all samples in which the first dilution (1:4) was negative.
Statistical analysis.
Count data (quantity of ASC) were analyzed using Poisson regression via generalized linear models (GLM) (33). For antibody titers to A/Wyoming H3N2 in older children and adults, baseline cross-sectional data were analyzed by fitting the mixture model (MM) (36), assuming a Poisson instead of a log-normal distribution, and longitudinal data were analyzed using generalized estimating equations (GEE) (19), assuming a conditional Poisson distribution for quantity of dilutions. For antibody titers to A/Wyoming H3N2 in younger children, longitudinal data were fit using GEE for a Poisson outcome. Mean percentages of ASC were compared among age groups using GLM, assuming a conditional gamma distribution and over time using paired t tests. Also, percentages of ASC were regressed on age in years, assuming a linear increase from birth to an age after which the mean percentage of ASC remained constant (a “plateau” model). Nonlinear regression (NLR), using the Marquardt algorithm (41), was employed to estimate the slope as well as the age at which the plateau began.
To identify the threshold ASC “take rate,” we assumed that all subjects' counts were truly zero at baseline, that any nonzero counts strictly represented residual background noise after corrections for negative controls, and that the distribution of this measurement error was the same for all subjects and visits. On this basis, from the data obtained at baseline, we calculated that the probability of observing two or more counts under a Poisson distribution was 1/1,309 for IgA and 1/20,537 for IgG, both of which are sufficiently small probabilities to safely assume that any counts above 1 are unlikely due to measurement error.
For Table 1, “take” rates were compared within a group between effector ASC assays and serology assays using an exact McNemar's test (1, 18) and between groups within each assay type using logistic regression (LR) or exact chi-square tests.
TABLE 1.
Comparison of immunization “take” rates after LAIV or TIV immunization measured by ASC, neutralization assay, and HAI response
| Assay antigen | Assay | % with significant increase over baselined
|
|||
|---|---|---|---|---|---|
| Adults
|
5- to 9-yr-old children
|
||||
| LAIV | TIV | LAIV | TIV | ||
| TIVa | Effector IgA ASC | 47.4 § | 52.4 | 28.6 § | 52.6 #,§ |
| TIV | Effector IgG ASC | 79.0 #,§ | 81.0 | 86.7 | 100.0 |
| One of three strainsb | Neutralizing Abse | 26.3 †,‡ | 66.7 ‡ | 66.7 † | 94.7 |
| One of four strainsc | HAI | 15.8 †,‡ | 61.9 ‡ | 78.6 | 94.7 |
| A/Wyoming H3N2 | Neutralizing Abs | 21.1 † | 52.2 | 37.5 † | 78.9 |
| A/Wyoming H3N2 | HAI | 15.8 † | 43.5 ‡ | 26.7 † | 78.9 |
Influenza virus-specific ASC were assayed against TIV vaccine containing A/Wyoming, A/New Caledonia, B/Jiangsu, and B/Hong Kong as the immobilized antigen.
Significant increase in neutralizing antibody titer was detected against at least one virus strain of three strains tested: A/Wyoming, A/New Caledonia, and B/Shanghai.
A significant increase in HAI titer was detected against at least one virus strain of four strains tested: A/Wyoming, A/New Caledonia, B/Shanghai, and B/Jilin.
†, P < 0.05, percentage of antibody responders after LAIV was significantly lower than after TIV vaccination; ‡, P < 0.05, percentage of antibody responders among adults was significantly lower than in children after TIV or LAIV vaccination; # and §, P < 0.05, percentages of flu-specific IgA and IgG ASC responders were significantly higher or lower than the percent neutralizing antibody responders against one of three strains of influenza virus (#) or among HAI responders against one of four strains of influenza virus (§).
Neutralizing Abs, neutralizing antibodies.
Throughout, data are summarized by group as the mean ± 1 standard error. Attained significance levels of P < 0.05 are considered to be statistically significant. Due to the exploratory nature of this study, no correction was made for compounding of type I error across multiple hypothesis tests. Analyses were conducted with SAS version 9.1 (SAS Institute, Cary, NC), S-Plus version 7.0 (Insightful Corp., Seattle, WA), and Mathematica version 5.2 (Wolfram Research, Champaign, IL).
RESULTS
Effector B-cell responses induced after TIV or LAIV immunization of adults and 5- to 9-year-old children.
We measured the quantity of influenza virus-specific IgA and IgG circulating ASC by ELISPOT prior to immunization in 42 adults and 36 older children, ages 5 to 9 years. In agreement with previous studies (9, 15, 21), almost all of these subjects (97%) had no detectable (less than 1 ASC in 1 million PBMC) influenza virus-specific IgA or IgG ASC in their blood on day zero (Fig. 1). One adult had one IgG ASC and one child had one IgA ASC before vaccination.
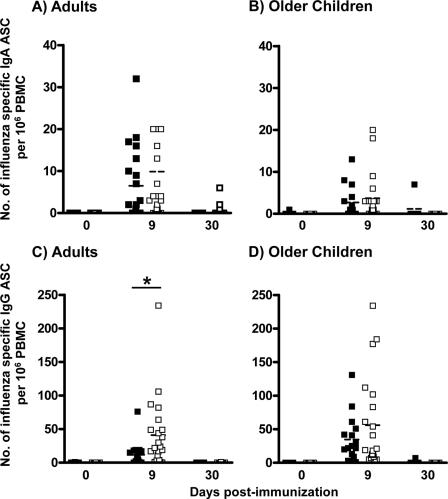
FIG. 1.
Quantities of circulating influenza virus-specific ASC present on day 0, day 9 (range, day 7 to 12), and day 30 (range, day 27 to 47) following vaccination with LAIV (▪) or TIV (□) in adults (A and C) and 5- to 9-year-old children (B and D). Influenza virus-specific IgA ASC (A and B) and IgG ASC (C and D) were measured by ELISPOT assay. The dashed horizontal line in each column indicates the mean value. Asterisks indicate a highly significant difference (P < 0.01, GLM).
Most subjects had detectable influenza virus-specific ASC in the samples obtained between 7 and 12 days after TIV or LAIV immunization (Fig. 1). Here, we provide the first report of ASC detection in LAIV-immunized individuals. Additionally, these data are consistent with previous studies of TIV in adults in which the influenza virus-specific ASC response was detected 4 to 15 days after vaccination and peaked at 7 to 8 days (9, 15, 21).
Older children and adults had similar quantities of circulating IgA ASC per million PBMC when comparing the effects of LAIV and TIV immunizations in these groups (Fig. 1A and B). We detected 3 ± 1 (mean ± standard error of the mean [SEM]) and 4 ± 1 IgA ASC per million PBMC in older children immunized with LAIV and TIV, respectively. Similarly, in adults the IgA ASC response averaged 7 ± 2 cells and 10 ± 5 cells after LAIV and TIV immunization, respectively. After LAIV immunization, adults and children did not have detectably different effector IgA B-cell responses (P = 0.125, GLM); in contrast, adults had a significantly higher effector IgA B-cell response than children following TIV immunization (P = 0.024, GLM).
IgG ASC responses in adults were significantly higher after TIV immunization than after LAIV. The average quantity of IgG ASC/million PBMC was 41 ± 11 versus 12 ± 4 in adults immunized with TIV or LAIV, respectively (Fig. 1 C) (P = 0.005, GLM). In children, no significant difference in the average quantities of IgG ASC was detected between TIV (56 ± 15) and LAIV (35 ± 9) (Fig. 1D) (P = 0.152, GLM). The IgG ASC responses in adults and older children were not significantly different after TIV immunization (P = 0.287, GLM), but children had a significantly higher IgG ASC response on average than adults after LAIV immunization (Fig. 1) (P = 0.028, GLM).
Of note, after TIV immunization in adults and in older children the effector IgG B-cell response was numerically greater than the IgA ASC response (P ≤ 0.011, GEE). This predominant effector IgG B-cell response was also observed after LAIV immunization in children (P = 0.004, GEE). However, after LAIV immunization in adults, IgA and IgG ASC responses were not detectably different (Fig. 1) (P = 0.109, GEE).
We also evaluated the effector response in samples drawn 1 month after immunization (days 27 to 47) from 36 adults and 17 older children. As expected, very few subjects had influenza virus-specific IgA and/or IgG ASC (only four adults who received TIV had detectable IgA or IgG ASC, averaging 3 ± 1 and 1 ± 0, respectively; 7 IgA and IgG ASC were observed in one child immunized with LAIV) (Fig. 1).
Effector B-cell responses after TIV immunization in different age groups.
Younger children (6 months to 4 years old) were immunized only with TIV, because LAIV was not licensed in this age group at the time of the study. Because they were influenza vaccine naïve, these younger children received two pediatric doses (7.5 μg each antigen) of TIV 1 month apart (28 to 35 days). We measured the effector B-cell response 9 days (range, 9 to 11 days) after the first TIV dose in seven children and 9 days (range, 9 to 11 days) after the second vaccine dose in an additional seven children. Significant differences were not detected in either effector IgA or IgG B-cell responses after the first versus after the second dose of TIV (Fig. 2A and B). Average quantities of IgA ASC per million PBMC were 4 ± 3 and 6 ± 3 after dose one and dose two, respectively (P = 0.698, GLM). Average quantities of IgG ASC per million PBMC were 20 ± 11 and 11 ± 3 after the first and second dose of TIV, respectively, and the difference in these means was not statistically significantly (P = 0.554, GLM) (Fig. 2).
FIG. 2.
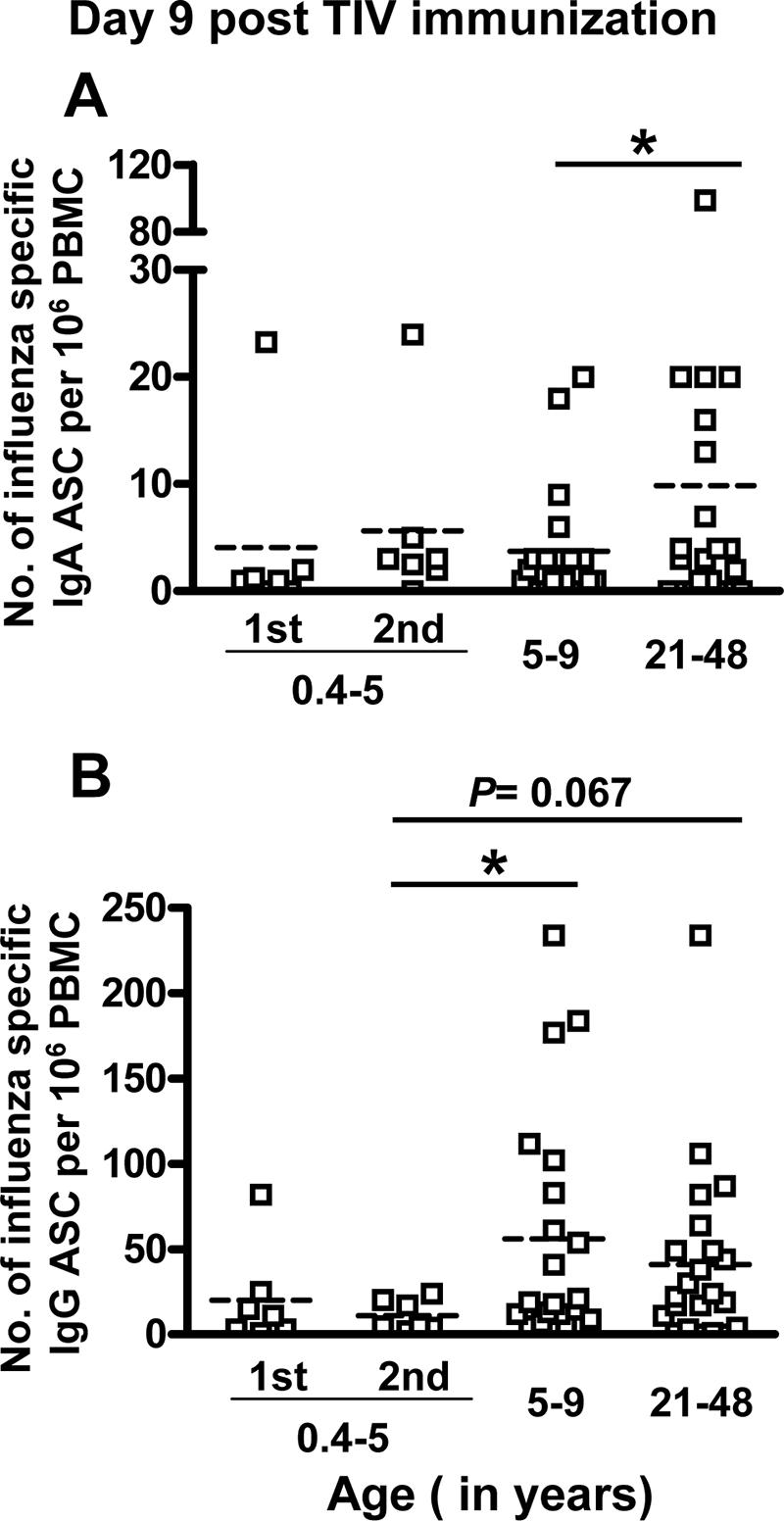
Comparison of the quantity of circulating influenza virus-specific ASC 9 days (range, day 7 to 12) after TIV vaccination in 0.5- to 4-year-old children, 5- to 9-year-old children, and adults. Influenza virus-specific IgA (A) and IgG (B) ASC were measured by ELISPOT. Adults and 5- to 9-year-old children received one dose of TIV as shown in Fig. 1. In 0.5- to 4-year-old children, influenza virus-specific ASC were measured 9 days (range, 9 to 11 days) after the first or second dose of TIV immunization. The horizontal dashed line in each column indicates the mean value. An asterisk indicates a significant difference (P < 0.05, GLM).
The effector B-cell response following TIV immunization was compared across the three age groups (Fig. 2). We detected a higher average effector IgA B-cell response in adults compared to older children (P = 0.037, GLM), but we did not detect significant differences between the younger children (either after one or two doses) and the other two age groups (P > 0.181, GLM). In contrast the influenza virus-specific IgG ASC response average was lower in the youngest children (20 ± 11 and 11 ± 3 IgG ASC/million PBMC after the first and second dose, respectively) compared to older children (56 ± 15) and adults (41 ± 11), although the difference was only statistically significant when comparing the average response after the second dose in the youngest children to the response in older children (Fig. 2B) (P = 0.015 and P ≥ 0.067 for the other comparisons [GLM]).
Baseline serum titers of neutralizing and HAI antibodies by age group.
Measurement of the serum neutralizing or HAI antibody titers after vaccination is the most common method for predicting the efficacy of TIV. We measured the neutralizing antibody and HAI titers against all the strains present in the vaccines, but for simplicity, only data for responses to the influenza H3N2 subtype viruses that have been drifting over the past few years are shown (http://www.cdc.gov/flu/weekly/fluactivity.htm).
Prior to immunization, no children of ages 6 months to 1 year had detectable neutralizing antibodies to H3N2, but 65% of the 2- to 4-year-old children and all to nearly all of the older children (100%) and adults (97.7%) had significant titers (Fig. 3A). The proportion of subjects having positive antibody titers was higher among older children and adults compared to children 6 months to 4 years old (P < 0.001, MM). Among the subjects with positive antibody titers, the 5- to 9-year-old children had significantly higher neutralizing antibody titers than the adults (P < 0.001, MM) (Fig. 3A). Similar results in the HAI-positive proportion and in the averages of the HAI titer were obtained when analyzing the HAI baseline titers (Fig. 3B).
FIG. 3.
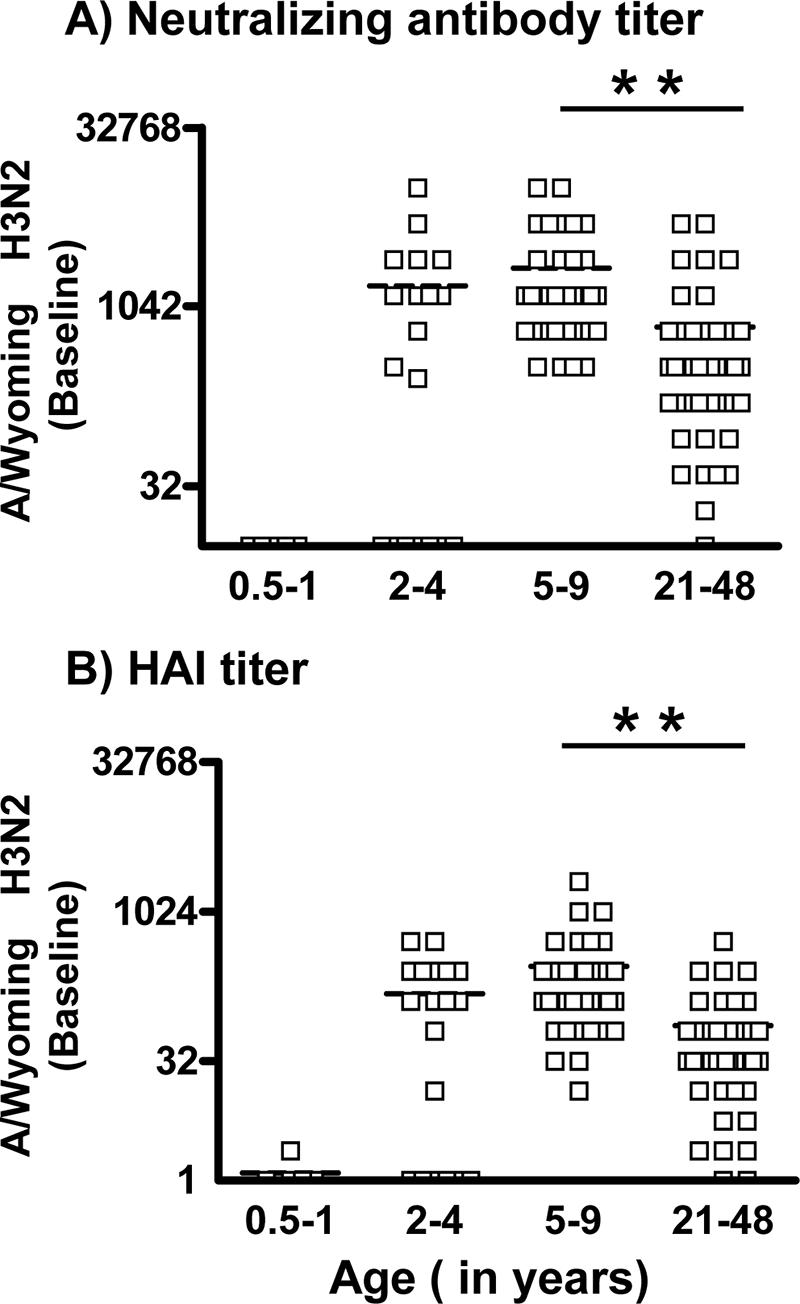
Comparison of day zero (baseline) titers of neutralizing antibody and HAI to the A/Wyoming H3N2 strain. Neutralizing antibody (A) and HAI (B) titers in the serum of children 0.5 to 1 years old, 2 to 4 years old, and 5 to 9 years old and of adults 21 to 48 years old were measured by neutralizing and HAI assay. The horizontal dashed line in each column indicates the mean value. Asterisks indicate a highly significant difference (P < 0.01, MM).
Average baseline neutralizing antibody titers in the older children that received either LAIV (1,942 ± 431 [mean ± SEM]) or TIV (2,288 ± 662) were similar (P = 0.932, GLM) (Fig. 4). In contrast, the mean baseline neutralizing antibody titer in the adults that received TIV was significantly higher than the one in the LAIV recipients (1,077 ± 298 versus 242 ± 64, respectively; P = 0.0003, GLM). It is important to point out here that, as mentioned in Materials and Methods, each adult received the same type of vaccine (either TIV or LAIV) as he/she had been administered in the prior year.
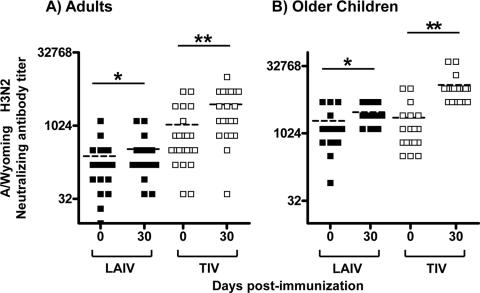
FIG. 4.
Comparison of the titers of neutralizing antibody to the A/Wyoming H3N2 strain before and 30 days (range, 27 to 47 days) after vaccination. Neutralizing antibody titers in the serum of adults (A) and 5- to 9-year-old children (B) at baseline (day 0) and 30 days after vaccination with LAIV (▪) or TIV (□) vaccination were measured. The horizontal dashed line in each column indicates the mean value. One asterisk or two asterisks indicate significant and highly significant differences (P < 0.05 and P < 0.01, respectively; GEE).
Serum antibody responses after TIV or LAIV immunization in younger children, older children, and adults.
The serological response was evaluated 1 month after TIV or LAIV in older children and adults (Fig. 4A and B). On average, TIV vaccination significantly increased serum neutralizing titers against H3N2 in adults (1,077 ± 298 at day 0 versus 2,798 ± 525 at day 30) and in older children (2,288 ± 662 at day 0 versus 12,288 ± 2,332 at day 30) (P < 0.001, GEE). LAIV vaccination also increased average serum neutralizing titers from 242 ± 64 to 337 ± 83 in adults and from 1,942 ± 431 to 3,040 ± 385 in older children (P ≤ 0.018, GEE); however, this increase was not as pronounced as was observed after TIV immunization, especially in older children (P = 0.009 and P = 0.201 [GEE] in children and adults, respectively, for the increase after TIV versus the increase after LAIV).
Because of limitations on the quantity of venipunctures in the youngest children (6 months to 4 years old), specimens were obtained 9 to 11 days after either the first or second dose of TIV immunization. As shown in Fig. 5, the H3N2 average antibody titers increased significantly above baseline levels in both subgroups (P ≤ 0.008, GEE); however, average titers achieved after either one or two TIV doses were very similar, which suggests that no booster effect occurred after the second dose (8,277 ± 2,747 and 8,373 ± 2,712, respectively, P = 0.254 [GEE], when comparing the antibody titer response in the subgroups tested after the first versus second dose) (Fig. 5).
FIG. 5.
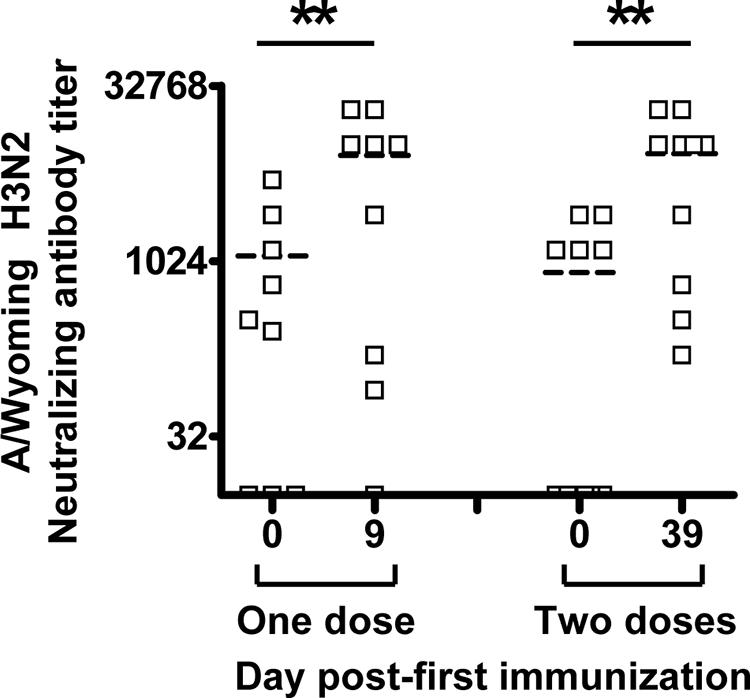
Comparison of titers of neutralizing antibody to the A/Wyoming H3N2 strain 9 days (range, 9 to 11 days) after dose one or two of TIV in 0.5- to 4-year-old children. Neutralizing antibody titers were determined before (day 0) and after one dose (day 9) or two doses (day 39 or day 9 after second vaccination) of TIV. The horizontal dashed line in each column indicates the mean value. Asterisks indicate a highly significant difference (P < 0.01, GEE).
Comparison of vaccine take rates measured by effector B-cell responses or standard serologic assay after TIV or LAIV immunization of adults and children 5 to 9 years old.
Vaccine “take” following influenza virus immunization is usually defined as a fourfold or greater increase in the HAI or neutralization titer postvaccination (seroconversion). It has been shown previously that the magnitude and seroconversion response rate of influenza virus-specific antibody responses in the serum after LAIV immunization is lower than after TIV immunization, especially in adults (7, 20). Our data are in agreement with these previous findings (Table 1), since the quantity of adults with a significant serological (HAI and/or neutralization) response after LAIV immunization (15.8 and 21.1% for anti-H3N2 HAI and neutralization, respectively) was significantly lower than the quantity after TIV immunization (43.5 to 52.2%) (P ≤ 0.048, LR). Furthermore, in children, as in adults, the percentage of serum responders after LAIV immunization (26.7 to 37.5%) was significantly lower than after TIV immunization (each 78.9%) (P ≤ 0.012, LR).
Using a Poisson model as described in Materials and Methods, we estimated that detecting two or more ASC spots for IgA and IgG at 7 to 12 days after vaccination constituted a significant increase over the baseline prevaccination level (see Materials and Methods). Using this threshold for “take,” we found an effector IgA B-cell response following immunization with LAIV or TIV in 47.4% or 52.4% (P = 0.758, exact chi-square) of the adults, respectively, while 28.6% of older children immunized with LAIV and 52.6% of the older children immunized with TIV had a response (P = 0.500, exact chi-square). The percentages of IgG ASC responses were considerably higher than the IgA responses: 79.0% and 81.0% (P = 0.714, exact chi-square) of the adults immunized with LAIV and TIV, respectively, had a response, and 86.7% and 100% (P = 0.180, exact chi-square) of the older children immunized with LAIV or TIV, respectively, had a significant response to vaccination.
Given the high percentage of responders that were detected with the threshold of two IgG ASC, we assessed how the estimated take rates change across threshold criteria of three and four spots as well. From this analysis we determined that the percentage of IgG responders at the doubled threshold of four spots does not materially change take rates, since 63.2% and 76.2% of the adults immunized with LAIV and TIV, respectively, remained in the responder group, as did 78.6% and 94.7% of the older children immunized with LAIV or TIV, respectively.
The ELISPOT assay does not distinguish between influenza A virus- and B virus-specific effector B cells, since TIV vaccine, which contains all three HA and NA serotypes, was used as antigen. Hence, in order to more directly compare the “take” rates after TIV and LAIV immunizations measured by ELISPOT versus by serology, we calculated the total “take” rate by either HAI or neutralization assay in adults and children, defined as seroconversion to at least one of the three influenza virus strains used in the neutralization assays or at least one of the four virus strains used for the HAI assay. In adults given TIV, the IgG ASC “take” rate, compared to the total “take” rate measured by neutralization or HAI serology, was not significantly different (Table 1) (P ≥ 0.289, McNemar's test). In contrast, after LAIV immunization, the percentage of vaccine responders detected by IgG ASC was significantly higher than the percentage based on neutralization or HAI titers when the IgG ASC threshold was either two or four spots (P ≤ 0.039, McNemar's test).
The IgG ASC “take” rate and total serology “take” rates were also similar among older children immunized with TIV (P = 1, McNemar's test). In contrast to adults, take rates measured by the IgG ELISPOT assay and by HAI or neutralization assay were not significantly different after LAIV (P ≥ 0.250, McNemar's test).
Baseline percentages of influenza virus-specific memory B cells by age group.
In order to evaluate the influenza virus-specific memory B-cell responses, we stimulated PBMC obtained before immunization with a mixture of B-cell mitogens that allowed memory B cells to differentiate into ASC. Since the nonspecific stimulation procedure allows all memory B cells to proliferate and differentiate into ASC, the influenza virus-specific responses are reported as percentages of the total memory B-cell pool detected by ELISPOT. Since baseline percentages of memory B cells were likely to depend on age and prior exposure to influenza virus antigens, the group of youngest children (6 months to 4 years old) was analyzed as two subgroups consisting of those who were 6 months to 1 year old (n = 5) and those who were 2 to 4 years old (n = 19). Children under the age of 1 were least likely to have had prior influenza virus exposure.
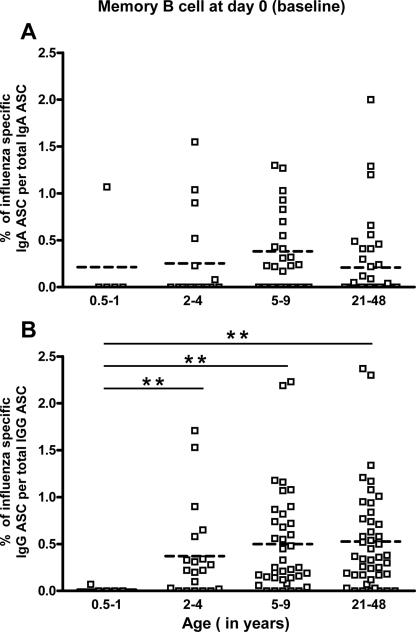
The percentage of influenza virus-specific memory IgA B cells detected prior to immunization in each age group is shown in Fig. 6. This average percentage was low and similar across the four age groups: 0.21% ± 0.21% (mean ± SEM) in children 0.5 to 1 year old, 0.25% ± 0.11% in children 2 to 4 years old, 0.38% ± 0.14% in children 5 to 9 years old, and 0.21% ± 0.07% in adults (P ≥ 0.120 for all age comparisons, GLM).
FIG. 6.
Comparison of prevaccination percentages of influenza virus-specific memory B cells between age groups. Influenza virus-specific memory IgA cells (A) and IgG cells (B) in individuals 0.5 to 1 year old, 2 to 4 years old, 5 to 9 years old, and 21 to 48 years old were measured by a memory B-cell assay. The horizontal dashed line in each column indicates the mean value. Asterisks indicate a highly significant difference (P < 0.01. GLM).
In contrast to memory IgA B cells, the percentage of circulating influenza virus-specific memory IgG B cells increased significantly with age. Among children 6 months to 1 year, this percentage averaged only 0.01% ± 0.01%, while in children 2 to 4 years old it averaged 0.34% ± 0.11%; the average percentage was 0.50% ± 0.09% in children 5 to 9 years old and 0.53% ± 0.08% in adults (P < 0.001 when comparing children 6 months to 1 year old with all of the other groups; GLM). No significant differences were detected in the percentage of influenza virus-specific memory IgG B cells before immunization between the children 2 to 4 years old, the older children (5 to 9 years old), and the adults (P ≥ 0.203, GLM). Only one (20%) of the youngest children (6 months to 1 year) had circulating influenza virus-specific memory IgA and/or IgG B cells, whereas these cells were detected in a higher percentage of subjects in the other age groups: 35 to 46% of all other subjects had detectable influenza virus-specific memory IgA B cells and 74 to 87% had influenza virus-specific memory IgG B cells. It is important to point out here that since flow cytometry data indicate that, on average, 8% and 6% of CD19-positive small lymphocytes express IgG and IgA on their surface, respectively (26), one might expect, after stimulation of memory IgA and IgG B cells, to retrieve similar quantities of IgA and IgG ASC. However, in our assay, we found that, on average, after a 5-day stimulation of 5 × 105 PBMC from adults prior to immunization, 2,063 ± 80 IgA ASC versus 20,423 ± 564 IgG ASC were generated, indicating an approximately 1:9 ratio of total IgA to IgG ASC. These results suggest that the polyclonal stimulation cocktail used in this study primarily induced proliferation of memory IgG B cells.
Our data suggest that the quantity of circulating memory IgA and IgG B cells increases from 0.5 to 6 years of age and then reaches a plateau. The estimated age at which the influenza virus-specific memory IgG B-cell response first plateaus was estimated to be 4.6 ± 1.6 years old (P = 0.006, NLR). In contrast, due to substantial variability in the influenza virus-specific memory IgA B-cell response across ages, we could not identify a plateau age for the IgA memory B-cell response (P = 0.130, NLR). When we fit a second set of more flexible models that allowed the “plateau” to have a nonzero slope, we found no evidence that this slope actually differed from zero (P = 0.581 for IgA; P = 0.993 for IgG). Thus, our data indicate that the percentage of memory B cells remains stable between approximately 5 years and 48 years, regardless of the extent of antigen exposure by natural infection or immunization.
Effect of TIV or LAIV immunization on the percentage of influenza virus-specific memory B cells.
In order to determine the effects of TIV and LAIV, we measured the percentages of influenza virus-specific memory IgA and IgG B cells 1 month after immunization of adults and older children (5 to 9 years old). Since all adults in this study had been immunized with the same type of vaccine (LAIV or TIV) during the 2003-2004 season and half of the older children had received TIV in 2003-2004, we determined the impact of previous immunizations on the baseline percentage of memory-specific B cells. The percentages of influenza virus-specific memory IgA and IgG B cells at baseline were similar in these different groups when compared between children and adults receiving the same vaccine and between individuals of the same age group receiving LAIV or TIV (Fig. 7). Average percentages of circulating influenza virus-specific memory IgA B cells were 0.15% ± 0.07% (adults/LAIV), 0.27% ± 0.11% (adults/TIV), 0.37% ± 0.11% (older children/LAIV), and 0.39% ± 0.25% (older children/TIV) (P ≥ 0.103 for all four group comparisons, GLM). Average percentages of circulating influenza virus-specific memory IgG B cells were as follows: 0.38% ± 0.09% (adults/LAIV), 0.65% ± 0.13% (adults/TIV), 0.57% ± 0.16% (older children/LAIV), and 0.44% ± 0.09% (older children/TIV) (P ≥ 0.125 for all four group comparisons, GLM).
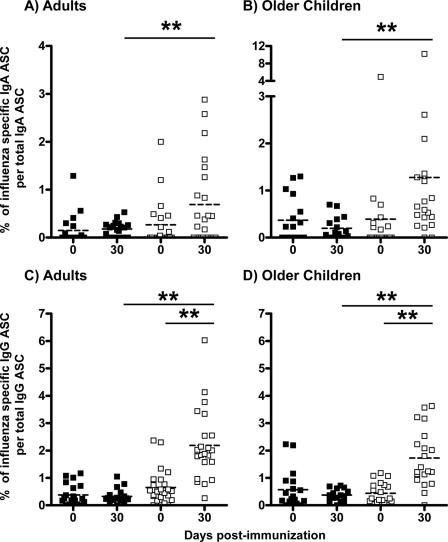
FIG. 7.
Percentage of influenza virus-specific memory B cells in the circulation before and 30 days (range, 27 to 47 days) after vaccination. Influenza virus-specific memory IgA cells (A and B) and IgG cells (C and D) in adults (A and C) and 5- to 9-year-old children (B and D) before and after LAIV (▪) or TIV (□) vaccination were measured by memory B-cell assay as described in the text. Asterisks indicate a highly significant difference (P < 0.01, GLM and paired t test).
After immunization with LAIV, significant changes in the percentages of influenza virus-specific memory IgA and IgG B cells were not detected in older children or adults (Fig. 7). Differences in average percentages of circulating influenza virus-specific memory B cells detected prior to and after LAIV immunization in older children and adults, respectively, were −0.19% ± 0.14% (P = 0.195, paired t test) and 0.03% ± 0.07% (P = 0.648, paired t test) for IgA B cells and −0.24% ± 0.17% (P = 0.188, paired t test) and −0.06% ± 0.08% (P = 0.473, paired t test) for IgG B cells. In contrast, after TIV immunization, significant increases in percentages of circulating memory IgG B cells were detected in older children and in adults (P < 0.001, paired t test); the difference in average percentages detected before and after TIV vaccination was 1.55% ± 0.25% in adults and 1.35% ± 0.23% in children. Of note, while some of the TIV vaccine recipients (50% of adults and 78% of older children) showed an increase in the percentage of influenza virus-specific memory IgA B cells, overall a significant difference was not detected before and after vaccination of adults (0.49% ± 0.26%; P = 0.069, paired t test) or children (0.93% ± 0.60%; P = 0.136, paired t test).
Average percentages of circulating influenza virus-specific memory IgA B cells elicited after TIV immunization in adults (0.69% ± 0.19%) and in older children (1.28% ± 0.52%) were significantly higher than after LAIV immunization (0.18% ± 0.03% and 0.20% ± 0.06% in adults and older children, respectively) (Fig. 7A and B) (P ≤ 0.002, GLM). Moreover, the circulating memory IgG B-cell response elicited by TIV immunization was also significantly higher than the response elicited by LAIV immunization (Fig. 7C and D). After TIV immunization, the mean percentages of circulating memory IgG B cells were 2.19% ± 0.27% and 1.72% ± 0.24% in adults and children, respectively, and, in contrast, after LAIV these means were only 0.32% ± 0.06% and 0.37% ± 0.05% (P < 0.001, GLM) in adults and children, respectively. Comparing adults versus children, average percentages of influenza virus-specific memory IgA and IgG B cells induced either after LAIV or TIV immunizations were not detectably different (P ≥ 0.135, GLM).
DISCUSSION
The goal of this study was to compare effector and memory B-cell and antibody responses induced by TIV and LAIV immunizations and to assess differences in the responses of children and adults. Although both of these vaccines have proven efficacy against influenza virus infection and disease, we detected important differences in the B-cell and antibody responses elicited after immunization with these two vaccine formulations in healthy adults and children ages 5 to 9 years (Table 2).
TABLE 2.
Summary comparisons across multiple groups
| Comparison | Constant | Marker | Outcomeb |
|---|---|---|---|
| TIV versus LAIV | Age | Effector IgA | Similar |
| TIV versus LAIV | Age | Effector IgG | TIV (adults) |
| Adults versus 5-9 yrs | LAIV | Effector IgA | Similar |
| Adults versus 5-9 yrs | LAIV | Effector IgG | 5-9 yrs |
| Adults versus 5-9 yrs | TIV | Effector IgA | Adults |
| Adults versus 5-9 yrs | TIV | Effector IgG | Similar |
| Adults versus 0.5-4 yrs | TIV | Effector IgA | Similar |
| Adults versus 0.5-4 yrs | TIV | Effector IgG | Adults |
| 5-9 versus 0.5-4 yrs | TIV | Effector IgA | Similar |
| 5-9 versus 0.5-4 yrs | TIV | Effector IgG | 5-9 yrs |
| TIV versus LAIV | Age | Memory IgA | TIV |
| TIV versus LAIV | Age | Memory IgG | TIV |
| Adults versus 5-9 yrs | TIV | Memory IgA | Similar |
| Adults versus 5-9 yrs | LAIV | Memory IgG | Similar |
| TIV versus LAIV | Age | Serum Abs | TIV |
| Adults versus 5-9 yrs | LAIV | Serum Abs | 5-9 yrs |
| Adults versus 5-9 yrs | TIV | Serum Abs | 5-9 yrs |
| Effector B cells versus serum Absa | LAIV | Take rate | Effector B cells (adults) |
| Effector B cells versus serum Abs | TIV | Take rate | Similar |
Abs, antibodies.
The group with a significantly higher response for a given comparison is indicated. Similar, similar responses for the groups in the indicated comparison.
Previous studies have shown that detection of circulating influenza virus-specific effector B cells following TIV immunization is transient, peaking around 1 week after immunization and then dropping rapidly, so that very few if any influenza virus-specific cells are detected 30 days after immunization (15, 21). Our results are in agreement with these observations. In addition we were able to determine that the effector B-cell response occurring after LAIV immunization has relatively similar kinetics to that of TIV, since we detected circulating influenza virus-specific ASC in significant quantities 7 to 12 days after LAIV immunization. Future studies will be needed to compare further the kinetics of the influenza virus-specific effector IgA and IgG B-cell responses elicited after TIV and LAIV in both adults and children. In addition, we only compared TIV to LAIV in older children and adults, so that virtually all recipients had some level of preexisting immunity to influenza virus prior to vaccination. In future studies it will be important to determine if the time course for effector B-cell appearance in the circulation is similar in vaccine-naïve children compared to subjects previously immunized and whether the ASC response in the naïve host differs significantly between TIV and LAIV. This latter aspect is not only important for evaluating immune responses in children but also could be very important in evaluating vaccine immunogenicity to novel, pandemic flu antigens in adults.
In this study we were able to directly compare, for the first time, the effector B-cell responses induced after TIV or LAIV immunization in primed children and in adults. We observed a greater influenza virus-specific effector IgG B-cell response to TIV than to LAIV immunization in adults and in older children, although this difference was only significant in adults. This difference can be at least partially explained by the different routes of immunizations used and the fact that systemic immunization will generally induce a greater effector IgG B-cell response than mucosal immunization, especially in previously immunized individuals. The fact that the influenza virus-specific effector IgG B-cell response did not significantly differ in children given LAIV or TIV may well be due to the enhanced replication capacity of the live vaccine in the younger age group (10). Increased replication would be expected to induce a more robust immune response, including effector IgA and IgG B-cell responses. However, in contrast to the IgG B-cell responses, the IgA B-cell responses were similar to each other in both adults and older children but lower than the IgG responses in both the TIV and LAIV recipients. It is not clear why TIV was as effective as LAIV at inducing circulating IgA ASC in the older children and adults, but this might be related to the fact that all vaccines in the older children and adult groups, including the TIV recipients, had probably experienced previous influenza virus infections that could have primed the IgA response. It will be of interest to determine whether the effector IgA B-cell responses are also comparable in vaccine-naïve children given either of the two vaccine preparations.
At least two mechanisms could account for the lower effector B-cell response detected after LAIV immunization. It is possible that we missed the peak response for effector IgA and for IgG B cells, since our study was not designed to identify the optimal time at which ASC circulate following mucosal immunization with LAIV. Additionally, the ASC response induced after LAIV immunization may be predominantly local, taking place in the nasal mucosa and the upper respiratory tract, and only a restricted proportion of the responding ASC actually enter the systemic circulation. The ability to induce a significant local response after intranasal immunization has been demonstrated previously (27, 28, 35). On the other hand, parenteral influenza virus immunization did not enhance the quantities of ASC in the nasal mucosa, while these quantities were increased in the blood and tonsils (9). Hence, it will be of great interest to determine the extent of the local response induced after LAIV immunization in future studies by looking at the effector B-cell response in nasal washes and tonsils and comparing these responses to the circulating response. In addition, while quantities of circulating IgA ASC were similar after TIV and LAIV immunization, it will be important to determine if the repertoire of lymphocyte trafficking molecules on these cells varies as it does after intestinal versus systemic immunization (29). Finally, it is also important to take into consideration the fact that responses to proteins other than HA and NA, like the M protein, are probably elicited after LAIV immunization and, thus, by using TIV as antigen in our ELISPOT assay, we could be underestimating the total ASC response to LAIV (14). Moreover, it is of interest to compare the quality (i.e., avidity, breadth of antibodies) of the antibody response elicited by the two vaccines in both adults and children. For instance, a previous report showed that LAIV vaccination in unprimed children can elicit higher cross-reactive antibody responses against H3N2 than TIV (2).
Although we detected a significant effector IgG B-cell response after TIV immunization in older children and adults, the responses observed were lower than those previously described (15, 21). For instance, Cox et al. and el-Madhun et al. detected an average of 1,200 ± 600 IgG ASC per million PBMC at 7 to 8 days post-TIV immunization in healthy adults. In our study, in the same age group, we were only able to detect 41 ± 11 circulating IgG ASC per million PBMC, 7 to 12 days postimmunization. The reason for this discrepancy could be related to differences in the timing of sample collection, since in our study, blood samples were drawn within a range of 7 to 12 days after immunization and, for the detection of circulating antigen-specific ASC, this is rather a broad range. For example, we observed that the quantity of cells detected in the samples drawn at day 9 postimmunization were higher than in the samples collected at day 12 postimmunization (data not shown) (14). Another difference with previous studies is the antigen used for capture in the ELISPOT assay: Cox et al. used purified influenza virus or surface HA and NA purified from different strains (A/Beijing/353/89 H3N2, A/Taiwan/1/86 H1N1, and B/Yamagata/16/88) while we used commercial TIV vaccine. However, we believe that using TIV as antigen allowed us to detect most responses directed against HA and NA and, in preliminary experiments, we were able to detect identical quantities of influenza virus-specific ASC using either TIV or purified H3N2 virus. Other possible reasons for the differences in the immune responses observed could be differences in the composition of the actual TIV vaccines used in the different studies as well as differences in the immune status of the populations enrolled in ours and previous studies at the time of vaccination.
Interestingly, we were able to detect significant differences in the effector B-cell responses between different age groups after TIV immunization. For instance, the quantity of effector IgG B cells detected in the group of youngest children (6 months to 4 years old) was significantly lower than the quantity detected in older children and adults. This result suggests that the ASC response in young children is not optimal, and repeated exposures to influenza virus antigen are likely to enhance the effector B-cell responses (21). Alternatively, or in addition, the immune systems of the youngest children might still be immature, which could contribute to a diminished response. Although our analysis of the effector response in the youngest children was limited by the fact that we were not able to test the same child after both the first and the second TIV dose, and though the quantity of children in this group was quite small, we found virtually identical average quantities of ASC elicited after either one or two doses of vaccine. This finding appears to suggest that the second vaccine dose did not have a booster effect on the effector B-cell response or on the neutralization response, although future studies looking at the response in the same child after each dose are needed to confirm this hypothesis. In addition, it will be interesting to perform a detailed time course analysis following the second TIV immunization, since it is possible that the kinetics of the primary and booster responses are different and, therefore, we missed the peak response after the second dose. Also, we might have missed some component of the primary response in these very young children by not measuring the effector IgM B-cell response, although a previous study showed that the IgG and IgM responses are similar in very young children (21).
Our study confirms previous reports that have shown that the frequency of antibody responses and postvaccination HAI and neutralization titers are significantly higher after TIV immunization compared to LAIV in children and in adults (7, 20, 46). It is also important to point out that the baseline and postimmunization antibody titers detected in our study are higher than the ones previously reported (15, 21). We believe this difference could be explained by the fact that all the adults and at least half of the older children enrolled in our study were vaccinated the preceding year, whereas in other studies most of the subjects had not been previously vaccinated. However, the average rates of seroconversion detected in our study are in agreement with other TIV or LAIV vaccination studies (20, 30, 46). Since the significant serological response elicited after TIV immunization is considered to be an important mediator of immunity to influenza illness, HAI or neutralization antibody titers are used as surrogate markers for TIV efficacy. However, it has also been shown that both vaccines are highly efficacious; hence, the exclusive consideration of the serological response results in a bias in favor of the parenteral vaccine and underestimates the efficacy of LAIV. Some authors have proposed that the levels of IgA in nasal secretions, which are significantly higher after LAIV compared to TIV immunization, can also account for vaccine efficacy (7). Our study suggests that the response or “take” rate as measured by the presence of a significant quantity of circulating IgG ASC detected 1 week after immunization is also likely to be a useful marker of vaccine efficacy in children and in adults after either LAIV or TIV immunization. Our findings document that there is, at least transiently, evidence of a systemic B-cell anti-HA/NA response to LAIV vaccination, even in adults without detectable serum neutralization or HAI responses. After LAIV immunization in adults, the percentage of IgG responders is significantly higher than the percentage of serum neutralizing antibody responders and correlates well with the previously determined rate of vaccine efficacy. In addition, in adults and older children, the IgG ASC response correlates well with the serum antibody response following TIV immunization. Future studies will be aimed at the following: (i) confirming the potential use of circulating IgG ASC as surrogate marker of protection following influenza vaccine immunization; (ii) determining more precisely the optimal time to measure the IgG ASC response after both LAIV and TIV immunizations; (iii) correlating the IgG circulating ASC response and or the B-cell trafficking marker phenotype on circulating IgG and IgA cells; and (iv) detecting the nasal antibody response in LAIV recipients.
Memory B cells are generally induced after immunization or infection and frequently persist for long periods of time (5, 17). We were able to detect influenza virus-specific memory IgG B cells, prior to immunization, in the majority of adults (86%) and older children (87%) examined. These results are in agreement with the study by Yarchoan et al., where influenza virus-specific memory IgG B cells were detected in at least 75% of the adults tested (47). Furthermore, in our study, we were able to measure, for the first time, the percentage of these cells across different age groups. Interestingly, we detected similar levels of influenza virus-specific circulating memory IgG B cells prior to vaccination in children 5 to 9 years old and adults (0.50% and 0.53% of total memory IgG B cells, respectively). These memory B-cell levels reflect prior immunizations and/or exposure to natural infection, since we were also able to detect influenza virus-specific memory B cells in children older than 2 years of age who did not have a history of prior influenza virus immunizations. It also appears from our cross-sectional analysis of the memory B-cell levels in children and adults of different ages that the percentage of influenza virus-specific memory IgG B cells increases during the first 4 to 5 years of life and then reaches a plateau, remaining relatively stable for over 40 years despite the increased likelihood of reexposure to infectious virus or immunization in older subjects. Moreover, although we demonstrated a significant increase in influenza virus-specific memory IgG B cells 30 days after TIV immunization in children and adults (from 0.44 to 0.65% to 1.72 to 2.19% in children and adults, respectively), such increases do not appear to be long lived, since the baseline levels observed prior to vaccination in older children and in adults were similar, despite obvious differences in the quantity of exposures to influenza virus antigens in the older subjects. Taken together, these results lead us to hypothesize that the levels of circulating influenza virus-specific memory B cells remain relatively constant over time. It remains to be determined if repetitive antigen exposure and/or immunization has a more significant effect in replenishing or renewing long-lived plasma cells in bone marrow or other sites as opposed to in the circulation.
In limited studies, memory B-cell responses to other vaccinations appear to be quantitatively and qualitatively similar to those described in the present study of TIV immunization. For example, Crotty et al. showed that, 4 weeks after vaccinia virus vaccination for smallpox (Dryvax), 1.5% of circulating memory IgG B cells were vaccinia virus specific and that after this time point this percentage declined and reached a steady level (0.1%) that was maintained for more than 50 years without antigen reexposure. In addition, percentages of specific memory IgG B cells were 0.02 to 0.2% and 0.3%, measured at least 2 years after tetanus and anthrax vaccination, respectively (5, 17). Taken together, these results suggest that the plateau levels of memory IgG B cells for various antigens are relatively similar even though the vaccination schedule and the chance of reexposure to antigens are frequently different. The mechanisms that are responsible for maintaining these relatively constant levels remain to be elucidated. Also, since to our knowledge most of the studies of memory B cells in humans have been done using peripheral blood, it remains to be determined if the levels of memory B cells are also highly regulated at other tissue sites.
In contrast to the effect of TIV, LAIV administration did not induce a significant increase in the percentage of memory B cells in children or in adults. This is in contradiction to a previous report, where Yarchoan et al. showed induction of circulating memory IgG B cells in adults after intranasal immunization with cold-adapted influenza virus (H3N2) (48). This difference might be explained by differences in methods between studies and the fact that Yarchoan et al. did not look at the proportion of influenza virus-specific antibody-producing cells among total antibody-producing cell populations, as we did for the memory B cells. It is clear from our data, however, that a significant difference exists between TIV and LAIV effects on the circulating memory B-cell response. One hypothesis that could potentially explain this difference is that, as previously suggested for the effector response, LAIV vaccination induces a memory B-cell response predominantly at the site of immunization (i.e., upper respiratory tract) and/or that the circulating memory B cells are actively recruited to their effector site and hence are only transiently circulating. Influenza virus-specific memory B cells localized in the nose and upper respiratory tract would contribute to long-term local antibody responses but would not be detected in our study of peripheral blood memory B cells. Previous studies also support this hypothesis. For example, it has been shown that when naïve children are immunized with live or inactivated influenza vaccine, nasal IgA antibody responses are observed up to 1 year after live virus vaccination, but inactivated influenza vaccination failed to induce a long-lived nasal IgA response (28). The different homing potentials of memory B cells elicited after these two immunization strategies are likely to be related to the induction of different homing and chemokine receptors on the surface of these cells. It has been shown that circulating human memory B cells are heterogeneous in their homing profiles and can express a diversity of homing and chemokine receptors, like α4β7, cutaneous lymphocyte antigen, L-selectin, CXCR3, CCR4, and CCR5 (26, 40). Considering these results, it will be of great interest to determine the phenotype of both memory and effector B cells following either mucosal or parenteral influenza virus vaccination. Since both TIV and LAIV effectively induce protective immunity to wild-type influenza virus infection, other cellular subsets, such as long-lived plasma cells and T cells, and not just circulating memory B cells, are likely to play an important role in protection after LAIV immunization. In this regard, it is important to point out that in experiments performed in parallel to the present study to assess the T-cell and NK cell responses, significant differences were observed in the responses elicited by the two vaccines (25). Interestingly, the mean percentage of influenza virus-specific IFN-γ+ CD4 and CD8 T cells increased significantly after LAIV but not TIV in older children. In contrast, no increases in the mean levels of influenza virus-reactive IFN-γ+ T cells and NK cells were observed in adults given either LAIV or TIV. However, although the adults as a group did not respond to vaccination, their T-cell responses were highly variable at the individual level, with a significant increase after vaccination occurring in a subset of adults with lower levels of baseline T-cell reactivity. In younger children, TIV induced a significant increase of influenza virus-reactive T cells and, as with the B-cell response, no T-cell booster effect was detected after the second TIV dose. Further comparisons for a correlation between humoral and cellular immune response will be done in subsequent studies.
In conclusion, we detected significant differences in the B-cell and antibody responses elicited after LAIV or TIV immunization. Each vaccine induced significant effector B-cell responses 7 to 12 days postimmunization that were detected more frequently than HAI or neutralization responses, especially in adult LAIV recipients. In contrast, TIV, but not LAIV, increased the percentage of circulating memory B cells 1 month after vaccination. Future experiments will be aimed at extending these findings to vaccine-naïve children and adults, exploring their functional consequences, particularly as related to the different mechanisms involved in generating protective immunity by the two vaccines, and at comparing these results to the immune response occurring after natural influenza virus infection in both adults and children.
Acknowledgments
We specially thank Pam Stepick Biek and Kira Y. Dionis for their help in the PBMC isolations and Nancy Bouvier, Judy Hallagan, and Karen Webb, for enrolling subjects and drawing the blood samples.
This work was supported by NIH grants AI057229 and DK56339 and by grant 5M01RR00070 from the National Center for Research Resources, National Institutes of Health.
H. B. Greenberg and A. M. Arvin are paid consultants of Medimmune Vaccines, the manufacturer of the live attenuated influenza vaccine used in this study.
Footnotes
Published ahead of print on 18 October 2006.
REFERENCES
- 1.Agresti, A. 1992. A survey of exact inference for contingency tables. Statistical Sci. 7:131-177. [Google Scholar]
- 2.Belshe, R. B., and W. C. Gruber. 2000. Prevention of otitis media in children with live attenuated influenza vaccine given intranasally. Pediatr. Infect. Dis. J. 19:S66-S71. [DOI] [PubMed] [Google Scholar]
- 3.Belshe, R. B., W. C. Gruber, P. M. Mendelman, H. B. Mehta, K. Mahmood, K. Reisinger, J. Treanor, K. Zangwill, F. G. Hayden, D. I. Bernstein, K. Kotloff, J. King, P. A. Piedra, S. L. Block, L. Yan, and M. Wolff. 2000. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J. Infect. Dis. 181:1133-1137. [DOI] [PubMed] [Google Scholar]
- 4.Belshe, R. B., P. M. Mendelman, J. Treanor, J. King, W. C. Gruber, P. Piedra, D. I. Bernstein, F. G. Hayden, K. Kotloff, K. Zangwill, D. Iacuzio, and M. Wolff. 1998. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine in children. N. Engl. J. Med. 338:1405-1412. [DOI] [PubMed] [Google Scholar]
- 5.Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199-2202. [DOI] [PubMed] [Google Scholar]
- 6.Beyer, W. E., A. M. Palache, M. Baljet, and N. Masurel. 1989. Antibody induction by influenza vaccines in the elderly: a review of the literature. Vaccine 7:385-394. [DOI] [PubMed] [Google Scholar]
- 7.Beyer, W. E., A. M. Palache, J. C. de Jong, and A. D. Osterhaus. 2002. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine 20:1340-1353. [DOI] [PubMed] [Google Scholar]
- 8.Block, S. L. 2004. Role of influenza vaccine for healthy children in the US. Paediatr. Drugs 6:199-209. [DOI] [PubMed] [Google Scholar]
- 9.Brokstad, K. A., J. C. Eriksson, R. J. Cox, T. Tynning, J. Olofsson, R. Jonsson, and A. Davidsson. 2002. Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosa. J. Infect. Dis. 185:878-884. [DOI] [PubMed] [Google Scholar]
- 10.Buonagurio, D. A., R. E. O'Neill, L. Shutyak, G. A. D'Arco, T. M. Bechert, Y. Kazachkov, H. P. Wang, J. DeStefano, K. L. Coelingh, M. August, C. L. Parks, T. J. Zamb, M. S. Sidhu, and S. A. Udem. 2006. Genetic and phenotypic stability of cold-adapted influenza viruses in a trivalent vaccine administered to children in a day care setting. Virology 347:296-306. [DOI] [PubMed] [Google Scholar]
- 11.Callard, R. E. 1979. Specific in vitro antibody response to influenza virus by human blood lymphocytes. Nature 282:734-736. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 1998. The 1998-99 WHO influenza reagent kit for the identification of influenza isolates. Centers for Disease Control and Prevention, Atlanta, Ga.
- 13.Clements, M. L., R. F. Betts, E. L. Tierney, and B. R. Murphy. 1986. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J. Clin. Microbiol. 24:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couch, R. B. 2003. An overview of serum antibody responses to influenza virus antigens. Dev. Biol. (Basel) 115:25-30. [PubMed] [Google Scholar]
- 15.Cox, R. J., K. A. Brokstad, M. A. Zuckerman, J. M. Wood, L. R. Haaheim, and J. S. Oxford. 1994. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine 12:993-999. [DOI] [PubMed] [Google Scholar]
- 16.Crotty, S., and R. Ahmed. 2004. Immunological memory in humans. Semin. Immunol. 16:197-203. [DOI] [PubMed] [Google Scholar]
- 17.Crotty, S., R. D. Aubert, J. Glidewell, and R. Ahmed. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286:111-122. [DOI] [PubMed] [Google Scholar]
- 18.Daniel, W. 1990. Applied nonparametric statistics. PWS-Kent Publishing Company, Boston, Mass.
- 19.Diggle, P. J., K.-Y. Liang, and S. L. Zeger. 1994. Analysis of longitudinal data. Clarendon Press, Oxford, United Kingdom.
- 20.Edwards, K. M., W. D. Dupont, M. K. Westrich, W. D. Plummer, Jr., P. S. Palmer, and P. F. Wright. 1994. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J. Infect. Dis. 169:68-76. [DOI] [PubMed] [Google Scholar]
- 21.el-Madhun, A. S., R. J. Cox, A. Soreide, J. Olofsson, and L. R. Haaheim. 1998. Systemic and mucosal immune responses in young children and adults after parenteral influenza vaccination. J. Infect. Dis. 178:933-939. [DOI] [PubMed] [Google Scholar]
- 22.Esposito, S., P. Marchisio, S. Bosis, L. Lambertini, L. Claut, N. Faelli, C. Bianchi, G. L. Colombo, and N. Principi. 2006. Clinical and economic impact of influenza vaccination on healthy children aged 2-5 years. Vaccine 24:629-635. [DOI] [PubMed] [Google Scholar]
- 23.Glezen, W. P. 1996. Emerging infections: pandemic influenza. Epidemiol. Rev. 18:64-76. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin, K., C. Viboud, and L. Simonsen. 2006. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24:1159-1169. [DOI] [PubMed] [Google Scholar]
- 25.He, X.-S., T. H. Holmes, C. Zhang, K. Mahmood, G. W. Kemble, D. B. Lewis, C. L. Dekker, H. B. Greenberg, and A. M. Arvin. 2006. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J. Virol. 80:11756-11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson, C., I. Ahlstedt, S. Furubacka, E. Johnsson, W. W. Agace, and M. Quiding-Jarbrink. 2005. Differential expression of chemokine receptors on human IgA+ and IgG+ B cells. Clin. Exp. Immunol. 141:279-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson, P. R., S. Feldman, J. M. Thompson, J. D. Mahoney, and P. F. Wright. 1986. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J. Infect. Dis. 154:121-127. [DOI] [PubMed] [Google Scholar]
- 28.Johnson, P. R., Jr., S. Feldman, J. M. Thompson, J. D. Mahoney, and P. F. Wright. 1985. Comparison of long-term systemic and secretory antibody responses in children given live, attenuated, or inactivated influenza A vaccine. J. Med. Virol. 17:325-335. [DOI] [PubMed] [Google Scholar]
- 29.Kantele, A., M. Westerholm, J. M. Kantele, P. H. Makela, and E. Savilahti. 1999. Homing potentials of circulating antibody-secreting cells after administration of oral or parenteral protein or polysaccharide vaccine in humans. Vaccine 17:229-236. [DOI] [PubMed] [Google Scholar]
- 30.Lee, M. S., K. Mahmood, L. Adhikary, M. J. August, J. Cordova, I. Cho, G. Kemble, K. Reisinger, R. E. Walker, and P. M. Mendelman. 2004. Measuring antibody responses to a live attenuated influenza vaccine in children. Pediatr. Infect. Dis. J. 23:852-856. [DOI] [PubMed] [Google Scholar]
- 31.Linder, J. A. 2006. Influenza-associated deaths among children. N. Engl. J. Med. 354:1317-1318. [PubMed] [Google Scholar]
- 32.Manz, R. A., A. E. Hauser, F. Hiepe, and A. Radbruch. 2005. Maintenance of serum antibody levels. Annu. Rev. Immunol. 23:367-386. [DOI] [PubMed] [Google Scholar]
- 33.McCullagh, P., and J. A. Nelder. 1989. Generalized linear models, 2nd ed. Chapman & Hall, London, United Kingdom.
- 34.Mendelman, P. M., R. Rappaport, I. Cho, S. Block, W. Gruber, M. August, D. Dawson, J. Cordova, G. Kemble, K. Mahmood, G. Palladino, M. S. Lee, A. Razmpour, J. Stoddard, and B. D. Forrest. 2004. Live attenuated influenza vaccine induces cross-reactive antibody responses in children against an A/Fujian/411/2002-like H3N2 antigenic variant strain. Pediatr. Infect. Dis. J. 23:1053-1055. [DOI] [PubMed] [Google Scholar]
- 35.Moldoveanu, Z., M. L. Clements, S. J. Prince, B. R. Murphy, and J. Mestecky. 1995. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine 13:1006-1012. [DOI] [PubMed] [Google Scholar]
- 36.Moulton, L. H., and N. A. Halsey. 1995. A mixture model with detection limits for regression analyses of antibody response to vaccine. Biometrics 51:1570-1578. [PubMed] [Google Scholar]
- 37.Nichol, K. L., K. P. Mallon, and P. M. Mendelman. 2003. Cost benefit of influenza vaccination in healthy, working adults: an economic analysis based on the results of a clinical trial of trivalent live attenuated influenza virus vaccine. Vaccine 21:2207-2217. [DOI] [PubMed] [Google Scholar]
- 38.Nichol, K. L., P. M. Mendelman, K. P. Mallon, L. A. Jackson, G. J. Gorse, R. B. Belshe, W. P. Glezen, and J. Wittes. 1999. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA 282:137-144. [DOI] [PubMed] [Google Scholar]
- 39.Plotkin, J. B., J. Dushoff, and S. A. Levin. 2002. Hemagglutinin sequence clusters and the antigenic evolution of influenza A virus. Proc. Natl. Acad. Sci. USA 99:6263-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rott, L. S., M. J. Briskin, and E. C. Butcher. 2000. Expression of α4β7 and E-selectin ligand by circulating memory B cells: implications for targeted trafficking to mucosal and systemic sites. J. Leukoc. Biol. 68:807-814. [PubMed] [Google Scholar]
- 41.Sen, A., and M. Srivastava. 1990. Regression analysis: theory, methods and applications. Springer-Verlag, New York, N.Y.
- 42.Szucs, T. D. 1999. Influenza. The role of burden-of-illness research. Pharmacoecon. 16(Suppl. 1):27-32. [DOI] [PubMed] [Google Scholar]
- 43.Thompson, W. W., D. K. Shay, E. Weintraub, L. Brammer, C. B. Bridges, N. J. Cox, and K. Fukuda. 2004. Influenza-associated hospitalizations in the United States. JAMA 292:1333-1340. [DOI] [PubMed] [Google Scholar]
- 44.Thompson, W. W., D. K. Shay, E. Weintraub, L. Brammer, N. Cox, L. J. Anderson, and K. Fukuda. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179-186. [DOI] [PubMed] [Google Scholar]
- 45.Treanor, J. J., F. G. Hayden, P. S. Vrooman, R. Barbarash, R. Bettis, D. Riff, S. Singh, N. Kinnersley, P. Ward, R. G. Mills, et al. 2000. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA 283:1016-1024. [DOI] [PubMed] [Google Scholar]
- 46.Treanor, J. J., K. Kotloff, R. F. Betts, R. Belshe, F. Newman, D. Iacuzio, J. Wittes, and M. Bryant. 1999. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 18:899-906. [DOI] [PubMed] [Google Scholar]
- 47.Yarchoan, R., L. A. Barrow, C. Kurman, W. Strober, and D. L. Nelson. 1985. Human peripheral blood mononuclear cells produce IgA anti-influenza virus antibody in a secondary in vitro antibody response. J. Immunol. 135:1033-1039. [PubMed] [Google Scholar]
- 48.Yarchoan, R., B. R. Murphy, W. Strober, M. L. Clements, and D. L. Nelson. 1981. In vitro production of anti-influenza virus antibody after intranasal inoculation with cold-adapted influenza virus. J. Immunol. 127:1958-1963. [PubMed] [Google Scholar]