Abstract
The relationship between the function of human immunodeficiency virus (HIV)-specific CD8 T-cell responses and viral load has not been defined. In this study, we used a panel of major histocompatibility complex class I tetramers to examine responses to frequently targeted CD8 T-cell epitopes in a large cohort of antiretroviral-therapy-naïve HIV type 1 clade C virus-infected persons in KwaZulu Natal, South Africa. In terms of effector functions of proliferation, cytokine production, and degranulation, only proliferation showed a significant correlation with viral load. This robust inverse relationship provides an important functional correlate of viral control relevant to both vaccine design and evaluation.
Virus-specific CD8 T cells play an essential role in control of chronic viral infections (4, 12). The strong association between HLA class I molecules and protection against disease progression (5, 10), together with data from models of simian immunodeficiency virus (9, 15), suggest that such responses can be protective and support current efforts to design T-cell-based vaccines for human immunodeficiency virus type 1 (HIV-1). However, despite such T-cell responses, the virus is not controlled long term and disease progression in untreated persons is almost inevitable. Understanding the mechanisms behind the failure of CD8 T-cell-based immune containment and how the T-cell quality, function, and specificity correlate with viral load is a crucial step in vaccine design.
To address issues of immune control in HIV infection, we established a cohort of over 600 adults with chronic, untreated clade C HIV infection from KwaZulu Natal, South Africa (10), the epicenter of the global HIV pandemic. Comprehensive screening by a gamma interferon (IFN-γ) Elispot assay using overlapping peptides spanning all expressed viral proteins, together with high-resolution HLA typing, revealed a number of peptides frequently targeted by CD8 T cells in the context of frequently expressed HLA alleles in this cohort (reference 10 and data not shown). From this set we identified eight peptides with strong linkage between the presence of a specific HLA allele and the presence of a CD8 T-cell response to the peptide. Major histocompatibility complex class I tetramers corresponding to highly targeted epitopes restricted by HLA-A*0205, A*3002, B*0801, B*4201, B*8101, and Cw*0304 were therefore constructed to enable direct visualization of antigen-specific cells from freshly isolated peripheral blood mononuclear cells (PBMC) by flow cytometry (Table 1). A total of 113 subjects, selected based on expressed HLA alleles, were evaluated, and a total of eight epitopes examined. The median viral load of the 113 subjects analyzed was 51,700 HIV-1 RNA copies/ml plasma (range, <50 to 784,000), and the median absolute CD4 count was 329 (range, 23 to 1,273). Information regarding duration of infection was not available, although all the patients had established chronic HIV infection at the time of analysis. The median magnitude of each of the epitope-specific responses was 0.75% of CD8+ T cells (range, 0.01 to 10.68%), with the strongest responses being detected to B*4201 TL9 and Cw*0304 YL9 (Table 1 and data not shown).
TABLE 1.
Description of major histocompatibility complex class I tetramers
| Tetramer | Amino acid sequence | HIV-1 protein | No. of patients tested | Median ex vivo tetramer frequencya |
|---|---|---|---|---|
| A*0205 GL9 | GAFDLSFFL | Nef | 11 | 0.48 |
| A*3002 KLY9 | KIQNFRVYY | Integrase | 20 | 0.45 |
| B*0801 DI8 | DIYKRWII | p24 | 27 | 0.55 |
| B*0801 FL8 | FLKEKGGL | Nef | 26 | 0.40 |
| B*4201 RM9 | RPQVPLRPM | Nef | 34 | 0.22 |
| B*4201 TL9 | TPQDLNTML | p24 | 38 | 2.23 |
| B*8101 TL9 | TPQDLNTML | p24 | 15 | 1.00 |
| Cw*0304 YL9 | YVDRFFKTL | p24 | 24 | 2.26 |
Percentage of CD8+ tetramer+ cells.
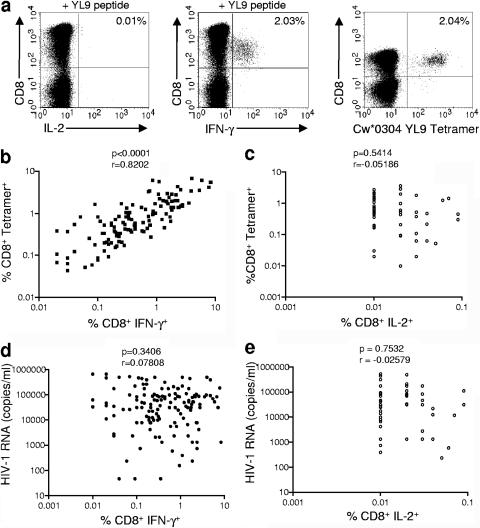
There have been conflicting reports from previous studies in terms of whether HIV induces cytotoxic T lymphocytes that are dysfunctional in their ability to produce cytokines upon in vitro stimulation with peptides (1, 7, 8, 11, 16). By using tetramer staining in conjunction with intracellular cytokine staining for multiple responses in a large cohort of individuals, we were able to address what proportion of HIV-specific CD8 T cells are able to make IFN-γ or interleukin-2 (IL-2) in response to stimulation with cognate antigen. Representative data are indicated in Fig. 1a. We found a strong correlation between the frequency of CD8+ tetramer+ cells and the frequency of CD8+ IFN-γ+ cells following a 6-hour stimulation with peptide (r = 0.8202, P < 0.0001; Fig. 1b). In contrast, CD8 T cells made very little if any detectable IL-2 in the same assay (range, 0.00 to 0.09% of CD8 T cells), and no correlation was observed between the frequency of CD8+ tetramer+ cells and the frequency of CD8+ IL-2+ cells (Fig. 1c). We analyzed the relationship between IFN-γ and IL-2 production by HIV-specific CD8+ T cells and viral load and found no significant correlations (Fig. 1d and e). Furthermore, no significant correlation was observed between the fraction of tetramer+ cells that produce IFN-γ and viral load (P = 0.4011; data not shown).
FIG. 1.
Frequency of IFN-γ- and IL-2-producing HIV-specific CD8+ T cells is not associated with plasma HIV viral load. (a) Representative intracellular cytokine staining and tetramer staining data from a Cw*0304+ individual with a Cw*0304-restricted YL9 response. Freshly isolated PBMC were stimulated for 6 h with YL9 peptide in the presence of brefeldin A and stained intracellularly with IFN-γ and IL-2 antibodies. PBMC from the same PBMC preparation were stained concurrently with Cw*0304 YL9 tetramer. (b and c) The percentage of CD8+ tetramer+ cells is correlated with the percentage of CD8+ IFN-γ+ cells (b) but not with the percentage of CD8+ IL-2+ cells (c). (d and e) One hundred twenty-five tetramer and intracellular cytokine staining pairs were evaluated for the correlations between tetramer (populations, >0.03% of CD8 T cells) and IFN-γ production and tetramer and IL-2 production. Neither the frequency of CD8+ IFN-γ+ cells (d) nor the frequency of IL-2+ cells (e) correlated with HIV plasma viral load.
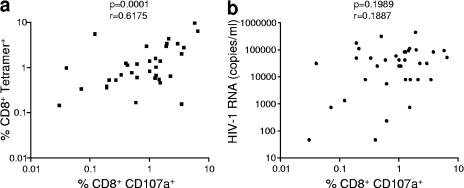
We next addressed the ability of HIV-specific CD8 T cells to degranulate upon stimulation with cognate antigen. PBMC were stimulated with peptide in the presence of anti-CD107a antibodies for 6 h, followed by surface staining with anti-CD8 and anti-CD107a antibodies (3). Tetramer staining was performed in parallel with the CD107a assay on the same samples, and a significant positive correlation was found between the frequency of CD8+ tetramer+ cells and the frequency of CD8+ CD107a+ cells following stimulation with the cognate peptide (P = 0.0001; Fig. 2a). However, when the frequency of CD8+ CD107a+ cells was analyzed in relation to viral load of these subjects, no correlation was observed (Fig. 2b).
FIG. 2.
HIV-specific CD8+ T cells maintain the ability to degranulate upon short-term stimulation with specific peptide, although this is not linked to HIV plasma viral load. (a) A significant correlation exists between the frequency of CD8+ tetramer+ cells and the frequency of CD8+ CD107a+ cells following a 6-hour stimulation with the specific peptide. (b) The frequency of CD8+ CD107a+ cells does not correlate with HIV plasma viral load (P = 0.1989). Forty-six CD107a degranulation assays where the tetramer+ frequency was >0.03% of CD8 T cells were evaluated for the correlations between ex vivo frequency of tetramer+ cells and HIV-1 viral load.
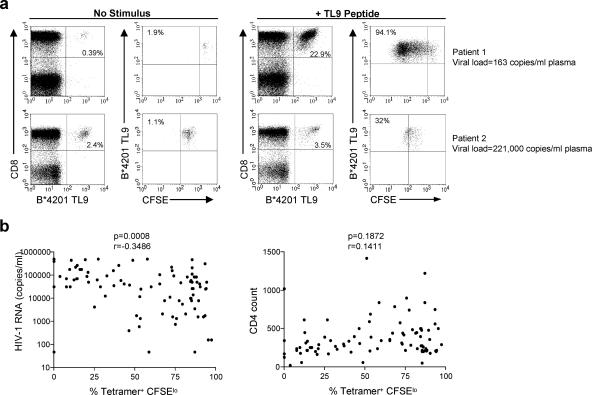
Recent studies indicate that the proliferative capacity of HIV-specific CD8 T cells is gradually lost following acute infection and is thus compromised during chronic infection in individuals with high-level viremia (13, 14, 17). In order to further investigate the proliferative capacity of CD8 T cells specific for dominant clade C epitopes, we labeled freshly isolated PBMC with 0.5 μM carboxyfluorescein diacetate, succinimidyl ester (CFSE), and stimulated them for 6 days in the presence or absence of 0.2 μg/ml of the specific peptide. Representative staining showing proliferation of TL9-specific CD8+ cells from a B*4201+ individual with low viral load (top panel) and a B*4201+ individual with high viral load (bottom panel) is shown in Fig. 3a. The proliferative response to each of the eight tetramer epitopes described in this study was tested in a total of 89 samples, and we found a strong inverse correlation between the percentage of CD8+ tetramer+ CFSElo cells and the viral load of these subjects (P = 0.0008; Fig. 3b, left panel). Although there were variations between proliferative responses within individual epitopes, when analyzed individually, we did not observe a significant difference in proliferative capacity between responses to the eight different epitopes (analysis of variance, P = 0.2012). Despite the fact that we observed a significant inverse correlation between the proliferative capacity of tetramer+ cells and viral load, we did not see a significant correlation between the absolute CD4 count and proliferative capacity of tetramer+ cells in these subjects (P = 0.1872; Fig. 3b, right panel). Multivariate regression analysis confirmed that the inverse correlation between proliferation and viral load was independent of CD4 count (P = 0.007; data not shown).
FIG. 3.
Proliferative capacity of virus-specific CD8+ T cells is inversely correlated with plasma HIV viral load. (a) Representative CFSE proliferation assay from a B*4201+ individual with low viral load (top panels) and a B*4201+ individual with high viral load (bottom panels). The left two panels indicate the proliferation of B*4201 TL9+ cells after 6 days in the absence of antigenic stimulation, whereas the right two panels indicate the percentage of B*4201 TL9+ cells following a 6-day incubation with TL9 peptide. The percentage in the upper right quadrant of the CD8-versus-tetramer plots indicates the percentage of CD8+ tetramer+ cells. The tetramer-versus-CFSE plots are gated on CD8+ tetramer+ cells, and the percentage in the upper left quadrant indicates the percentage of CFSElo CD8+ tetramer+ cells. (b) The frequency of tetramer+ CFSElo cells following a 6-day stimulation with peptide in vitro correlates inversely with HIV viral load but not with absolute CD4 count (n = 89).
HIV infection is associated with a strong adaptive cellular immune response, and yet the majority of infected persons progress to AIDS in the absence of treatment. In this study, we assessed the impact of T-cell function on viral load in persons with untreated clade C HIV infection from KwaZulu Natal, South Africa, where the seroprevalence of HIV infection is over 35% in some age groups. Using a panel of eight HLA class I tetramers to frequently targeted epitopes in this population, we find that the proliferative capacity of epitope-specific responses may be a correlate of viral control in chronic HIV infection.
Previous studies have addressed similar relationships but have been done in small cohorts and for the most part have not been linked to viral load. To our knowledge this is the largest study of its kind, and the data set is unique in that it is derived from a highly characterized, untreated population of adults with chronic HIV clade C infection. This analysis is not entirely comprehensive, in that not all responses in each individual were assessed. However, the responses analyzed represent the dominant response against HIV in this population.
Functional studies were performed for analysis of IFN-γ and IL-2 secretion, proliferation, and degranulation. IFN-γ secretion and degranulation appeared to be well maintained following a short-term stimulation with peptide, with no evidence of a significant functional defect. In contrast, IL-2 secretion was weak throughout, with little secretion even at relatively low viral loads. The proliferative capacity of epitope-specific CD8+ T cells showed a clear inverse correlation with viral load. These data therefore provide a functional assay of HIV tetramer+ cells that is associated with viral load in a large, untreated, chronically infected cohort. PBMC from HIV-infected individuals with a wide range of viral loads were assayed, and a substantial number of relevant tetramer responses were evaluated. Thus, the result appears robust and biologically significant—analysis of proliferative function using a combined CFSE tetramer assay is technically relatively simple and provides critical information relevant to both vaccine design and evaluation. In particular, these data highlight the importance of assessing the proliferative capacity of HIV-specific T cells in addition to quantifying the number of IFN-γ-producing cells when evaluating the function of vaccine-induced cellular immune responses.
Overall these data provide important support for the analysis of the role of proliferative function of antigen-specific CD8 T cells as a key measure of the exhaustion of T cells in chronic viral infections in general and HIV in particular. The mechanism behind this functional loss is not yet fully established, although recent data on the expression of the inhibitory receptor PD-1 (2, 6) may provide important links between function, phenotype, and disease progression.
Acknowledgments
This work was supported by a Royal Society postdoctoral fellowship (C.L.D.), the Wellcome Trust (P.K. and P.J.R.G.), and the Doris Duke Charitable Foundation (B.D.W.).
We thank Chantal de Pierres, Nompumelelo Mkhwanazi, Eshia S. Moodley, Zenele Mncube, and Sharon Reddy for technical assistance.
Footnotes
Published ahead of print on 18 October 2006.
REFERENCES
- 1.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. [DOI] [PubMed] [Google Scholar]
- 3.Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78. [DOI] [PubMed] [Google Scholar]
- 4.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrington, M., G. W. Nelson, M. P. Martin, T. Kissner, D. Vlahov, J. J. Goedert, R. Kaslow, S. Buchbinder, K. Hoots, and S. J. O'Brien. 1999. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283:1748-1752. [DOI] [PubMed] [Google Scholar]
- 6.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. R. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 7.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 74:10249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goulder, P. J., Y. Tang, C. Brander, M. R. Betts, M. Altfeld, K. Annamalai, A. Trocha, S. He, E. S. Rosenberg, G. Ogg, C. A. O'Callaghan, S. A. Kalams, R. E. McKinney, Jr., K. Mayer, R. A. Koup, S. I. Pelton, S. K. Burchett, K. McIntosh, and B. D. Walker. 2000. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J. Exp. Med. 192:1819-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiepiela, P., A. J. Leslie, I. Honeyborne, D. Ramduth, C. Thobakgale, S. Chetty, P. Rathnavalu, C. Moore, K. J. Pfafferott, L. Hilton, P. Zimbwa, S. Moore, T. Allen, C. Brander, M. M. Addo, M. Altfeld, I. James, S. Mallal, M. Bunce, L. D. Barber, J. Szinger, C. Day, P. Klenerman, J. Mullins, B. Korber, H. M. Coovadia, B. D. Walker, and P. J. Goulder. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432:769-775. [DOI] [PubMed] [Google Scholar]
- 11.Kostense, S., G. S. Ogg, E. H. Manting, G. Gillespie, J. Joling, K. Vandenberghe, E. Z. Veenhof, D. van Baarle, S. Jurriaans, M. R. Klein, and F. Miedema. 2001. High viral burden in the presence of major HIV-specific CD8+ T cell expansions: evidence for impaired CTL effector function. Eur. J. Immunol. 31:677-686. [DOI] [PubMed] [Google Scholar]
- 12.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichterfeld, M., D. E. Kaufmann, X. G. Yu, S. K. Mui, M. M. Addo, M. N. Johnston, D. Cohen, G. K. Robbins, E. Pae, G. Alter, A. Wurcel, D. Stone, E. S. Rosenberg, B. D. Walker, and M. Altfeld. 2004. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J. Exp. Med. 200:701-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 16.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8+ T cells in chronic human immunodeficiency virus infection. Blood 96:3094-3101. [PubMed] [Google Scholar]
- 17.Zimmerli, S. C., A. Harari, C. Cellerai, F. Vallelian, P. A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA 102:7239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]