Abstract
Early clearance of a thymidine kinase-deficient strain of herpes simplex virus type 2 from the female genital tract required T-cell-produced gamma interferon (IFN-γ). Transfer of activated CD8+ T cells to irradiated C57BL/6 mice resulted in rapid virus clearance, but clearance was greatly delayed in recipients deficient in the IFN-γ receptor (IFN-γR). Early virus clearance was demonstrated in radiation chimeras in which IFN-γR expression was limited to parenchymal cells, but resolution was significantly delayed in chimeras deficient in IFN-γR expression and chimeras expressing IFN-γR only on hematopoietic cells. Together, these results suggest that early IFN-γ-mediated protection was manifested mainly by stimulation of genital parenchymal cells.
Resolution of herpes simplex virus (HSV) lesions from epithelial sites of infection is achieved in healthy individuals by cellular immune mechanisms, but this process may be impaired in immunocompromised individuals. Understanding the cellular and molecular events involved in lesion resolution may be important for the development of therapies to decrease the severity of HSV lesions in these individuals. However, the exact mechanisms involved in clearance of HSV-2 from the genital epithelium are not fully understood. CD8+ T cells have been identified as important for clearance of genital herpetic lesions, a process that involves both gamma interferon (IFN-γ) secretion and cytolytic mechanisms (6, 7, 12, 13). IFN-γ is a major mediator of HSV-2 clearance (5, 17, 18, 20, 22, 24), presumably due to the activation of multiple immune cell types and/or initiation of numerous antiviral pathways in somatic cells. However, the cell types responding to IFN-γ in the female genital tract and the antiviral mechanisms relevant to the resolution of HSV-2 lesions are not understood.
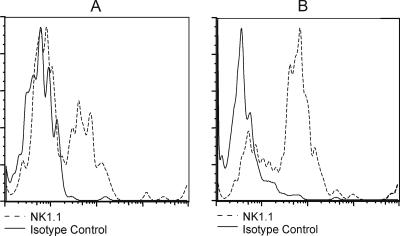
IFN-γ in the genital secretions of fully immunocompetent mice is derived primarily from NK cells and antigen-specific T lymphocytes, with NK-cell-produced IFN-γ peaking at day 2 after intravaginal (ivag) inoculation and T-cell-produced IFN-γ present after day 3 (2, 17). To test if non-T-cell sources of IFN-γ were sufficient for HSV-2 clearance, Rag1-deficient mice (Rag1−/−) genetically deficient in adaptive immune cells but possessing an intact innate immune system, including macrophages, dendritic cells, and NK cells (19), were utilized as recipients of activated wild-type OT-I or IFN-γ-deficient OT-I (OT-I IFN−/−) T cells. Mice were treated with medroxyprogesterone in all experiments to induce susceptibility to genital HSV-2 inoculation (15), most likely reflecting hormonal induction of the HSV entry receptor, nectin-1, on vaginal epithelial cells (14). Rag1−/− mice were injected intravenously (i.v.) with 3 × 106 activated OT-I or OT-I IFN−/− T cells and then challenged ivag with 5 × 103 PFU of an ovalbumin-expressing virus, HSV-2 tk− OVA (7). HSV-2 tk− strains replicate similarly to wild-type HSV-2 in genital epithelial cells but do not replicate well in neurons (15), therefore development of encephalitis and mortality does not occur following ivag inoculation, as is common following inoculation with wild-type HSV-2 (15, 18). The use of a tk− HSV-2 strain in the present study therefore allowed a focused examination of the T-cell-mediated mechanisms of virus clearance from the genital tract. To confirm the presence of NK cells in the genital epithelia of recipient mice, the vaginae and cervices were dissected from groups of uninfected or HSV-2 tk− OVA-infected Rag1−/− mice three days after virus inoculation and mechanically dissociated. The leukocyte fraction was isolated over Histopaque and stained with fluorescein isothiocyanate-anti NK1.1 or isotype control antibodies. A small population of NK1.1+ cells was detected in the genital tracts of uninfected Rag1−/− mice (Fig. 1A); however, this population increased following ivag HSV-2 tk− OVA inoculation (Fig. 1B).
FIG. 1.
Detection of NK 1.1+ cells in the genital tracts of uninfected and HSV-2 tk− OVA-infected Rag1−/− mice. Leukocytes were isolated from the genital tracts of naive (n = 8) (A) or HSV-2 tk− OVA-infected Rag1−/− mice on day 3 after intravaginal inoculation (n = 6) (B) and stained with phycoerythrin-conjugated NK1.1 or isotype control antibodies. Data were acquired on a Becton Dickson FACS Canto flow cytometer at the University of Texas Medical Branch Flow Cytometry Core Facility and analyzed using FlowJo software (Treestar Inc., Ashland, OR). Results from a representative experiment of two performed are shown. NK1.1+ cell numbers were as follows. Naive mice: total vaginal cell count, 2 × 105; 1.2% NK1.1+ cells; total number of NK1.1+ cells, 2,400. HSV-2-inoculated mice: total vaginal cell count, 3 × 105; 4.9% NK1.1+ cells; total number of NK1.1+ cells, 14,700.
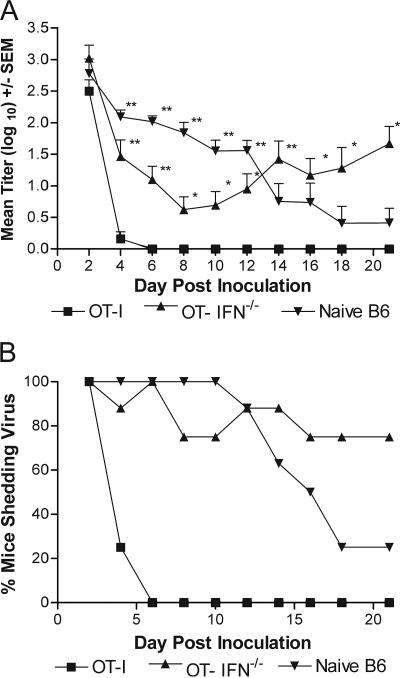
Rag1−/− mice receiving activated wild-type OT-I cells cleared virus by day 6 postchallenge (Fig. 2A). However, activated OT-I IFN-γ−/− T-cell recipients were unable to clear HSV-2 tk− OVA from the genital tract, and 75% (n = 8) of these mice continued to shed virus through day 21 postinoculation (Fig. 2B). These data, together with previous results demonstrating that less IFN-γ is produced by NK cells than with antigen-specific T cells (17), suggest that insufficient NK-cell-produced IFN-γ was available to achieve virus clearance and that T-cell-produced IFN-γ was required for resolution of the genital infection. These results do not preclude the possibility that other proinflammatory cytokines were also involved in HSV-2 clearance such as tumor necrosis factor alpha or interluekin-15 (8, 11, 21).
FIG. 2.
Rapid HSV-2 clearance from the genital epithelium requires T-cell-produced IFN-γ. A. Groups of eight Rag1−/− mice were injected i.v. with 3 × 106 activated OT-I cells, OT-IFN−/− cells, or CD8+ T cells from naive B6 mice (naive B6 CD8+ T cells) and then inoculated ivag with 5 × 103 PFU HSV-2 tk− OVA. Mice were swabbed for virus quantification on the indicated day. Values marked with an asterisk are significantly different compared to same-day values for activated OT-I T-cell recipients (**, P < 0.01; *, P < 0.05; one-way ANOVA). Representative data from one of two experiments performed are shown. B. Percentage of mice from panel A shedding virus on the indicated days calculated as the number of mice with virus present in the vaginal vault divided by the number of mice in each group. SEM, standard error of the mean.
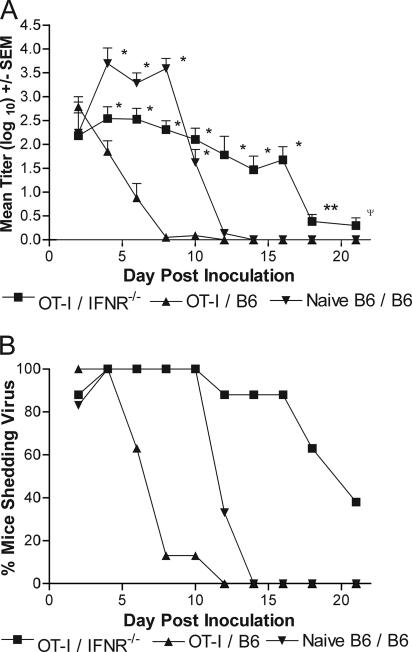
Cantin et al. showed that mice genetically deficient in the IFN-γ receptor (IFN-γR−/−) were significantly more susceptible to ocular challenge with virulent HSV-1 than were wild-type controls (5). Therefore, as a further test for the importance of IFN-γ in virus clearance, IFN-γR−/− mice (10) and C57BL/6 (B6) mice were sublethally irradiated (650 rads), repopulated with activated OT-I T cells, and inoculated ivag with HSV-2 tk− OVA. The majority of B6 mice receiving activated OT-I T cells cleared virus by day 8, and all these recipients cleared by day 10 (Fig. 3A). Virus titers in IFN-γR−/− recipient mice were significantly greater than in B6 OT-I recipients through day 21, although virus titers began dropping by day 18. Eighty-eight percent (n = 8) of the IFN-γR−/− mice were still shedding high titers of virus on day 16, but only 38% were on day 21 (Fig. 3B). Together these results demonstrate a requirement for recipient expression of IFN-γR to achieve rapid virus clearance but suggest an IFN-γR-independent antiviral mechanism may have been acting much later in infection.
FIG. 3.
Tissue expression of the IFN-γR is necessary for early clearance of HSV-2 tk− OVA. A. Groups of eight irradiated B6 or IFN-γR−/− mice were reconstituted i.v. with 3 × 106 activated OT-I or naive B6 CD8+ T cells. The recipient mice were subsequently challenged ivag with 5 × 103 PFU HSV-2 tk− OVA and swabbed on the indicated days. Marked values are significantly different compared to same-day values for OT-I B6 recipients (*, P < 0.001; **, P < 0.004; Ψ, P < 0.05; one-way ANOVA). Representative data from one of two experiments performed are shown. B. Percentage of mice from panel A shedding virus on the indicated days calculated as the number of mice with virus present in the vaginal vault divided by the number of mice in each group. SEM, standard error of the mean.
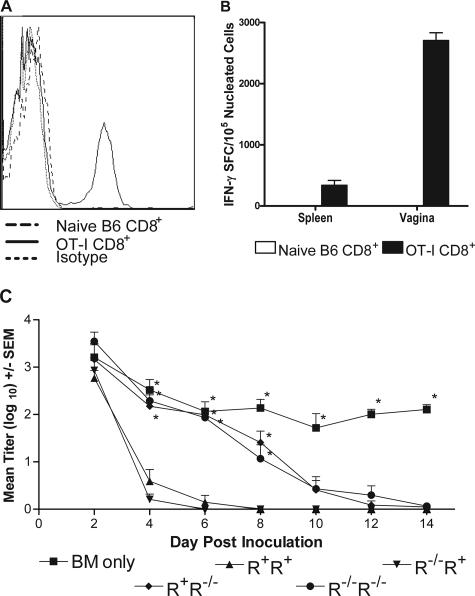
We previously showed that activated OT-I T cells infiltrated the vaginal tracts of HSV-2 tk− OVA-infected, but not uninfected, mice (7). In agreement with our previous results, CD8+ T cells were detected in the vaginae of OT-I recipient mice on day 7 after ivag inoculation with HSV-2 tk− OVA (Fig. 4A). Further, T cells obtained from the vaginae of identically treated mice produced IFN-γ upon culture with mitomycin C-treated syngeneic spleen cells pulsed with the immunogenic OVA peptide, SIINFEKL (Fig. 4B). Thus, IFN-γ-producing OT-I T cells were present at the site of genital infection at a time concurrent with virus clearance.
FIG. 4.
Expression of the IFN-γR on the genital parenchymal cells is important for early resolution of a genital HSV-2 tk− OVA infection. A. Flow cytometric analysis of vaginal cells from irradiated B6 mice reconstituted with naive B6 CD8+ T cells or activated OT-I T cells taken 7 days after inoculation with 5 × 103 PFU HSV-2 tk− OVA. B. Quantification of SIINFEKL peptide-specific T cells in spleen and vaginal cells from irradiated B6 mice receiving either naive B6 CD8+ T cells or activated OT-I T cells. Cells were obtained for analysis on day 7 after inoculation with 5 × 103 PFU HSV-2 tk− OVA. C. Irradiated IFN-γR+ or IFN-γR−/− recipients were reconstituted with 2 × 107 T-cell-depleted IFN-γR+ or IFN-γR−/− bone marrow and splenocytes to form chimeras of at least eight mice per group. Recipients were rested for one week, injected i.v. with 3 × 106 activated OT-I T cells, and challenged with 5 × 103 PFU HSV-2 tk− OVA. Values marked with an asterisk are significantly different compared to same-day values for R+ R+ mice (P < 0.05; one-way ANOVA). Representative data from one of three studies performed are shown. SEM, standard error of the mean; BM, bone marrow; SFC, spot-forming cell.
The IFN-γR is expressed on nearly all nucleated cells (1, 3), and studies have shown that vaginal epithelial cells expressed class II major histocompatibility complex proteins upon exposure to T-cell-produced IFN-γ (20), thereby demonstrating the ability of genital tissue to respond to IFN-γ. Large numbers of neutrophils and monocytes/macrophages have been shown to infiltrate the vaginal epithelium following genital HSV-2 inoculation, and these cells play an as-yet-undefined role in rapid virus clearance (16). To determine if rapid resolution of HSV-2 infection of the genital epithelium correlated with IFN-γ activation of these infiltrating innate cells or of genital parenchymal cells, we constructed IFN-γR chimeras. B6 or IFN-γR−/− mice were lethally irradiated (900 rads) to fully deplete immune cells and then repopulated i.v. with 1.2 × 107 T-cell-depleted bone marrow and spleen cells from B6 or IFN-γR−/− donors. T-cell depletion was confirmed by flow cytometric analysis of donor cell preparations (G. N. Milligan, unpublished results). The resulting chimeras expressed the IFN-γR on all cells (R+ R+), on the parenchymal cells only (R−/− R+), on hematopoietic immune cells only (R+ R−/−), or they lacked the receptor on both cell types (R−/− R−/−). The mice were rested for one week, injected i.v. with 3 × 106 activated OT-I CD8+ T cells, and challenged ivag with 5 × 103 PFU HSV-2 tk− OVA virus. As shown in Fig. 4C, R+ R+ and R−/− R+ mice cleared virus by day 8 compared to R+ R−/− and R−/− R−/− chimeras, which were unable to clear virus until day 14. Mice receiving T-cell-depleted hematopoietic cells only were unable to clear virus and were shedding significantly higher titers of virus on day 14 (P < 0.05; analysis of variance [ANOVA]). These data suggest rapid clearance of HSV-2 is correlated with IFN-γR expression on genital parenchymal cells. These results are consistent with a recent report that clearance of lymphocytic choriomeningitis virus was dependent on parenchymal cell expression of the IFN-γR (9).
The delayed clearance in R−/− R−/− chimeras (Fig. 4C) and IFN-γR−/− recipients (Fig. 3A) suggests the presence of a less-efficient, IFN-γR-independent antiviral mechanism, distinct from the IFN-γR-dependent mechanism responsible for early resolution of infection. This late-acting mechanism apparently did not require IFN-γR expression on either transferred hematopoietic cells or on the genital parenchymal cells and may have involved enhanced production of cytokines such as IFN-α/β, tumor necrosis factor alpha, or interleukin-15 which are thought to be involved in controlling HSV infections (4, 8, 11, 21). Because the transfer of accessory cells alone did not mediate clearance (Fig. 4), the process also involved the activity of T lymphocytes. The difference in clearance kinetics between IFN-γR−/− recipient mice (Fig. 3) and IFN-γR−/− R−/− chimeras (Fig. 4C) most likely reflects differences in experimental design. The IFN-γR−/− recipients (Fig. 3) were irradiated and reconstituted only with activated OT-I T cells immediately before virus inoculation, whereas radiation chimeras received T-cell-depleted accessory cells from bone marrow and spleen one week before T-cell reconstitution and virus inoculation. It is possible that the irradiation and cell reconstitution regimen used to construct chimeras may have resulted in expansion of critical accessory cell populations involved in important interactions with T cells or responsible for production of higher amounts of antiviral cytokines or other effector molecules relative to the IFN-γR−/− recipients described in Fig. 3.
Although alternative IFN-γ-independent mechanisms may ultimately result in a delayed resolution of the genital tract infection, early resolution of the infection required interaction of IFN-γ with the genital parenchymal cells and not the recruited hematopoietic immune cells present in the HSV-infected vagina. IFN-γ may therefore be necessary for promoting an antiviral state in the genital epithelial cells, possibly by initiating antiviral gene cascades or through increasing the expression of molecules necessary for enhancing antigen processing and presentation which in turn may promote recognition and cytolysis of HSV-infected cells.
Acknowledgments
We thank Nigel Bourne and Mark Estes for critical reading of the manuscript and Mark Griffin of the University of Texas Medical Branch Flow Cytometry Core Facility for assistance with flow cytometry.
This work was supported by research grants AI42815 and AI054444 from the National Institutes of Health. M. D. Bird was supported by a predoctoral fellowship from The James W. McLaughlin Fellowship Fund.
The authors have no conflicting financial interests.
Footnotes
Published ahead of print on 25 October 2006.
REFERENCES
- 1.Aguet, M., Z. Dembic, and G. Merlin. 1988. Molecular cloning and expression of the human interferon-gamma receptor. Cell 55:273-280. [DOI] [PubMed] [Google Scholar]
- 2.Ashkar, A. A., and K. L. Rosenthal. 2003. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77:10168-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach, E. A., M. Aguet, and R. D. Schrieber. 1997. The IFN-gamma receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 15:563-591. [DOI] [PubMed] [Google Scholar]
- 4.Biron, C. A. 2001. Interferons alpha and beta as immune regulators—a new look. Immunity 14:661-664. [DOI] [PubMed] [Google Scholar]
- 5.Cantin, E., B. Tanamachi, H. Openshaw, J. Mann, and K. Clarke. 1999. Gamma interferon (IFN-γ) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFN-γ ligand null-mutant mice. J. Virol. 73:5196-5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham, A. L., R. R. Turner, A. C. Miller, M. F. Para, and T. C. Merigan. 1985. Evolution of recurrent herpes simplex lesions. An immunohistologic study. J. Clin. Investig. 75:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobbs, M. E., J. E. Strasser, C. F. Chu, C. Chalk, and G. N. Milligan. 2005. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J. Virol. 79:14546-14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill, N., K. L. Rosenthal, and A. A. Ashkar. 2005. NK and NKT cell-independent contribution of interleukin-15 to innate protection against mucosal viral infection. J. Virol. 79:4470-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henrichsen, P., C. Bartholdy, J. P. Christensen, and A. R. Thomsen. 2005. Impaired virus control and severe CD8+ T-cell-mediated immunopathology in chimeric mice deficient in gamma interferon receptor expression on both parenchymal and hematopoietic cells. J. Virol. 79:10073-10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 11.Ito, M., and J. A. O-Malley. 1987. Antiviral effects of recombinant human tumor necrosis factor. Lymphokine Res. 6:309-318. [PubMed] [Google Scholar]
- 12.Koelle, D. M., H. Abbo, A. Peck, K. Ziegweid, and L. Corey. 1994. Direct recovery of HSV-specific T lymphocyte clones from human recurrent HSV-2 lesions. J. Infect. Dis. 169:956-961. [DOI] [PubMed] [Google Scholar]
- 13.Koelle, D. M., C. M. Posavad, G. R. Barnum, M. L. Johnson, J. M. Frank, and L. Corey. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Investig. 101:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linehan, M. M., S. Richman, C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and A. Iwasaki. 2004. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. J. Virol. 78:2530-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott, M. R., J. R. Smiley, P. Leslie, J. Brais, H. E. Rudzroga, and J. Bienenstock. 1984. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J. Virol. 51:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milligan, G. N. 1999. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J. Virol. 73:6380-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milligan, G. N., and D. I. Bernstein. 1997. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259-268. [DOI] [PubMed] [Google Scholar]
- 18.Milligan, G. N., D. I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093-60100. [PubMed] [Google Scholar]
- 19.Mombaerts, P., J. Iacomini, R. S. Johnson, K. Herrup, S. Tonegawa, and V. E. Papaioannou. 1992. RAG-1 deficient mice have no mature B and T lymphocytes. Cell 68:869-877. [DOI] [PubMed] [Google Scholar]
- 20.Parr, M. B., and E. L. Parr. 1999. The role of gamma interferon in resistance to vaginal infection by herpes simplex type 2 in mice. Virology 258:282-294. [DOI] [PubMed] [Google Scholar]
- 21.Rossol-Voth, R., S. Rossol, K. H. Schutt, W. de Cian, and D. Falke. 1991. In vivo protective effect of tumor necrosis factor alpha against experimental infection with herpes simplex virus type 1. J. Gen. Virol. 72:143-147. [DOI] [PubMed] [Google Scholar]
- 22.Smith, P. M., R. M. Wolcott, R. Chervenak, and S. R. Jennings. 1994. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-gamma). Virology 202:76-88. [DOI] [PubMed] [Google Scholar]
- 23.Reference deleted.
- 24.Yu, Z., E. Manickan, and B. T. Rouse. 1996. Role of interferon-γ in immunity to herpes simplex virus. J. Leukoc. Biol. 60:528-532. [DOI] [PubMed] [Google Scholar]